Open Access Article
Open Access ArticleN-Doped graphene/C60 covalent hybrid as a new material for energy harvesting applications†
Myriam
Barrejón‡
 ab,
Luis M.
Arellano‡
ab,
Luis M.
Arellano‡
 a,
Habtom B.
Gobeze
a,
Habtom B.
Gobeze
 c,
María J.
Gómez-Escalonilla
c,
María J.
Gómez-Escalonilla
 a,
Jose Luis G.
Fierro
a,
Jose Luis G.
Fierro
 d,
Francis
D'Souza
d,
Francis
D'Souza
 *c and
Fernando
Langa
*c and
Fernando
Langa
 *a
*a
aUniversidad de Castilla-La Mancha, Instituto de Nanociencia, Nanotecnología y Materiales Moleculares (INAMOL), 45071-Toledo, Spain. E-mail: Fernando.Langa@uclm.es
bUniversità degli Studi di Trieste, Dipartimento di Scienze Chimiche e Farmaceutiche, Via Licio Giorgeri, 1 Edifizio C11, 34127 Trieste, Italy
cDepartment of Chemistry, University of North Texas, 1155 Union Circle, #305070, 76203-5017, Denton, TX, USA. E-mail: Francis.DSouza@UNT.edu
dInstituto de Catálisis y Petroleoquímica, CSIC, Cantoblanco, 28049, Madrid, Spain. E-mail: jlgfierro@icp.csic.es
First published on 27th August 2018
Abstract
N-Doped graphene (N-G) was chemically functionalized by N-alkylation with the well-known electron acceptor C60. The degree of functionalization and the key structural features of the N-G/C60 hybrid were systematically investigated by a number of techniques including thermogravimetric analysis, X-ray photoelectron and Raman spectroscopies and transmission electron and atomic force microscopies. Absorption and electrochemical studies revealed interactions between the N-G and C60 while the fluorescence of C60 within the hybrid was found to be fully quenched. Evidence for the occurrence of excited state charge transfer from the singlet excited C60 to N-G in the hybrid was obtained from femtosecond transient absorption studies covering the visible–near-IR regions. Electron-pooling experiments performed in the presence of a sacrificial electron donor and a second electron acceptor, methyl viologen, revealed the accumulation of the one-electron reduced product of methyl viologen upon continuous irradiation of the N-G/C60 nanohybrid, thus revealing the utility of this material in photocatalytic energy harvesting applications.
Introduction
Chemical doping, which has been demonstrated to be operative in carbon nanotubes,1 is an essential approach to tailor the properties of graphene (G) in order to broaden the applications by creating a band gap.2 One approach to achieve this goal is substitutional doping by introducing heteroatoms, such as boron, sulfur, phosphorus or nitrogen, into the carbon framework of graphene.3 In this way, it is possible to modulate the electronic properties of these carbon nanostructures. For example, graphene doped with boron or nitrogen shows p- and n-type semiconducting properties with a shift in the Fermi level below and above the Dirac point, respectively.4 In particular, N-doped graphene (N-G) has been extensively used in various fields including catalysis,5–7 electrocatalysis,8 biosensors,9 capacitors10 and field-effect transistors.11When G is doped with nitrogen, three types of N atoms are incorporated into the carbon lattice: pyridine-type N, pyrrole-type N and quaternary N. Both pyridine and pyrrole types are suitable to be functionalized by N-alkylation12 to allow the synthesis of a new family of functionalized G.
The promising field of graphene nanocomposites for energy applications13 has stimulated the study of supramolecular and covalent hybrids between G or graphene oxide (GO) and donor14 or acceptor15,16 organic addends with the aim of promoting photo-induced electron transfer for application in solar cells.17 However, hybrid materials that take advantage of both the excellent properties of N-G and the electroactive moiety (donor or acceptor) covalently linked to the N atom have not been widely explored.
In the work reported here, we studied the formation of a novel N-G/C60 hybrid 4 by N-alkylation of N-G with a fullerene derivative; the structural properties of the new hybrid were investigated by thermogravimetric analysis (TGA), X-ray photoelectron (XPS) and Raman spectroscopies, and transmission electron (TEM) and atomic force (AFM) microscopies. In an effort to shed light on the electronic interactions between the two carbon nanostructures, we studied the photophysical properties by absorption and emission spectroscopic techniques including femtosecond transient absorption spectroscopy covering both the visible and near-infrared regions.
Results and discussion
Synthesis and characterization of hybrid N-G/C604
Prior to the preparation of the graphene material 4, the synthesis of fulleropyrrolidine building block 3 was carried out by the widely used approach involving decarboxylation of iminium salts obtained by the condensation of N-(3,5-didodecyloxybenzyl)glycine 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 and aldehyde 2
18 and aldehyde 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (see Scheme 1). Compound 3 was obtained in 32% yield, the structure was confirmed by means of 1H and 13C NMR spectroscopies, and MALDI-TOF MS (see the Experimental section in the ESI†).
19 (see Scheme 1). Compound 3 was obtained in 32% yield, the structure was confirmed by means of 1H and 13C NMR spectroscopies, and MALDI-TOF MS (see the Experimental section in the ESI†).
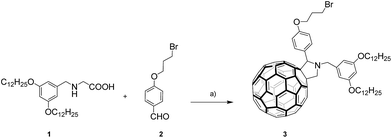 | ||
| Scheme 1 Synthetic route to fulleropyrrolidine 3. Reagents and conditions: (a) C60, dry toluene, 3 h, reflux, (32%). | ||
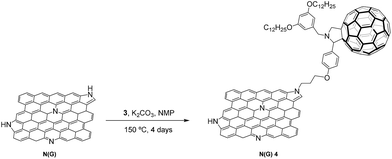
The synthesis of the nitrogen-doped graphene–C60 nanoensemble, N-G/C604, was performed by N-alkylation of pyrrole-type nitrogen atoms onto the graphene surface using potassium carbonate (K2CO3) as the base (Scheme 2).12 For this purpose, commercial nitrogen-doped graphene (http://www.timesnano.com), N-G, was initially exfoliated in N-methyl-2-pyrrolidone (NMP)20 for 15 hours in a sonication bath (37 kHz) at constant temperature (25 °C). The sample was then centrifuged at an appropriate speed to remove the non-exfoliated nitrogen-doped graphene and heavier layers to give predominantly a dispersion of few-layer graphene sheets (3–4 layers) (see further details in the AFM section and ESI†).
N-Alkylation of exfoliated N-G with fulleropyrrolidine 3 was carried out at 150 °C for 4 days in the presence of K2CO3 as the base to afford the corresponding N-G/C60 hybrid 4, which was purified by several cycles of sonication, centrifugation and filtration with water, acetone, methanol and dichloromethane to remove all unreacted components (Scheme 2).
The new material was characterized by several complementary techniques in order to confirm that the chemical functionalization of the N-G material had taken place.
The first evidence for the successful covalent functionalization of N-G was obtained from thermogravimetric analysis (TGA).
The TGA plot, measured at 10 °C min−1 under nitrogen, of nanoensemble 4 is shown in Fig. S1 (see the ESI†) along with those for fulleropyrrolidine 3 and the starting N-G for the sake of comparison. The thermogram of N-G showed a weight loss of around 10.3% between 300 and 650 °C and this is attributed to defects in the starting material. Nanoensemble 4 displayed an additional weight loss of 33.2% in the first derivative curve in this temperature range, which is directly related to the thermal decomposition maxima of the fulleropyrrolidine 3 units at around 350 °C. These losses matched the desorption in the N-G/C604 material.
Raman studies revealed the characteristic D and G bands of graphitic materials at 1356 cm−1 and 1590 cm−1, respectively (see Fig. 1). As previously reported for nitrogen-doped graphene,12 the existence of significant defects related to the presence of nitrogen within the graphitic network increases the intensity of the D band and broadens the Raman bands, thus hampering the observation of any difference after the functionalization process. Evidence for the presence of the C60 cage in the new nanohybrid 4 was provided by the peak at 1471 cm−1, assigned to the C60 Ag (2) pentagonal pinch mode (Fig. 1a), which is shifted by 11 cm−1 compared to the fulleropyrrolidine 3 precursor.16,21–23 Importantly, in N-G/C604 (Fig. 1b) an upshift in the G band due to the p-doping effect24,25 of C60 was observed, as previously reported in the literature for graphene–C60 hybrids.16,23 Finally, a distortion of the 2D band caused by the intercalation of the nitrogen atoms was also observed.26,27
FT-IR spectroscopy corroborated the results obtained by the previously described techniques (Fig. S2 in the ESI†). In the spectrum of 4, the characteristic vibrational modes of C60 at 1458, 1164 and 525 cm−1 are clearly observed with slight shifts when compared to the precursor 3, thus suggesting the existence of C60 units attached to the hybrid. Interestingly, fulleropyrrolidine 3 presents at 833 cm−1 the stretching vibrational mode of the C–Br bond, which disappears after the reaction with N-G, thus confirming that C60 units had been covalently bonded to the nitrogen atoms of N-G; in addition, N-G/C604 exhibits peaks at around 2950 and 2850 cm−1, assigned to C–H stretching vibrations of alkyl chains,16,28 along with a strong peak at 1242 cm−1 attributed to the asymmetric stretching vibration of Ar–O–C in the ether group.
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that allows the determination of the elemental composition within a material by giving information about the binding energy, the electronic states and the type of hybridization.29 A high-resolution N 1s XPS spectrum of the starting nitrogen-doped graphene (N-G) is shown in Fig. S3 and S4 (see the ESI†) and this is deconvoluted into three components located at 398.2, 400.2 eV and 401.8 eV due to pyridinic-N, pyrrolic-N and quaternary nitrogen atoms, respectively30 (see Fig. S4b in the ESI†). This spectrum contrasts with the single N 1s component observed, as expected, for the bromo-fulleropyrrolidine precursor 3, with a binding energy of 398.9 eV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 (see Fig. S6b in the ESI†). After N-alkylation, the analysis of the high-resolution N 1s core-level spectrum peak of nanoensemble 4 was satisfactorily fitted to three components (see Fig. 2 and S7 in the ESI†) even though the N 1s component of the anchored fulleropyrrolidine moiety should also be present. However, as the intensity of this peak is expected to be very low, no attempt was made to include this fourth component in the fitting of N 1s emission of nanohybrid 4. Also, concerning the N 1s component, we observed a shift (∼0.3 eV) of the lower binding energies in the XPS N 1s spectrum of the nanohybrid 4 as compared with the N-G material (see Fig. S8 in the ESI†). This shift suggests a charge transfer from nitrogen-doped graphene to fullerene derivative 3; similar results employing the XPS technique have been obtained for carbon nanotube materials.31 It should also be noted that the C 1s spectrum of the nanohybrid 4 shows a small contribution at 285.2 eV,32 which indicates the presence of sp3 C-atoms of the alkyl chains in fulleropyrrolidine 3 (see Fig. S6a and Table S1 in the ESI†), as observed in previous studies12,16,28 (Fig. 2).
23 (see Fig. S6b in the ESI†). After N-alkylation, the analysis of the high-resolution N 1s core-level spectrum peak of nanoensemble 4 was satisfactorily fitted to three components (see Fig. 2 and S7 in the ESI†) even though the N 1s component of the anchored fulleropyrrolidine moiety should also be present. However, as the intensity of this peak is expected to be very low, no attempt was made to include this fourth component in the fitting of N 1s emission of nanohybrid 4. Also, concerning the N 1s component, we observed a shift (∼0.3 eV) of the lower binding energies in the XPS N 1s spectrum of the nanohybrid 4 as compared with the N-G material (see Fig. S8 in the ESI†). This shift suggests a charge transfer from nitrogen-doped graphene to fullerene derivative 3; similar results employing the XPS technique have been obtained for carbon nanotube materials.31 It should also be noted that the C 1s spectrum of the nanohybrid 4 shows a small contribution at 285.2 eV,32 which indicates the presence of sp3 C-atoms of the alkyl chains in fulleropyrrolidine 3 (see Fig. S6a and Table S1 in the ESI†), as observed in previous studies12,16,28 (Fig. 2).
 | ||
| Fig. 2 XPS analysis of N-G/C60 hybrid 4. The insets contain the XPS high-resolution spectra of C 1s and N 1s showing all the components. | ||
In addition, the surface composition (atom percentages) was evaluated from peak intensities normalized with atomic sensitivity factors,33,34 (see Table S2 in the ESI†). It was found that the percentage of nitrogen in N-G/C604 decreases with respect to the N-G precursor. This result is expected due to the dilution effect of the C atoms incorporated during the anchoring of the bromo-fulleropyrrolidine moiety. Finally, the absence of bromine in nanohybrid 4 unambiguously confirms the covalent linkage of pyrrolidine[60]fullerene 3 to the N-G material.
Atomic force microscopy (AFM) was used to study the morphology of the final nanoensemble N-G/C604 in comparison with the starting N-G. Nitrogen-doped graphene sheets had an average thickness of 3 nm, which corresponds to few-layer graphene (3–4 layer graphene). In addition, the presence of thinner sheets corresponding to single-layer graphene was confirmed in the sample and these had an average height of about 1.4 nm (see ESI, Fig. S9a†). After the N-alkylation, the presence of C60 aggregates on the new nanoensemble N-G/C604 was clearly evidenced by the appearance of brightened zones mainly on the edges of the graphene sheets. N-G/C604 displayed step heights ranging from 8 to 24 nm due to the presence of the aforementioned fullerene aggregates (Fig. S9b in the ESI†).
In order to gain further insights into the microstructure of the samples, Transmission Electron Microscopy (TEM) experiments were performed. Suspensions of nitrogen-doped graphene and N-G/C604 in ethanol were drop-cast onto a lacey carbon grid and dried under vacuum. The study of nitrogen-doped graphene revealed the presence of stacked thin sheets with folded edges of several hundreds of nanometers in lateral dimensions (Fig. 3). After the functionalization process, a clear change in the morphology was observed due to the presence of C60 aggregates on the surface of the graphene sheets. In this case, zoomed images clearly revealed the existence of spherical species with diameters of ∼1 nm attached to the edges of the N-G/C604 nanoensemble, which are highlighted with red circles in Fig. 3b. It should be noted that, in contrast to these results, isolated C60 units were not observable by AFM because of the large uncertainty of this technique during the determination of small structures, which is attributed mainly to the substrate roughness, and the low signal-to-noise ratio.35–37 In addition, given that the individual C60 molecules were mainly present at the outside edge of the graphene sheets, the detection of graphene/C60 step heights normally detected in overlapped structures by AFM was not possible in this case.
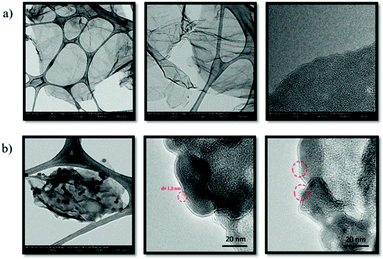 | ||
| Fig. 3 TEM images of (a) nitrogen-doped graphene and (b) N-G/C604 showing spherical C60 units highlighted in red circles. | ||
Further studies on the electronic interaction between fullerene derivative 3 and the nitrogen-doped graphene material were carried out by absorption and fluorescence spectroscopies on samples in o-dichlorobenzene (o-DCB). The UV-vis absorption spectrum of N-G was almost featureless (Fig. 4a); however, after the functionalization process, a new broad band was observed with a maximum at 416 nm, which is blue-shifted when compared with the characteristic absorption peak of the precursor fullerene derivative 3 (432 nm).
Based on these results, the following conclusions can be drawn: (1) C60 units were successfully incorporated onto the graphene surface and, (2) electronic communication exists between the two-electroactive components16 (fullerene and graphene material).
In order to study the existence of excited-state interactions between the two forms of carbon in the final hybrid, the emission of the materials was studied using isoabsorbent dispersions of nanoensemble 4 and fulleropyrrolidine 3 and using o-DCB as solvent (Fig. 4b). Compound 3 exhibits a weak emission band (upon 432 nm excitation) at 711 nm. This band is dramatically quenched (by nearly 81%) in N-G/C604 and this suggests the occurrence of excited-state events such as electron transfer or energy transfer.16
Electrochemical studies were performed to compare the redox potentials of the nanoensemble 4 and fulleropyrrolidine 3. The Differential Pulse Voltammograms (DPVs) of the different species in o-DCB/acetonitrile solutions containing tetra-n-butylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte are shown in Fig. 5.
 | ||
Fig. 5 DPV (reduction run) of fulleropyrrolidine 3 ( ) and N-G/C604 ( ) and N-G/C604 ( ) in o-DCB/ACN (4 ) in o-DCB/ACN (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 0.1 M TBAPF6. Scan rate = 1 mV s−1, pulse width = 0.2 s. 1) containing 0.1 M TBAPF6. Scan rate = 1 mV s−1, pulse width = 0.2 s. | ||
In the hybrid N-G/C604, three reduction potentials at −1.18 V, −1.58 V and −2.12 V appeared in the observation window. These potentials are shifted by 30–40 mV with respect to those of fulleropyrrolidine 3 (−1.15 V, −1.54 V and −2.06 V), respectively.
This shift is attributed to the existence of intramolecular electronic interactions in the ground state between the nitrogen-doped material and the fullerene cages, as observed previously in related materials.16
Photochemical studies
The steady-state fluorescence studies discussed above revealed quantitative quenching of the fulleropyrrolidine emission in the N-G/C60 hybrid 4, thus suggesting the occurrence of excited state events. This is conceivable since a bandgap of ∼0.16 eV exists in N-G due to N-doping.38 Under such circumstances, the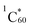 in the N-G/C60 hybrid could undergo charge transfer either by donating the electron from the LUMO level of
in the N-G/C60 hybrid could undergo charge transfer either by donating the electron from the LUMO level of  to the valence band of N-G or the half-filled HOMO of
to the valence band of N-G or the half-filled HOMO of  could abstract an electron from the conduction band of N-G. The latter charge transfer path is preferred due to the superior electron acceptor behavior of C60 (facile reduction); thus, formation of the N-G˙+–C60˙− charge-separated state is expected in the N-G/C60 hybrid 4. In order to provide evidence for such a photochemical event, femtosecond transient absorption studies were performed followed by photocatalytic electron pooling studies,39,40 wherein the N-G˙+–C60˙− charge separated state generated upon photoexcitation was used to produce the one-electron reduced product of methyl viologen, MV2+. We have successfully used this strategy in the past to demonstrate charge separation and utilization of a charge-separated state in photocatalytic applications in nanocarbon-derived donor–acceptor hybrids.33,41,42
could abstract an electron from the conduction band of N-G. The latter charge transfer path is preferred due to the superior electron acceptor behavior of C60 (facile reduction); thus, formation of the N-G˙+–C60˙− charge-separated state is expected in the N-G/C60 hybrid 4. In order to provide evidence for such a photochemical event, femtosecond transient absorption studies were performed followed by photocatalytic electron pooling studies,39,40 wherein the N-G˙+–C60˙− charge separated state generated upon photoexcitation was used to produce the one-electron reduced product of methyl viologen, MV2+. We have successfully used this strategy in the past to demonstrate charge separation and utilization of a charge-separated state in photocatalytic applications in nanocarbon-derived donor–acceptor hybrids.33,41,42
The femtosecond transient absorption spectra of pristine N-G dispersed in DMF are shown in Fig. 6a and b and these cover both the visible and near-IR regions. The features of the spectra are largely similar to the transient features of graphene dispersions in organic solvents.43,44 At the excitation wavelength of 432 nm, the instantaneous formation of optical phonon peaks with peak minima at 506 and 1120 nm was observed. The near-IR signal recovered with a time constant of 1.5 ps. In the case of the N-G/C60 hybrid, at the excitation wavelength of 432 nm, where  was also expected to form, the spectral features were similar to those of pristine N-G (see Fig. 6c and d). Weak spectral features of
was also expected to form, the spectral features were similar to those of pristine N-G (see Fig. 6c and d). Weak spectral features of  in the 850–1000 nm range were largely buried under the strong phonon peaks of N-G (see Fig. S10 in the ESI†) for the femtosecond transient spectra of fulleropyrrolidine. Rapid decay of the
in the 850–1000 nm range were largely buried under the strong phonon peaks of N-G (see Fig. S10 in the ESI†) for the femtosecond transient spectra of fulleropyrrolidine. Rapid decay of the  signals was also accompanied by weak absorbance in the 1000 nm region, which is attributable to C60˙−, a product of charge separation. Interestingly, the time profiles for recovery of the visible and near-IR peak were almost identical to that of pristine N-G (see Fig. 6e and f for overlapping time profiles). This suggests that the excited N-G phonons are probably not involved in promoting charge separation but only the
signals was also accompanied by weak absorbance in the 1000 nm region, which is attributable to C60˙−, a product of charge separation. Interestingly, the time profiles for recovery of the visible and near-IR peak were almost identical to that of pristine N-G (see Fig. 6e and f for overlapping time profiles). This suggests that the excited N-G phonons are probably not involved in promoting charge separation but only the 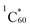 is involved in producing the N-G˙+–C60˙− charge-separated state.
is involved in producing the N-G˙+–C60˙− charge-separated state.
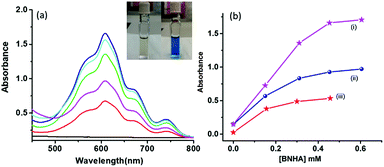
As pointed out earlier, the accumulation of electron transfer products using the donor–acceptor hybrids as a photocatalyst not only serves as proof of the occurrence of excited state charge separation in these systems, where it is difficult to characterize spectrally the electron transfer products, but also their usefulness in energy harvesting applications. In the present study, we performed electron pooling experiments involving a sacrificial electron donor, 1-benzyl-1,4-dihydronicotinamide (BNAH), and the electron acceptor methyl viologen (MV2+).33,42 Direct electron transfer from BNAH to MV2+ is an uphill process; however, a charge-separated state from a donor–acceptor hybrid could make this process energetically feasible. Here, continuous irradiation of a mixture of the N-G/C60 nanohybrid in the presence of MV2+ and BNAH led to the accumulation of MV˙+ with a characteristic peak at 610 nm (see Fig. 7a inset for solution color before and after the experiment). In the present study, the samples were excited using a xenon lamp with a 400 nm filter to excite selectively the N-G/C60 nanohybrid and not the sacrificial electron donor or electron acceptor (BNAH and MV2+) used in the pooling experiment. As shown in Fig. 7a, the addition of more BNAH increased the intensity of MV˙+ in an experiment involving the N-G/C60 nanohybrid. The efficiency of MV˙+ production with the N-G/C60 nanohybrid is shown in Fig. 7b along with the data for pristine N-G and additives alone for the sake of comparison. A higher efficiency was clearly observed when N-G/C60 hybrid 4 was used. The results obtained here complement outcomes of a recent study by Jia and co-workers, where N-G/CdS nanocomposites were shown to produce enhanced photocatalytic hydrogen from water under visible light irradiation.45
Conclusions
The newly synthesized and characterized N-doped graphene–C60 nanohybrid 4 was found to be both electroactive and photoactive, which are useful characteristics for different applications. The synthetic methodology developed here gave reasonable yields of the nanohybrids and it was possible to estimate the degree of functionalization. Ground state interactions between N-G and C60 were revealed by absorption and electrochemical studies. Excited state interactions were monitored by fluorescence and femtosecond transient absorption spectral studies. The quenching of C60 fluorescence in the N-G/C60 hybrids due to excited state charge separation from the state was supported by femtosecond transient absorption studies. The photochemically active N-G/C60 hybrid was also found to be a good photocatalyst in an electron pooling experiment, which suggests the usefulness of this system in solar energy harvesting applications.
state was supported by femtosecond transient absorption studies. The photochemically active N-G/C60 hybrid was also found to be a good photocatalyst in an electron pooling experiment, which suggests the usefulness of this system in solar energy harvesting applications.
Compared to pristine graphene derived donor–acceptor hybrids in the literature, the present study involving N-doped graphene stands out in several aspects. Some of these include (i) the N atom in N-G provides a site for facile chemical functionalization, (ii) it would be easier to control the distance between the donor and acceptor, and the type of spacer groups controlling the electronic communication between the entities due to the availability of N sites in the graphene sheet, (iii) as demonstrated in the present study, due to better exfoliation, characterization of mono- or few-layer functionalized NG would be relatively easier, and finally, (iv) control of photochemical events in the donor–acceptor hybrid has been possible to some extent. Further work on functionalized hetero-atom doped graphene derived donor–acceptor hybrids is underway in our laboratories.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Spanish Ministry of Economy and Competitiveness of Spain (CTQ2015-71936-REDT and CTQ2016-79189-R) and the US-National Science Foundation (Grant No. 1401188 to FD) financially supported this research. L. M. A. thanks the Spanish Ministry of Economy and Competitiveness for a doctoral FPI grant. Thanks to Prof. M. Prato for his kind support with the TEM equipment.Notes and references
- C. Zhou, J. Kong, E. Yenilmez and H. Da, Science, 2000, 290, 1552 CrossRef PubMed.
- H. Zhang, E. Bekyarova, J.-W. Huang, Z. Zhao, W. Bao, F. Wang, R. C. Haddon and C. N. Lau, Nano Lett., 2011, 11, 4047 CrossRef PubMed.
- S. Navalón, A. Dhakshinamoorthy, M. Álvaro and H. García, Chem. Rev., 2014, 114, 6179 CrossRef PubMed.
- L. S. Panchakarla, K. S. Subrahmanyam, S. K. Saha, A. Govindaraj, H. R. Krishnamurthy, U. V. Waghmare and C. N. R. Rao, Adv. Mater., 2009, 21, 4726 Search PubMed.
- H. Wang, T. Maiyalagan and X. Wang, ACS Catal., 2012, 2, 781 CrossRef.
- C. Hua, D. Liub, Y. Xiao and L. Dai, Prog. Nat. Sci.: Mater. Int., 2018, 28, 121 CrossRef.
- Y. Cao, S. Mao, M. Li, Y. Chen and Y. Wang, ACS Catal., 2017, 7, 8090 CrossRef.
- H. Cui, Z. Zhou and D. Jia, Mater. Horiz., 2017, 4, 7 RSC.
- H. Fan, Y. Li, D. Wu, H. Ma, K. Mao, D. Fan, B. Du, H. Li and Q. Wei, Anal. Chim. Acta, 2012, 711, 24 CrossRef PubMed.
- H. M. Jeong, J. W. Lee, W. H. Shin, Y. J. Choi, H. J. Shin, J. K. Kang and J. W. Choi, Nano Lett., 2011, 11, 2472 CrossRef PubMed.
- U. A. Palnitkar, R. V. Kashid, M. A. More, D. S. Joag, L. S. Panchakarla and C. N. R. Rao, Appl. Phys. Lett., 2010, 97, 063102 CrossRef.
- M. Barrejón, A. Primo, M. J. Gómez-Escalonilla, J. L. García-Fierro, H. García and F. Langa, Chem. Commun., 2015, 51, 16916 RSC.
- I. V. Lightcap and P. V. Kamat, Acc. Chem. Res., 2012, 46, 2235 CrossRef PubMed.
- C. B. KC, S. K. Das, K. Ohkubo, S. Fukuzumi and F. D'Souza, Chem. Commun., 2012, 48, 11859 RSC.
- L. Wibmer, L. M. O. Lourenço, A. Roth, G. Katsukis, M. G. P. M. S. Neves, J. A. S. Cavaleiro, J. P. C. Tomé, T. Torres and D. M. Guldi, Nanoscale, 2015, 7, 5674 RSC.
- M. Barrejón, M. Vizuete, M. J. Gómez-Escalonilla, J. L. García-Fierro, I. Berlanga, F. Zamora, G. Abellán, P. Atienzar, J.-F. Nierengarten, H. García and F. Langa, Chem. Commun., 2014, 50, 9053 RSC.
- G. Bottari, M. Á. Herranz, L. Wibmer, M. Volland, L. Rodríguez-Pérez, D. M. Guldi, A. Hirsch, N. Martín, F. D'Souza and T. Torres, Chem. Soc. Rev., 2017, 46, 4464 RSC.
- F. Ajamaa, T. M. Figueira Duarte, C. Bourgogne, M. Holler, P. W. Fowler and J. F. Nierengarten, Eur. J. Org. Chem., 2005, 3766 CrossRef.
- E. Shibu, A. Sonoda, Z. Tao, Q. Feng, A. Furube, S. Masuo, L. Wang, N. Tamai, M. Ishikawa and V. Biju, ACS Nano, 2012, 6, 1601 CrossRef PubMed.
- S. P. Ogilvie, M. J. Large, G. Fratta, M. Meloni, R. Canton-Vitoria, N. Tagmatarchis, F. Massuyeau, C. P. Ewels, A. A. K. King and A. B. Dalton, Sci. Rep., 2017, 7, 16706 CrossRef PubMed.
- M. Vizuete, M. J. Gómez-Escalonilla, J. L. García-Fierro, K. Ohkubo, S. Fukuzumi, M. Yudasaka, S. Iijima, J.-F. Nierengarten and F. Langa, Chem. Sci., 2014, 5, 2072 RSC.
- X. Zhang, Y. Huang, Y. Wang, Y. Ma, Z. Liu and Y. Chen, Carbon, 2008, 47, 313 Search PubMed.
- M. Barrejón, H. B. Gobeze, M. J. Gómez-Escalonilla, J. L. García-Fierro, M. Zhang, M. Yudasaka, S. Iijima, F. D'Souza and F. Langa, Nanoscale, 2016, 8, 14716 RSC.
- G. Jnawali, Y. Rao, J. H. Beck, N. Petrone, G. Jnawali, Y. Rao, J. H. Beck, N. Petrone, I. Kymissis, J. Hone and T. F. Heinz, ACS Nano, 2015, 9, 7175 CrossRef PubMed.
- D. García, L. Rodríguez-Pérez, M. Á. Herranz, D. Peña, E. Guitián, S. Bailey, Q. Al-Galiby, M. Noori, C. J. Lambert, D. Pérez and N. Martín, Chem. Commun., 2014, 52, 6677 RSC.
- N. Mahmood, C. Zhang, H. Yin and Y. Hou, J. Mater. Chem. A, 2014, 2, 15 RSC.
- C. Chang, S. Kataria, C. Kuo, A. Ganguly, B. Wang, J. Hwang, K. Huang, W. Yang, S. Wang, C. Chuang, C. Huang, W. Pong, K. Song, S. Chang, J. Guo, Y. Tai, M. Tsujimoto, S. Isoda, C. Chen, L. Chen and K.-H. Chen, ACS Nano, 2013, 7, 133 Search PubMed.
- A. Criado, M. J. Gómez-Escalonilla, J. L. García-Fierro, A. Urbina, D. Peña, E. Guitián and F. Langa, Chem. Commun., 2010, 46, 7028 RSC.
- M. E. Lipinska, S. L. H. Rebelo, M. F. R. Pereira, J. A. N. F. Gomes, C. Freire and J. L. Figueiredo, Carbon, 2012, 50, 3280 CrossRef.
- Z. Tian, S. Dai and D. Jiang, Chem. Mater., 2015, 27, 5775 CrossRef.
- V. O. Koroteev, L. G. Bulusheva, I. P. Asanov, E. V. Shlyakhova, D. V. Vyalikh and A. V. Okotrub, J. Phys. Chem. C, 2011, 115, 21199 CrossRef.
- M. Biswal, X. Zhang, D. Schilter, T. K. Lee, D. Y. Hwang, M. Saxena, S. H. Lee, S. Chen, S. K. Kwak, C. W. Bielawski, W. S. Bacsa and R. S. Ruoff, J. Am. Chem. Soc., 2017, 139, 4202 CrossRef PubMed.
- L. M. Arellano, M. Barrejón, H. B. Gobeze, M. J. Gómez-Escalonilla, J. L. García-Fierro, F. D'Souza and F. Langa, Nanoscale, 2017, 9, 7551 RSC.
- L. M. Arellano, L. Martín-Gomis, H. B. Gobeze, M. Barrejón, D. Molina, M. J. Gómez-Escalonilla, J. L. García-Fierro, M. Zhang, M. Yudasaka, S. Iijima, F. D'Souza, F. Langa and Á. Sastre-Santos, J. Mater. Chem. C, 2015, 3, 10215 RSC.
- P. Nemes-Incze, Z. Osváth, K. Kamarás and L. P. Biró, Carbon, 2008, 46, 1435 CrossRef.
- C. H. Lui, L. Liu, K. F. Mak, G. W. Flynn and T. F. Heinz, Nature, 2009, 462, 339 CrossRef PubMed.
- A. Mechler, J. Kopniczky, J. Kokavecz, A. Hoel, C.-G. Granqvist and P. Heszler, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 125407 CrossRef.
- C. Zhang, L. Fu, N. Liu, M. Liu, Y. Wang and Z. Liu, Adv. Mater., 2011, 23, 1020 CrossRef PubMed.
- S. Fukuzumi, H. Imahori, K. Ohkubo, H. Yamada, M. Fujitsuka, O. Ito and D. M. Guldi, J. Phys. Chem. A, 2002, 106, 1903 CrossRef.
- F. D'Souza, A. S. D. Sandanayaka and O. Ito, J. Phys. Chem. Lett., 2010, 1, 2586 CrossRef.
- M. Barrejón, S. Pla, I. Berlanga, M. J. Gómez-Escalonilla, L. Martín-Gomis, J. L. García-Fierro, M. Zhang, M. Yudasaka, S. Iijima, H. B. Gobeze, F. D'Souza, Á. Sastre-Santos and F. Langa, J. Mater. Chem. C, 2015, 3, 4960 RSC.
- L. M. Arellano, L. Martín-Gomis, H. B. Gobeze, D. Molina, C. Hermosa, M. J. Gómez-Escalonilla, J. l. García-Fierro, Á. Sastre-Santos, F. D'Souza and F. Langa, Nanoscale, 2018, 10, 5205 RSC.
- A. Stergiou, H. B. Gobeze, I. D. Petsalakis, S. Zhao, H. Shinohara, F. D'Souza and N. Tagmatarchis, Nanoscale, 2015, 7, 15840 RSC.
- M. E. Ragoussi, J. Malig, G. Katsukis, B. Butz, E. Spiecker, G. de la Torre, T. Torres and D. M. Guldi, Angew. Chem., Int. Ed., 2012, 51, 6421 CrossRef PubMed.
- L. Jia, D.-H. Wang, Y.-X. Huang, A.-W. Xu and H.-Q. Yu, J. Phys. Chem. C, 2011, 115, 11466 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and synthetic procedures and compound characterization data, along with Fig. S1–S10 and Tables S1 and S2. See DOI: 10.1039/c8sc02013b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |