One-pot synthesis of ordered nanoporous amorphous H-Zn-aluminosilicate for catalysis of bulky molecules†
Jitendra
Diwakar
ab,
Nagabhatla
Viswanadham
 *ab,
Saurabh
Kumar
ab,
Adarsh
Kumar
ac and
Sandeep K.
Saxena
b
*ab,
Saurabh
Kumar
ab,
Adarsh
Kumar
ac and
Sandeep K.
Saxena
b
aAcademy of Scientific and Innovative Research (AcSIR) at CSIR-Indian Institute of Petroleum, Dehradun-248005, Uttarakhand, India
bConversions & Catalysis Processes Division, Indian Institute of Petroleum, Council of Scientific and Industrial Research, Dehradun-248005, India. E-mail: nvish@iip.res.in; Fax: +91-135-2525702; Tel: +91-135-2525856
cBiofuel Division, Indian Institute of Petroleum, Council of Scientific and Industrial Research, Dehradun-248005, India
First published on 5th June 2018
Abstract
We are reporting a simple one-pot synthesis method of nanoporous H-zinc-aluminosilicate (HZnAlSi) from a Na-free chemical mixture. The material exhibits strong acidity required to catalyse bulky molecular reactions generally restricted in micro-porous zeolites. The material also exhibits superior catalytic properties giving as high as 99% glycerol conversion in glycerol acetylation, with stability and reusability.
In the present scenario which calls for cleaner fuel and chemicals, the demand for green processes is increasing for industrial applications.1 For catalytic processes, the trend is to switch over from liquid to solid acid catalysed processes that have the advantages of ease of catalyst separation, ease of catalyst handling, being economical and avoiding additional process steps involved in product separation.2 Aluminosilicates are one such material that have potential catalytic applications by virtue of their having valence differences to create the acid sites. Generally, an aluminosilicate (AlSi) is an inorganic polymer which consists of aluminium and silicon along with oxygen atoms. They are found in the earth’s crust in many forms such as zeolite, kaolinite, clay etc.3 Due to their natural abundance and stability, aluminosilicates have attracted much attention for their wide application, especially in the area of catalysis for decades.4–6 The advantage lies in the flexible and tunable catalytic properties that include acidity, surface area, pore size etc. during pre- as well as post-synthesis processes.7,8 This material has a further advantage of being suitable for loading a variety of metal atoms for performing acid, redox and base catalytic reactions.9,10 Functionalization of AlSi with Zn was observed to improve the catalytic properties by modifying the acidity and providing additional dehydrogenation properties.11 Zinc functionalized zeolites such as Zn/ZSM-5 have been successfully applied for hydrocarbon conversion, but the narrow micropores limit the bulky catalytic reactions. Hence synthesizing ZnAlSi with larger pores is an interesting approach. The nanoporous aluminosilicates are generally synthesized by using bulky organic templates such as micelles, polymers and macro molecular adducts. Triethanolamine (TEA),12 polystyrene,13 citric acid,14 and CTAB alone or in combination with a beta zeolite precursor15 have been successfully employed to synthesize nanoporous aluminosilicates. However, these methods involve the use of costly templates and cumbersome procedures, and simple and effective methods for the synthesis of nanoporous aluminosilicates are in demand. Achieving stable zinc aluminosilicates is another challenge as the synthesis of zinc aluminosilicate (ZnAlSi) having all the zinc in its integral framework is not easy since Zn is too reactive to form zinc oxide (ZnO) on the surface of AlSi.16,17 In order to have ZnO-free ZnAlSi for catalytic applications, the focus is on the synthesis of such materials along with the creation of nanopores to facilitate bulky molecular reactions such as those with bulky fossil and bio-derived feedstocks for the production of fuels and fine chemicals. The main objective of the present study is to achieve direct synthesis of H–Zn–aluminosilicates possessing nanopores from a simple single step method so as to avoid additional treatments with ammonia followed by calcination that may cause instability to the Zn in the structure.
Glycerol is one of the low-value bio-derived feeds available for value addition. Recently biodiesel production and its demand have increased significantly, which leads to the formation of a large number of by-products, especially glycerol, in the biodiesel industry.18,19 According to the OECD report, the global production of biodiesel is estimated to reach 42 billion liters by 2020, from which 10 wt% of glycerol will be formed.20 So glycerol is an easily available low-cost feedstock that can be used for the synthesis of a variety of value-added chemicals.21 Glycerol can be converted into acetals, ketals, esters, and carbonates, which have huge applications as fuel additives, solvents and many others.22 Acetylation of glycerol using appropriate acylation agents has gained attention due to the formation of important chemicals i.e. monoacetin (MA), diacetin (DA) and triacetin (TA).23 TA and DA have wide application in the field of petrochemicals, and they can be used as bio-additives for gasoline and biodiesel, which improves properties such as cetane number, anti-knocking, anti-freezing and viscosity and also lowers the NO emission, whereas MA and DA are used as raw materials for biodegradable polyester formation and all acetylation products also have wide application in the food, cosmetic, and pharmaceutical industries.24,25
The aim of the present study is to develop a promising catalyst that can effectively convert glycerol into industrially important products. The acetalization products of glycerol, called acetals, require acidity and space availability in the micro-environment of the catalyst for reaction. Zeolites, a class of crystalline aluminosilicates, have been proven as well known industrial catalysts, but the narrow micropores limit their applications to the present reaction. Hence we aimed our study at amorphous aluminosilicates and Zn-aluminosilicates possessing larger nanopores in the present work. Here we synthesized nanoporous HAlSi and HZnAlSi by a simple one-step method (1.1 Catalyst preparation, ESI†) to obtain an acidic, framework-interacting Zn containing nanoporous catalyst suitable for the processing of bio-originated bulky molecule glycerol. The direct Na-free synthesis of the material provides a greener way of catalyst preparation by having an advantage of obtaining the proton form of the catalyst that does not require any additional NH4-exchange and decomposition treatments to evolve ammonia gas. Green aspects are also involved in the process selection where the acetylation of glycerol has been chosen to produce industrially important and bio-degradable di- and tri-acetin molecules whose formation is expected to increase in the larger nanopores of the present catalyst system.26 The presence of Zn is anticipated to alter the acidity and porosity properties of the catalyst.
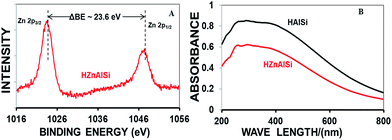
The composition of the synthesized samples was analysed by SEM EDX (Fig. S1, ESI†) and ICP-MS (Table S2 ESI†). The results confirmed the presence of Al and Si in HAlSi and Al, Si and Zn in the HZnAlSi catalyst samples respectively. Further, the HZnAlSi sample was analysed by XPS (Fig. S2 ESI†) and UV spectroscopy. In XPS the appearance of two peaks at 1024 eV and 1048 eV confirmed the +2 oxidation state of zinc in the sample (Fig. 1A),27 whereas in the UV spectra HZnAlSi did not show the adsorption band at 360 nm related to ZnO revealing the absence of the oxide form of Zn in the sample (Fig. 1B).16,17 Here the UV adsorption patterns were observed to be similar for both HAlSi and HZnAlSi samples. This gives indirect evidence to believe that all the Zn present in HZnAlSi chemically interacted with Al and Si and did not exist as ZnO. The presence of zinc metal in the chemical interaction with Si and Al was further confirmed by EDS mapping (Fig. S3 ESI†). This aspect is further confirmed by HRTEM.
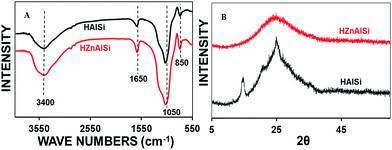
The structural elucidation of the materials was conducted by FTIR and Raman spectroscopy methods (Fig. S5 ESI†). The FTIR spectra of both the samples (Fig. 2A) show a broad band centered around 3400 cm−1 representing the O–H stretching.28,29 The band at 850 cm−1 is due to –Si–OH bending, while that of 1050 cm−1 is related to the Si–O–Al stretching vibration. A low-intensity band at around 1650 cm−1 related to the hydration of the samples is also observed in both the spectra. Overall, the FTIR spectra of the samples indicate the formation of the alumina-silicate material.
In wide angle XRD the materials show no definite crystalline nature: while HZnAlSi shows an amorphous nature, HAlSi shows some bands at about 15–25 2θ, indicating its semi-crystalline nature. The difference in the wide angle patterns of the two materials may be due to the presence of Zn and its participation in the chemical structure in HZnAlSi during the hydrothermal synthesis (Fig. 2B).
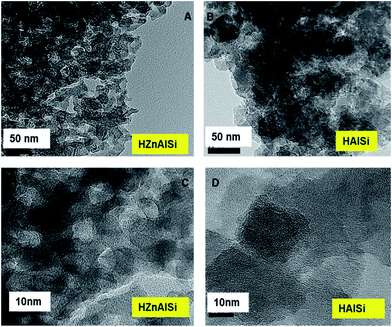
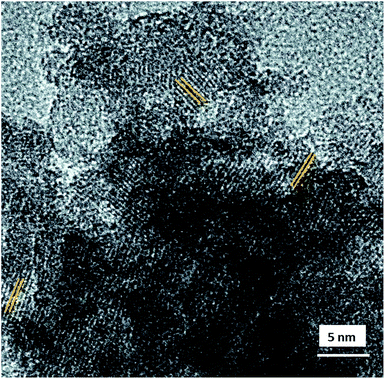
The morphology and internal structure of the material was analysed using SEM, TEM and HRTEM images. SEM analysis of both materials showed a well defined morphology (Fig. S6 ESI†), and TEM images showed that HZnAlSi has a well defined uniform particle nature, while the corresponding HAlSi showed a layer type morphology (Fig. 3A and 4B). In the HRTEM of HZnAlSi the presence of ∼50 nm size particles with a spherical morphology can be clearly seen (Fig. 3C). The HRTEM also confirms the presence of lattice springs related to zinc metal which is also support the presence of zinc metal in the chemical interaction with Si and Al (Fig. 4).
The material also exhibited a porous morphology in HRTEM, whereas in HAlSi, the pores were not visible even at such a high resolution, perhaps due to the formation of the multilayered structure that suppresses the visibility of such pores (Fig. 3D). However, the nanoporous nature of both the samples was reflected in the low angle XRD patterns of the samples, where both HAlSi and HZnAlSi materials showed a sharp single diffraction band at 0.5 2θ, representing the nanoporous nature of these materials (Fig. S7 ESI†).
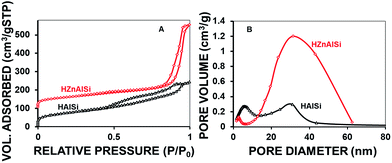
Detailed studies on the porous nature of these materials were conducted using N2 adsorption–desorption isotherms (Fig. 5). The type IV isotherm exhibited by both samples clearly indicates the presence of nanosized pores in the materials. For HAlSi, the adsorption of pores started at P/P0 of ∼0.5 with a distinct loop formation that shows the filling of pores up to the P/P0 of 1, representing the distribution of pores with a range of pore sizes. Unlike this, the HZnAlSi showed a very sharp adsorption loop between 0.8 and 1.0P/P0, representing the presence of almost uniformly sized nanopores (Fig. 5A). The isotherm also gives an indication that the chemical structure of HZnAlSi is more ordered and organized to create uniform pores. This again supports the participation of Zn in the chemical structure of HZnAlSi through chemical bonding.
This aspect is further analysed using BJH pore size distribution patterns. As shown in Fig. 5B, the pores are distributed from 5 nm to 40 nm sizes in HAlSi with two distinct populations at ∼5 nm and 30 nm. For the corresponding HZnAlSi an increased nanopore volume with a huge peak at ∼40 nm was observed. This observation suggests that not only are the pores uniformly sized but also the increased pore volume is created in the material by the presence of Zn. Accordingly, the total pore volume of HZnAlSi is observed to be 0.70 cm3 g−1, which is almost double that of HAlSi (0.37 cm3 g−1) (Table S3 ESI†). The pore volume distribution data in the table also indicate that the micropore volumes of both the samples are comparable and the significant increase in pore volume observed in HZnAlSi is completely due to the nanopores. Overall the TEM and N2-sorption analysis revealed that both the materials have a porous structure but HZnAlSi has a morphology and porosity different from HAlSi, which can be ascribed to the molecular level interaction of Zn with Si and Al in the initial synthesis gel used during the hydrothermal treatment.
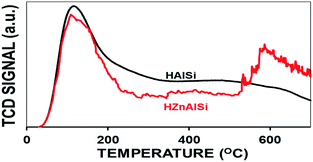
The acidity of the materials was determined by temperature programmed desorption (TPD) of ammonia (Fig. 6). HAlSi exhibited two distinct desorption peaks at 150 °C and 550 °C related to weak and strong acidity, respectively. In the case of HZnAlSi, the strong acidity peak was not distinct but displayed a continuous desorption from 300 °C to 550 °C related to medium to strong acidity. Since both HZnAlSi and HAlSi have comparable amounts of Si and Al, the difference in acidity, especially the acid sites of medium strength created in HZnAlSi, can be ascribed to the presence of chemically interacting Zn in this sample. Overall, both the materials exhibited comparable total acidity values, but there are more strong acid sites in the HAlSi sample (Table S2 ESI†).
The synthesized HAlSi and HZnAlSi materials also show good thermal stability, as confirmed by TG/DTA analysis (Fig. S8 ESI†). In TGA the weight loss of around 4 wt% that occurred below 200 °C with the corresponding endothermic peak in DTA reveals the removal of moisture from the materials. Above this temperature, no considerable weight loss or exothermic peak was observed up to the studied temperature of 600 °C, which confirms the suitability of the synthesized materials for high temperature catalytic applications. It is interesting to see that zinc incorporation does not affect the thermal stability of the material as HZnAlSi exhibited similar DT/TGA patterns to those of HAlSi.
Glycerol acetalization is an esterification reaction where the three hydroxyl groups in the glycerol act as three alcohol moieties to individually react with three acetic acid molecules to form a tri-ester of glycerol. As shown in Scheme S1 (ESI†), the acid sites on the catalyst can facilitate the reaction between the –OH group of glycerol and acetic acid to undergo dehydration to form an ester group.30 Unlike a simple alcohol, the tri-hydroxy molecule glycerol makes the reaction molecularly bulky, which requires a larger free-space environment around the active sites, i.e. acid sites. The acetylation reaction is an acid catalysed reaction but the acidity should be optimum so as to control the undesired de-acetylation reactions that lower the selectivity to bulky products such as di- and tri-acetin.31 Finally, the bulky molecular acetylation reactions are thermodynamically favoured at lower temperatures and thus the catalyst materials should possess low-temperature activity to produce the desired product. Overall, the reaction requires moderate acidity and reaction space on the catalyst to favour bulky acetal products. The synthesized materials of the present study exhibiting larger nanopores and higher acidity have been applied for the glycerol acetylation reaction that is carried out in a liquid phase batch reactor, where the reaction temperatures are chosen between 100 and 150 °C.32
The studies indeed indicated the promising catalytic functionality of the synthesized materials. Data given in Table 1 indicate that both HAlSi and HZnAlSi are suitable for the effective production of acetins from glycerol. At the studied reaction temperatures of >100 °C, the catalysts exhibited more than 95% conversion. At lower reaction temperatures the glycerol conversion is low. On the other hand, higher reaction temperatures (>120 °C) also caused a decrease in the selectivity towards bulky products DA and TA. A common phenomenon observed is that a low temperature favoured the formation of bulky products i.e. DA and TA on both the catalysts. This may be due to the thermodynamically favourable branch formation at lower temperatures. At higher temperatures, one of the reactants, acetic acid, exists in the vapour phase while the other reactant glycerol is in the liquid phase that may limit the reactivity towards acetin formation. Another important factor is the equilibrium shift from the acetylation to de-acetylation reactions favoured at higher temperatures. The optimum reaction temperature observed for the catalyst of the present study seems to be 110 °C to produce the desired bulky acetylated products.
| S. no. | Catalyst | Temp (°C) | Ratios (glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH) AcOH) |
Ratios (feed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst) catalyst) |
Conversion of glycerol (%) | Product yield (wt%) | ||
|---|---|---|---|---|---|---|---|---|
| Monoacetin | Diacetin | Triacetin | ||||||
| a Performance of the catalyst after three hours of reaction time. b Regenerated catalyst performance. c After five reaction cycles. | ||||||||
| 1 | HAlSi | 110 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
13.3 | 85.3 | 73.4 | 11.9 | — |
| 2 | " | 100 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
22.16 | 27.3 | 23.4 | 3.9 | — |
| 3 | " | 110 | " | " | 96.4 | 76.4 | 7.9 | 12.0 |
| 4 | " | 120 | " | " | 99.9 | 59.9 | 40.0 | — |
| 5 | " | 150 | " | " | 99.9 | 99.1 | 0.9 | — |
| 6 | " | 110 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
44.33 | 99.9 | 78.0 | 21.9 | — |
| 7a | " | 110 | " | " | 98.4 | 72.8 | 25.6 | — |
| 8b | " | 110 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
22.16 | 99.9 | 76.2 | 23.7 | — |
| 9c | " | 110 | " | " | 99.8 | 65.9 | 33.9 | — |
| 10 | HZnAlSi | 110 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
13.3 | 91.2 | 67.8 | 6.4 | 17.0 |
| 11 | " | 100 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
22.16 | 38.2 | 28.6 | 9.6 | — |
| 12 | " | 110 | " | " | 99.9 | 16.5 | — | 83.4 |
| 13 | " | 120 | " | " | 99.8 | 5.8 | 76.2 | 17.8 |
| 14 | " | 150 | " | " | 85.3 | 79.4 | 5.9 | — |
| 15 | " | 110 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
44.33 | 99.9 | 82.7 | 17.2 | — |
| 16a | " | 110 | " | " | 99.9 | 84.3 | 15.6 | — |
| 17b | " | 110 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
22.16 | 99.9 | 58.3 | — | 41.6 |
| 18c | " | 110 | " | " | 99.9 | 55.7 | — | 44.2 |
Between HAlSi and HZnAlSi, the later one exhibited significant TA formation (as high as 83 wt%, entry 12), while the yield of this particular product is minimal for HAlSi under similar reaction conditions (12 wt%, entry 3). Upon increasing the temperature to 120 °C, the TA yield decreased along with the simultaneous formation of DA on the HZnAlSi catalyst (entry 13). This particular feature of HZnAlSi can be ascribed to the highly porous nature of the HZnAlSi when compared to the corresponding HAlSi. Since the strong acid sites possessing a higher heat of adsorption are expected to enhance the de-acetylation reactions (cracking at the alkyl branch), a medium strength of acidity seems to be useful for improving the bulky DA and TA product selectivity. HZnAlSi possessing a higher number of medium strength acid sites may be responsible for the formation of higher multiple-branched products in the present study. HAlSi also exhibited reasonably high conversions of glycerol but the main product obtained here is less bulky MA. The difference in the product selectivity of the two catalysts can be ascribed to the difference in their acid strength. As can be seen from the TPD patterns, HAlSi exhibits stronger acidity when compared to HZnAlSi. In other words, strong acid sites in HAlSi seem to catalyse the reversible de-acetylation of DA and TA to produce more MA.
Reaction parameters related to the amount of catalyst and the feed ratio (glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH) were also studied. The stoichiometric equation of glycerol acetalization demands a 1
AcOH) were also studied. The stoichiometric equation of glycerol acetalization demands a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mole ratio of glycerol to acetic acid to produce triacetin (TA). Excess acetic acid ratios are also studied in the present work. The conversion and product selectivity patterns obtained at various catalyst amounts and feed ratios indicate the 1
3 mole ratio of glycerol to acetic acid to produce triacetin (TA). Excess acetic acid ratios are also studied in the present work. The conversion and product selectivity patterns obtained at various catalyst amounts and feed ratios indicate the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 feed ratio as the optimum with a catalyst amount of 0.6 g in the present study.33
6 feed ratio as the optimum with a catalyst amount of 0.6 g in the present study.33
Stability of the active sites against leaching was also studied. For this, the spent catalyst was removed from the reaction mixture, washed with ethanol, dried and reused for the fresh run. Data given in entries 8, 9 and 17, 18 clearly indicate the comparable performance of the catalysts and their stable performance in reusability studies. This stability aspect of the catalysts was also checked by the characterization of the spent catalysts. As shown Fig. S9 and S10 ESI† the spent catalyst exhibited a comparable morphology in SEM and TEM. The FTIR spectra indicated the structural stability while the stability of acid sites was confirmed from ammonia TPD experiments. As shown in Fig. S11 and S12 ESI,† the spent catalysts exhibited structure and acidity properties comparable with those of the corresponding fresh ones, confirming the catalyst stability against leaching. The SEM-EDX analysis of the spent catalyst also shows a comparable elemental composition of HZnAlSi (Fig. S13 ESI†).
A comparison of the performance of the present catalysts with those of reported is given in Table S1 (ESI†). The glycerol conversions are almost more than 90% for all the catalysts but the selectivity towards mono-, di- and tri-acetin are different for these catalysts. The selectivity towards bulky tri-acetin is limited for most of the catalysts reported except for A15 (entry 9 in Table S1 ESI†). This may be either due to the space constraints around the active sites or due to the occurrence of reversible de-acetylation reactions. HAlSi of the present study also exhibited an inferior selectivity to the formation of tri-acetin. On the other hand, significant formation of tri-acetin was observed on the corresponding HZnAlSi catalyst of the present study. Since the difference in properties of the two catalysts is mainly in terms of porosity and strong acidity, these factors seem to be responsible for the difference in product selectivity. Acid sites are active sites for both acetylation and deacetylation reactions and hence the presence of moderate acidity may be suitable for favouring the formation of the acetylation product by minimizing the reversible reaction. Between the two catalysts studied in the present work, HZnAlSi possessing more porosity and moderate acidity could produce more bulky acetylation (tri-acetin) products. It is interesting to see that the cumulative yield of DA and TA turned out to be as high as 94 wt% on the HZnAlSi catalyst, which is useful for industrial applications.
Overall, the studies indicated that the formation of TA and DA is favoured by nanoporous HZnAlSi possessing moderate acidity at low reaction temperatures by virtue of favoured reaction equilibrium towards acetylation reaction to produce industrially important (bio-degradable fuel additive) bulky triacetin for green applications.26
Conclusions
In summary, a single-step method was successfully developed for the synthesis of nanoporous HAlSi and HZnAlSi materials from a Na-free gel mixture. The new concept of avoiding the use of NaOH to directly obtain the H-form HZnAlSi has been realized to reduce the additional steps and time involved in the traditional synthesis methods i.e. ion exchange of the Na-form to obtain the NH4-form of the material and its calcination to produce the H-form in the final step. The alkalinity required for establishing the chemical interaction between Si and Al has been achieved by the presence of TMAOH and TPAOH structure-directing agents. The present method is simple and reproducible for the production of thermally stable materials suitable for catalytic applications involving organic transformations. The HZnAlSi synthesized by a simple one-pot Na-free synthesis exhibited excellent textural properties such as large surface area, high pore volume, nanoporosity, moderate acidity, high temperature thermal stability and reusability. Since glycerol is a by-product obtained from bio-fuel production, present catalyst systems have potential application for the value addition of glycerol through the production of important oxygenated compounds acetins. Though there are several glycerol conversion reactions, value addition through acetin production is important as there is no oxygen removal involved in the process. Further, bulky di- and tri-acetin formation facilitated by nanoporous HZnAlSi of the present study is useful for wide industrial application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to the director, IIP, for his encouragement. JD is thankful to CSIR-New Delhi for awarding a junior research fellowship. We are thankful to XRD, IR, and SEM analysis groups at IIP for analysis.Notes and references
- C. J. Clarke, W. Tu, O. Levers, A. Bro and J. P. Hallett, Chem. Rev., 2018, 118, 747 CrossRef PubMed.
- F. Su and Y. Guo, Green Chem., 2014, 16, 2934 RSC.
- C. Ning, M. Xu, D. C. W. Hui, C. S. K. Lin and G. McKay, Chem. Eng. J., 2017, 311, 37 CrossRef.
- P. Djinović, T. Tomše, J. Grdadolnik, Š. Božič, B. Erjavec, M. Zabilskiy and A. Pintar, Catal. Today, 2015, 258, 648 CrossRef.
- M. Zendehdel, M. Ali, H. Behyar and Z. Mortezaei, Microporous Mesoporous Mater., 2018, 266, 83 CrossRef.
- M. Peng, D. Chen, J. Qiu, X. Jiang and C. Zhu, Opt. Mater., 2007, 29, 556 CrossRef.
- E. R. Cooper, C. D. Andrews, P. S. Wheatley, P. B. Webb, P. Wormald and R. E. Morris, Nature, 2004, 430, 1012 CrossRef PubMed.
- D. López, L. J. Giraldo, J. P. Salazar, D. M. Zapata, D. C. Ortega, C. A. Franco and F. B. Cortés, Catalysts, 2017, 7, 319 CrossRef.
- P. Gao, S. Li, X. Bu, S. Dang, Z. Liu, H. Wang, L. Zhong, M. Qiu, C. Yang, J. Cai, W. Wei and Y. Sun, Nat. Chem., 2017, 9, 1019 CrossRef PubMed.
- J. E. Samad, J. Blanchard, C. Sayag, C. Louis and J. R. Regalbuto, J. Catal., 2016, 342, 213 CrossRef.
- N. Musselwhite, K. Na, K. Sabyrov, S. Alayoglu and G. A. Somorjai, J. Am. Chem. Soc., 2015, 137, 10231 CrossRef PubMed.
- J. Zhou, Z. Hua, J. Zhao, Z. Gao, S. Zeng and J. Shi, J. Mater. Chem., 2010, 20, 6764 RSC.
- G. Zhu, S. Qiu, O. Terasaki and Y. Wei, J. Am. Chem. Soc., 2001, 123, 7723 CrossRef PubMed.
- L. Xu, S. Wu, J. Guan, Y. Ma, K. Song, H. Xu, C. Xu, Z. Wang and Q. Kan, Catal. Commun., 2008, 9, 1970 CrossRef.
- Y. Li, J. Shi, Z. Hua, H. Chen, M. Ruan and D. Yan, Nano Lett., 2003, 3, 609 CrossRef.
- S. Sogukkanli, K. Iyoki, S. P. Elangovan, K. Itabashi, N. Koike, M. Takano, Y. Kubota and T. Okubo, Microporous Mesoporous Mater., 2017, 257, 272 CrossRef.
- N. Koike, W. Chaikittisilp, K. Iyoki, Y. Yanaba, T. Yoshikawa, S. P. Elangovan, K. Itabashi and T. Okubo, Dalton Trans., 2017, 46, 10837 RSC.
- N. Bordoloi, R. Narzari, R. S. Chutia, T. Bhaskar and R. Kataki, Bioresour. Technol., 2015, 178, 83 CrossRef PubMed.
- C. H. Lam, A. J. Bloomfield and P. T. Anastas, Green Chem., 2017, 19, 1958 RSC.
- P. U. Okoye, A. Z. Abdullah and B. H. Hameed, Fuel, 2017, 209, 538 CrossRef.
- A. Shafiei, H. Rastegari, H. S. Ghaziaskar and M. Yalpani, Biofuel Res. J., 2017, 13, 565 CrossRef.
- N. J. Venkatesha, Y. S. Bhat and B. S. Jai Prakash, RSC Adv., 2016, 6, 45819 RSC.
- B. O. Dalla Costa, H. P. Decolatti, M. S. Legnoverde and C. A. Querini, Catal. Today, 2017, 289, 222 CrossRef.
- A. Behr, J. Eilting, K. Irawadi, J. Leschinski and F. Lindner, Green Chem., 2008, 10, 13 RSC.
- Y. Sun, J. Hu, S. An, Q. Zhang, Y. Guo, D. Song and Q. Shang, Fuel, 2017, 207, 136 CrossRef.
- X. Liu, H. Ma, Y. Wu, C. Wang, M. Yang and U. Welz-biermann, Green Chem., 2011, 13, 697–701 RSC.
- S. S. Mali, H. Kim, W. Y. Jang, H. S. Park, P. S. Patil and C. K. Hong, ACS Sustainable Chem. Eng., 2013, 1, 1207 CrossRef.
- A. Coloma, F. J. Rodríguez, J. E. Bruna, A. Guarda and M. J. Galotto, J. Chil. Chem. Soc., 2014, 59, 2409 CrossRef.
- M. Bartoszek, R. Eckelt, C. Jäger, H. Kosslick, A. Pawlik and A. Schulz, J. Mater. Sci., 2009, 44, 6629 CrossRef.
- A. Pankajakshan, S. M. Pudi and P. Biswas, Int. J. Chem. Kinet., 2017, 50, 98–111 CrossRef.
- L. Zhou, E. Al-zaini and A. A. Adesina, Fuel, 2013, 103, 617–625 CrossRef.
- M. S. Khayoon, S. Triwahyono, B. H. Hameed and A. A. Jalil, Chem. Eng. J., 2014, 243, 473–484 CrossRef.
- M. Balaraju, P. Nikhitha, K. Jagadeeswaraiah, K. Srilatha, P. S. S. Prasad and N. Lingaiah, Fuel Process. Technol., 2010, 91, 249–253 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, SEM, EDX, TEM, XRD etc. See DOI: 10.1039/c8se00150b |
| This journal is © The Royal Society of Chemistry 2018 |