Production of hydrocarbons from biomass-derived biochar assisted microwave catalytic pyrolysis
Lei
Zhu†
 ,
Yayun
Zhang†
,
Yayun
Zhang†
 ,
Hanwu
Lei
*,
Xuesong
Zhang
,
Lu
Wang
,
Quan
Bu
and
Yi
Wei
,
Hanwu
Lei
*,
Xuesong
Zhang
,
Lu
Wang
,
Quan
Bu
and
Yi
Wei
Department of Biological Systems Engineering, Washington State University, Richland, WA 99354-1671, USA. E-mail: hlei@wsu.edu; Fax: +1-509-372-7690; Tel: +1-509-372-7628
First published on 5th June 2018
Abstract
In the present study, in situ catalytic pyrolysis of Douglas fir pellets was performed in a microwave reactor. A biochar catalyst derived from corn stover biochar was prepared for the experiment. The results showed that the highest amounts of hydrocarbons (52.77% of bio-oil) were achieved from microwave-assisted catalytic pyrolysis over the biochar catalyst at a reaction temperature of 480 °C. A non-condensable gas enriched in H2, CO, and CO2 was observed and analyzed by micro-GC. The amounts of H2 and CO increased during catalytic pyrolysis compared to the non-catalytic runs. GC/MS analysis results showed that the quantity of lignin-derived guaiacols decreased dramatically with the increase of the ratio of catalyst to biomass. The biochar catalyst exhibited good selectivity towards hydrocarbon and phenol compounds, simplifying the chemical composition, reducing undesirable compounds and producing pyrolysis oil in an acceptable yield. The reaction mechanism for hydrocarbon production from catalytic pyrolysis was also analyzed.
Introduction
Renewable energy is produced from an energy resource that is replaced or replenished rapidly by a natural process. Bioenergy has been identified as a green and renewable solution to decrease fossil fuel consumption and greenhouse gas (GHG) emission. A major objective of bioenergy research is the production of liquid biofuels as substitutes for crude oil products. Due to the negative implication of first-generation biofuels on food resources and energy safety, lignocellulosic biomass is being studied worldwide as a feedstock for second-generation biofuel production.1,2Lignocellulosic biomass is mainly composed of cellulose, hemicellulose, and lignin, and can be converted into liquid fuels and chemicals via biochemical or thermochemical methods.3 Thermochemical methods, in general, can convert all fractions of biomass while biochemical conversions are limited to the carbohydrate fraction. A higher utilization of biomass resources could be achieved when using thermochemical methods. Pyrolysis, gasification and direct combustion are the three major thermochemical conversion processes.4 Fast pyrolysis is one of the simplest and most promising methods of processing one fuel in order to produce a better fuel. In this process, biomass is heated to intermediate temperatures (400–600 °C) at high heating rates in an oxygen-free atmosphere and it thermally decomposes to biochar, syngas, and bio-oil.
Bio-oil is the major liquid product from fast pyrolysis, and usually has only half the energy content of crude oil. In addition, the product is highly oxygenated and contains acid contaminants that must be removed. The presence of acids in bio-oil catalytic polymerization reactions further decreases the stability of the bio-oil. Furthermore, high acid content makes bio-oil corrosive and difficult to use in engines.4 Researchers have been seeking an approach to refine and upgrade raw bio-oil for producing transportation fuels and other high-value chemicals.2,5 One of the most promising processes to achieve this is the in situ catalytic upgrading of biomass pyrolysis vapors.6 The catalysts can be added directly to the pyrolysis reactor for in situ vapor upgrading.
Zeolite catalysts have been studied for upgrading pyrolysis oil since the 1980s.7 ZSM-5 has been found to change the composition of raw bio-oil dramatically by reducing the amounts of oxygenated compounds via deoxygenation reactions and simultaneously increasing the aromatic species, producing a lighter fraction.8 Catalytic upgrading of pyrolysis vapors using zeolites is a promising method for removing oxygen from organic compounds and converting them to hydrocarbons.9,10 The highest yield of hydrocarbons (approximately 16 wt%, including 3.5 wt% of toluene) was achieved using nickel, cobalt, iron, and gallium-substituted ZSM-5.11 However, the deposition of coke and tar on the catalyst caused a gradual decrease in the activity of the catalyst up to complete poisoning. The catalysts used in catalytic pyrolysis and bio-oil upgrading are expensive as they need to be doped with costly noble metals to promote oxygen removal and ring-opening reactions.3
Biochar can be produced by several thermal decomposition processes of biomass like pyrolysis and gasification.12 It has a high porosity and surface area, high chemical stability, and is cost-effective. Besides the intrinsic nature of the biomass feedstock, the pyrolysis process conditions could greatly affect the biochar quality and determine its resultant properties.13 Biochar contains a high quantity of minerals and functional groups anchored on its surface, which make it suitable for soil amendment, activated carbon production, and water treatment.14 However, the use of biochar as a biochar catalyst for biofuel production is still in its infancy with only a few studies on biogas reforming and biodiesel production.15,16 Therefore, developing an appropriate biochar based catalyst with the desired characteristics could be a new approach for biomass pyrolysis and bio-oil upgrading.
The goal of the research presented here was to investigate the feasibility of a low-cost biochar catalyst for in situ catalytic biomass pyrolysis and to obtain upgraded bio-oil with increased hydrocarbon yields and lower oxygen content. The effects of biochar catalyst loadings on product yields were determined. The bio-oils obtained from the biomass in situ catalytic pyrolysis using different catalyst loadings were characterized and a possible chemical reaction mechanism of this process was proposed.
Experimental section
Materials
Douglas fir sawdust pellets (7 mm in diameter and 15 mm in length) were purchased from Bear Mountain Forest Products Inc. (USA) and used in the experiments. The pellets were made from 100% natural Douglas fir wood sawdust, the elemental analysis data of which are presented in Table 1.| Feedstock | Douglas fir sawdust pellets |
|---|---|
| Main compositions, dry basis wt% | |
| Carbon | 47.8 |
| Hydrogen | 6.6 |
| Oxygen | 45.4 |
| Nitrogen | 0.08 |
| Sulfur | 0.01 |
| Ash | 0.2 |
| Moisture, wt% as received | 4.8 |
| Higher heating value (HHV), MJ kg−1 | 19.4 |
Catalytic materials preparation and characterization
All biochar catalysts used in the study were produced from corn stover microwave pyrolysis. The corn stover was obtained from Brookings, South Dakota, USA and dried in air at room temperature. The proximate analysis of the corn stover is shown in Table 2. Prior to the experiments, it was then ground to 1/8 inch (3.175 mm) and pyrolyzed at a temperature of 550 °C with a power input of 700 W. The cold biochar was collected after microwave pyrolysis and stored in a desiccator.| Characteristics | Corn stover | |
|---|---|---|
| Proximate analysis (dry, wt%) | Volatile matter | 74.46 |
| Fixed carbon | 20.06 | |
| Ash | 5.48 | |
| Moisture (wt%) | 6.29 | |
| HHV (MJ kg−1) | 16.82 |
Table 3 lists the characteristics of the biochar catalyst. The surface areas were obtained from N2 adsorption–desorption isotherms measured at −196 °C on a Quantachrome Autosorb-1 analyzer with all samples outgassed at 200 °C prior to analysis for 16 h. The BET surface areas are taken from a multipoint plot over a P/P0 range of 0.05–0.35.
| Properties | Biochar catalyst |
|---|---|
| BET surface area (m2 g−1) | 44.16 |
| Average pore diameter (nm) | 6.5 |
| Single point total volume pores (cm3 g−1) | 0.02 |
| Particle size (mesh) | 6–30 |
| Apparent density (g mL−1) | 0.12 |
| Total number of carbon surface functional acidic groups (mmol g−1) | 1.85 |
| Total number of carbon surface functional basic groups (mmol g−1) | 0.48 |
The total titrable surface functional groups were quantified based on Boehm's method.17 It involved suspending 0.5 g of biochar catalyst in 25 mL of a 0.1 N solution of sodium ethoxide, sodium hydroxide or hydrochloric acid. The HCl/NaOH neutralized the unreacted base/acid and prevented further reaction between atmospheric carbon dioxide and the various bases from occurring. The acidified solutions were bubbled with N2 for 1 h to expel dissolved CO2 from solution. All samples were then back-titrated with 0.1 N NaOH/HCl, and the endpoints were potentiometrically determined using a pH meter (Oakton PC – 400). All titrations were carried out at room temperature (22 ± 3 °C).
Microwave-assisted catalytic pyrolysis system
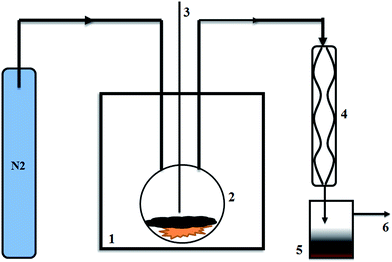
A Sineo MAS-II batch microwave oven (Shanghai, China) was used to conduct biomass catalytic pyrolysis at a reaction temperature of 480 °C for a duration of 15 min. The pyrolytic temperature was selected based on our previous study, which indicated that the optimized bio-oil yield could be obtained at this temperature.18 The reaction time was set to 15 min in order to obtain the maximum bio-oil yield. A constant power input of 700 W at a microwave frequency of 2450 MHz was used for each batch. Five different ratios of biochar catalyst to biomass (10%, 20%, 40%, 80%, and 100%) were tested with a fixed biomass loading of 20 g. The Douglas fir pellets were first introduced in a 500 mL quartz flask and then the biochar catalyst was added to the flask to cover the feedstock. The system was purged with N2 at a flow rate of 1000 mL min−1 for 30 min prior to microwave pyrolysis to create an oxygen-free environment. The temperature of the biomass was measured by an infrared sensor through a dead-end quartz tube which penetrated to the center of the flask. After reaching the desired reaction temperatures, the microwave reactor (equipped with automatic temperature/power control) used a minimum power (e.g. 0–100 W) to maintain the desired reaction temperatures. Fig. 1 shows the schematic diagram of the system. The volatiles from the flask passed through the condensation system and the condensable liquid was collected as bio-crude oil. The catalyst and pellet biochar were left in the quartz flask. The biochar catalyst and biochar pellets were separated due to their difference in particle size. All experiments were carried out in triplicate. The mass of syngas from the biomass catalytic pyrolysis was calculated based on a mass balance using the following equation:| Weight of syngas = initial pellet mass − bio oil mass − pellet char mass | (1) |
GC/MS analysis of bio-oils
The chemical compositions of the bio-oil were determined using an Agilent gas chromatograph-mass spectrometer (GC, Agilent 7890A; MS, Agilent 5975C) with a DB-5 capillary column. The GC temperature was first maintained at 45 °C for 3 min and then increased at 10 °C min−1 to 300 °C and finally held at this value for 10 min. The injector temperature was 280 °C and the injection amount was 1 μL. The flow rate of the carrier gas (helium) was 0.6 mL min−1. The ion source temperature was 230 °C for the mass selective detector. The NIST Mass Spectral libraries were used for the identification of the compounds found in the bio-oil.19 In order to obtain the concentration of some typical hydrocarbons in bio-oils, different standard solutions with various concentrations of hydrocarbons were also injected into the GC/MS and the obtained data (absolute peak area) were used to calculate the concentration of hydrocarbons in the obtained bio-oil.Micro-GC analysis of non-condensable gas (NCG)
The NCG released from the microwave catalytic pyrolysis apparatus was collected and analyzed using a dual channel Micro-GC (INFICON, 3000). The Micro-GC contains two channels and the operating conditions are as follows: Channel A with a molecular sieve 5A column (MS-5) was set at 95 °C for determination of H2, CO, and CH4; Channel B with Porapak Q (PPQ) was set at 60 °C for checking the release properties of CO2, C2H6, C2H4, C3H6, and C3H8. During the stable operation period, the NCG was sampled every 3 min and each gas sample was measured 3 times to get the average.Results and discussion
Product fractional yield
The yields (wt% based on biomass) of the three fractions of products, i.e., bio-oil, syngas and biochar, obtained by the microwave-assisted in situ catalytic pyrolysis are shown in Fig. 2. These values are compared to the yields obtained by the use of the non-catalytic Douglas fir pellets. The result shows that the catalysts used in our study affected the fractional yields to different degrees.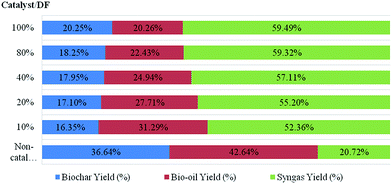 | ||
| Fig. 2 Yield distribution of catalytic pyrolysis of Douglas fir pellets with different loadings of biochar catalysts. | ||
In this study, the average heating rate observed was about 100 °C min−1. The highest bio-oil (42.64 wt%) and biochar (36.64 wt%) yields were achieved in the non-catalytic run. The use of biochar catalysts led to a decrease in the bio-oil and biochar yields with a simultaneous increase in syngas yield. Depending on the loading of biochar catalysts, the bio-oil yield with catalyst addition was between 20.26 wt% and 31.29 wt% of the biomass and the biochar yield with catalyst addition ranged from 16.35 wt% to 20.25 wt%. However, the syngas yields with catalyst addition were in the range of 52.36 wt% to 59.49 wt%, which were much higher than the results of non-catalytic runs (20.72 wt%).
A similar phenomenon was found and reported by other studies as well.20–22 It may be related to two factors. One is that some catalysts may increase the heating rate and temperature. The other is that some catalysts may actually promote biomass thermal decomposition. Graphite and char have been used and reported as microwave absorbers in the pyrolysis of biomass.22 In this study, the biochar catalyst used in biomass microwave pyrolysis has both functions. As a good microwave absorber, the temperature of the biochar catalyst is much higher than that of the surrounding solids and gases during microwave heating, which creates heated areas called “hot spots”. As a result, the target catalytic and pyrolysis temperatures can be obtained in the reactor, where biomass decomposition and bio-oil secondary cracking reactions can occur smoothly.23,24
According to Fig. 2, the loading ratio of biochar catalysts to feedstock significantly influenced the product yields. The biochar catalysts favored syngas production. The bio-oil yield decreased significantly with the increase of the loading ratio of catalysts to feedstock, while the syngas yield and biochar yield increased slightly. This could be explained by the catalytic cracking of biomass and bio-oil secondary cracking reactions that occurred due to the higher temperature achieved by using the biochar catalyst in microwave-assisted pyrolysis.
GC/MS analysis of the bio-oils
In order to further understand the chemical reaction of catalytic biomass pyrolysis, the bio-oils were analyzed by GC/MS. In addition, the water content of the obtained bio-oils was in the range of 30–40 wt%, and was calculated from the mass difference before and after organic compound extraction from bio-oils by using an organic solvent. The obtained water in bio-oils mainly came from catalytic pyrolysis reactions and moisture in the original feedstock. The identified organic compounds were classified into 10 groups: acids, ketone/aldehydes, esters, alcohols, hydrocarbons, phenols, guaiacols, furans, sugars and others. The chemical composition of bio-oils by GC-MS analysis was categorized and depicted according to the differences of functional groups as shown in Fig. 3, which indicates that the chemical composition of bio-oils was greatly influenced by the addition of the biochar catalyst and its loading ratio.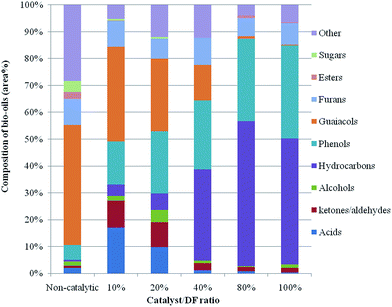 | ||
| Fig. 3 Chemical composition of bio-oils produced from different biochar catalyst loadings determined by GC-MS analysis. | ||
The raw bio-oil from the non-catalytic microwave pyrolysis has a very high content of guaiacols, plus a certain amount of furans, phenols, acids, sugars and other unclassified compounds. Guaiacols and acids are considered undesirable, especially for liquid transportation fuel production purposes. Highly oxygenated compounds like guaiacols, esters, and ethers reduce the heating value of the bio-oil and make it chemically unstable. Organic acids are corrosive and make the bio-oils difficult to use in engines.4 Acids catalyze polymerization reactions and their presence further decreases the stability of bio-oils.25 Hence, upgrading is necessary for a more stable and energy-intensive liquid product.
As seen in Fig. 3, a much higher content of hydrocarbons and phenols was detected in the bio-oils from in situ catalytic microwave pyrolysis of biomass with the biochar catalyst. However, in our previous research, only a few hydrocarbons were reported in Douglas fir pellet microwave assisted pyrolysis without any catalyst or with activated carbon (AC).26,27 The presence of hydrocarbons (including aromatic hydrocarbons and aliphatic hydrocarbons) is highly desirable for liquid transportation fuel production. Phenols are high value-added chemicals which can be used for phenol formaldehyde resin production. These compounds can help to make the pyrolysis process more economically attractive.
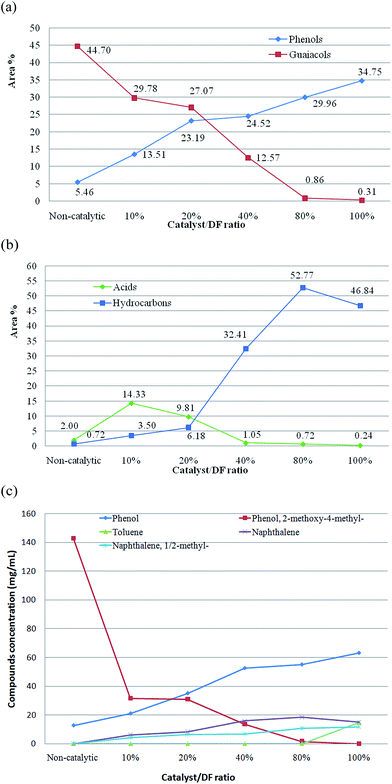
More experiments were conducted on Douglas fir pellets using the biochar catalyst at different loadings. The results of the main chemical compounds of bio-oils from in situ catalytic microwave pyrolysis of biomass are shown in Table 4 and Fig. 4. A gradual increase in phenols and a decrease in guaiacols were noticed. It was observed that the main components of the phenols were phenol, 2-methyl-phenol (o-cresol), and 4-methyl-phenol (p-cresol), and the main components of guaiacols were 2-methoxy-phenol, 2-methoxy-4-methyl-phenol, 4-ethyl-2-methoxy-phenol, 2-methoxy-4-vinylphenol and 2-methoxy-4-(1-propenyl)-phenol. Fig. 4(a) shows that the phenols were about 5.46 to 34.75% and gradually increased with the amount of the biochar catalyst loading in the catalytic microwave pyrolysis compared to the raw bio-oil from non-catalytic pyrolysis. The maximum phenol yield of 34.75% (including 11.89% phenol, 5.01% o-cresol and 10.16% p-cresol) was obtained with the catalyst-to-biomass ratio of 100%. However, the guaiacol content decreased dramatically with increasing catalyst loading ratios.
| Categories | Compounds name | Retention time (min) | Non-catalytic (%) | Cat/DF 10% (%) | Cat/DF 20% (%) | Cat/DF 40% (%) | Cat/DF 80% (%) | Cat/DF 100% (%) |
|---|---|---|---|---|---|---|---|---|
| Furans | Furfural | 6.43 | 4.98 | 4.06 | 4.97 | 5.31 | 2.51 | 1.38 |
| Furan, tetrahydro-2,5-dimethoxy- | 7.69 | 3.47 | 0.48 | 0.27 | — | — | — | |
| Benzofuran | 11.31 | — | 0.31 | 1.07 | 2.29 | 2.98 | 3.46 | |
| Benzofuran, 2-methyl- | 14.85 | — | — | — | 0.27 | 1.14 | 1.20 | |
| Dibenzofuran | 26.23 | — | 0.31 | 0.31 | 0.58 | 1.13 | 1.04 | |
| Dibenzofuran, 4-methyl- | 29.10 | — | — | — | 0.40 | 0.38 | 0.40 | |
| Other furan compounds | 1.44 | 2.98 | 0.79 | 1.10 | 1.32 | 0.87 | ||
| Total | 9.80 | 8.14 | 7.41 | 9.55 | 9.08 | 7.95 | ||
| Phenols | Phenol | 10.82 | 1.68 | 4.25 | 6.23 | 8.03 | 12.97 | 11.89 |
| Phenol, 2-methyl- | 13.12 | 0.97 | 1.58 | 2.88 | 3.24 | 4.85 | 5.01 | |
| Phenol, 4-methyl- | 13.81 | — | 2.25 | 5.80 | 6.04 | 8.63 | 10.16 | |
| Phenol, 2/3/4-ethyl- | 14.81/16.58 | — | 0.65 | 1.04 | 1.72 | 2.23 | 0.20 | |
| Phenol, 2,3/4/6-dimethyl- | 14.82/16.89 | 0.89 | 2.28 | 3.02 | 3.07 | 0.20 | 4.19 | |
| Phenol, 3,4/5-dimethyl- | 16.70/17.41 | — | 0.67 | 0.52 | 0.32 | 0.15 | 2.11 | |
| Phenol, 2/3-ethyl-4/5-methyl- | 18.66/16.67 | — | — | — | 0.59 | 0.30 | 0.52 | |
| Other phenol compounds | 1.92 | 1.83 | 3.70 | 1.51 | 0.11 | 0.67 | ||
| Total | 5.46 | 13.51 | 23.19 | 24.52 | 29.96 | 34.75 | ||
| Guaiacols | Phenol, 2-methoxy- | 14.15 | 5.32 | 4.19 | 3.98 | 2.64 | — | — |
| Phenol, 2-methoxy-4-methyl- | 17.29 | 14.03 | 10.03 | 7.43 | — | — | — | |
| 1,2-Benzenediol | 17.50 | 3.67 | 0.97 | 1.68 | 0.55 | — | — | |
| 1,2-Benzenediol, 4-methyl- | 19.37 | 0.93 | 1.23 | 1.98 | — | — | — | |
| Phenol, 4-ethyl-2-methoxy- | 19.72 | 4.98 | 2.30 | 2.25 | 1.50 | 0.45 | 0.31 | |
| 2-Methoxy-4-vinylphenol | 20.76 | 5.93 | 3.69 | 4.13 | 3.94 | — | — | |
| Phenol, 2-methoxy-4-(1-propenyl)- | 23.27 | 9.10 | 5.85 | 4.87 | 2.55 | — | — | |
| Other guaiacols compounds | 0.74 | 1.52 | 0.75 | 1.39 | 0.41 | — | ||
| Total | 44.70 | 29.78 | 27.07 | 12.57 | 0.86 | 0.31 | ||
| Hydrocarbons | Toluene | 4.30 | — | — | — | — | 0.69 | 2.94 |
| p-Xylene | 7.42 | — | — | — | — | 0.28 | 0.67 | |
| Styrene | 8.06 | — | — | — | — | 1.46 | 3.63 | |
| Indene | 12.83 | 0.11 | 0.37 | 1.40 | 5.40 | 7.92 | 5.95 | |
| Undecane | 14.56 | — | 1.41 | 1.28 | 1.43 | 1.00 | 1.13 | |
| Naphthalene | 17.20 | — | — | — | 10.69 | 12.84 | 7.66 | |
| Naphthalene, 1,2-dihydro-3-methy- | 19.54 | — | — | — | — | 0.20 | 0.23 | |
| Naphthalene, 1-methyl- | 20.40 | — | — | — | 2.33 | 3.83 | 3.52 | |
| Naphthalene, 2-methyl- | 20.82 | 0.21 | 0.43 | 0.62 | 1.04 | 3.57 | 3.06 | |
| Naphthalene, 1-ethyl- | 23.07 | — | — | — | 0.22 | 0.42 | 0.46 | |
| Naphthalene, 1,3/6/7-dimethyl- | 23.37/24.24 | — | — | — | 0.17 | 0.98 | 0.66 | |
| Naphthalene, 2,3/6-dimethyl- | 23.82/24.24 | 0.12 | 0.18 | 0.24 | 0.40 | 0.65 | 1.06 | |
| Naphthalene, 2-ethenyl- | 24.03 | — | — | — | 0.74 | 0.84 | 0.67 | |
| Naphthalene, 1,4,5/6-trimethyl- | 25.93/26.57 | — | — | — | — | 0.10 | 0.22 | |
| Biphenyl | 22.68 | — | — | 0.36 | 0.56 | 0.78 | 0.69 | |
| Acenaphthylene | 24.50 | — | — | — | 1.05 | 3.69 | 0.25 | |
| Biphenylene | 24.51 | — | — | — | — | 0.02 | 2.88 | |
| Acenaphthene | 25.36 | — | — | — | 0.68 | 0.79 | 0.76 | |
| 1,1′-Biphenyl, 3-methyl- | 25.67 | — | — | — | 0.13 | 0.79 | 0.74 | |
| Fluorene | 27.85 | 0.16 | 0.44 | 0.44 | 2.41 | 3.18 | 2.58 | |
| 9H-Fluorene, 4/2/1-methyl- | 30.59 | 0.02 | 0.12 | 0.19 | 0.88 | 1.22 | 1.24 | |
| Anthracene | 32.46 | — | — | — | 1.51 | 2.25 | 2.26 | |
| Phenanthrene | 32.72 | — | — | 0.34 | 1.27 | 1.10 | 0.56 | |
| Anthracene, 1-methyl- | 35.13 | — | — | — | 0.10 | 0.16 | 0.21 | |
| Fluoranthene | 38.63 | — | — | 0.07 | 0.10 | 0.72 | 0.52 | |
| Pyrene, 1-methyl- | 41.97 | — | — | 0.04 | 0.40 | 0.51 | 0.62 | |
| Other hydrocarbon compounds | 0.10 | 0.55 | 1.20 | 0.90 | 2.78 | 1.67 | ||
| Total | 0.72 | 3.50 | 6.18 | 32.41 | 52.77 | 46.84 |
For catalyst-to-biomass ratios of 80% and 100%, the guaiacol content is only 0.86 and 0.31%, respectively, indicating that most guaiacols were converted to phenols and hydrocarbons via cracking and hydrogenolysis reactions. However, the content of phenols and guaiacols for the raw bio-oil from non-catalytic pyrolysis was 5.46 and 44.70%, respectively. Therefore, the results revealed that the biochar catalyst used in the Douglas fir pellet microwave catalytic pyrolysis acts selectively towards the hydrogenolysis of the oxygen–aromatic bonds of methoxyl or hydroxyl functionalities. Table 4 shows that the primary hydrocarbons from the in situ microwave catalytic pyrolysis with biochar catalyst addition are aromatics, which mainly include indene, naphthalene, biphenyl and fluorine compounds. The typical results of hydrocarbon selectivity versus biochar catalyst loadings are shown in Fig. 4(b). The yield of hydrocarbons increased significantly from 0.72 to 52.77%. Increases in the catalyst-to-biomass ratio resulted in an increase of hydrocarbon yield for microwave catalytic pyrolysis of Douglas fir pellets. However, when the catalyst-to-biomass ratio increased to 100%, the hydrocarbon content decreased slightly. The maximum hydrocarbon yield of 52.77% (including 12.84% naphthalene, 7.92% indene, and 7.40% methylnaphthalene) was obtained with the catalyst-to-biomass ratio of 80%. At a higher catalyst-to-biomass ratio, xylene and toluene are gradually produced, whereas the yield of indene, naphthalene and biphenyl is decreased. This result can be explained in terms of the biochar catalyst hydrogenation of biphenyl and naphthalene, respectively, using hydrogen generated in situ.
At low levels of biochar catalyst loading, the results showed either a sudden increase in the appearance of acids, ketones, and aldehydes, which were higher than in the original bio-oil from non-catalytic pyrolysis. A possible explanation for these atypical results is that for low catalyst loading, the bio-oil cracking process and secondary reactions are not fully complete, leading to a high yield of acid compounds. And when the catalyst-to-biomass ratio is higher than 40%, the acid amount is decreased dramatically.
The concentrations of the typical compounds (phenol, phenol,2-methoxy-4-methyl-, toluene, naphthalene, and naphthalene,1/2-n) were also quantified by GC/MS, and are depicted in Fig. 4(c) with respect to different catalyst loading ratios. It can be seen that the concentration of phenol,2-methoxy-4-methyl- decreased gradually with the increasing catalyst/DF ratio from 142.97 mg mL−1 without any catalyst to 0.0 mg mL−1 with the catalyst/DF being 100%. The decreased phenol,2-methoxy-4-methyl- was proposed to undergo deoxygenation and demethylation reactions induced by biochar catalysts during catalytic processes, which was evidenced by the increase in phenol production. For the concentration of phenol, the maximum was 63.14 mg mL−1 with the catalyst/DF ratio of 100%. The concentration of aromatic hydrocarbons such as naphthalene and naphthalene,1/2-n also increased gradually with more catalyst applied. Neither of them were detected in the bio-oil obtained in the absence of a catalyst, whereas these compounds were generated by using the biochar catalyst and the highest concentrations of naphthalene and naphthalene,1/2-n were 18.48 and 11.76 mg mL−1 with a catalyst loading ratio of 80% and 100%, respectively. It is worth noting that a slight drop in the concentration of naphthalene was observed when increasing the catalyst/DF ratio from 80 to 100%, which can be ascribed to the further decomposition caused by additional catalyst loading. On the other hand, with a high enough catalyst loading ratio, toluene was detected and the concentration of that was 14.92 mg mL−1 at the catalyst/DF ratio of 100%.
These results indicated that the loading amount of biochar catalyst is a significant factor affecting the bio-oil composition and hydrocarbon production, which also indicates that the bio-oil chemical profiles can be altered by adjusting the loading of catalysts, suggesting that it is possible to target specific desired products by controlling the loading. The valuable phenols obtained in the upgraded bio-oil by using the bio-char catalyst could be applied in various industrial processes such as the production of plastics, phenol resins, caprolactam, and even pharmaceutical drugs.28 Additionally, aromatics such as toluene, indene, and naphthalene are jet-fuel range hydrocarbons, which can be used directly or subjected to further hydrogenation to cycloalkanes for aviation fuels.29,30 Currently, zeolite-based catalysts are regarded as promising catalysts for hydrocarbon production from raw biomass and up to 24.68% carbon yield of aromatics could be obtained, which was dominated by mono-ring and double-ring compounds.30,31 The bio-char catalyst used here also showed comparable catalytic performance in hydrocarbon generation. In addition, the bio-char catalysts are produced in an economical and environmentally friendly manner. Besides, various modifications are accessible via facile methods to fully release their potential as promising alternative catalysts in bio-oil upgrading. Therefore, in this study, the biochar catalyst exhibited a high selectivity towards hydrocarbons and phenols, which offered a promising route to generate better-quality bio-oils for fuel production.
Micro-GC analysis of non-condensable gas (NCG)
A Micro-GC was used for determining the quantitative NCG compound yields from the microwave catalytic pyrolysis of biomass. The Douglas fir pellet microwave pyrolysis and catalytic pyrolysis gas product distributions are shown in Fig. 5. The NCG products from the microwave pyrolysis of biomass mainly contain CO2, CO, H2, CH4, and some other hydrocarbons (<C4) such as C2H6, C2H4, C3H6, and C3H8. These results indicate that the NCG generated from the Douglas fir pellet catalytic pyrolysis has higher amounts of CO, H2, CH4 and short chain hydrocarbons but not CO2 when compared to non-catalytic runs. In previous research, smaller hydrogen yields (<1%) were observed in the biomass pyrolysis. This is because hydrogen is evolved at a higher pyrolysis temperature and longer residence time.9,24 However, in raw Douglas fir pellet microwave pyrolysis, about 22.64 vol% hydrogen was observed and a higher hydrogen yield (30.15 vol%) was obtained in the catalytic runs with biochar catalysts.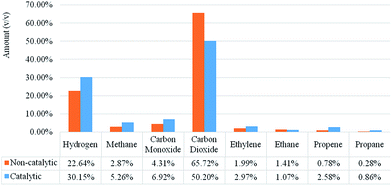 | ||
| Fig. 5 Non-condensable gas product distributions from Douglas fir pellet pyrolysis and catalytic pyrolysis (catalyst-to-biomass ratio: 80%). | ||
CO2 and CH4 mainly evolved at 400–600 °C. CH4 can be formed from methoxyl functionality decomposition and ring hydrogenation. The higher yield of H2 and CH4 can be attributed to the higher content of aromatic rings and O–CH3 functional groups in the lignin-derived compounds. The H2 from organic pyrolysis mainly comes from the cracking and deformation of aromatics (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H) while the CH4 mainly comes from the cracking of methoxyl.32 Furthermore, at higher temperatures, the endothermic steam reforming reaction also contributes to a higher hydrogen production. The pyrolysis temperature in this study might not be high enough to promote the reforming reaction. However, the biochar catalyst derived from biochar is a good microwave absorber, and much higher temperatures in solid matter could be reached due to the formation of hot spots caused by microwave heating.22,33 These results also suggested that the biochar catalyst derived from biochar favors hydrogen production, and furthermore, that endothermic reforming reaction might play a significant role during microwave in situ catalytic pyrolysis.
C and C–H) while the CH4 mainly comes from the cracking of methoxyl.32 Furthermore, at higher temperatures, the endothermic steam reforming reaction also contributes to a higher hydrogen production. The pyrolysis temperature in this study might not be high enough to promote the reforming reaction. However, the biochar catalyst derived from biochar is a good microwave absorber, and much higher temperatures in solid matter could be reached due to the formation of hot spots caused by microwave heating.22,33 These results also suggested that the biochar catalyst derived from biochar favors hydrogen production, and furthermore, that endothermic reforming reaction might play a significant role during microwave in situ catalytic pyrolysis.
The Fig. 5 results also indicated that Douglas fir pellet pyrolysis with the biochar catalyst leads to a lower yield of CO2 (50.20 vol%) compared to the 65.72 vol% obtained by raw Douglas fir pellet pyrolysis. These results are ascribed primarily to the secondary cracking of volatiles, followed by the increase of CO and hydrocarbons. A similar phenomenon was reported and analyzed in the catalytic pyrolysis over H-ZSM5.34 The strong acid sites in the H-ZSM5 catalyst have proven to be more active for decarbonylation to produce CO than decarboxylation to CO2 from oxygenates during catalytic pyrolysis.8
As the literature indicates, the surface functional groups anchored on/with carbon were found to be responsible for the variety of catalytic and physicochemical properties of the material considered.35 Hence, it is reasonable to speculate that the oxygen acidic sites on the surface of the biochar catalyst promote the formation of CO by catalyzed decarbonylation and formation of aromatics.36 The metal elements contained in the biochar catalyst might also enhance the secondary reaction. This indicates that oxygenates primarily went through decarbonylation other than decarboxylation to form reaction intermediates which were subsequently converted to aromatic hydrocarbons over the biochar catalyst.
Reaction mechanism analysis
The in situ upgrading of biomass pyrolysis vapors over the biochar catalyst was studied in the microwave assisted catalytic pyrolysis system. In general, the catalytic pyrolysis process goes through a series of pyrolysis reactions followed by catalytic conversion of biomass-derived oxygenates.2Since the biochar catalysts were placed on the top of the Douglas fir pellets, the volatiles from biomass decomposition first passed through the biochar catalyst zone before condensing. The cracking and reforming of the volatiles might occur within this catalysis zone, which involves a series of the heterogeneous solid–gas reactions and gas–gas reactions. A reaction pathway was proposed by integrating the findings from the different catalyst to biomass loadings and the analysis of chemical profiles of bio-oils for in situ microwave assisted catalytic pyrolysis of Douglas fir pellets. The reaction network of microwave assisted in situ catalytic pyrolysis of lignocellulosic biomass is summarized in Fig. 6.
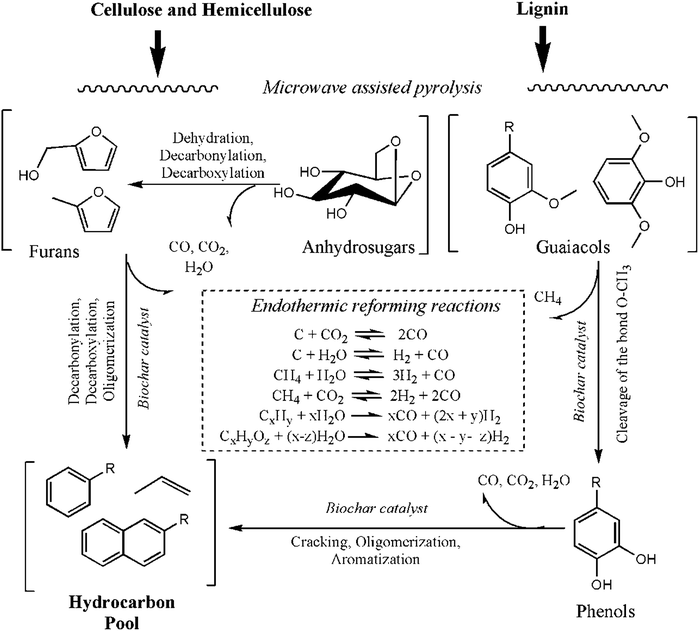 | ||
| Fig. 6 Proposed reaction pathway for guaiacol and phenolic aromatics to hydrocarbons over the biochar catalyst under microwave pyrolysis. | ||
Cellulose, hemicellulose, and lignin are the major components of biomass, and they decompose at different temperatures. At low temperatures (200–400 °C), cellulose and hemicellulose decompose into small molecule compounds such as acids, ketones, aldehydes, sugars, and furans.28,37,38 Due to the effects of catalyst, anhydrosugars will undergo dehydration, decarbonylation or decarboxylation reactions to form smaller furanic compounds. Then the acid sites present on the biochar catalyst surface promote a series of decarbonylation and oligomerization reactions inside the pores of the biochar catalyst, which further convert these furans to aromatic hydrocarbons and olefins.
However, lignin decomposes more slowly over a broader temperature range (200–500 °C) than cellulose and the hemicellulose components of biomass. It primarily generates guaiacols and phenolic compounds, which could also contribute to the production of aromatic hydrocarbons.39,40 One reaction pathway is lignin and furan derived guaiacol cracking by the cleavage of methyl from O–CH3 to phenols over the biochar catalysts. This demethoxylation reaction of guaiacols can be verified by the increased presence of CH4 in NCG. A further microwave specific mechanism must also be considered. Dominguez and Russell observed that particular types of carbon “microplasmas” could be generated during the interaction of the electromagnetic microwave field, which creates charge imbalances.41,42 The pyrolysis vapors passing through this area will be cracked to a greater extent due to the considerably higher temperature of these microplasmas compared to the surroundings.
The high amount of H2 gas generated by endothermic reforming reactions promotes the deoxygenation reactions of the lignin-derived oxygenate during the in situ catalytic pyrolysis. The phenols could be further cracked to aromatic hydrocarbons by the cleavage of –OH. The olefins produced from alkyl-phenol cracking might be other intermediates for aromatic hydrocarbon production.32 The biochar catalyst with high-energy surface functional groups (e.g. acidic sites) also contributes to the extra cracking and upgrading of pyrolysis oils.
The proposed pathway illustrated in Fig. 6 may also be confirmed by the GC/MS analysis of the chemical composition of bio-oils. This GC/MS analysis showed that the concentrations of the small molecule compounds, such as acid, sugars, ketones, and aldehydes, were all significantly decreased compared to the raw pyrolysis oils by conventional pyrolysis, while the production of aromatic hydrocarbons and phenols was significantly increased with the significant decrease of guaiacol content. The bio-oil chemical profile from microwave-assisted catalytic pyrolysis over biochar catalysts was simplified to phenols and hydrocarbons, and their concentrations were increased by increasing the ratios of biochar catalyst to biomass.
Conclusions
This study investigated the production of hydrocarbons from biomass through in situ microwave-assisted catalytic pyrolysis with a biochar catalyst. High hydrocarbon concentrations (52.77%) of the bio-oil were obtained from catalytic pyrolysis over the biochar catalyst, as compared to 0.72% for the bio-oil from non-catalytic runs. The biochar catalyst deoxygenated the raw bio-oil and in situ upgrading occurred, resulting in a narrowly targeted hydrocarbon and phenol product, with a higher potential commercial value. The increased amounts of H2 and CO observed in NCG from biomass catalytic pyrolysis also demonstrated the biochar catalyst selectivity towards hydrocarbons. A possible reaction pathway was proposed for this in situ microwave assisted catalytic pyrolysis. The results indicated that the corn stover derived biochar catalyst might be a cost-competitive catalyst in fuel and chemical production from biomass-derived feedstocks. Further studies are needed to investigate the effect of functional group sites on the carbon surface and catalyst regeneration, which could also help better understand the catalytic mechanism and optimize this biochar catalyst. The commercial application of lignocellulose biomass to hydrocarbons through in situ microwave-assisted catalytic pyrolysis processes will depend on the development of technologies for hydrodeoxygenation and on the market price of crude oil.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partially supported by The Agriculture and Food Research Initiative of the National Institute of Food and Agriculture, United States Department of Agriculture (Award Numbers 2016-67021-24533 and 2018-67009-27904) and the China Scholarship Council (CSC). The authors are also grateful to Dr Ahamed Aftab of the Bioproducts, Science and Engineering Laboratory and Michel Gray of the Pacific Northwest National Laboratory for their contributions and assistance in GC/MS and Micro-GC analysis.Notes and references
- S. Thangalazhy-Gopakumar, S. Adhikari, R. B. Gupta, M. Tu and S. Taylor, Bioresour. Technol., 2011, 102, 6742–6749 CrossRef PubMed.
- T. R. Carlson, G. a. Tompsett, W. C. Conner and G. W. Huber, Top. Catal., 2009, 52, 241–252 CrossRef.
- M. Stöcker, Angew. Chem., Int. Ed., 2008, 47, 9200–9211 CrossRef PubMed.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106(9), 4044–4098 CrossRef PubMed.
- A. V. Bridgwater, Biomass Bioenergy, 2011, 38, 1–27 CrossRef.
- S. D. Stefanidis, K. G. Kalogiannis, E. F. Iliopoulou, a. a. Lappas and P. a. Pilavachi, Bioresour. Technol., 2011, 102(17), 8261–8267 CrossRef PubMed.
- P. Chantal, S. Kaliaguine, J. L. Grandmaison and A. Mahay, Appl. Catal., 1984, 10, 317–332 CrossRef.
- Y. T. Cheng and G. W. Huber, ACS Catal., 2011, 1, 611–628 CrossRef.
- C. a. Fisk, T. Morgan, Y. Ji, M. Crocker, C. Crofcheck and S. a. Lewis, Appl. Catal., A, 2009, 358, 150–156 CrossRef.
- L. Wang, H. Lei, S. Ren, Q. Bu, J. Liang, Y. Wei, Y. Liu, G. S. J. Lee, S. Chen and J. Tang, J. Anal. Appl. Pyrolysis, 2012, 98, 194–200 CrossRef.
- R. French and S. Czernik, Fuel Process. Technol., 2010, 91, 25–32 CrossRef.
- J. Lehmann and S. Joseph, Biochar for environmental management, Science and Technology, Earthscan, London, 2009, pp. 85–105 Search PubMed.
- J. J. Manyà, Environ. Sci. Technol., 2012, 46, 7939–7954 CrossRef PubMed.
- C. A. Mullen, A. A. Boateng, N. M. Goldberg, I. M. Lima, D. A. Laird and K. B. Hicks, Biomass Bioenergy, 2010, 34(1), 67–74 CrossRef.
- A. M. Dehkhoda, A. H. West and N. Ellis, Appl. Catal., A, 2010, 382, 197–204 CrossRef.
- R. Azargohar and A. K. Dalai, Appl. Biochem. Biotechnol., 2006, 131, 762–773 CrossRef PubMed.
- H. Boehm, Carbon, 2002, 40(2), 145–149 CrossRef.
- Y. Zhang, H. Lei, Z. Yang, K. Qian and E. Villota, ACS Sustainable Chem. Eng., 2018, 6, 5349–5357 CrossRef.
- H. Lei, S. Ren and J. Julson, Energy Fuels, 2009, 11, 3254–3261 CrossRef.
- Q. Bu, H. Lei, L. Wang, Y. Wei, L. Zhu, X. Zhang, Y. Liu, G. Yadavalli and J. Tang, Bioresour. Technol., 2014, 162, 142–147 CrossRef PubMed.
- A. A. Salema and F. N. Ani, J. Anal. Appl. Pyrolysis, 2012, 96, 162–172 CrossRef.
- Y. Fernández, A. Arenillas, M. a. Díez, J. J. Pis and J. a. Menéndez, J. Anal. Appl. Pyrolysis, 2009, 84, 145–150 CrossRef.
- Y. Wang, Y. Li, L. Tang, J. Lu and J. Li, Electrochem. Commun., 2009, 11, 889–892 CrossRef.
- S. Ren, H. Lei, L. Wang, Q. Bu, S. Chen and J. Wu, RSC Adv., 2014, 4, 10731 RSC.
- M. Boucher, A. Chaala, H. Pakdel and C. Roy, Biomass Bioenergy, 2000, 19, 351–361 CrossRef.
- Q. Bu, H. Lei, S. Ren, L. Wang, Q. Zhang, J. Tang and R. Ruan, Bioresour. Technol., 2012, 108, 274–279 CrossRef PubMed.
- S. Ren, H. Lei, L. Wang, Q. Bu, S. Chen, J. Wu, J. Julson and R. Ruan, J. Anal. Appl. Pyrolysis, 2012, 94, 163–169 CrossRef.
- J. Michalowicz and W. Duda, Pol. J. Environ. Stud., 2007, 16, 347–362 Search PubMed.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef PubMed.
- X. S. Zhang, H. W. Lei, L. Zhu, M. Qian, J. C. Chan, X. L. Zhu, Y. P. Liu, G. Yadavalli, D. Yan, L. Wang, Q. Bu, Y. Wei, J. Wu and S. L. Chen, Catal. Sci. Technol., 2016, 6, 4210–4220 Search PubMed.
- G. Kabir and B. Hameed, Renewable Sustainable Energy Rev., 2017, 70, 945–967 CrossRef.
- H. Yang, R. Yan, H. Chen, D. H. Lee and C. Zheng, Fuel, 2007, 86, 1781–1788 CrossRef.
- Q. Bu, H. Lei, L. Wang, Y. Wei, L. Zhu, Y. Liu, J. Liang and J. Tang, Bioresour. Technol., 2013, 142, 546–552 CrossRef PubMed.
- A. Corma, G. W. Huber, L. Sauvanaud and P. Oconnor, J. Catal., 2007, 247, 307–327 CrossRef.
- S. Biniak, G. Szymański, J. Siedlewski and A. Światkowski, Carbon, 1997, 35, 1799–1810 CrossRef.
- K. Wang, K. H. Kim and R. C. Brown, Green Chem., 2014, 16, 727 RSC.
- Y. Zhang, C. Liu and H. Xie, J. Anal. Appl. Pyrolysis, 2014, 105, 23–34 CrossRef.
- Y. Zhang, C. Liu and X. Chen, J. Anal. Appl. Pyrolysis, 2015, 113, 621–629 CrossRef.
- C. Liu, Y. Zhang and X. Huang, Fuel Process. Technol., 2014, 123, 159–165 CrossRef.
- M. Brebu and C. Vasile, Cellul. Chem. Technol., 2010, 44, 353–363 Search PubMed.
- A. Dominguez, B. Fidalgo, Y. Fernandez, J. Pis and J. Menendez, Int. J. Hydrogen Energy, 2007, 32, 4792–4799 CrossRef.
- A. D. Russell, E. I. Antreou, S. S. Lam, C. Ludlow-Palafox and H. a. Chase, RSC Adv., 2012, 2, 6756 RSC.
Footnote |
| † The authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2018 |