Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rhodium-catalyzed ortho-heteroarylation of phenols: directing group-enabled switching of the electronic bias for heteroaromatic coupling partner†
Yimin
Wu
,
Wei
Li
,
Linfeng
Jiang
,
Luoqiang
Zhang
,
Jingbo
Lan
 and
Jingsong
You
and
Jingsong
You
 *
*
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu 610064, PR China. E-mail: jsyou@scu.edu.cn
First published on 18th July 2018
Abstract
The directed oxidative C–H/C–H cross-coupling reactions between a functionalized arene and a heteroarene typically exhibit an electronic bias for the heteroaromatic coupling partner. Disclosed herein is a conception of directing group enabled-switching of the electronic bias for coupling partner from the electron-deficient to electron-rich heteroarene, demonstrating that the modification of the directing group may match the latent reactivity of heteroarene substrates caused by the distinctly different electronic nature. In this work, we develop a Rh(III)-catalyzed ortho-heteroarylation of phenols with greatly important electron-rich heteroarenes such as benzothiophene, benzofuran, thiophene, furan and pyrrole via two-fold C–H activation, which presents broad substrate scopes of both phenols and electron-rich heteroarenes and shows the advantage of tolerance of reactive functional groups, especially halogen. This work also provides a new strategy for the construction of π-conjugated furan-fused heteroacenes prevalent in materials science in dramatically simplified procedures, which makes the protocol highly applicable.
Introduction
Aryl-heteroaryl scaffolds containing 2-hydroxyphenyl units, especially for electron-rich heteroaryl-2-hydroxyphenyl, are prevalent in the field of natural products, pharmaceuticals and materials science (Scheme 1).1,2 Over the past decades, significant efforts have been devoted to develop various reliable and efficient methods for the synthesis of such structural linkages, including conventional transition metal-catalyzed C–X/C–M cross-coupling reactions3 and recently developed ortho-C–H (hetero)arylations of phenols with (hetero)arylating reagents such as organic halides and pseudohalides through the chelation assistance.4 From the viewpoint of step and atom economy, transition metal-catalyzed oxidative C–H/C–H cross-coupling would undoubtedly be one of the most straightforward routes to assemble these aryl-heteroaryl skeletons,5 which obviates wasteful prefunctionalization of coupling reactants and the use of organometallic reagents. | ||
| Scheme 1 Selected examples of natural products, pharmaceuticals and organic functional material molecules. | ||
Although the past few years have witnessed tremendous advancements in the realm of C–H bond activation, there remain persistent obstacles associated with the latent reactivity of substrates and the regioselectivity issue. Recently, the directing group strategy has proved to be a very useful solution to such challenges through a cyclometalation.6 Through judicious choice of directing group, the modification of the coordinating capability as well as the ring size of forming metallacycle species enables to dramatically influence the reactivity of substrates caused by the distinctly different electronic nature and further match a variety of substrates, thus efficiently extending the compatibility of protocols for coupling reactants.
Recent research has shown that the directed oxidative C–H/C–H cross-coupling reactions between a functionalized arene and a heteroarene typically exhibit an electronic bias for the heteroaromatic coupling partner. Using the oxyacetamide (O–NHAc) as the directing group, You's and Zhao's groups disclosed the Rh(III)-catalyzed mono/bis-ortho-heteroarylation of phenols with electron-deficient heteroarenes such as azoles through internal and external oxidative C–H activation strategies, respectively (Scheme 2).7 However, transition metal-catalyzed oxidative C–H/C–H cross-coupling reactions between phenols and greatly important electron-rich heteroarenes, such as thiophene, furan and pyrrole, remain unsolved so far. Thus, it is required to develop an innovative system to overcome such a limitation caused by the distinctly different electronic nature of substrates. Herein, we wish to present a convenient strategy to achieve the switching of the electronic bias for coupling partner from the electron-deficient to electron-rich heteroarenes by judicious choice of directing group.
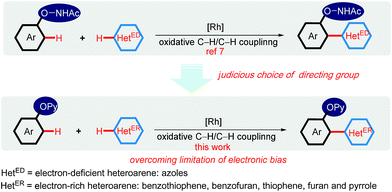 | ||
| Scheme 2 Directing group-enabled switching of the electronic bias for coupling partner from the electron-deficient to electron-rich heteroarenes. | ||
Results and discussion
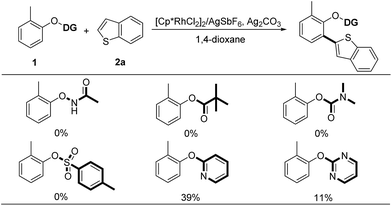
Considering the wide application of thiophene-based scaffolds,1e,2 we initially selected the cross-coupling between phenol derivatives (1) and electron-rich benzothiophene (2a) as a model reaction.8 At the outset of our investigation, we tested the feasibility of oxidative C–H/C–H cross-coupling between phenol and benzothiophene under the privileged [Cp*RhCl2]2/AgSbF6 catalytic system, using the commonly used O-carbamate, acyloxy, oxyacetamide and tosyl as the directing groups (Scheme 3). However, these directing groups were incapable of promoting the oxidative coupling reactions. Among various directing groups in C–H bond activation, the 2-pyridyl has exhibited a powerful potential because of its impressive coordination ability to transition metal center.9 Furthermore, the 2-pyridinyl group is readily introduced to phenols and the resulting 2-pyridyloxyl moiety is easily cleavable to produce the free phenol.4c,4d,9 Thus, we turned to investigate the 2-pyridyl as the directing group. It was delighted to observe that the desired ortho-heteroarylated product could be obtained in 39% yield (Scheme 3).Subsequently, we tried to screen several parameters as shown in Tables S1–S4.† Among the oxidants investigated, we found that copper salts were superior to other inorganic salts (Table S1†). Among the solvents used, 1,4-dioxane was the best choice and a concentrated reaction system was more efficient (Table S2†). Further optimization of additives showed that the combination of acid with base could improve the yield of 3a (Table S4†). Finally, the reaction delivered 3a in 82% yield when [Cp*RhCl2]2 (5 mol%) was used in combination with AgSbF6 (20 mol%), PivOH (1.0 equiv.), CsOPiv (30 mol%) and Cu(OAc)2 (3.0 equiv.) in 1,4-dioxane at 150 °C for 24 h (Table S4,† entry 6).
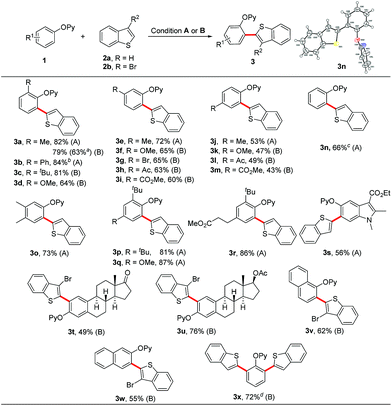
With the optimal system in hand, we assessed the scope of this coupling reaction. As shown in Scheme 4, a variety of phenol substrates reacted efficiently with benzothiophene to give the corresponding ortho-heteroarylated products in moderate to high yields. For the meta-substituted and 3,4-disubstituted phenols, the heteroarylation occurred at the less hindered site (Scheme 4, 3e–3i and 3o). It is emphasized that the phenols bearing a more bulky group at the ortho position such as tert-butyl could also deliver the coupled products in good yields (Scheme 4, 3c and 3p–3r). In the presence of extra Ag2CO3 (20 mol%), the ortho-unsubstituted 2-phenoxypyridine could selectively undergo the ortho-heteroarylation to afford the monosubstituted product 3n in 66% yield without observation of the diarylated product 3x (Scheme 4, 3n). The symmetrical bis-heteroarylated product could also be obtained in one step in 72% yield using Ag2O as the oxidant and Zn(OTf)2 as the additive at 100 °C (Scheme 4, 3x). It is worthy of note that the reaction of 1a with 2a could be carried out on a 4.0 mmol scale with an acceptable yield of 63% (Scheme 4, 3a), thus demonstrating the applicability of this method for mass production. Gratefully, phenol-containing natural products and pharmaceuticals such as estrone, estradiol and mecarbinate and naphthalenol derivatives smoothly underwent the ortho-heteroarylation, providing the corresponding cross-coupled products in satisfactory yields (Scheme 4, 3s–3w).
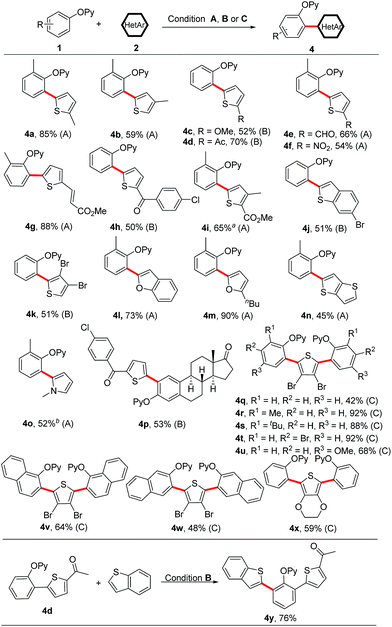
Next, we turned our attention to heteroarene substrates (Scheme 5). To our delight, a broad range of electron-rich heteroarenes including benzothiophene, benzofuran, thiophene, furan and pyrrole engaged in this reaction in moderate to good yields (Scheme 5, 4a–4o). Thiophenes bearing both the electron-donating and electron-withdrawing groups could afford the coupled products (Scheme 5, 4a–4i). 3-Methylthiophene participated in the coupling reaction exclusively at the less sterically hindered 5-position (Scheme 5, 4b). The synthetic utility of this protocol is underscored by its tolerance towards a wide range of reactive functionalities such as chloro, bromo, formyl, nitro, acetyl, ester, alkenyl and methoxy groups, which easily undergo further transformations. Even 3,4-dibromothiophene could also furnish the coupled product 4k in 51% yield. Especially, starting from 3,4-dibromothiophene, an array of symmetrical 2,5-diaryl thiophenes could be successfully obtained in moderate to excellent yields in one-pot synthesis (Scheme 5, 4q–4x). Thieno[3,2-b]thiophene and N-methyl pyrrole were also suitable substrates, delivering the coupled products in synthetically useful yields (Scheme 5, 4n and 4o). In addition, it is grateful to obtain the coupled product 4p, which supports the robustness of our protocol in the functionalization of steroid derivatives. Treatment of 4d with benzothiophene further yielded the unsymmetrical diheteroaryl-substituted product 4y. Notably, while a broad range of electron-rich heteroarenes smoothly underwent the coupling reaction, the electron-deficient heteroarenes did not provide any desired products, which makes the reaction complementary to the previously reported protocols by You's and Zhao's groups.
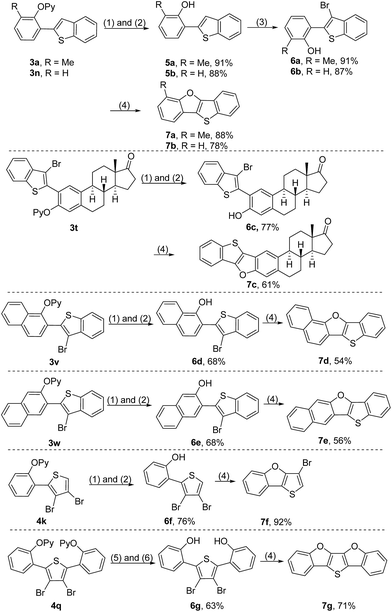
In order to further illustrate the synthetic utility of this protocol, a series of transformations of the resultant ortho-heteroarylated phenol derivatives were explored. The 2-pyridyl group of the coupled products could be removed by sequential treatment with methyl trifluoromethanesulfonate (MeOTf) in dry toluene and a refluxing Na/MeOH solution, producing 2-heteroarylated phenols (Scheme 6).4c,4d,9 Considering that extended π-conjugated furan-fused heteroacenes are promising functional organic materials for organic field-effect transistors (OFETs), organic light-emitting diodes (OLEDs) and liquid crystals,2 we attempted to convert ortho-heteroarylated phenols to furan-fused heteroarenes. Starting from 3a and 3n, benzo[4,5]thieno[3,2-b]benzofurans were rapidly constructed by sequential removal of the directing group, bromination and intramolecular cyclization in satisfactory total yields (Scheme 6, 7a and 7b). Because the oxidative coupling reaction developed herein enables tolerance of the reactive bromide groups, the direct use of bromo-substituted thiophenes as a starting material could significantly streamline synthetic routes (Scheme 6, 7c–7g). For example, starting from diarylated 3,4-dibromothiophene 4q, a new extended π-conjugated molecule, thieno[3,2-b:4,5-b′]bis[1]benzofuran (7g), could be constructed rapidly, highlighting the charm of this protocol in the construction of furan-fused heteroacenes.
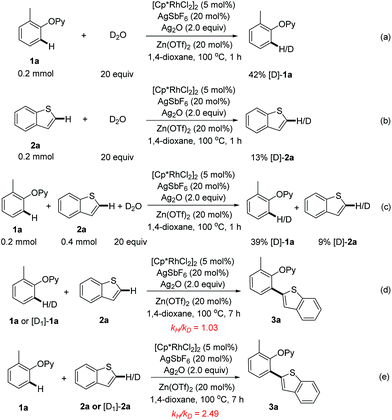
Finally, for insight into this rhodium-catalyzed reaction, the H/D exchange control experiments were conducted. When 2-(o-tolyloxy)pyridine (1a) was treated with D2O for 1 h under the optimized catalytic conditions, 42% of 1a was deuterated (Scheme 7a), while a 13% H/D scrambling was observed with benzothiophene (2a) (Scheme 7b). The exposure of 1a and 2a to D2O could produce similar ratios of deuterated products (Scheme 7c). These observations implied that the C–H activation processes of both 1a and 2a could be reversible. Next, the kinetic isotope effect (KIE) experiments were performed. A KIE value of 1.03 for 1a was obtained while 2.49 was observed for 2a (Scheme 7d and 7e), indicating that the C2–H cleavage of benzothiophene might be involved in the rate-limiting step. Based on the above results and the previously reported literature,10 we speculated that a tentative mechanism could consist of (1) the coordination of the 2-phenoxypyridine to [Cp*Rh(III)] and subsequent ortho-C–H activation of arene, (2) the reaction of the resulting rhodacycle intermediate with a heteroarene to form the key aryl-Rh(III)-heteroaryl, (3) the reductive elimination to deliver the ortho-heteroarylated product, and (4) the re-oxidation of Rh(I) to close the catalytic cycle (Scheme S2†).
Conclusions
In summary, we have developed a rhodium-catalyzed ortho-heteroarylation of phenols with greatly important electron-rich heteroarenes such as benzothiophene, benzofuran, thiophene, furan and pyrrole via two-fold C–H activation. This protocol features the readily installed and removable directing group, broad substrate scope and excellent functional group tolerance. From oxyacetamide to 2-pyridyloxyl as the directing group,7 the scope of coupling substrates switches from electron-deficient to electron-rich heteroarenes, which would bring inspiration for the solution of the electronic bias of heteroaromatic coupling partner in the directed oxidative C–H/C–H cross-coupling reactions between two (hetero)arenes. Additionally, the coupled products can further be transformed to furan-fused heteroacenes in good yields, which makes the method highly applicable.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the financial support from the National NSF of China (No. 21432005) and the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, Sichuan University.Notes and references
- (a) J. He, L. Chen, D. Heber, W. Shi and Q.-Y. Lu, J. Nat. Prod., 2006, 69, 121 CrossRef PubMed; (b) M. Preller, K. Chinthalapudi, R. Martin, H.-J. Knölker and D. J. Manstein, J. Med. Chem., 2011, 54, 3675 CrossRef PubMed; (c) S.-Y. Cho, S. Cho, E. Park, B. Kim, E. J. Sohn, B. Oh, E.-O. Lee, H.-J. Lee and S.-H. Kim, Bioorg. Med. Chem. Lett., 2014, 24, 2560 CrossRef PubMed; (d) H. J. Lacey, D. Vuong, J. I. Pitt, E. Lacey and A. M. Piggott, Aust. J. Chem., 2016, 69, 152 CrossRef; (e) R. Heuvel, J. J. Van Franeker and R. A. J. Janssen, Macromol. Chem. Phys., 2017, 218, 1600502 CrossRef PubMed.
- (a) K. Černovská, J. Svoboda, I. Stibor, M. Glogarová, P. Vaněk and V. Novotná, Ferroelectrics, 2000, 241, 231 CrossRef; (b) J.-J. Aaron, C. Párkányi, A. Adenier, C. Potin, Z. Zajíčková, O. R. Martínez, J. Svoboda, P. Pihera and P. Váchal, J. Fluoresc., 2011, 21, 2133 CrossRef PubMed; (c) Y. S. Yang, T. Yasuda and C. Adachi, Bull. Chem. Soc. Jpn., 2012, 85, 1186 CrossRef; (d) R. Chen, Y. Wang, T. Chen, H. Li, C. Zheng, K. Yuan, Z. Wang, Y. Tao, C. Zheng and W. Huang, J. Phys. Chem. B, 2015, 119, 583 CrossRef PubMed; (e) L. Zhang, W. Shen, R. He, X. Tang, Y. Yang and M. Li, Mater. Chem. Phys., 2016, 175, 13 CrossRef; (f) M. Matsumura, A. Muranaka, R. Kurihara, M. Kanai, K. Yoshida, N. Kakusawa, D. Hashizume, M. Uchiyama and S. Yasuike, Tetrahedron, 2016, 72, 8085 CrossRef.
- (a) S. Ishikawa and K. Manabe, Tetrahedron, 2011, 67, 10156 CrossRef; (b) M. Noreen, N. Rasool, M. E. Khatib and G. A. Molander, J. Org. Chem., 2014, 79, 7243 CrossRef PubMed; (c) T. Komiyama, Y. Minami, Y. Furuya and T. Hiyama, Angew. Chem., Int. Ed., 2018, 57, 1987 CrossRef PubMed.
- (a) S. Gu, C. Chen and W. Chen, J. Org. Chem., 2009, 74, 7203 CrossRef PubMed; (b) B. Xiao, Y. Fu, J. Xu, T.-J. Gong, J.-J. Dai, J. Yi and L. Liu, J. Am. Chem. Soc., 2010, 132, 468 CrossRef PubMed; (c) J.-H. Chu, P.-S. Lin and M.-J. Wu, Organometallics, 2010, 29, 4058 CrossRef; (d) L. Ackermann, E. Diers and A. Manvar, Org. Lett., 2012, 14, 1154 CrossRef PubMed.
- For selected reviews, see: (a) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef PubMed; (b) X. Bugaut and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 7479 CrossRef PubMed; (c) D. Zhao, J. You and C. Hu, Chem.–Eur. J., 2011, 17, 5466 CrossRef PubMed; (d) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef PubMed; (e) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef PubMed; (f) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef PubMed; (g) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (h) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef PubMed; (i) C. Liu, J. Yuan, M. Gao, S. Tang, W. Li, R. Shi and A. Lei, Chem. Rev., 2015, 115, 12138 CrossRef PubMed; (j) Y. Yang, J. Lan and J. You, Chem. Rev., 2017, 117, 8787 CrossRef PubMed.
- For recent reviews on directing group, see: (a) G. Rousseau and B. Breit, Angew. Chem., Int. Ed., 2011, 50, 2450 CrossRef PubMed; (b) F. Zhang and D. R. Spring, Chem. Soc. Rev., 2014, 43, 6906 RSC; (c) M. Zhang, Y. Zhang, X. Jie, H. Zhao, G. Li and W. Su, Org. Chem. Front., 2014, 1, 843 RSC; (d) Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 1107 RSC.
- (a) B. Li, J. Lan, D. Wu and J. You, Angew. Chem., Int. Ed., 2015, 54, 14008 CrossRef PubMed; (b) Q. Wu, Y. Chen, D. Yan, M. Zhang, Y. Lu, W.-Y. Sun and J. Zhao, Chem. Sci., 2017, 8, 169 RSC.
- For our previous works in the construction of bi(hetero)aryl scaffolds via dual C–H activation, see: (a) J. Dong, Z. Long, F. Song, N. Wu, Q. Guo, J. Lan and J. You, Angew. Chem., Int. Ed., 2013, 52, 580 CrossRef PubMed; (b) Y. Huang, D. Wu, J. Huang, Q. Guo, J. Li and J. You, Angew. Chem., Int. Ed., 2014, 53, 12158 CrossRef PubMed; (c) X. Qin, X. Li, Q. Huang, H. Liu, D. Wu, Q. Guo, J. Lan, R. Wang and J. You, Angew. Chem., Int. Ed., 2015, 54, 7167 CrossRef PubMed; (d) D. Qin, J. Wang, X. Qin, C. Wang, G. Gao and J. You, Chem. Commun., 2015, 51, 6190 RSC; (e) G. Tan, S. He, X. Huang, X. Liao, Y. Cheng and J. You, Angew. Chem., Int. Ed., 2016, 55, 10414 CrossRef PubMed; (f) Y. Cheng, Y. Wu, G. Tan and J. You, Angew. Chem., Int. Ed., 2016, 55, 12275 CrossRef PubMed; (g) Y. Ran, Y. Yang, H. You and J. You, ACS Catal., 2018, 8, 1796 CrossRef.
- (a) W. Ma and L. Ackermann, Chem.–Eur. J., 2013, 19, 13925 CrossRef PubMed; (b) W. Zhang, J. Zhang, S. Ren and Y. Liu, J. Org. Chem., 2014, 79, 11508 CrossRef PubMed; (c) Y. Xu, P. Liu, S.-L. Li and P. Sun, J. Org. Chem., 2015, 80, 1269 CrossRef PubMed; (d) A. J. Borah, G. Yan and L. Wang, Eur. J. Org. Chem., 2015, 4782 CrossRef; (e) K. Raghuvanshi, K. Rauch and L. Ackermann, Chem.–Eur. J., 2015, 21, 1790 CrossRef PubMed.
- (a) M. U. Raja, R. Ramesh and K. H. Ahn, Tetrahedron Lett., 2009, 50, 7014 CrossRef; (b) W.-W. Chan, S.-F. Lo, Z. Zhou and W.-Y. Yu, J. Am. Chem. Soc., 2012, 134, 13565 CrossRef PubMed; (c) N. Kuhl, M. N. Hopkinson and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 8230 CrossRef PubMed; (d) J. Wencel-Delord, C. Nimphius, H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 13001 CrossRef PubMed; (e) W. Ai, X. Yang, Y. Wu, X. Wang, Y. Li, Y. Yang and B. Zhou, Chem.–Eur. J., 2014, 20, 17653 CrossRef PubMed; (f) X.-F. Yang, X.-H. Hu, C. Feng and T.-P. Loh, Chem. Commun., 2015, 51, 2532 RSC; (g) S. Allu, M. Ravi and K. C. K. Swamy, Eur. J. Org. Chem., 2016, 5697 CrossRef. For selected reviews, see: (h) Y.-F. Han and G.-X. Jin, Chem. Soc. Rev., 2014, 43, 2799 RSC; (i) N. Kuhl, N. Schröder and F. Glorius, Adv. Synth. Catal., 2014, 356, 1443 CrossRef; (j) G. Song and X. Li, Acc. Chem. Res., 2015, 48, 1007 CrossRef PubMed; (k) K. Shin, H. Kim and S. Chang, Acc. Chem. Res., 2015, 48, 1040 CrossRef PubMed; (l) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900 RSC; (m) S. Vásquez-Céspedes, X. Wang and F. Glorius, ACS Catal., 2018, 8, 242 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information on experimental procedures, characterization data and crystallographic, and X-ray crystal structures (CIF) of CCDC 1837385 (3n). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc02529k |
| This journal is © The Royal Society of Chemistry 2018 |