Economics & carbon dioxide avoidance cost of methanol production based on renewable hydrogen and recycled carbon dioxide – power-to-methanol
Christoph
Hank
 ab,
Svenja
Gelpke
a,
Andrea
Schnabl†
a,
Robin J.
White
ab,
Svenja
Gelpke
a,
Andrea
Schnabl†
a,
Robin J.
White
 *a,
Johannes
Full
*a,
Johannes
Full
 a,
Nikolai
Wiebe
a,
Nikolai
Wiebe
 a,
Tom
Smolinka
a,
Tom
Smolinka
 a,
Achim
Schaadt
a,
Achim
Schaadt
 a,
Hans-Martin
Henning
a,
Hans-Martin
Henning
 a and
Christopher
Hebling
a
a and
Christopher
Hebling
a
aFraunhofer Institute for Solar Energy Systems ISE, Heidenhofstraße 2, 79110 Freiburg, Germany. E-mail: robin.white@ise.fraunhofer.de
bKarlsruhe Institute of Technology (KIT), Engelbert-Arnold-Straße 4, 76131 Karlsruhe, Germany
First published on 14th March 2018
Abstract
The synthesis of sustainable methanol based on renewable electricity generation, sustainable hydrogen (H2) and recycled carbon dioxide (CO2) represents an interesting sustainable solution to integrated renewable energy storage and platform chemical production. However, the business case for this electricity based product (denoted hereafter as eMeOH) under current market conditions (e.g. vs. conventional fossil methanol (fMeOH) production cost) and the appropriate implementation scenarios to increase platform attractiveness and adoption have to be highlighted. The aim of the following study was to perform a dynamic simulation and calculation of the cost of eMeOH production (where electricity is generated at a wind park in Germany), with comparison made to grid connected scenarios. Consideration of these scenarios is made with particular respect to the German energy market and potential for the reduction in fees/taxes (i.e. for electrolyser systems). This evaluation and indeed the results can be viewed in light of European Union efforts to support the implementation of such technologies. In this context, CO2 is sourced from EU relevant sources, namely a biogas or ammonia plant, the latter profiting from the resulting credit arising from CO2 certificate trading. Variation in electricity cost and the CO2 certificate price (in the presented sensitivity study) demonstrate a high cost reduction potential. Under the energy market conditions of Germany it is found that eMeOH production costs vary between €608 and 1453 per tonne based on a purely grid driven scenario, whilst a purely wind park supplied scenario results in €1028–1067 per tonne. The reported results indicate that the eMeOH production cost in Germany is still above the present (although variable) market price, with the economical evaluation indicating that electrolyser and H2 storage represent the lion share of investment and operational cost. Substitution of fMeOH results in CO2 avoidance costs between €365 and 430 per tonne of CO2eq avoided for green methanol produced in Germany. The presented assessment indicates that the eMeOH production cost in Germany will reach market parity in ca. 2030–2035 with the price for the avoidance of CO2eq turning from a cost to a benefit at around the same time. Optimistically, the cost is predominantly influenced by rapidly reducing renewable electricity costs as is already the case in South American and Arabic countries offering the potential for methanol production at a cost of <€500 per tonne.
Introduction
With the steadily increasing contribution of renewable energy (RE) to the electrical grid, efficient and economic solutions to fluctuating energy supply storage are currently required. For example, the European Union (EU) aspires to reach a contribution of renewables to primary energy supply >80% by 2050.1–3 Currently storage at the TWh level is needed to avoid curtailing of RE power output (Germany: 4.7 TWh in 2015; 3.7 TWh in 2016)4 during periods of high supply from solar and wind in combination with the continuous operation of large scale fossil and nuclear power plants. Stationary storage technologies need to provide large capacities whilst simultaneously being efficient and economically attractive. The conversion of electrical into chemical energy is one solution in this context that can also provide a route to clean synthetic fuels, potentially resulting in a reduction of greenhouse gas (GHG) emissions in the mobility sector. Future mobility, besides battery and fuel cell electric vehicles for private transport, will also be dependent on liquid fuels with energy densities suitable for heavy road freight, aviation and shipping. It would be an extremely attractive proposition if this could be achieved whilst generating suitable sustainable platform chemicals for the chemical industry and the decoupling of energy production from energy use.5,6 Such a scheme, if based on the conversion of CO2 will contribute to GHG emission reduction targets established through a number of national and international agreements (e.g. COP21).7There has been much interest in the literature and at the industrial level regarding the interface between RE storage and chemical/fuel production schemes such as Carbon Capture & Utilisation (CCU) and Emissions-to-Liquids.8 Currently in Germany, this concept is generally referred to as “Power-to-X” (PtX). X equals gas (H2 or CH4 – Power-to-Gas (PtG)), chemicals and liquid energy carriers (e.g. MeOH, Fischer–Tropsch diesel – Power-to-Liquid (PtL)). Regarding PtL, this scheme is based on the recycling of CO2 from industrial exhaust gases or biomass plants and its hydrogenation (with H2 produced from RE powered H2O electrolysis) to produce long lasting chemical molecules (Fig. 1). Taking MeOH as the desired liquid product, this C1 alcohol offers numerous potential benefits:9
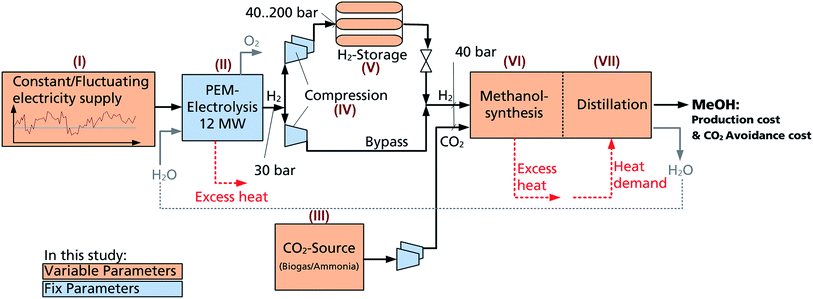 | ||
| Fig. 1 The production of methanol based on renewable energy, water electrolysis and recycled carbon dioxide, commonly referred to as the “Power-to-Liquid” (PtL) process chain. | ||
(1) An industrially established synthesis under moderate conditions such as T < 270 °C and p < 80 bar.
(2) A relatively high energy density (at ambient conditions; 16.9 MJ L−1) and suitability as a storage molecule for RE (i.e. via 12.6 wt% (H2)).
(3) A highly versatile platform molecule (e.g. olefins and acetic acid) for higher fuel production.6,10
(4) High volume, existing market (ca. 75 Mt a−1; 2016) and a high ratio of market price to production cost in the case of conventional fossil production.11
However, the extremely low price of CH4 (the main basis for current industrial MeOH production via reforming and syngas conversion6) makes adoption and market entry somewhat difficult for PtL.12
The option to recycle CO2 is attractive as it could reduce industrial costs related to CO2-certification, whilst holistically elevating CO2 from a liability to a potential commercial asset.5 It is important to note that currently the capture of industrial CO2 and its utilisation (CCU) in chemical processes is not included in the EU Emission Trading System (EU ETS)‡ and, for this reason, does not lead to CO2 certificate savings (i.e. not as for Carbon Capture and Storage (CCS)).12,13 For the current energy system, the regulatory context is extremely important to support the economical implementation of CCU.13–15 In January 2017 the European Court of Justice pronounced that in the case of CCU the transferred CO2 is not to be seen as an emission, as it is not released into the atmosphere.16,17 Hereby the emitter (i.e. a lime producing company implementing CCU for the production of precipitated calcium carbonate) is not obliged to purchase CO2 certificates. In this ruling, the court annulled the second sentence of Article 49(1) of the monitoring regulation with reference to the existence of sufficient means of monitoring for the avoidance of feared CO2 loopholes. This case is foreseen as a critical legal precedent for any CCU project which, after careful assessment of its CO2 mitigation potential, is found to result in reduced CO2 emissions. Therefore a comprehensive LCA is mandatory. Therefore, in this study it is assumed that imminent legislation will consider CCU as a part of the EU ETS market resulting in a credit due to the saving of CO2 certificates. For any large scale implementation of CO2 converting PtL processes it is important to shift focus towards biomass based CO2 sources. Only these (besides the still cost intensive direct air capture methods) enable the recycling of CO2 from distributed emitters. For the following economic evaluation we consider an industrial/fossil and a biogenic source of CO2, namely an ammonia and a biogas plant.
Regarding the synthesis of fossil based methanol (denoted hereafter as fMeOH), three key stoichiometric equations are important (eqn (1)–(3)). The reforming and gas cleaning involved in the syngas preparation give rise to high specific CO2 equivalent emissions. These range from 0.50 to 0.77 t(CO2eq) t(fMeOH)−1, ref. 18 and 19 respectively, including direct specific CO2 emissions of 0.24 t(CO2dir) t(fMeOH)−1.20 These emissions are coupled with a very high environmental impact as a consequence of fossil fuel exploration and processing.21 Positively, the synthesis of electricity based methanol (denoted hereafter as eMeOH) based on the direct hydrogenation of a pure CO2 feed is also known (eqn (1)).22,23 According to the equilibrium reaction, the production of 1 metric tonne of MeOH (CH3OH) requires 1.370 kg(CO2) and 188 kg(H2) are converted (producing 558 kg(H2O) as a side product) (N.B. this value ignores CO2 and H2 consumption via the Reverse Water Gas Shift reaction (eqn (3)) which becomes increasingly favoured with rising reaction temperature). This equilibrium reaction occurs under exothermal reaction conditions and as a consequence is favoured at low T and because the volume reducing reaction requires high p. Efficient compression of the feed stream(s) as well as integration of the exothermal reaction heat are important factors determining the overall process efficiency.
| CO2 + 3H2 ⇌ CH3OH + H2O, ΔHR(298 K,50 bar) = −40.9 kJ mol−1 | (1) |
| CO + 2H2 ⇌ CH3OH, ΔHR(298 K,50 bar) = −90.7 kJ mol−1 | (2) |
| CO2 + H2 ⇌ CO + H2O, ΔHR(298 K,50 bar) = +42.0 kJ mol−1 | (3) |
The commercial demonstration of PtL is exemplified by the George Olah Renewable Methanol Plant (Carbon Recycling International, Iceland), operating at 5 million litres per year production. Plant operation here is based on a very low electricity cost due to inexpensive renewable geothermal power and associated low cost H2/CO2 sourcing.24
One significant distinction and possible advantage of developing CH3OH production based on direct CO2 hydrogenation is the avoidance of the cost intensive syngas production step (e.g. CH4 reforming at T > 700 °C). It typically accounts for ca. 60% of total plant investment.22 Whilst replacement of syngas production with CO2 and H2 sources can be relatively capital intensive, depending on the overall process conditions, investment in such a scheme can result in a “multi-benefit” system: MeOH production, recycling of CO2 emissions, system services for the electrical power grid and indirect avoidance of fossil CO2 emissions. In this regard, a number of recent reports have sought to evaluate the cost-effectiveness of PtL, focusing predominantly on evaluation of eMeOH synthesis with little consideration given to the preceding process steps in the process chain.3,11,25–34 Therefore, it is the aim of the following work to investigate different electricity and CO2 purchase options, the fundamental influence of total and partially dynamic system operation and, in turn, the impact on eMeOH production cost, H2 storage requirements and associated investment costs. Furthermore, a complementary parametric sensitivity study is presented, highlighting the most important factors concerning eMeOH production cost (e.g. in a future RE system). Concerning the environmental impacts of PtL-schemes, the economic evaluation presented is concluded with the CO2 avoidance cost – i.e. an important key performance indicator when evaluating technologies for CO2 emission mitigation.
Power-to-liquid – MeOH production: a brief process description
The PtL scheme for eMeOH production, denoted hereafter as Power-to-Methanol (PtM), can be divided into six primary process steps (Fig. 1):(I) Electricity as the main power source for the process is generated from renewables on-site or purchased from the grid.
(II) H2, the first educt for eMeOH synthesis, is obtained via H2O electrolysis or industrial streams providing high concentrations of H2 (e.g. coke oven gas from steel mills). The PtM process is investigated with the intent to minimise CO2 emissions. For this purpose, H2 used in hydrogenation should not be generated (in-)directly from fossil resources (e.g. steam reforming of CH4 and H2O electrolysis powered by the electricity grid mix). Please note that a valuable by-product of H2O electrolysis is high purity O2, which will be discussed in the following evaluation.
(III) CO2, the second educt for eMeOH production, is captured from industrial processes, biogas or even ambient air.35–38 Source dependent purification of the CO2 is necessary.
(IV) Compression of the feed H2 and CO2 and the associated energy demand. This will be source dependent.
(V) Temporary storage, the size of which will be related to the fluctuation of the electricity production and the range of the systems' dynamics.
(VI) Catalytic conversion of H2 and CO2 to MeOH is based on established technology. Recent studies focus on higher efficiency and dynamic operation.23,31,39,40
(VII) Synthesis is followed by a purification step based here on distillation.
The PtM process steps that are varied within this study are marked in orange (i.e. power supply, CO2 source, H2 storage, and MeOH synthesis; Fig. 1). The non-variable components of the PtM process steps are marked in blue (i.e. polymer electrolyte membrane electrolysis (PEMEL), compression and pressure levels, and distillation).
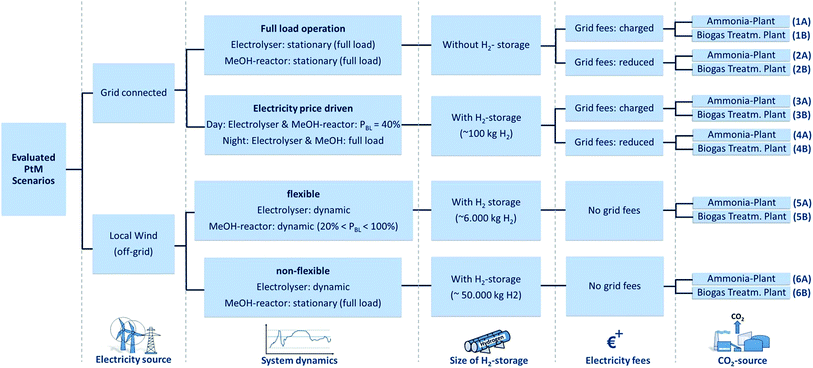
Technical analysis – general framework, examined PtM scenarios and process variables
A total of six different PtM production scenarios (each with two different CO2 sources) are considered (Fig. 2). In this study, two different electricity sources are considered: a constant grid supply (scenarios 1–4) and a local power supply (scenarios 5 and 6) from a 36 MW onshore wind park (which in turn provides a dynamic, fluctuating electricity supply for this study). Grid connected energy is further differentiated as a constant full load power supply (scenarios 1 and 2) or a price dependent power supply (scenarios 3 and 4), (i.e. dual tariff based on electricity availability). Wind park electricity supplies a 100% dynamic electrolyser and a MeOH reactor differentiated into a dynamic (20% < PRL < 100%, scenario 5) and a stationary model (PRL = 100%, scenario 6; PRL = rated load). A dynamic MeOH synthesis reactor is at present not realised at the industrial scale as a consequence of the unknown effects on catalyst degradation/behaviour and the challenges of efficient heat integration (i.e. when operating under partial load). Dynamic simulation of each system leads to H2 storage capacities between ‘zero H2 storage’ and a ‘50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg H2 storage’ reflecting the synthesis ability of flexible operation. The investigated scenarios are additionally differentiated regarding the application of electricity fees. Finally, all six resulting scenarios are evaluated based on the utilisation of two different CO2 sources, namely from a biogas treatment plant (biogenic CO2, indicated by ‘B’) and from an ammonia production plant (fossil CO2, indicated by ‘A’). CO2 from biomass offers, besides air capture methods, the only possibility to close the global carbon-loop and offer a long-term solution for a PtL-process independent from fossils. Regarding ammonia plant-derived CO2, a high purity is assumed and is considered an exemplary scenario for other industrial processes (e.g. cement, steel or bioethanol production). In the subsequent section a down-stream modular overview of the evaluated PtM process is provided. The various parameters identified for the generated economic evaluation are discussed and simulation results based on a combined Matlab/Simulink-based analysis and previously reported data are used.41,42
000 kg H2 storage’ reflecting the synthesis ability of flexible operation. The investigated scenarios are additionally differentiated regarding the application of electricity fees. Finally, all six resulting scenarios are evaluated based on the utilisation of two different CO2 sources, namely from a biogas treatment plant (biogenic CO2, indicated by ‘B’) and from an ammonia production plant (fossil CO2, indicated by ‘A’). CO2 from biomass offers, besides air capture methods, the only possibility to close the global carbon-loop and offer a long-term solution for a PtL-process independent from fossils. Regarding ammonia plant-derived CO2, a high purity is assumed and is considered an exemplary scenario for other industrial processes (e.g. cement, steel or bioethanol production). In the subsequent section a down-stream modular overview of the evaluated PtM process is provided. The various parameters identified for the generated economic evaluation are discussed and simulation results based on a combined Matlab/Simulink-based analysis and previously reported data are used.41,42
Electricity purchase
Purchase from the German electricity market (scenarios 1–4): these scenarios refer to the spot market (i.e. short-term purchase, normally the next day) whereby the final purchase price is dependent on the actual spot market price plus taxation, fees and apportionments (electricity, grid and concession fee, the EEG, ‘KWK’ and offshore apportionment and the apportionment according to §19 ‘StromNEV’ and §18 ‘AbLaV’).Low spot market prices are available in times of high production from RE (e.g. low or even negative residual load),43 or during periods of low energy demand (e.g. at night). Therefore, electricity purchase during such periods can aid in the reduction of the final production cost. The stock price is, however, not the biggest share since ca. 80% can be attributed to taxation and fees. Under certain circumstances (e.g. amount of energy consumed/total utilisation hours/specific electricity cost intensity), it is commonplace in Germany, as an industrial customer, to receive a tax reduction down to 15% (EEG 2017). For this study, electricity prices are calculated either with tax and fees (scenarios 1 and 3) or based on the aforementioned reduction of fees and taxes (scenarios 2 and 4). The respective reduction was calculated depending on the specific electric energy demand in the scenarios. Furthermore the calculation model differs between electric energy demand of the electrolyser and the ‘rest’ of the plant (i.e. compressors and eMeOH-plant). According to ‘Energiewirtschaftsgesetz’ (EnWG, §118 (6)) and ‘Stromsteuergesetz’ (StromStG, §9a), electrolysers are found to be exempt from the grid fee and electricity tax, respectively. The exemption from EEG apportionment for electrolysers and the energy demand of additional components in the PtM system takes effect after the first consumed GWh of electrical energy. After analysing the specific tax and fee reduction potential for each scenario, electricity prices are found to be between 3.11 and 3.28 ct€ per kWh with reduction, or 14.29–14.63 ct€ per kWh without the reduction. The partial reduction of EEG apportionment (6.88 ct€ per kWh full/0.18 ct€ per kWh reduced), grid fees (2.26–2.77 ct€ per kWh full/0.00 ct€ per kWh reduced) and electricity tax (2.05 ct€ per kWh full/0.00 ct€ per kWh reduced) is the most significant since they have the largest reduction potentials.
Another possibility for electricity purchase is based on participation in an operating reserve market (support of grid stability), where negative reserve (increase of load/H2 production) and positive reserve (decrease of load/H2 production) could both be provided. In our study, the German operating reserve market has been utilised as a model. Since market entry in this case is simplified (e.g. pooling is possible), there are more and more participants in the market, resulting in decreasing revenues. The exact economic benefit of participation in the control energy market, whilst an important global consideration, is not discussed in this study. Kopp et al. had a first evaluating insight into this topic in their work regarding the PtG plant in Mainz, Germany.44
It is important to note that PtM scenarios using grid electricity only produce eMeOH, if mainly RE is fed into the grid. The RE share will keep increasing following German/EU Climate Targets (>80% RE in 2050).44
Electricity purchase from a local onshore wind park with a rated power of 36 MW (scenarios 5 and 6): for the simulation, values at minute intervals have been applied. If insufficient wind electricity is available for H2 production (i.e. the amount required for eMeOH production), the difference is covered by either the flexible adaption of the eMeOH production rate (scenario 5) and/or an H2 storage (scenarios 5 and 6). A limited flexible operation of eMeOH synthesis is a novelty, and therefore for the purposes of this study is assumed technically feasible. For the wind electricity driven scenarios 5 and 6 an additional grid connection to acquire electricity in periods of low wind supply is not considered for the purpose of a remote renewable scenario. The levelized cost of electricity (LCOE) from onshore wind energy is assumed to be 4.4 ct€ per kWh,45 without any additional fees or taxes since wind electricity is used for personal consumption and without grid connection (as defined by the “Renewable Energy Act” (“Erneuerbare Energiengesetz” EEG) of the German Government in 2014 (§61, subsection 2, sentence no. 2)).46
H2 production and O2 valorisation
A PEM electrolyser (PEMEL) is adopted in this study for H2 production as a consequence of its ability to operate under both dynamic and steady state conditions with only a marginal reduction in efficiency.1,47 During dynamic operation, a PEMEL is capable of following fast load changes and load-ramp curves with high inclination (±138% PRL per min).42 Large-scale PEMELs with production capacities >10 N m3(H2) h−1 do offer efficiencies as high as alkaline electrolysers (AELs) (PEMEL: <ca. 5.3 kWhel per Nm3(H2); AEL (pressurised): <ca. 5.0 kWhel per Nm3(H2); both system-related); a positive technical learning curve has been traversed over the last few years, indicating that further efficiency improvements can be expected in the coming decade.48–50 Additionally, PEMELs operate very well under variable loads, down to PRL = 0% and an overload of up to PRL = 150% (for up to 15 min).51,52 The product purity from the PEMEL operation is high grade (i.e. H2 purity > 99.9 vol%), whilst the operating temperature is typically low (50–100 °C), facilitating a faster start-up (i.e. in comparison to an alkaline electrolyser).52 The operating PEMEL parameters evaluated in this study are displayed in Table 1.| Rated load, PRL [MW] | 12 |
| Efficiency, ηEly [kWh per Nm3] | 4.76 |
| Output pressure, PH2 [bar] | 30 |
| Grid connected scenario | P RL: 40% (day), 100% (night) |
| Wind electricity driven scenario | 0% < PRL < 100% |
Molecular O2 is produced as a high purity by-product and as such is included in this study as a consequence of its valorisation potential. The selling price depends on purity, with O2 purities > 99.99% (and without S or CO impurities) making it useful for many high-tech applications (e.g. medical use in hospitals, electronics industry, and oxy-fuel processing).53 If possible, O2 is utilised locally to avoid the associated energy intensive liquefaction and transport needs.3 For these reasons, the use of O2 from large electrolysers in nearby chemical/energy plants is considered the most appropriate option (e.g. in epoxide/polycarbonate production via oxidation of propylene and CO2 addition).54
CO2 sourcing
Technical processes for the capture of CO2 include both chemical and physical absorption processes (e.g. amine scrubbing, Rectisol®, Selexol®, cryogenic methods, etc.) based on the use of a variety of solvents, membranes and solid capture media.9,35,36,55–57 Likewise, there are various CO2 sources, which can be distinguished on the basis of volumetric output, CO2 concentration, presence of impurities, pressure level, CO2 origin (biogenic/fossil), impact on up-stream processes due to carbon capture, and CO2-price (prices from negative to positive possible).35,55 Which CO2 source is captured by which method depends on the operating conditions of the emitting process. Concurrently, the downstream CCU process has its own requirements regarding required purity, tolerable impurities and volumetric demand.In this study, two different CO2 sources are considered, namely a biogas treatment plant and an ammonia plant. In the case of biogas, CO2 is separated during a treatment process of the raw biogas. This is conventionally composed of CH4 (70–50 vol%) and CO2 (30–50 vol%), with the exact composition influenced by the biomass or waste being converted, which also dictates the presence of other compounds and impurities (e.g. N2, H2S, H2O, and organosulfur compounds). Taking Germany again as the example, the quality requirements for the feed-in of treated biogas to the gas grid are regulated.58,59 CO2 sourced from biomass is designated as biogenic.
For CO2 capture from an ammonia plant, it is assumed that CO2 is already a component of an existing ammonia production process and therefore is designated as fossil-based (from steam methane reforming as a process related step in ammonia production).60
H2 storage
All process scenarios examined in this study, apart from the full-load power supply scenario, include H2 storage facilities. In this study, the incorporated H2 storage units consist of medium pressure vessels operating dependent on a load capacity in the range 30 bar < PH2,storage < 200 bar. The required size and range of this storage has been identified, based on dynamic simulation via Matlab, to be in the range 100 kg(H2) < mH2,storage < 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg(H2). It is important to mention that H2 storage with a 50 tonne capacity would rather be realised as an underground cavern facility. However, due to a strict dependence on local conditions, the possibility of an environmentally sound disposal of the resulting brine, and socio-economic conditions in the case of underground cavern storage, and for a better comparability, the installation of storage vessels is assumed for all the evaluated scenarios in the present study.
000 kg(H2). It is important to mention that H2 storage with a 50 tonne capacity would rather be realised as an underground cavern facility. However, due to a strict dependence on local conditions, the possibility of an environmentally sound disposal of the resulting brine, and socio-economic conditions in the case of underground cavern storage, and for a better comparability, the installation of storage vessels is assumed for all the evaluated scenarios in the present study.
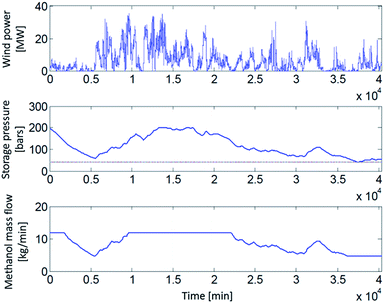
In the first of the examined scenarios 1 and 2 (i.e. grid connected, constant supply), no storage is necessary as a consequence of a constant H2 production. The resulting H2 mass flow of 5.47 t(H2) d−1 is directly converted to MeOH. For the other scenarios, H2 storage requirements depend on the assumed dynamics of the MeOH synthesis reactor (Fig. 2). In wind powered scenarios, MeOH mass flow is a function of the actual wind power, the H2 storage pressure and the possible dynamics of the MeOH synthesis reactor (Fig. 3).
Compressor
Both H2 and CO2 streams require compression since MeOH synthesis is favoured under pressure (here designated at 40 bar). The output pressure of the PEMEL is 30 bar. In the case of direct feed via a by-pass to the MeOH synthesis reactor, a single-stage H2 compression step is deemed technically appropriate. If sourced from H2 storage, a maximum pressure of 200 bar is available. For the compression of the PEMEL H2 product stream for storage (i.e. 30 to 200 bar), a two-stage reciprocating compressor is considered. For the CO2 stream where a compression from ambient to 40 bar pressure is necessary, a two-stage reciprocating compressor is utilised. The compressor specifications are based on the manufacturer's operational details (e.g. in terms of energy demand).MeOH production and distillation
The hydrogenation of CO2 takes place under exothermal reaction conditions (eqn (1)–(3)). The final theoretical MeOH yield is based on a stationary simulation model generated using the ChemCAD® software platform. The selected reactor type is designated as an ideal adiabatic tube reactor with a reaction pressure of 40 bar.42 For calculation of the CO2 amount needed for MeOH production, a per pass conversion efficiency of 90% is adopted resulting in 10% of the CO2 feed not being converted to MeOH. Evaluation of the simulation is based on the results produced from the hydrogenation of CO2 on a lab scale MeOH production facility operated at Fraunhofer ISE. In this study, the total theoretical amount of MeOH produced varies between 4000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tMeOH a−1.
000 tMeOH a−1.
Economic analysis – general framework
To establish a fair economic evaluation, the framework conditions for the study have to be defined (Table 2). Referring to the energy needed for eMeOH production, calculations include the electric energy demand. A large share of thermal energy demand results from distillation and is mainly covered by the exothermal MeOH synthesis. Cooling duties and advanced heat integration are not yet considered and will be addressed in a later report. Where possible, the economic evaluation is based on price data from manufacturers within the model market (i.e. Germany). Otherwise, values are taken from literature sources as indicated and adjusted accordingly for use with eqn (4).61 | (4) |
| Recovery period | 10 years | — |
| Interest rate | 2.5% p.a. | Ref. 62 |
| PEMEL specific investment | 800€ kW−1 | Ref. 48, 49, 63 and 64 |
| PEMEL grid connection | 66€ kW−1 | Ref. 48 |
| PEMEL stack life-time | 50k hours | Ref. 64 |
| PEMEL stack reconditioning cost | 2.0% of spec. invest. | Ref. 64 |
| PEMEL maintenance & insurance | 2.0% of spec. invest. | Ref. 64 |
| H2 storage specific investment | Depending on storage size | Ref. 65 |
| H2 storage maintenance & insurance | 2.0% of spec. invest. | Ref. 62 |
| MeOH synthesis specific investment | 810€ t−1 a−1 | Ref. 66 |
| MeOH synthesis maintenance & insurance | 10.0% of spec. invest. | Ref. 66 |
| Other installation maintenance & insurance | 2.0% of spec. invest. | Ref. 62 |
| Technical staff | 14 h per week at 100€ h−1 | Own estimate |
| Plant buildings and facilities | 7.0% of spec. invest. | Ref. 64 |
| Engineering, planning, delivery, and setup | Inspired by internal experiences | — |
If not included in the component's investment cost, additional costs for delivery and setup are added at a proportionate share of the total investment cost (TIC). Maintenance costs are either based on literature values for the specific component or comparable processes. Not considered in this economic evaluation are costs associated with land, buildings or employees. As mentioned earlier, alongside MeOH, the by-product of H2O electrolysis, namely O2, is also considered as a valuable product in the plant and its potential sales are also included. Credit for O2 is considered at 50€ t(O2)−1,67 which is a relatively conservative value. Atsonios et al.29 and Matzen et al.30 considered a credit of ca. 75€ t(O2)−1 for their calculations (see Table 5) (NB: any possible future increase of installed electrolysis capacity may act to decrease the impact of the O2 market value, unless a large increase in oxidation chemistry is anticipated).
The supply cost of CO2 differs for different sources. From the considered biogas plant source, the CO2 production cost is assumed to be 0€ t(CO2,capt.)−1, as this is essentially a waste product from biogas cleaning and therefore, the cost for separating CO2 is assigned to CH4 production cost. Based on ammonia production, CO2 capture costs are 3€ t(CO2,capt.)−1.36,68 Furthermore, assuming that avoided CO2 emissions will also be considered within the European Emission Allowances (EUA) in the near future, a projected price of −10€ t(CO2,emitt.)−1 is obtained. These allowances are tradable and the resulting CO2 feedstock price is −7€ t(CO2)−1 (Table 3). Biogas treatment plants do not take part in the EUA trade, since the emitted CO2 is of biogenic origin.
| Biogas treatment plant | Ammonia plant | |
|---|---|---|
| Capture cost [€ t(CO2,capt.)−1] | Excluded | 3 |
| CO2 certificate price [€ t(CO2,emitt.)−1] | 0 | −10 |
| CO2 feedstock price [€ t(CO2)−1] | 0 | −7 |
| Output pressure [bar] | Atmosph. | Atmosph. |
| Environmental burden | Biogenic origin | Fossil origin |
The operating and full load hours of the PEMEL and the MeOH synthesis reactor differ for the different scenarios. For purely wind electricity driven scenarios, the PEMEL full load hours are in the range of 3585 to 3896 h a−1 and the operating hours (the period during which the PEMEL is operational at any load factor) at 7044 h a−1 (Table 4). The costs for maintenance and replacement of the PEMEL stacks do reflect the specific operating hours and a predicted stack life-time of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h. Furthermore, the yearly amortisation payments are calculated based on a linear depreciation over 10 years.
000 h. Furthermore, the yearly amortisation payments are calculated based on a linear depreciation over 10 years.
| Grid connected constant supply | Grid connected electricity price driven | Wind electricity driven flexible | Wind electricity driven non-flexible | |
|---|---|---|---|---|
| PEM-electrolysis – production capacity [t a−1] | 1906 | 992 | 791 | 1210 |
| PEM-electrolysis – energy demand [GWh a−1] | 100.80 | 55.44 | 43.02 | 46.75 |
| PEM-electrolysis – operating/full load hours [h] | 8400/8400 | 8400/4620 | 7044/3585 | 7044/3896 |
| Hydrogen storage – volume [m3] | — | 6 | 345 | 2840 |
| Carbon capture – capturing capacity [t a−1] | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 225 |
7765 | 6392 | 9780 |
| Methanol reactor – production capacity [t a−1] | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031 031 |
5139 | 4188 | 6408 |
Results and discussion
Investment cost
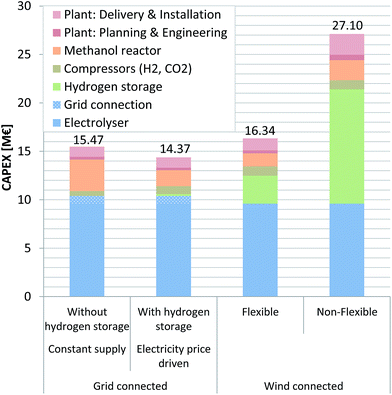
The TIC for every investigated scenario is found to be within the margin of 14.4–27.1 M€ (Fig. 4). The TIC is strongly influenced by the investment cost related to PEMEL use. The PEMEL is a necessary procurement, with an assumed cost of 800€ kWinst−1 without and 866€ kWinst−1 with grid connection related to installed capacity (kWinst). Considering that the mid-term (for the year 2023–2030) cost of a PEMEL on the MW-scale ranges from 750 to 1600€ kWinst−1,48,49,63,64 these values can be seen as an optimistic target value. The European Multi-Annual Implementation Plan of the Fuel Cells and Hydrogen Joint Undertaking (FCH JU) targets 720€ kWinst−1 for PEMEL systems with production capacities <1000 kg(H2) d−1.51 Schmidt et al., based on interviews with experts from industry and academia, quantify PEMEL specific investment in 2030 between 850 and 1650€ kWinst−1 under the assumption of no changes in technology funding and without a production scale-up. Intensified R&D funding as well as a production scale-up could lead to a further 24% reduction in specific investment.52 Saba et al. reported even lower cost estimations of 397–955€ kWHHV-output−1.50All scenarios take a PEMEL as a basis, even though the grid-connected scenarios would be able to function reasonably using an AEL electrolyser. As mentioned earlier, the TIC will likely decrease in the future as a consequence of further technology development, efficiency improvements, and more widespread use, with a target value of 440€ kW−1 considered desirable for large water electrolysis systems >10 MW.67 The 12 MW PEMEL included in this investigation has a TIC of 9.6 M€ (without a grid connection) and 10.4 M€ (with grid connection), representing ca. 60–70% of the TIC of the overall process. An exception to this observation is the wind-driven scenario using a non-flexible MeOH reactor (scenario 6), since a large H2 storage is included, representing a significant expense in itself (i.e. TIC (PEMEL) = 35%; TIC (PEMEL + H2 storage) = 79%). Mignard et al. arrived at a similar conclusion in a previous report where the electrolyser's share of the TIC is within 65–82%, by far the biggest share, followed by the H2 storage and compression cost, with a share of 9–16%.27 The impact of investment in the MeOH reactor depends on the annually produced MeOH, which is assumed in this study to be 810€ t−1 a−1.66 Therefore, the grid connected scenario without H2 storage (scenario 1) has the highest MeOH reactor cost (3.3 M€), since this scenario produces the greatest amount of product. The potential of additional cost for a flexible reactor is not considered in detail. Excluding this mentioned scenario, the MeOH synthesis reactor's influence is comparatively low. For future assessments a detailed investigation of the cost incurred by a dynamic reactor design should be included. Additionally, since methanol from syngas with high CO2 content and H2 potentially offers higher purity (free of most ketones, paraffins and other by-products) compared to methanol from syngas, a reduced demand for distillation could have a cost decreasing effect.23,69
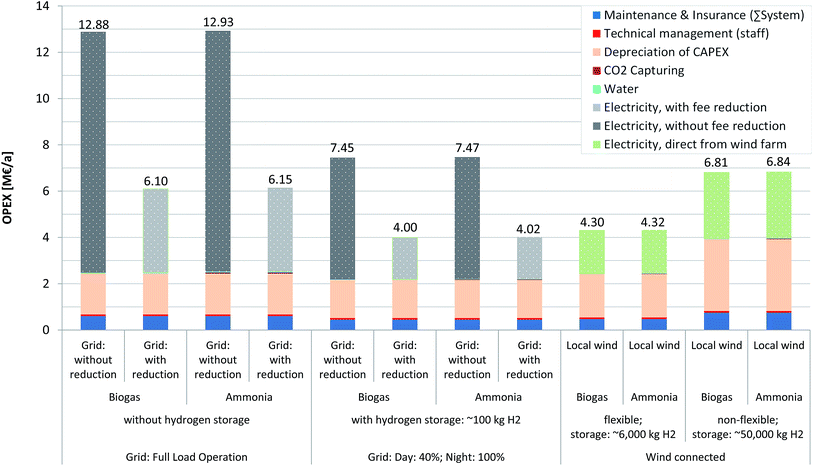
Operational & annual costs
Each scenario has a different operational mode with different resulting operating costs, ranging between 4.00 and 12.93 M€ a−1 (Fig. 5). It can be seen that the main factor is electricity cost, as long as a reduction in taxation/fees is not possible (left bar of each scenario). In that case, the operational cost (OPEX) is ca. 2.5 times higher than in scenarios including a reduction, which results in a share of electricity cost of ca. 70–81% of the OPEX (with 45–59% reduction). Wind energy-based scenarios however indicate that the electricity cost share lies approximately between 42 and 44% and therefore represents a reduction relative to grid-connected scenarios. Due to the high electricity demand of the PEMEL, slight changes in the electricity price have significant impacts on the electricity cost. A similar observation was made by Atsonios et al.29 Although this report assumed a low electricity price of 2.9–5.0 ct€ per kWh, electricity still represents the largest share of the OPEX. The CO2 feedstock costs by comparison (e.g. for ammonia plant scenarios) are very low (i.e. 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–46
000–46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 € a−1). As mentioned earlier, the trade of the EUA in the amount of recycled CO2 is assumed to be possible in the near future. Since this generates a negative value, it may even lead to positive revenues (Table 3). However, the assumed certificate price is very low at the current time (ca. 10€ t(CO2)−1) and the revenue, based on current values, is accordingly small. It is important to consider that the EUA value is increasing and a rise to just 30€ t(CO2)−1 would lower the OPEX by between 1.7 and 5.0% (or 0.17–0.3 M€ a−1).
000 € a−1). As mentioned earlier, the trade of the EUA in the amount of recycled CO2 is assumed to be possible in the near future. Since this generates a negative value, it may even lead to positive revenues (Table 3). However, the assumed certificate price is very low at the current time (ca. 10€ t(CO2)−1) and the revenue, based on current values, is accordingly small. It is important to consider that the EUA value is increasing and a rise to just 30€ t(CO2)−1 would lower the OPEX by between 1.7 and 5.0% (or 0.17–0.3 M€ a−1).
Methanol production cost
The eMeOH production cost (MPC) is calculated using eqn (5). | (5) |
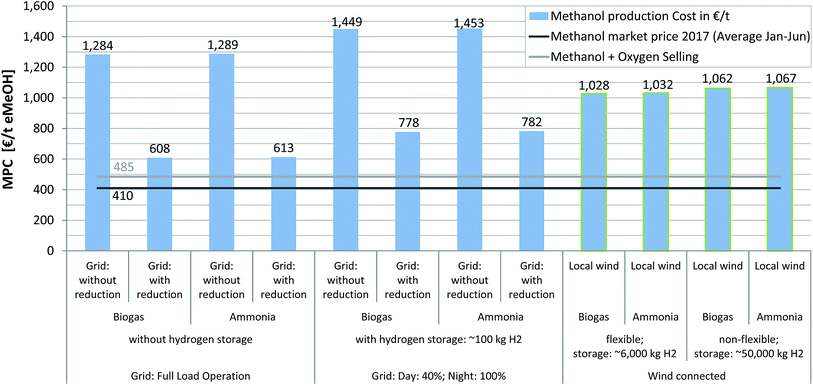
Currently, the MPC of all considered scenarios (Fig. 6) is above the actual market price (i.e. Methanex average 2017 (Jan to June):70 410€ t(eMeOH)−1), which has featured a low-level upward trend of +3.20€ per month over the last 2.5 years. The scenario with the lowest MPC (scenario 2B, grid connected with constant supply, fee reduction, without H2 storage, CO2 from biogas, 608€ t(eMeOH)−1) costs 1.5 times the possible market revenues, whereas the most uneconomical scenario (scenario 3A, electricity price driven, without fee reduction, with H2 storage, ammonia, 1453€ t(eMeOH)−1) is 254% above the 2017 market price level. The electricity price has a huge impact on the MPC: where a fee reduction is not possible, MPCs are in the range of 1284 to 1453€ t(eMeOH)−1. If taxation and fees do not have to be paid in full, the MPC will decrease to 608–782€ t(eMeOH)−1. The wind driven scenarios feature MPCs in the range of 1028–1067€ t(eMeOH)−1, which are characterised by large H2 storage capacities and associated high investment costs.
In this study, a positive value of 50€ t(O2)−1 for selling the electrolyser by-product was used. Previously Atsonios et al. considered an O2 revenue of 87€ t(O2)−1 reflected in a decrease in eMeOH production cost (ca. 10% reduction as a consequence of O2 sales).29 Matzen et al. also included O2 sales in their process evaluation and concluded that a price of 75€ t(O2)−1 had a positive impact on the economic feasibility of their process.30 Likewise, Rivarolo et al. assumed a much higher market value of 150€ t(O2)−1.34 Compared to the 50€ t(O2)−1 used in this study, this results in a difference of 150€ t(eMeOH)−1 (1.5 t(O2) t(eMeOH)−1 produced). For further analysis a detailed evaluation of the necessary process steps for pressurizing, bottling and transportation of O2 has to be included as well as a discussion of the development of the O2 market price in the case of increasing market penetration for electrolyser technology.
Sensitivity study
The economic feasibility of PtM is influenced by a number of parameters, and it is essential to know which parameters, besides the obvious electricity price, have a high impact on the process economy. Therefore, a sensitivity study was performed for the wind-powered, flexible MeOH synthesis based on biogas-derived CO2 (scenario 5B). Based on a biogenic CO2 source and a local supply of wind power the chosen scenario represents the most preferable option up to now regarding the establishment of a sustainable eMeOH production route. Only one parameter has been varied at a time; the unchanged parameters are based on the one set for the economic evaluation of scenario 5B (see also Table 2). Displayed are the costs (negative slope) and benefits (positive slope); the steeper the slope the stronger the influence of the parameter on the process economy (Fig. 7). The most important factor is identified to be the MeOH selling price, since it has a direct impact on the specific profit. Increasing MeOH selling prices are consequently directly visible in the process economics. The electrolyser investment is another important impact factor. At 12 MW, a reduction of the specific investment has a large impact on the TIC. A specific investment reduction of only −10% leads to an investment decrease of 1 M€ (−6.2% of the TIC). If an investment target value of 440€ kW−1 for water electrolysis systems could be reached, the TIC would decrease by an amount of ca. 4.3 M€ (−27% of the TIC). Regarding the expected price decrease of the PEMEL, eMeOH production costs should decrease in the long term. Thirdly, as already discussed in the context of operational cost, the electricity price has a strong impact on the overall process economy. Marginal changes in electricity price are directly visible in the eMeOH production cost. Therefore, the availability of green but inexpensive electricity (e.g. in periods of excess or over-production and regions with significantly lower RE generation costs) is a basic prerequisite for an economic elaboration of PtM. Although the investment in the methanol synthesis reactor is not a key indicator for the TIC, scaling-up of the methanol reactor will also have a decreasing effect on the reactor specific investment cost and hence on the TIC of the PtL systems. Hence, to improve the economic viability of PtM and decrease the difference between the current market price level and the production cost, the above-mentioned parameters are important starting points to be considered and in turn optimised.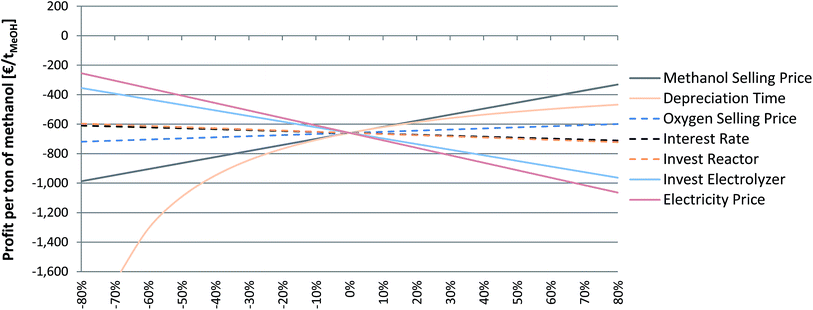 | ||
| Fig. 7 Sensitivity study for the wind powered, flexible synthesis based on biogas-derived CO2 (scenario 5B). | ||
Evaluation
To provide an overview and classification of the results generated from this study, in comparison with previously reported studies and data, Table 5 has been compiled. In this comparison, monetary values that were not given in € were calculated based on a time-dependent currency exchange rate (the average exchange rate in the year of the study). For a better comparability of the values, eMeOH production costs were also converted based on the current currency exchange rate (average 2017). For the calculation of production capacity, 350 days per annum were assumed if no value for working h a−1 was available. All values not directly stated in the studies were calculated on the basis of the available values (where possible).| Electricity | Electrolyser | CO2 | Storage | Methanol reactor + distillation | Source | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Price [ct€ per kWh] | Type | Spec. invest [€ kW−1] | O2 price [€ tO2−1] | Source | Spec. costa [€ tCO2−1 a−1] | H2 | CO2 | Spec. investb [€ tMeOH−1] | Plant capacity [t d−1] | Pressure level [bar] | Plant depreciation time [a] | Methanol production cost [€ tMeOH−1] | |
| a Without including CO2 certificate revenues. b Whole methanol plant. c Currency exchange rate in the year of the study (average). d Currency exchange rate of 2017 (average). e Calculated with the daily needed hydrogen amount for methanol production. f Outside system boundaries. g Assumed purchase price for hydrogen: 3090€ t−1. h Assumed purchase price for hydrogen: 3000€ t−1. i Conversion with low heating value (LHV), RES = renewable energy sources, CPP = coal power plant, and CHP = combined heat and power plant. | ||||||||||||||
| Grid | 3.74–13.43 | PEM 12 MW | 800 | 50 | Biogas/ammonia | 0/3 | No | No | 1514 | 29 | 40 | 10 | 608–1615 | This study |
| Grid | 3.67–14.13 | PEM 12 MW | 800 | 50 | Biogas/ammonia | 0/3 | 6 m3 | No | 2745 | 15 | 40 | 10 | 816–1969 | This study |
| Wind onshore | 4.4 | PEM 12 MW | 800 | 50 | Biogas/ammonia | 0/3 | 345 m3 | No | 3830 | 12 | 40 | 10 | 1040–1051 | This study |
| Wind onshore | 4.4 | PEM 12 MW | 800 | 50 | Biogas/ammonia | 0/3 | 2840 m3 | No | 4144 | 19 | 40 | 10 | 956–967 | This study |
| Hydro power | 1.3 | — | — | — | Flue gas/atmosphere | — | — | — | — | 200 | — | 15 | 258/387 | 1999-Specht and Bandi |
| Hydro power | 2.5 | — | — | — | CPP/atmosphere | — | — | — | — | 200 | — | 15/20 | 529/717 | 1999-Specht et al. |
| RES | 1.5–2.2 | AEL 100/500 MW | 422–1409 | 102 | Flue gas (CPP) | — | Floating head/pressurised tanks | — | 1299–2420 | 178–372 | 50 | 15 | 498–711c | 2003-Mignard et al. |
| 429–613d | ||||||||||||||
| Grid/RES | 4 | AEL 2 MW | 200 | Sold/used | Flue gas (CHP) | 15 | 100–900 tH2 underground gas storage | No | — | 890 | 144 | — | 555 | 2010-Clausen et al. |
| Grid/CPP | 2.9–5 | AEL 140 MW | 376 | 87 | Flue gas (CPP) | 44 | Partially | — | — | 318 | 65 | 25 | 870–913 | 2015-Atsonios et al. |
| Wind | 6.3 | AEL 41 MWe | 440 | 75 | Ethanol plant | — | Pressurised storage | Liquid storage | 850 | 97 | 50 | 10 | 324–781c | 2015-Matzen et al. |
| 389–938d | ||||||||||||||
| CPP | 9.5–12.1 | —f,g | —f | —f | Flue gas (CPP) | 0 | — | — | 497 | 1320 | 76 | 20 | 724 | 2015-Perez-Fortes et al. |
| —f | 9.3 | —f,h | —f | —f | Purchased | 50 | Yesf | No | 1329 | 138 | 75 | 10 | 980 | 2015-Tremel et al. |
| RES | 2.6/3.3 | SOEC 50 MW | — | Used | Flue gas (CPP) | 20 | — | Buffer storage | 1000 | 96/235 | — | 20 | 450/484 | 2015-Varone et al. |
| — | 5 | SOEC 20 MW | 1469–5785 (SOEC) | Sold | — | 3–10 | — | — | 6115 (SOEC) | 58 (SOEC) | 78 | 20 | 5459 (SOEC) | 2016-Rivera-Tinoco et al. |
| PEM 24 MW | 600 (PEM) | 883 (PEM) | 49 (PEM) | 891 (PEM) | ||||||||||
| RES | 1–5 | PEM 2190 MW | 700 | 40 | Flue gas | 40 | — | — | 1247–2495 | 5003 | — | 15 | 400–2775i | 2016-Bertau et al. |
| RES + grid | 3–5 | AEL 1 MW | — | 150 | Biogas/purchase | 10 | — | — | — | — | 80 | 10 | — | 2016-Rivarolo et al. |
Due to the different parameter assumptions used in the calculation of eMeOH production costs, the results vary considerably from study to study. Therefore selection of system boundaries, system components and framework conditions is critical as they have a significant impact on the final results and as a consequence, different studies should be compared carefully.
In this context, CO2 sourcing differs from study to study. Typically, flue gas of a Coal Power Plant (CPP) is used. Varone et al. evaluated an integrated oxy-fuel/co-electrolysis process, where the oxy-fuel coal plant delivers the CO2 and the O2 by-product from the electrolysis is used for the oxy-fuel process.32 Such combined processes are indeed logical but have not been considered thus far by other studies. Besides CO2 captured from coal fired power plants is of fossil origin and should therefore not be part of a future energy system with the intention to provide sustainable fuels. The use of CO2 from the atmosphere has been considered by Specht et al.,25,26 and was recently revisited by the German-Swiss initiative of Audi, Sunfire and Climeworks.71 More recent studies have considered this possibility but assess the acquisition cost to be still too high (e.g. Bertau et al.3). The EUA has, except for this study, so far not been considered by other authors. Depending on the source, the assumed costs to provide CO2 lie between 0 and 50€ t(CO2)−1.
Compared to the actual MeOH market price of 410€ t(MeOH)−1 (Methanex, average 2017),70 almost all reported production costs are above the market price and the production via H2 and CO2 is as such not considered economical (when excluding additional O2 sales). The cost of producing fMeOH from conventional processes via natural gas or coal varies depending on the geographical location of the production site between 51€ t(fMeOH)−1 and 408€ t(fMeOH)−1. They are therefore less expensive than any process based on RE, H2 and CO2.6 If legislation or regulation regarding either RE or CO2 conversion is established (e.g. within the EU), it remains to be seen how long these relatively low prices will be maintained. The smallest difference compared to the actual price level was reported in 1999, by Specht and Bandi (i.e. an eMeOH production cost of 258–387€ t(eMeOH)−1).25 This report was based on an unrealistically low electricity price of 1.3 ct€ per kWh (in 1999), leading in turn to low eMeOH production costs. However, recent reports demonstrate the rapid reduction in renewable electricity costs (e.g. solar power in Dubai72,73 or Chile74,75 with prices <3 ct€ per kWh).
The highest eMeOH production costs (excluding the production via High Temperature Solid Oxide Electrolysis)33 with >2000€ t(eMeOH)−1 were reported by Bertau et al.6 In this work, eMeOH production costs depended on the electricity price and operating hours per year. With less than 2000 h a−1, the MeOH production costs rise sharply, reaching values up to 2800€ t(eMeOH)−1. Their calculations show clearly that the maximisation of operation is desirable regarding an economical production. A comprehensive PtL-overview focussing on electrofuels for the transport sector is provided by Brynolf et al.(2018).76
CO2 avoidance cost
The economic inefficiency of the evaluated PtM scenarios on the one hand results from the energy and capital intensive structure of the PtM process itself. On the other hand cheap production conditions in the case of fossil based methanol production are reflected in a low market price. The lack of performing legislation taking into account the environmental burdens resulting from the production and provision of fossil based syngas leads to a ‘free-lunch’ for coal and methane based production of methanol. The internalization of direct and indirect environmental damage would mean further expenses for (up-stream) production processes which are very intense in terms of, for example, CO2-, CFC-11- and SO2-equivalents (eq)§ and can result in thorough land-occupation and -change. In terms of climate change and curtailing global warming CO2eq-emissions are in the focus of scientific and political discussions. We evaluated the global warming potential (besides other impact categories) in a profound life-cycle-assessment (LCA) for the ‘green’ methanol production. In the frame of the present study we establish a first interconnection between the higher production costs and the possibly lower CO2eq-footprint of the green methanol pathway compared to the fossil reference¶ by calculating the CO2 avoidance cost: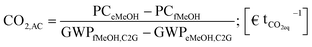 | (6) |
The CO2 avoidance cost (CO2,AC) (eqn (6)) is expressed as the ratio of the differences between the production cost (PC) of green (‘electricity based’) and fossil based methanol (PCeMeOH and PCfMeOH respectively) and their respective global warming potentials (GWPs).
The CO2,AC can be seen as a key performance indicator when discussing the future of CCU processes and the establishment of an economy covering the majority of its C-demand with recycled carbon. It is important to note that the CO2 avoidance cost is an indicator for the connection of economic and ecological efficiency besides many other ecological parameters such as water demand and indirect land use change or occupation. For a holistic evaluation of PtM and other CCU processes and their comparison to fossil based references a multitude of economic and, even more importantly, ecological parameters have to be included in a process' evaluation.
For the production cost of green methanol the results obtained in scenarios 5B and 6B with production cost of 1028 and 1062€ t(eMeOH)−1, respectively, have been used. The production costs for fossil methanol depend on geographical location, ranging from 51€ t(fMeOH)−1 (Saudi Arabia) to 408€ t(fMeOH)−1 (Europe) and are dominated by the cost for the syngas feedstocks.77 In order to put a price on the amount of avoided CO2eq not emitted within the European region we decided to compare ‘our’ green methanol pathway (mainly dominated by the German electricity prices) with any fossil-based methanol production facility located within European borders. Therefore for this evaluation the production costs for fossil methanol are set to a ‘European level’ of 400€ t(fMeOH)−1.|| It could be argued that for other regions featuring significantly lower production costs for fossil methanol such as Saudi Arabia or China the difference between cheap fossil and cost-expensive green methanol production would increase. But likewise a lower levelised cost of renewable electricity would have a clear decreasing effect on green methanol production cost and would partly balance the cheap market conditions for the fossil production.
Regarding the GWP for the green methanol production the values are based on a life cycle assessment based on hydrogen from a wind-powered PEM electrolyser in combination with CO2 from a biogas upgrading plant** (representing scenarios 5B and 6B). The LCA was performed using the Umberto NXT Universal software with the Ecoinvent-v3.3 database. With a GWP of 506 kg(CO2eq) t(eMeOH)−1 during the production phase and an uptake (‘molecular binding’) of 1374 kg(CO2) t(eMeOH)−1†† the PtM process can be seen as ‘netto-negative’ (506–1347 = −868 kg(CO2eq) t(eMeOH)−1) in terms of CO2eq-emissions when evaluated by a cradle-to-gate approach. Expanding the system boundaries by containing also the utilisation of the produced methanol (cradle-to-grave, C2G),‡‡ all C-content will be oxygenated (1388 kg(CO2eq) t(MeOH)−1) again and released into the environment. Utilisation could be in the MeOH-form as a partial fuel substitute or further processed to downstream derivatives such as DME or OME3–5. In this case the resulting cradle-to-grave emissions would add up to 520 kg(CO2eq) t(eMeOH)−1.§§
As a reference for the GWP of a European based fossil methanol production process we used the Ecoinvent process ‘methanol production [GLO] from natural gas’.¶¶ Where possible the contained activities have been adjusted to an assumed methanol production in Europe. This accounted for the supply of natural gas for syngas production and the market for electricity as well as the provision of tap water. The adjusted EU-based fossil methanol production results in slightly higher specific CO2eq-emissions (cradle-to-gate: 526 kg(CO2eq) t(fMeOH)−1 (global) vs. 623 kg(CO2eq) t(fMeOH)−1 (EU-based)) which mainly arise due to the transport intensive provision for the syngas production with natural gas/process heat in Europe (GWP100a: +18.3 and +33.4% resp.). Concurrently the electricity supply shows a lower GWP (GWP100a: −36.0%) presumably due to higher efficiencies in fossil electricity production and higher shares of renewables within the EU. For fMeOH also a complete oxygenation of the C-content is assumed for the utilization phase (1388 kg(CO2eq) t(fMeOH)−1) resulting in cradle-to-grave-CO2eq-emissions of 2011 kg(CO2eq) t(fMeOH)−1.
Based on these values the CO2 avoidance costs are 421€ t(CO2)−1 (scenario 5B) and 444€ t(CO2eq)−1 (scenario 6B) giving emitted CO2 significantly higher prices than the recent prices envisioned by the European Emission Trading Scheme (EU-ETS) with CO2-certificate prices of ca. 5€ t(CO2eq)−1 for the last 12 months (Sept 16 to Aug 17).||||
Future development of methanol production cost & the corresponding CO2 avoidance cost
For future development of the parameters until 2035 we assumed the development of some central sensitive parameters. The initial point for this assessment sets scenario 5A. Parameters are set considering in-house studies for the decrease of large-scale PEMEL specific cost (2035 @ 300€ kWinst−1), a significant increase in CO2 certificate prices (2035 @ 80€ t(CO2)−1),78 and a further decrease in the levelised cost of renewable electricity in general and PV-electricity in particular (2035 @ 1.8 ct€ per kWhel).72–75 The resulting predictions in green methanol production costs and the corresponding CO2 avoidance costs are compared with a constant (business as usual, ‘bau’) and the exponential forward projection of the methanol market prices (‘exp’) based on the Methanex European posted contract price for the last 15 years.70Fig. 8 contrasts the resulting development of eMeOH with an increasing methanol market price, demonstrating a prediction for market parity in ca. the year 2032. At around the same time the CO2 avoidance cost will turn negative (based on the assumptions made regarding investment and operational costs) which can be interpreted as the market offering an economic incentive to the industry for utilising their CO2 emissions instead of emitting them. These points of time fit the published results for the development of the market demand for electrolyser technology showing a clear market launch from the year 2032.78,79 Furthermore a future increase of the GWP for fMeOH production could be assumed due to the necessity of a more intensive exploration of fossil resources and longer transport distances. This would have an additional lowering effect on CO2 avoidance cost, which was not considered here.
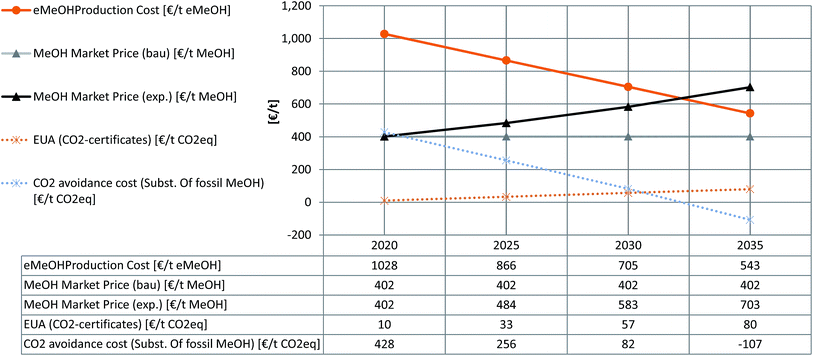 | ||
| Fig. 8 Estimation for the future development of green methanol production cost and CO2 avoidance cost in the case of fossil methanol substitution. | ||
Conclusions
The presented evaluation shows that the economic feasibility of a specific PtL process (i.e. for eMeOH) strongly depends on electricity and H2 production cost, CO2 cost (also reflecting any introduced carbon taxes), specific electrolyser cost and the possible dynamics of the methanol reactor (having an impact on the necessity and size of H2 storage). Furthermore, indirect parameters such as carbon taxes will have an impact on the willingness of the market to pay for eMeOH (and derived sustainable chemicals) and as such can be considered desirable.Under the evaluated process conditions in this study, the production of eMeOH in Germany is currently not economically competitive, if one presupposes the competition with fossil based large capacity methanol production and its current low price. The investment and operational costs are currently inhibitory and therefore, the eMeOH production price exceeds the expected revenues. Currently, conventional large scale methanol production based on CH4 reforming has a very low price due to very inexpensive fossil supply (e.g. from increased fracking in the USA and increased production of fMeOH from coal in China). A high degree of capacity utilisation is advantageous and favourable for low eMeOH production cost. Therefore maximised plant operation (e.g. in terms of hours) is also to be highlighted as an important factor in achieving eMeOH economic feasibility.
The presented sensitivity study demonstrates that a decrease in the specific investment costs of the PEMEL leads to a major economic efficiency improvement for the described PtM process. Heading towards the target value for specific electrolyser investment (target value: 440€ kW−1) is in general an important step towards the economic feasibility of PtL-systems. The low-emission eMeOH production scenario (wind energy driven) had a higher production cost due to the additional requirement for H2 storage (i.e. 2nd largest share of investment costs). A reduction in specific H2 storage size and costs would therefore be beneficial. The possibility of underground H2 storage in salt caverns (e.g. in northern Germany) depends on local conditions but offers a promising way for mitigating the strong interlinkage between H2 storage volume and its associated investment costs.64 In the long-term it will be necessary to offer possibilities for a more dynamic operation of the synthesis step to reduce the storage demand for H2.
To successfully introduce any PtL process, it is initially important to think of potential synergies: the methanol production of Carbon Recycling International is underpinned by low-cost geothermal electricity and CO2 from a geothermal power plant (located adjacent to the plant). Thyssenkrupp AG is aiming to significantly cut their CO2 emissions by utilising the waste gases from steel production (coupled with additional H2 production) to generate side streams of fuels and chemicals.80 The presented PtL scenarios are all on a small-scale compared to fossil based plants on the Mt-scale. Future large-scale PtL-plants, coupled with existing chemical or heavy industry, would also have a number of advantages: instant access to low-cost grid electricity, availability of (highly concentrated) CO2 streams (which still need purification) or unused H2 in off-gases, if necessary access to HT-process heat/steam and last but not least, the vast cost-reducing effect of a PtL plant scale-up.
Regarding policy instruments to support the introduction of economically viable PtL schemes, amendment of general policy frameworks and taxation systems (e.g. special grid fees for energy storage and grid-supporting technologies, and an improved European Emission Trading System) would be beneficial. A modified taxation system for CO2 emissions could generate a CO2 certificate price and market conditions appropriate for an industrial business case for CCU. Another approach could be a tax reduction for “renewable fuels”. The gradual reduction of fossil fuel subsidies would also aid in the long term attractiveness of PtM schemes, particularly as the amount of the RE share in the electric grid increases. The proposed ‘double counting’*** of 2nd generation biofuels according to the revision (iLUC Directive 2015/1513/EC81) of the RE directive RED (2009/28/EC82) is a first step when also including CO2-based fuels. Regarding fossil-based production, environmental costs and impacts are generally not internalised and therefore factoring environmental burdens would lead to a higher price of fMeOH. One example is the introduced foundation for climate protection and CO2-compensation (Foundation for Climate Protection and Carbon Offset (Stiftung Klimaschutz und CO2-Kompensation, Klik)),83 following the revision of Swiss CO2-law. It commits producers and distributors of fossil fuels to provide compensation for the environmental impacts of their products and in turn provides investment in funding programs focusing on Swiss GHG emission reduction.
The presented analysis highlights that the CO2 avoidance costs for the evaluated wind electricity based scenarios are currently in the range of 421–444€ t(CO2eq)−1 avoided and strongly depend on the process and market conditions within the selected scenarios. The process conditions (electrolyser efficiency, synthesis pressure, source of CO2, etc.) not only influence eMeOH production cost but also the results for the CO2eq footprint and thus the CO2 avoidance cost. It has been shown that for future technological optimisation of some CCU key components (resulting in the improvement of central economic process parameters) even for cost intensive 100% RE based set-ups the green methanol production cost can be strongly decreased (543€ t(eMeOH)−1, −47%, scenario 5A) within the next two decades bringing market competitiveness with fossil based methanol within the realms of possibility. In the same time frame the CO2 avoidance cost will feature a clear drop (−124%) resulting in negative values and through this provide an incentive for industries to reinterpret their CO2 emissions from waste to feedstock.
It is important to consider that the CO2 avoidance cost should be used only as one key-criterion for the selection among the variety of possible CCU processes.84 In combination with the specific CO2 avoidance potential of each PtL-/CCU-technology, the most cost- and eco-efficient routes should therefore be selected. In this context, a detailed Life Cycle Assessment (LCA) is mandatory to identify the specific CO2 avoidance cost and potential. Positively, the analysed technology will be supported by (and also support) the rapidly growing and intended expansion of RE (e.g. in Germany and the EU in general) over the next few decades. This growth will necessitate consideration of technologies such as PtL, further supported in terms of economic readiness by the ever reducing RE price per ct€ per kWh. PtX technologies will be one important pillar of the integrated-energy concept (‘sector-coupling’) within the European markets of electricity, heat and mobility. Further postponement of integrating renewable energies in the mobility sector and chemical industries will further impede the climate protection programs.79
Our results demonstrate that off-grid wind electricity is promising for eMeOH production, but in the case of an isolated wind energy system and without large H2 storage it cannot ensure a high degree of capacity utilisation. For realisation of an off-grid renewable PtL process, the coupling of wind power with photovoltaic or solar thermal electricity generation and/or additional baseload supplies (e.g. biogas plants or combined heat and power plants based on biomass, offering also a biogenic CO2 point source) would lead to higher capacity utilisation and a reduced storage demand. Thinking globally, relocation to regions with higher solar irradiance (e.g. the Maghreb region, Australia or south western parts of North America) and/or reliable wind power (e.g. Chile, Peru, South Africa, or Scandinavia) offers the possibility of a low cost RE supply. Low cost RE electricity of around 2.5 ct€ per kWhel (reflecting recent price bids for PV electricity72–75) and a higher degree of capacity utilisation due to a less fluctuating RE potential MPC of ∼500€ t(eMeOH)−1 and below are in a realistic range. Regions with a current and prospectively low RE production cost provide opportunities for the further development of a business case for PtL (and PtX) technology within the coming decade. Further installation of pilot plants and the associated operational experiences, coupled with expected economies of scale, will lay a path to improved PtL economics, even in countries where RE electricity costs are currently prohibitive.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
RJW is grateful to the Fraunhofer Society and Fraunhofer ISE for providing financial support via the Attract programme.References
- Energiespeicher – Bedarf, Technologien, Integration, ed. M. Sterner and I. Stadler, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014 Search PubMed.
- A. Palzer and H.-M. Henning, Renewable Sustainable Energy Rev., 2014, 30, 1019 CrossRef.
- K. Räuchle, L. Plass, H.-J. Wernicke and M. Bertau, Energy Technol., 2016, 4(1), 193 CrossRef.
- Bundesnetzagentur für Elektrizität, Gas, Telekommunikation, Post und Eisenbahnen, Monitoringbericht 2017: Monitoringbericht gemäβ §63 Abs. 3 i. V. m. §35 EnWG und §48 Abs. 3 i. V. m. §53 Abs. 3 GWB, Bundesnetzagentur, 2017.
- R. Schlögl, Top. Catal., 2016, 59(8–9), 772 CrossRef.
- Methanol: The Basic Chemical and Energy Feedstock of the Future, ed. M. Bertau, H. Offermanns, L. Plass, F. Schmidt and H.-J. Wernicke, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014 Search PubMed.
- United Nations, Paris Agreement, Paris, 2015 Search PubMed.
- M. Z. Jacobson, Energy Environ. Sci., 2009, 2(2), 148 CAS.
- G. A. Olah, A. Goeppert and G. K. S. Prakash, Beyond Oil and Gas: The Methanol Economy, Wiley-VCH, Weinheim, 2009 Search PubMed.
- A. Otto, Chemische, verfahrenstechnische und ökonomische Bewertung von CO2 ans Rohstoff in der chemischen Industrie, Dissertation, Forschungszentrum Jülich, Aachen, 2015.
- A. Tremel, P. Wasserscheid, M. Baldauf and T. Hammer, Int. J. Hydrogen Energy, 2015, 40(35), 11457 CrossRef CAS.
- European Commission, Directive 2003/87/EC: Scheme for Greenhouse Gas Emission Allowance Trading (EU ETS), 2003 Search PubMed.
- SCOT Project, EU-ETS to Incentivise CO2 Utilisation: Scot Project Briefing Paper, 2016 Search PubMed.
- European Commission SETIS, Carbon Capture Utilisation and Storage, Brüssel, 2016 Search PubMed.
- Draft Report: on the Proposal for a Directive of the European Parliament and of the Council Amending Directive 2003/87/EC to Enhance Cost-effective Emission Reductions and Low-carbon Investments, (COM(2015)0337 – C8-0190/2015 – 2015/0148(COD)), ed. Ian Duncan, European Parliament, Brüssel, 2016 Search PubMed.
- European Court of Justice, curia.europa.eu, 2017, http://curia.europa.eu/juris/document/document.jsf;jsessionid=9ea7d2dc30d58212fbda909b459b9498e176cd9e54d9.e34KaxiLc3qMb40Rch0SaxyKbNv0?text=%26docid=186967%26pageIndex=0%26doclang=EN%26mode=req%26dir=%26occ=first%26part=1%26cid=679758.
- S. Altenschmidt, Recognition of Climate Protection Measures: Success at ECJ for the Lime Industry with Luther, 2017 Search PubMed.
- International Maritime Organization, DNV GL, 2016, 2016.
- Ecoinvent Centre – Swiss Centre for Life Cycle Inventories, ecoinvent LCA-database V3.3, ecoinvent, 2017.
- W. Kuckshinrich, P. Markewitz, J. Linssen, P. Zapp, M. Peters and W. Leitner, Weltweite Innovationen bei der Entwicklungvon CCS-Technologien und Möglichkeiten der Nutzung und des Recyclings von CO2: Studie im Auftrag des Bundesministeriums für Wirtschaft und Technologie (BMWi), Projektnummer 25/08 Endbericht März 2010, Jülich, 2010 Search PubMed.
- Hugill, Overbeek and Spoelstra, a Comparison of the Eco-efficiency of Two Production Routes for Methanol, 2001 Search PubMed.
- F. Pontzen, W. Liebner, V. Gronemann, M. Rothaemel and B. Ahlers, Catal. Today, 2011, 171(1), 242 CrossRef CAS.
- H. Goehna and P. Koenig, CHEMTECH, 1994, 1994(24/6), 36 Search PubMed.
- E. A. Quadrelli, G. Centi, J.-L. Duplan and S. Perathoner, ChemSusChem, 2011, 4(9), 1194 CrossRef CAS PubMed.
- M. Specht and A. Bandi, Forschungsverbund Sonnenenergie “Themen 98/99”, 1999, p. 59 Search PubMed.
- M. Specht, A. Bandi, M. elser and F. Staiss, Stud. Surf. Sci. Catal., 1998, 114(114), 363 CrossRef.
- D. Mignard, Int. J. Hydrogen Energy, 2003, 28(4), 455 CrossRef CAS.
- L. R. Clausen, N. Houbak and B. Elmegaard, Energy, 2010, 35(5), 2338 CrossRef CAS.
- K. Atsonios, K. D. Panopoulos and E. Kakaras, Int. J. Hydrogen Energy, 2016, 41(4), 2202 CrossRef CAS.
- M. Matzen, M. Alhajji and Y. Demirel, Energy, 2015, 93, 343 CrossRef CAS.
- M. Pérez-Fortes, J. C. Schöneberger, A. Boulamanti and E. Tzimas, Appl. Energy, 2016, 161, 718 CrossRef.
- A. Varone and M. Ferrari, Renewable Sustainable Energy Rev., 2015, 45, 207 CrossRef.
- R. Rivera-Tinoco, M. Farran, C. Bouallou, F. Auprêtre, S. Valentin, P. Millet and J. R. Ngameni, Int. J. Hydrogen Energy, 2016, 41(8), 4546 CrossRef CAS.
- M. Rivarolo, D. Bellotti, L. Magistri and A. F. Massardo, Int. J. Hydrogen Energy, 2016, 41(4), 2105 CrossRef CAS.
- N. v. d. Assen, L. J. Müller, A. Steingrube, P. Voll and A. Bardow, Environ. Sci. Technol., 2016, 50(3), 1093 CrossRef PubMed.
- CO2: Abtrennung, Speicherung, Nutzung: Ganzheitliche Bewertung im Bereich von Energiewirtschaft und Industrie, ed. M. Fischedick and K. Görner, Springer Berlin Heidelberg, Berlin, Heidelberg, 2015 Search PubMed.
- D. W. Keith, M. Ha-Duong and J. K. Stolaroff, Clim. Change, 2006, 74(1–3), 17 CrossRef CAS.
- https://www.nature.com/news/commercial-boost-for-firms-that-suck-carbon-from-air-1.18551, last accessed March 2018.
- L. K. Rihko-Struckmann, A. Peschel, R. Hanke-Rauschenbach and K. Sundmacher, Ind. Eng. Chem. Res., 2010, 49(21), 11073 CrossRef CAS.
- B. Stefansson, Power and CO2 Emissions to Methanol, Brüssel, 2015 Search PubMed.
- S. Gelpke, Wirtschaftlichkeitsanalyse der Power-to-liquid-Prozesskette zur Methanolherstellung aus CO2 und H2, Bachelor-thesis, Fraunhofer Institute for Solar Energy Systems ISE, Flensburg, 2016.
- A. Schnabl, Die Konversion von CO2 und H2 zu Methanol als nachhaltigem chemischen Energiespeicher, Master-thesis, Fraunhofer Institute for Solar Energy Systems ISE, München, 2015.
- Bundesverband der Energie und Wasserwirtschaft, Erneuerbare Energien und das EEG: Zahlen, Fakten, Grafiken (2013), Anlagen, installierte Leistung, Stromerzeugung, EEG-Auszahlungen, Marktintegration der Erneuerbaren Energien und regionale Verteilung der EEG-induzierten Zahlungsströme, BDEW Bundesverband der Energie- und Wasserwirtschaft e.V., Berlin, 2013.
- M. Kopp, D. Coleman, C. Stiller, K. Scheffer, J. Aichinger and B. Scheppat, Int. J. Hydrogen Energy, 2017, 42(19), 13311–13320 CrossRef CAS.
- C. Kost, J. Mayer, J. Thomsen, N. Hartmann, C. Senkpiel, S. Philipps, S. Nold, S. Lude and T. Schlegl, Stromgestehungskosten Erneuerbare Energien, Freiburg, 2013 Search PubMed.
- Bundesministerium für Wirtschaft und Energie BMWi, Gesetz für den Ausbau erneuerbarer Energien: Erneuerbare-Energien-Gesetz – EEG 2014, 2014.
- F. Ausfelder, C. Beilmann, M. Bertau, S. Bräuninger, A. Heinzel, R. Hoer, W. Koch, F. Mahlendorf, A. Metzelthin, M. Peuckert, L. Plass, K. Räuchle, M. Reuter, G. Schaub, S. Schiebahn, E. Schwab, F. Schüth, D. Stolten, G. Teßmer, K. Wagemann and K.-F. Ziegahn, Chem. Ing. Tech., 2015, 87(1–2), 17 CrossRef CAS.
- T. Smolinka, M. Günther and J. Garche, Stand und Entwicklungspotenzial der Wasserelektrolyse zur Herstellung von Wasserstoff aus regenerativen Energien: Kurzfassung des Abschlussberichts, NOW-Studie, Freiburg, 2011 Search PubMed.
- B. V. Mathiesen, I. Ridjan and D. Connolly, Technology Data for High Temperature Solid Oxide Electrolyser Cells, Alkali and PEM Electrolysers, Aalborg, 2013 Search PubMed.
- S. M. Saba, M. Müller, M. Robinius and D. Stolten, Int. J. Hydrogen Energy, 2018, 43(3), 1209 CrossRef CAS.
- Fuel Cell and Hydrogen Joint Undertaking – FCH JU, Study on Early Business Cases for H2 in Energy and More Broadly Power to H2 Applications: Final Report, Brüssel, 2017 Search PubMed.
- O. Schmidt, A. Gambhir, I. Staffell, A. Hawkes, J. Nelson and S. Few, Int. J. Hydrogen Energy, 2017, 42(52), 30470 CrossRef CAS.
- T. Kato, M. Kubota, N. Kobayashi and Y. Suzuoki, Energy, 2005, 30(14), 2580 CrossRef CAS.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed.
- D. Schüwer, K. Arnold, K. bienge and S. Bringezu, CO2 ReUse NRW: Evaluating Gas Sources, Demand and Utilization for CO2 and H2 within the North Rhine- Westphalia Area with Respect to Gas Qualities, Supported by Climate-kic Deutschland, Berlin, Berlin, 2015 Search PubMed.
- F. Ausfelder and A. Bazzanelle, Verwertung und Speicherung von CO2: Diskussionspapier, Frankfurt/Main, 2008 Search PubMed.
- M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell, J. R. Fernández, M.-C. Ferrari, R. Gross, J. P. Hallett, R. S. Haszeldine, P. Heptonstall, A. Lyngfelt, Z. Makuch, E. Mangano, R. T. J. Porter, M. Pourkashanian, G. T. Rochelle, N. Shah, J. G. Yao and P. S. Fennell, Energy Environ. Sci., 2014, 7(1), 130 CAS.
- S. Klinski, Einspeisung von Biogas in das Erdgasnetz, Leipzig, 2006 Search PubMed.
- DVGW Deutscher Verein des Gas- und Wasserfaches e. V, Technische Regel – Arbeitsblatt Nr. 260 (A) Zur Qualität von einzuspeisenden Gasen in das Erdgasnetz(260 (A)), DVGW, Bonn.
- W. Zhou, B. Zhu, Q. Li, T. Ma, S. Hu and C. Griffy-Brown, Energy Policy, 2010, 38(7), 3701 CrossRef CAS.
- Technische Chemie, ed. A. Behr, A. Brehm, H. Hofmann, J. Gmehling, M. Baerns and U. Onken, Wiley-VCH, Weinheim, 2nd edn, 2013, ISBN: 978-3-527-33072-0 Search PubMed.
- K. K. Humphreys, Project and Cost Engineers' Handbook, Marcel Dekker, New York, USA, 2005 Search PubMed.
- T. Smolinka, N. Wiebe and M. Thomassen, Cost Break Down and Cost Reduction Strategies for PEM Water Electrolysis Systems: Session B05: H2 from Electrolysers: Concepts & Costs, Luzern, Schweiz, 2017 Search PubMed.
- T. Smolinka, T. Raksha, T. Pregger, A. Friedrich and G.-S. Schneider, Studie über die Planung einer Demonstrationsanlage zur Wasserstoff-Kraftstoffgewinnung durch Elektrolyse mit Zwischenspeicherung in Salzkavernen unter Druck: Plan Delykad, Plan Delykad, Stuttgart, 2014 Search PubMed.
- T. Aicher, M. I. Gonzalez and M. Götz, Betrachtung des Gesamtsystems im Hinblick auf Dynamik und Prozessintegration: Arbeitspaket 5, 2014 Search PubMed.
- ADI Analytics LLC, Natural Gas Utilization via Small-scale Methanol Technologies, Commissioned by Ben Franklin Technology Partners' Shale Gas Innovation & Commercialization Center, Houston, Texas, 2015 Search PubMed.
- O. Machhammer, A. Bode and W. Hormuth, Chem. Ing. Tech., 2015, 87(4), 409 CrossRef CAS.
- C. Hendriks and W. Graus, Global Carbon Dioxide Storage Potential and Costs, Utrecht, 2004 Search PubMed.
- J. Ladebeck, J. P. Wagner and T. Matsuhisa, Stud. Surf. Sci. Catal., 1997, 1997(107), 73 CrossRef.
- https://www.methanex.com/our-business/pricing, last accessed December 2017.
- Universität Stuttgart, Verbundprojekt Sunfire – Herstellung von Kraftstoffen aus CO2 und H2O unter Nutzung regenerativer Energie, Bericht Arbeitspaket D Ökobilanz, 2015 Search PubMed.
- https://www.bloomberg.com/news/articles/2016-05-03/solar-developers-undercut-coal-with-another-record-set-in-dubai, last accessed March 2018.
- http://fortune.com/2016/09/19/world-record-solar-price-abu-dhabi/, last accessed March 2018.
- https://economictimes.indiatimes.com/industry/energy/power/chile-breaks-dubais-record-of-solar-power-output-at-low-cost/articleshow/53932595.cms, last accessed March 2018.
- https://www.bloomberg.com/news/articles/2016-08-19/solar-sells-in-chile-for-cheapest-ever-at-half-the-price-of-coal, last accessed March 2018.
- S. Brynolf, M. Taljegard, M. Grahn and J. Hansson, Renewable Sustainable Energy Rev., 2018, 81, 1887 CrossRef.
- A. Boulamanti and J. A. Moya, Renewable Sustainable Energy Rev., 2017, 68, 1205 CrossRef CAS.
- H.-M. Henning and A. Palzer, Was kostet die Energiewende?: Wege zur Transformation des Deutschen Energiesystems bis 2050, Freiburg, 2015 Search PubMed.
- G. Norman, F. Sandau, B. Zimmermann, C. Pape, S. Bofinger and C. Hofmann, Geschäftsmodell Energiewende: Eine Antwort auf das “Die-Kosten-der-Energiewende”-Argument, Kassel, 2014 Search PubMed.
- https://www.thyssenkrupp.com/de/carbon2chem/, last accessed March 2018.
- European Commission, Directive 2015/1513/EC. Quality of Petrol and Diesel Fuels, 2015 Search PubMed.
- European Commission, Directive 2009/28/EC. Promotion of the Use of Energy from Renewable Sources: 2009/28/EC, 2009 Search PubMed.
- https://www.klik.ch/, last accessed March 2018.
- A. W. Zimmermann and R. Schomäcker, Energy Technol., 2017, 5(6), 850 CrossRef.
Footnotes |
| † Current address: University of Oxford, Department of Engineering Science, Parks Road, Oxford, OX1 3PJ, UK. |
| ‡ Annex I of the EU Directive 2003/87/EC underlines that “under the EU ETS rules for any other transfer” than in the case of long-term geological storage “of CO2 out of the installation, no subtraction of CO2 from the installation's emissions is allowed”. |
| § Listed examples for equivalent (eq) emissions: CO2eq: climate change, CFC-11eq: stratospheric ozone depletion, and SO2eq: acidification. |
| ¶ In this evaluation: methanol production based on syngas from steam-reforming of natural gas. |
| || In the case of assuming lower fossil MPC for example for any production facility in Saudi Arabia or China, the green MPC would also decrease presumably because of lower renewable electricity generation cost (PV electricity in Abu Dhabi @ 2.45 ct€ per kWh; in Chile @ 2.91 ct€ per kWh; and levelised cost of electricity for large PV plants within the G20 states below 4.10 ct€ per kWh). |
| ** To be reported in a forthcoming article. |
| †† 41 kg of CO2 are vented during the synthesis step. Therefore 1388-41 = 1347 kg of CO2 per t(eMeOH) are ‘bound’ in the product. |
| ‡‡ The cradle-to-grave assessment does not consider impacts resulting from transportation of the produced methanol and any recycling of the infrastructure. However it is assumed that impacts resulting from these phases in the eMeOH and the fMeOH are in a comparable range. |
| §§ GWP100a, method CML 2001, allocation cut-off. |
¶¶ Original ecoinvent activity methanol production [GLO]: GWP100a: 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 545 kg(CO2eq); method CML 2001, allocation cut-off. 545 kg(CO2eq); method CML 2001, allocation cut-off. |
| |||| EU CO2 Emission Allowances (Sept 16 to Aug 17) according to the European Energy Exchange. |
| *** Double counting of advanced/second generation biofuels: regarding the extension of the Renewable Energy Directive (RED) 2009/28/EC (28.04.2015) member States must ensure that 10% of the final energy consumption in the transport sector is provided by renewable sources. The share provided with advanced/second and/or third generation biofuels is double weighted. |
| This journal is © The Royal Society of Chemistry 2018 |