Open Access Article
Open Access ArticleA bottom-up synthesis of rare-earth-hydrotalcite monolayer nanosheets toward multimode imaging and synergetic therapy†
Xuan
Mei
,
Jialing
Ma
,
Xue
Bai
,
Xin
Zhang
,
Shaomin
Zhang
,
Ruizheng
Liang
*,
Min
Wei
 *,
David G.
Evans
and
Xue
Duan
*,
David G.
Evans
and
Xue
Duan
State Key Laboratory of Chemical Resource Engineering, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: liangruizheng2000@163.com; weimin@mail.buct.edu.cn
First published on 29th May 2018
Abstract
Recently, ultrathin two-dimensional (2D) nanomaterials have attracted considerable research interest in biomedical applications, owing to their intriguing quantum size and surface effects. In this work, a one-step “bottom-up” method is developed to prepare rare-earth (Gd3+ and Yb3+) co-doped layered double hydroxide (LDH) monolayer nanosheets, with a precisely controlled composition and uniform morphology. Due to the successful introduction of Gd3+ and Yb3+ into the LDH host layer, the Gd&Yb-LDH monolayer nanosheets exhibit excellent magnetic resonance (MR)/X-ray computed tomography (CT) dual-mode imaging functionality. Moreover, the Gd&Yb-LDH monolayer nanosheets achieve an ultrahigh loading of a chemotherapeutic drug (SN38) with a loading content (LC) of 925%, which is a one order of magnitude enhancement compared with previously reported delivery systems of hydrophobic drugs. Interestingly, by further combination with indocyanine green (ICG), in vivo tri-mode imaging, including CT, MR and near infrared fluorescence (NIRF) imaging, is achieved, which enables a noninvasive visualization of cancer cell distribution with deep spatial resolution and high sensitivity. In addition, in vitro and in vivo therapeutic evaluations demonstrate an extremely high tri-mode synergetic anticancer activity and superior biocompatibility of SN38&ICG/Gd&Yb-LDH. Therefore, this work demonstrates a paradigm for the synthesis of novel multifunctional 2D monolayer materials via a facile “bottom-up” route, which shows promising applications in cancer synergetic theranostics.
Introduction
Recently, increasingly interdisciplinary research efforts have been devoted to exploring biomedical applications of ultrathin two-dimensional (2D) nanomaterials such as graphene,1 black phosphorus (BP),2 hexagonal boron nitride (h-BN)3 and transition-metal dichalcogenides (TMDs),4 due to their extraordinary quantum size and surface effects.5 Their intrinsic physicochemical and electronic properties make them promising candidates for a variety of imaging techniques (e.g. magnetic resonance (MR), X-ray computed tomography (CT), and photoacoustics (PA)) and photothermal therapy.6 Although much progress has been made,7 the most studied ultrathin 2D nanomaterials, normally synthesized by exfoliation of bulk precursors, suffer from fixed chemical composition with uncontrollable functionality. The lack of universal and gentle methods to prepare ultrathin or monolayer 2D nanomaterials is still the key scientific problem in 2D nanomaterials.8 Therefore, if monolayer 2D nanomaterials with a fine control over morphology, thickness, chemical composition and functionality can be prepared by a universal bottom-up chemical synthesis method, the intrinsic performance of 2D nanomaterials would be observably promoted.Layered double hydroxides (LDHs), a typical 2D nanomaterial with the formula [M1−x2+Mx3+(OH)2](An−)x/n·mH2O, are made up of positively charged, edge-sharing MO6 octahedral host layers and exchangeable interlayer anions.9 Due to the unique 2D structure with tunability in both host layer and interlayer anions, LDHs have shown potential for application as drug carriers in biomedicine.10 For example, LDHs have been demonstrated as an efficient drug formulation for delivery and controlled release of drug molecules owing to their low toxicity and good biocompatibility.11,12 Although a synthetic strategy for LDHs has been developed,13 the preparation of LDH monolayer nanosheets with precise control over chemical compositions and regulation of monolayer functionality is still an obstacle and hasn't been resolved yet. Moreover, hydrophobic drugs like SN38 possesses 100–1000 times stronger anticancer performance than their modified hydrophilic drugs (camptothecin-11);14 however, their application is greatly limited by their poor water solubility, and the current drug carriers usually exhibit poor loading content (LC). Enhancing the loading and controlled release of hydrophobic drugs like SN38 is highly needed but remains a big challenge.
Herein, we designed and prepared Gd3+ and Yb3+ co-doped LDH monolayer nanosheets via a one-step “bottom-up” method, which exhibited excellent dual-model imaging functionality. HRTEM and AFM show that Gd&Yb-LDH nanosheets possess a uniform lateral size of ∼50 nm and a thickness of ∼0.9 nm, corresponding to a single sheet of LDH matrix. EDX and XPS spectroscopy verify the successful co-doping of Gd3+ and Yb3+ into the LDH host layer. Moreover, Gd&Yb-LDH monolayer nanosheets achieve an ultrahigh loading of chemotherapeutic drug SN38 with a LC of 925%, which is a one order of magnitude enhancement compared with previously reported delivery systems of hydrophobic drugs.14 The as-prepared SN38&ICG/Gd&Yb-LDH shows a controlled drug release behavior with pH stimuli or NIR irradiation, as well as significantly enhanced photothermal and photodynamic performance. In vitro tests verify a superior anticancer efficacy of SN38&ICG/Gd&Yb-LDH with an IC50 value of 0.532 μg mL−1, significantly lower than that of pristine SN38 (5.80 μg mL−1) or ICG (1.89 μg mL−1). In vivo MR/CT/near infrared fluorescence (NIRF) tri-mode imaging exhibits an excellent passive targeted tumor accumulation of SN38&ICG/Gd&Yb-LDH, and a complete tumor ablation is realized after treatment with SN38&ICG/Gd&Yb-LDH, demonstrating a promising synergetic theranostics performance.
Results and discussion
Structural characterization
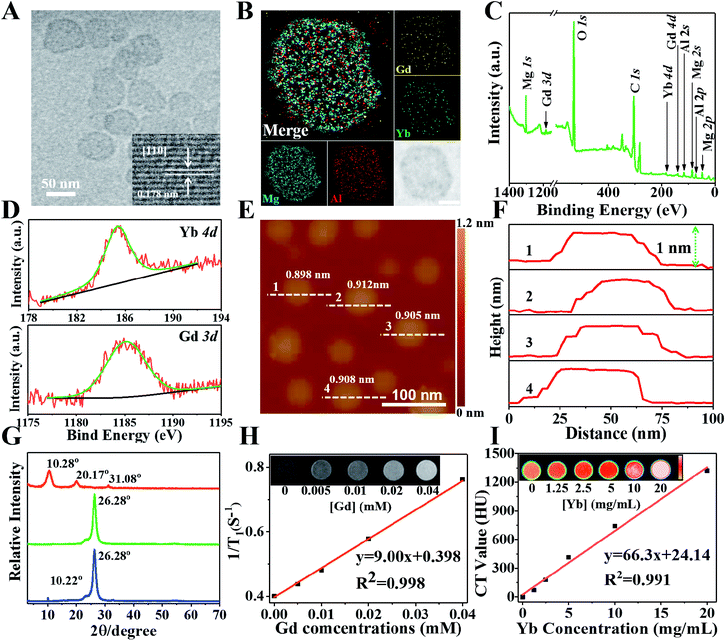
To realize MR and CT imaging, a new “bottom-up” method is developed to synthesize Gd&Yb-containing LDH monolayer nanosheets (see the Experimental section for details), where Gd3+ and Yb3+ are simultaneously introduced into an LDH matrix (molar content: 10% each). As shown in Fig. S1,† ultrathin Gd&Yb-LDH nanosheets are prepared through a bottom-up hydrothermal synthesis with a yield of 15.4%. Fig. 1A shows the high resolution transmission electron microscopy (HRTEM) image of monodisperse, hexagonal Gd&Yb-LDH nanosheets with a lateral size of ∼50 nm, and the lattice fringe of 0.178 nm in the inset can be ascribed to the LDH [110] plane. The dynamic light scattering technique gives an equivalent hydrodynamic diameter of 50 ± 9.5 nm in water, phosphate buffered saline (PBS) or culture medium (Fig. S2†). After storage for two weeks, no obvious change in the hydrodynamic size of Gd&Yb-LDH was found (Fig. S3†), demonstrating its long-term stability. The elemental distributions obtained from energy-dispersive X-ray (EDX) mapping (Fig. 1B) and the corresponding EDX line profile (Fig. S4†) reveal that Mg, Al, Gd and Yb are homogeneously distributed throughout the LDH nanosheets, indicating the co-doping of Gd and Yb into the LDH matrix. This is further confirmed by X-ray photoelectron spectroscopy (XPS) peaks at 1185.2 eV and 185.4 eV, corresponding to the characteristic peaks of Gd 3d and Yb 4d, respectively (Fig. 1C and D). Atomic force microscopy (AFM) images verify that the Gd&Yb-LDH nanosheets possess a monolayer thickness of ∼0.9 nm (Fig. 1E and F), close to that obtained using the liquid delamination method.15 The monolayer structure of Gd&Yb-LDH nanosheets is further confirmed by X-ray diffraction (XRD) analysis. The Gd&Yb-LDH colloid sample doesn't show the characteristic reflection of the LDH phase (Fig. 1G; green line), in contrast to the (003) peak at 2θ = 10.22° for an LDH bulk sample (Fig. 1G; blue line), indicating the absence of the multilayer structure. However, after drying the Gd&Yb-LDH colloid sample, a (003) reflection at 10.28° is observed, which is due to the re-stacking of the LDH monolayer to the bulk material (Fig. 1G; red line). Taking into account MRI and CT dual-mode contrast agents, the relevant parameters of Gd&Yb-LDH were evaluated. The T1-weighted relaxivity (r1, a key parameter in MRI) value of Gd&Yb-LDH is determined to be 9.00 mM−1 s−1 (Fig. 1H), while the CT value shows a positive enhancement against concentration with a slope of 66.3 (Fig. 1I), which are 2.47 and 2.20 fold respectively larger than that of commercial MRI (Gd-DOTA)16 and CT (iobitridol) contrast agents.17Drug loading performance
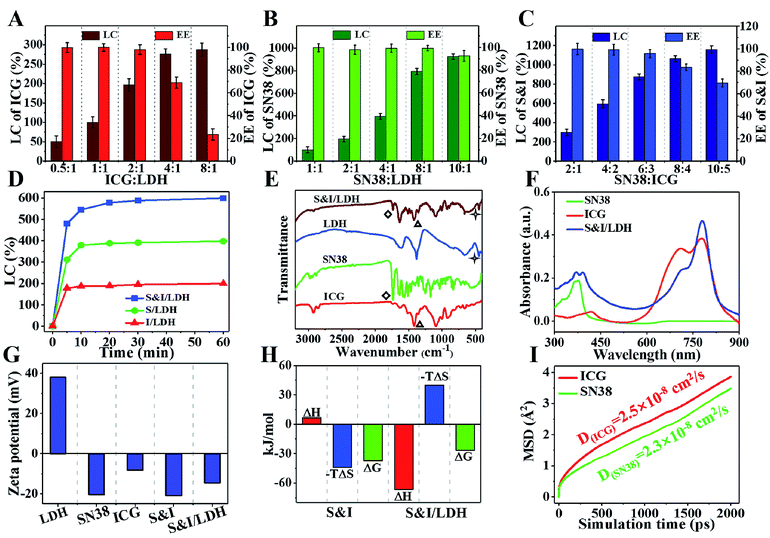
The loading performance of Gd&Yb-LDH nanosheets toward ICG and SN38 was studied. For separate loading of ICG, the loading content (LC) and encapsulation efficiency (EE) are 196% and 98.1% with a nominal mass ratio of drug![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH = 2
LDH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2A). The LC increases slightly but the EE decreases obviously along with the increase of ICG
1 (Fig. 2A). The LC increases slightly but the EE decreases obviously along with the increase of ICG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd&Yb-LDH mass ratio. For the loading of SN38, the LC and EE are 794% and 99.3% with a mass ratio of drug
Gd&Yb-LDH mass ratio. For the loading of SN38, the LC and EE are 794% and 99.3% with a mass ratio of drug![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDH = 8
LDH = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2B), giving an ultrahigh LC value at close to 100% EE. Most importantly, the LC of SN38 reaches 925% with a mass ratio of SN38
1 (Fig. 2B), giving an ultrahigh LC value at close to 100% EE. Most importantly, the LC of SN38 reaches 925% with a mass ratio of SN38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd&Yb-LDH = 10
Gd&Yb-LDH = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is a one order of magnitude enhancement compared with previously reported delivery systems of hydrophobic drugs (Table S1†).14 Subsequently, we further investigated the co-loading of SN38 and ICG (mass ratio of 2
1, which is a one order of magnitude enhancement compared with previously reported delivery systems of hydrophobic drugs (Table S1†).14 Subsequently, we further investigated the co-loading of SN38 and ICG (mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) onto the Gd&Yb-LDH nanosheets. The largest co-loading LC is found to be 1105% (SN38
1) onto the Gd&Yb-LDH nanosheets. The largest co-loading LC is found to be 1105% (SN38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ICG
ICG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd&Yb-LDH = 10
Gd&Yb-LDH = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); however, taking into account both LC and EE, the final mass ratio is determined to be SN38
1); however, taking into account both LC and EE, the final mass ratio is determined to be SN38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ICG
ICG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd&Yb-LDH = 4
Gd&Yb-LDH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, for which the LC and EE values are 595% and 99.1%, respectively (Fig. 2C). The time-dependent adsorption behavior (Fig. 2D) shows a very fast adsorption within the first 5 min and a gentle increment up to 60 min. As shown in the Fourier transform infrared (FT-IR) spectra (Fig. 2E), bands at 1735 cm−1 (stretching vibration of C
1, for which the LC and EE values are 595% and 99.1%, respectively (Fig. 2C). The time-dependent adsorption behavior (Fig. 2D) shows a very fast adsorption within the first 5 min and a gentle increment up to 60 min. As shown in the Fourier transform infrared (FT-IR) spectra (Fig. 2E), bands at 1735 cm−1 (stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) for SN38 and 1423 cm−1 (stretching vibration of C
O) for SN38 and 1423 cm−1 (stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) for ICG are observed in the final sample, accompanied by the characteristic band of LDH at 445 cm−1 (vibration absorption peak of O–M–O), indicating the co-loading of SN38 and ICG onto LDH nanosheets. The combination is further confirmed in the UV-vis-NIR spectra (Fig. 2F), where SN38&ICG/Gd&Yb-LDH exhibits both the absorption peaks of SN38 and ICG. Moreover, due to the monodisperse state of ICG on Gd&Yb-LDH nanosheets, SN38&ICG/Gd&Yb-LDH shows a 1.21-fold absorption enhancement compared to pristine ICG, which is beneficial to the photothermal conversion. In addition, the zeta potential of SN38&ICG/Gd&Yb-LDH is measured to be −14.5 mV (Fig. 2G), which is ascribed to the immobilization of negatively charged SN38&ICG (−21.1 mV) onto positively charged Gd&Yb-LDH nanosheets (+38.0 mV).
C) for ICG are observed in the final sample, accompanied by the characteristic band of LDH at 445 cm−1 (vibration absorption peak of O–M–O), indicating the co-loading of SN38 and ICG onto LDH nanosheets. The combination is further confirmed in the UV-vis-NIR spectra (Fig. 2F), where SN38&ICG/Gd&Yb-LDH exhibits both the absorption peaks of SN38 and ICG. Moreover, due to the monodisperse state of ICG on Gd&Yb-LDH nanosheets, SN38&ICG/Gd&Yb-LDH shows a 1.21-fold absorption enhancement compared to pristine ICG, which is beneficial to the photothermal conversion. In addition, the zeta potential of SN38&ICG/Gd&Yb-LDH is measured to be −14.5 mV (Fig. 2G), which is ascribed to the immobilization of negatively charged SN38&ICG (−21.1 mV) onto positively charged Gd&Yb-LDH nanosheets (+38.0 mV).
The HRTEM image (Fig. S5†) shows that the uniform morphology of SN38&ICG/Gd&Yb-LDH is well maintained after the co-loading of SN38 and ICG, with a diameter of ∼60 nm. Moreover, its thickness in aqueous solution, which was studied by molecular dynamics (MD) simulations (Fig. S6†), was determined to be 8.4 nm. Fig. S7† displays rather similar hydrodynamic diameters of SN38&ICG/Gd&Yb-LDH in deionized water, PBS and culture medium. Moreover, both Gd&Yb-LDH and SN38&ICG/Gd&Yb-LDH display an obvious Tyndall effect for 14 days (Fig. S8†), indicating high aqueous dispersibility and colloidal stability. The interactions of SN38–ICG and drug–LDH were investigated by isothermal titration calorimetry (ITC) measurements. The SN38–ICG system shows positive values of ΔH and ΔS, indicating hydrophobic interaction between SN38 and ICG, while in the case of SN38&ICG/Gd&Yb-LDH, negative values of both ΔH and ΔS are obtained, demonstrating the existence of van der Waals' force, electrostatic interaction or hydrogen bonding between drug and LDH (Fig. 2H and S9†). To further address the interaction mechanism of drug–LDH and the high drug loading performance, molecular dynamics (MD) simulations were employed (see details in Fig. S6†). As shown in Fig. 2I, both SN38 and ICG give obviously smaller diffusion coefficients (with an average binding energy of −31.1 kcal mol−1 for SN38 and −35.9 kcal mol−1 for ICG) on Gd&Yb-LDH than in water (Fig. S10†), which is ascribed to electrostatic interaction between drug molecules and the Gd&Yb-LDH monolayer. Fig. S11A† displays the O⋯H–O hydrogen bond with a bond length of 2.0–3.0 Å demonstrated by the radial distribution function (Fig. S11B and C†), indicating a strong hydrogen bond interaction between drug molecules and the Gd&Yb-LDH monolayer. In conclusion, both electrostatic interaction and hydrogen bond play a key role in determining the high drug loading content.
Photothermal study and stimulated drug release
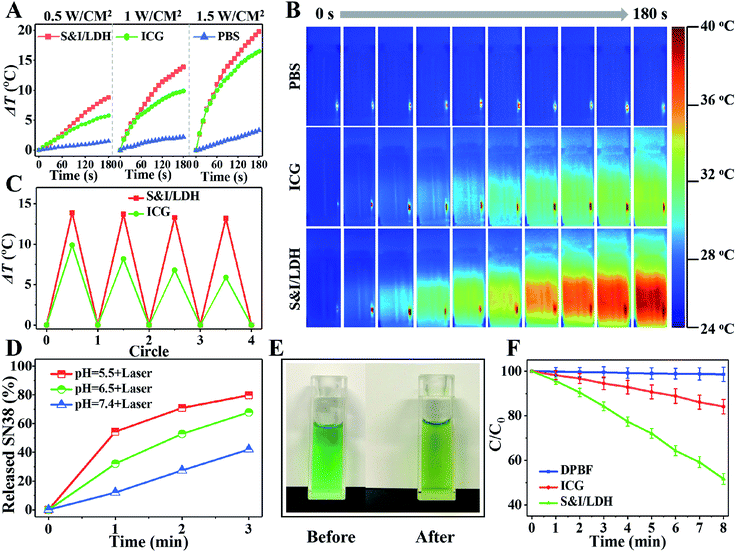
Owing to the intrinsic near-infrared (NIR) optical absorption of ICG, the photothermal conversion performance of SN38&ICG/Gd&Yb-LDH was studied. Fig. 3A shows the temperature elevation in the presence of SN38&ICG/Gd&Yb-LDH, ICG and PBS (concentration of ICG: 2.5 μg mL−1), respectively, upon 808 nm laser irradiation at three power densities (0.5 W cm−2, 1.0 W cm−2, and 1.5 W cm−2). Within 3 min of irradiation, the SN38&ICG/Gd&Yb-LDH suspension displays a much higher temperature increment (8.8 °C, 13.9 °C and 19.8 °C) in comparison with the pristine ICG suspension (5.8 °C, 9.9 °C and 16.5 °C) and PBS (less than 3 °C). The thermal imaging photographs give a visual observation for the temperature change at 1.0 W cm−2 (Fig. 3B), illustrating the superior photothermal performance of SN38&ICG/Gd&Yb-LDH. Moreover, SN38&ICG/Gd&Yb-LDH exhibits a more stable photothermal effect than pristine ICG (Fig. 3C): the latter shows a significant loss after four cycles.The release behavior of SN38 from SN38&ICG/Gd&Yb-LDH was studied through pH control and NIR laser irradiation. Less than 7.4% of SN38 is released at pH 7.4 after 12 h of incubation (Fig. S12†), indicating a high stability of SN38&ICG/Gd&Yb-LDH in a normal physiological environment. However, 17.3% and 21.3% of SN38 is released at pH 6.5 (tumor microenvironment) and pH 5.5 (cancer cell endosome) over 12 h, respectively, showing a pH triggered release behavior. We further investigated the release performance of SN38&ICG/Gd&Yb-LDH upon NIR laser irradiation at different pH values. As shown in Fig. 3D, with NIR irradiation for 3 min (808 nm, 1.0 W cm−2), the released amount reaches 42.0% (at pH = 7.4), 67.9% (at pH = 6.5) and 79.8% (at pH = 5.5) for SN38, which reveals a synergetic drug release behavior under acidic conditions plus NIR irradiation. In comparison, ICG exhibits a limited released amount under the same conditions with NIR irradiation (Fig. S13†), which is ascribed to the stronger binding energy of ICG with Gd&Yb-LDH nanosheets. The temperature induced SN38 release is confirmed in the MD simulations as well (Table S2 and Fig. S14†), from which the diffusion coefficient of SN38/Gd&Yb-LDH increases from 0.5 × 10−8 cm2 s−1 to 10.5 × 10−8 cm2 s−1 as the temperature increases from 298.15 K to 328.15 K. Moreover, the color of the SN38&ICG/Gd&Yb-LDH suspension gradually changes from green to yellow (Fig. 3E), accompanied by enhanced fluorescence emission from free SN38 (Fig. S15†), demonstrating a photothermal effect induced SN38 release. In addition, due to the intrinsic photodynamic properties of ICG, the reactive oxygen species (ROS) production of SN38&ICG/Gd&Yb-LDH was measured by using 1,3-diphenylisobenzofuran (DPBF) as a probe18 (Fig. 3F and S16†). Within 8 min of NIR irradiation, SN38&ICG/Gd&Yb-LDH exhibits a 3.06-fold larger ROS production (proportional to the photodynamic efficiency) than pristine ICG, indicating its better photodynamic performance. The enhanced mono-dispersion of ICG on Gd&Yb-LDH (Fig. 2F) is beneficial to both photothermal conversion and photodynamic therapy, accounting for the increase of ROS production.
In vitro study with Hela cells
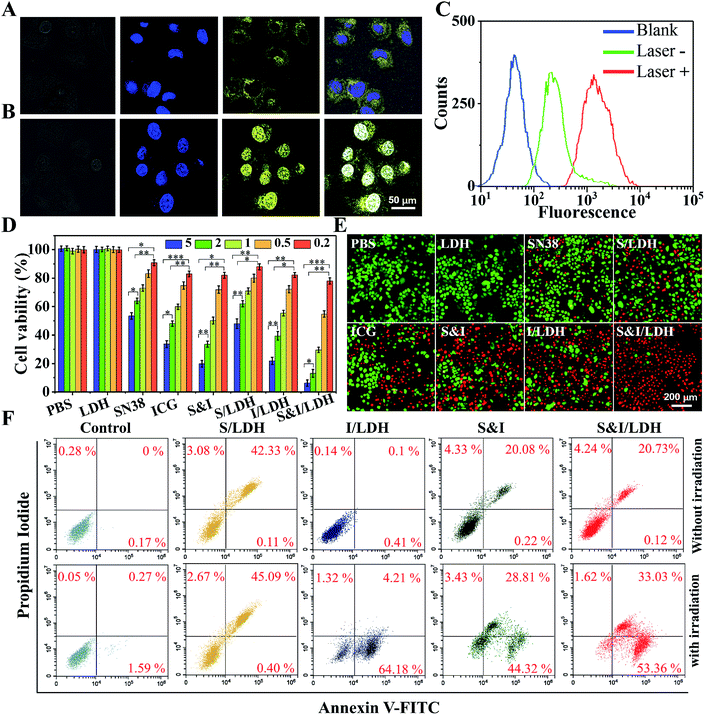
To further study the intracellular release behavior, Hela cells were incubated with SN38&ICG/Gd&Yb-LDH for 6 h, and then stained with Hoechst 33342 to mark nuclei.19Fig. 4A shows the yellow fluorescence signal of SN38 in the cytoplasm, indicating the successful endocytosis of SN38&ICG/Gd&Yb-LDH by Hela cells. After 808 nm NIR light irradiation for 3 min, the fluorescence signal is predominantly observed in nuclei, demonstrating an efficient release of SN38 into nuclei from SN38&ICG/Gd&Yb-LDH in the cytoplasm (Fig. 4B). As shown in Fig. S17,† free SN38 exhibits a much stronger fluorescence signal than SN38&ICG/Gd&Yb-LDH with an equal SN38 dose in PBS solution. After irradiation, an efficient release of SN38 is observed from SN38&ICG/Gd&Yb-LDH (in the cytoplasm, Fig. 4A) into nuclei (Fig. 4B), and the fluorescence intensity in nuclei becomes stronger than in the cytoplasm as a result of SN38 release (corresponding to the results in Fig. S17†). This is further confirmed by flow cytometer measurements (Fig. 4C).In vitro therapeutic performance of SN38&ICG/Gd&Yb-LDH was investigated by a standard methyl thiazolyl tetrazolium (MTT) assay.20 The cytotoxicity of the Gd&Yb-LDH nanosheets was firstly evaluated by incubation with three kinds of common cancer cells (U87MG, KB and Hela) at concentrations of up to 500 μg mL−1 and the MTT results showed little cytotoxicity (Fig. S18†), demonstrating an excellent biocompatibility of Gd&Yb-LDH nanosheets. Subsequently, Hela cells were respectively cultured with PBS, Gd&Yb-LDH, SN38, SN38/Gd&Yb-LDH, ICG, ICG/Gd&Yb-LDH, SN38&ICG and SN38&ICG/Gd&Yb-LDH for 24 h, with the equivalent drug concentration ranging from 0.2 to 5.0 μg mL−1, followed by a thorough washing with PBS. As illustrated in Fig. S19,† MTT results without irradiation show a good biocompatibility in the presence of Gd&Yb-LDH as a drug carrier. After irradiation with 808 nm NIR light (1.0 W cm−2, 3 min), the cellular viability decreases with the drug concentration rising from 0.2 to 5.0 μg mL−1 (Fig. 4D). The half maximal inhibitory concentration (IC50) values are calculated as below: SN38 (5.80 μg mL−1) > SN38/Gd&Yb-LDH (4.28 μg mL−1) > ICG (1.89 μg mL−1) > ICG/Gd&Yb-LDH (1.25 μg mL−1) > SN38&ICG (1.08 μg mL−1) > SN38&ICG/Gd&Yb-LDH (0.532 μg mL−1). Based on the IC50 value, the combination index (CI, a parameter for evaluation of the synergetic effect of drugs,21 see the Experimental section for details) of SN38&ICG/Gd&Yb-LDH was calculated to be 0.22 (much lower than 1.0), indicating a significant synergistic effect between SN38 and ICG in this chemo/PTT/PDT tri-mode therapy. To visualize the anticancer efficacy, Hela cells treated with 5 μg mL−1 of each of the above samples with irradiation were stained with calcein-AM and propidium iodide (calcein-AM/PI) (Fig. 4E), and SN38&ICG/Gd&Yb-LDH showed the optimal anticancer capability, which is consistent with the results of in vitro tests (Fig. 4D). In addition, cell apoptosis was studied using the PI/annexin V–FITC double staining method and analyzed by flow cytometry.22 As shown in Fig. 4F, Hela cells treated with SN38/Gd&Yb-LDH appear in the PI+/annexin V–FITC+ region, indicating a late-stage apoptosis; while an early-stage apoptosis of Hela cells is observed for the sample of ICG/Gd&Yb-LDH. In the case of SN38&ICG/Gd&Yb-LDH, it exhibits both early- and late-stage apoptosis with largely enhanced cell death, demonstrating the combination of chemo/PTT/PDT therapy.
In vivo tri-mode imaging and cancer therapy
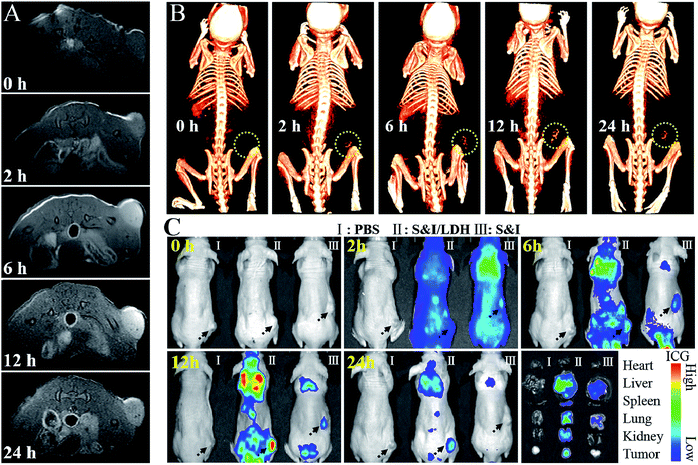
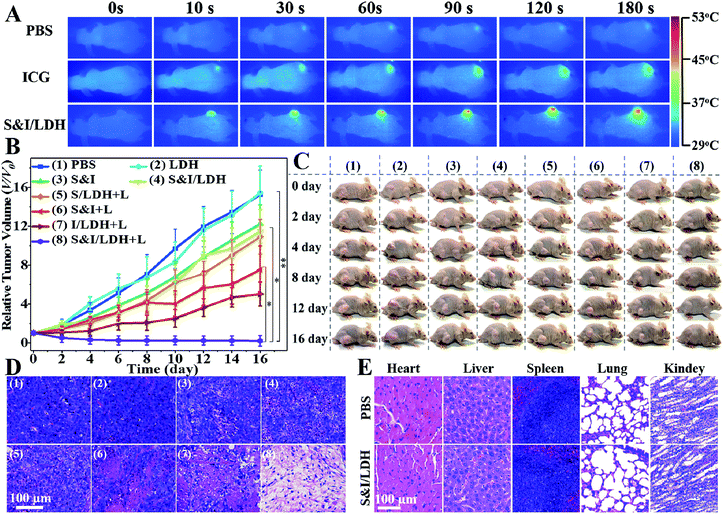
All animal procedures were performed in accordance with the National Institute of Health Guiding Principles for the Care and Use of Laboratory Animals and were approved by the Ethics Review Committee for Animal Experimentation of the Institute of Clinical Medicine, China-Japan Friendship Hospital, Beijing. As shown in the pharmacokinetics profile of SN38&ICG/Gd&Yb-LDH (Fig. S20†), the blood circulation of SN38&ICG/Gd&Yb-LDH obeys the typical two compartment model, where the first phase (distribution phase with a rapid decline) gives a half-life of 0.621 ± 0.163 h and the second phase (elimination phase, the predominant process for nanoparticle clearance) gives a half-life of 9.65 ± 0.97 h. Moreover, the area under the curve (AUC) is determined to be 337 μg h mL−1 and the volume of distribution (V) is measured to be 1.94 mL ± 0.68 mL. The long blood circulation may delay the clearance of SN38&ICG/Gd&Yb-LDH and thus contribute to drug accumulation in tumours. The in vivo bio-distribution and accumulation process of SN38&ICG/Gd&Yb-LDH in tumors were investigated by MR/CT/NIRF tri-mode imaging, which would offer both deep spatial resolution (MRI and CT) and high imaging sensitivity (NIRF). As shown in Fig. 5A, after the intravenous injection of SN38&ICG/Gd&Yb-LDH, the tumor site of Balb/c nude mouse displays a gradually enhanced MRI signal which reaches the strongest state after 12 h due to the enhanced permeability and retention (EPR) effects of SN38&ICG/Gd&Yb-LDH. Reconstructed three-dimensional (3D) CT imaging gives a similar result (Fig. 5B): the clearest tumor angiography is obtained at 12 h due to the preferential accumulation of SN38&ICG/Gd&Yb-LDH at the tumor site. Furthermore, the NIRF imaging performance was investigated in vivo. As shown in Fig. 5C, mice injected with SN38&ICG or SN38&ICG/Gd&Yb-LDH after 2 h exhibit NIRF signals throughout the whole body. Afterwards, a rather weak fluorescence signal at the tumor site is observed at 6 h for the former group, which fades quickly and disappears completely at 12–24 h. For the mice treated with SN38&ICG/Gd&Yb-LDH, however, a strong fluorescence signal at the tumor site is observed at 12 h and it remains constant even at 24 h. To further estimate the biodistributions of SN38&ICG/Gd&Yb-LDH, major organs and tumors were collected at different time points after intravenous injection of SN38&ICG/Gd&Yb-LDH. Fig. S21A† shows the ex vivo fluorescence images of various organs at 2 h, 6 h, 12 h, 24 h and 48 h, where the strongest fluorescence intensity of the tumor is found at 12 h. Furthermore, the time-dependent distribution of SN38&ICG/Gd&Yb-LDH in the tumor and various organs was determined in Fig. S21B.† A high average fluorescence intensity is observed in the tumor, as well as in reticuloendothelial systems (RES) such as the liver, lung, and kidney, demonstrating an excellent permeability and retention (EPR) effect of SN38&ICG/Gd&Yb-LDH. The results above indicate the excellent passive targeted properties of SN38&ICG/Gd&Yb-LDH as well as a prolonged tumor retention time.Based on the exciting results demonstrated above, the combined therapeutic ability of SN38&ICG/Gd&Yb-LDH was further investigated on mice bearing Hela tumors. Firstly, in vivo photothermal images were recorded after i.v. injection for 12 h (Fig. 6A). Within 3 min of NIR irradiation, the mice injected with ICG exhibit temperatures as high as 42 °C at the tumor site, while the mice injected with SN38&ICG/Gd&Yb-LDH show temperatures as high as 51 °C, which is beneficial to in vivo photothermal therapy. Subsequently, the mice were divided into eight groups: (1) PBS, (2) Gd&Yb-LDH, (3) SN38&ICG, (4) SN38&ICG/Gd&Yb-LDH, (5) SN38/Gd&Yb-LDH with irradiation, (6) SN38&ICG with irradiation, (7) ICG/Gd&Yb-LDH with irradiation, and (8) SN38&ICG/Gd&Yb-LDH with irradiation. The drug injection dose was 3 mg kg−1, the irradiation (808 nm laser, 1.0 W cm−2) was conducted for 3 min at 12 h post injection, and the tumor volume was monitored for 16 days (Fig. 6B). No obvious tumor inhibition is detected for the PBS or Gd&Yb-LDH group, while SN38&ICG, SN38&ICG/Gd&Yb-LDH without irradiation and SN38/Gd&Yb-LDH with irradiation inhibit the tumor growth partially due to the chemotherapy of SN38. A remarkably enhanced therapeutic effect is found in the group of SN38&ICG and ICG/Gd&Yb-LDH with irradiation. In the case of SN38&ICG/Gd&Yb-LDH with irradiation, a complete tumor ablation is observed, demonstrating an excellent in vivo synergistic therapeutic effect with a rather low drug dose. Fig. 6C displays the digital photos of dynamic tumor growth and the corresponding excised tumors are shown in Fig. S22,† in accordance with the tumor volume curves. On staining with hematoxylin and eosin (H&E),23 the tumor tissue collected from mice treated with SN38&ICG/Gd&Yb-LDH plus irradiation exhibits obvious necrosis while other groups only show partial necrosis or even retain their normal morphology (Fig. 6D). The weight of the mice increases slightly for every group during the treatment, indicating little side effect (Fig. S23†). In addition, in vivo toxicity of the SN38&ICG/Gd&Yb-LDH nanosheets was evaluated through i.v. injection of SN38&ICG/Gd&Yb-LDH into mice (200 μL, 10 mg kg−1), and the results showed that all the blood biochemistry and liver and kidney function markers were maintained in the normal range compared with the PBS control group (Fig. S24†). Moreover, no further potential signs of toxicity were observed by comparing the SN38&ICG/Gd&Yb-LDH group with the PBS group in the H&E analysis of major organs (heart, liver, spleen, lung, and kidney) (Fig. 6E), demonstrating a negligible toxicity of SN38&ICG/Gd&Yb-LDH.
Conclusions
In summary, we reported a facile “bottom-up” method for the preparation of monolayer Gd&Yb-LDH nanosheets, which serve as a promising drug carrier for imaging guided cancer therapy. The loading capacity of Gd&Yb-LDH nanosheets toward SN38 reaches 925%, far beyond that of previously reported drug carriers. In addition, by virtue of the co-doped rare earth elements (Gd3+ and Yb3+) in the LDH host layer, SN38&ICG/Gd&Yb-LDH can provide real time feedback through tri-mode MR/CT/NIRF imaging, which facilitates visualization of drug bio-distribution for further effective treatment. For the in vivo tests with Hela tumor-bearing mice, the synergistic chemo/PTT/PDT therapy of SN38&ICG/Gd&Yb-LDH results in a complete ablation of tumors with minimum side effects. Therefore, the LDH monolayer nanosheets with precise control over the chemical composition and functionality provide a strategy to develop novel theranostic systems, holding potential for application in clinical cancer therapy.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC), the 973 Program (2014CB932103), the Beijing Natural Science Foundation (2174082), and the Fundamental Research Funds for the Central Universities (buctylkxj01).Notes and references
- Y. Chen, C. Tan, H. Zhang and L. Wang, Chem. Soc. Rev., 2015, 44, 2681 RSC.
- H. Wang, X. Yang, W. Shao, S. Chen, J. Xie, X. Zhang, J. Wang and Y. Xie, J. Am. Chem. Soc., 2015, 137, 11376 CrossRef PubMed.
- L. Gao, Y. Wang, H. Li, Q. Li, N. Ta, L. Zhuang, Q. Fu and X. Bao, Chem. Sci., 2017, 8, 5728 RSC.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313 CrossRef PubMed.
- (a) J. D. Nunez, A. M. Benito, S. Rouziere, P. Launois, R. Arenal, P. M. Ajayan and W. K. Maser, Chem. Sci., 2017, 8, 4987 RSC; (b) B. R. Smith and S. S. Gambhir, Chem. Rev., 2017, 117, 901 CrossRef PubMed; (c) V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1226419 CrossRef; (d) J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807 CrossRef PubMed; (e) P. Sun, R. Ma and T. Sasaki, Chem. Sci., 2018, 9, 33 RSC.
- (a) D. Chimene, D. L. Alge and A. K. Gaharwar, Adv. Mater., 2015, 27, 7261 CrossRef PubMed; (b) M. Xiao, S. Wei, Y. Li, J. Jasensky, J. Chen, C. Brooks and Z. Chen, Chem. Sci., 2018, 9, 1769 RSC; (c) H. Zhang, ACS Nano, 2015, 9, 9451 CrossRef PubMed; (d) Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192 CrossRef PubMed; (e) G. Mikhaylov, U. Mikac, A. A. Magaeva, V. I. Itin, E. P. Naiden, I. Psakhye, L. Babes, T. Reinheckel, C. Peters, R. Zeiser, M. Bogyo, V. Turk, S. G. Psakhye, B. Turk and O. Vasiljeva, Nat. Nanotechnol., 2011, 6, 594 CrossRef PubMed.
- (a) H. Wang, J. Zhang, X. Hang, X. Zhang, J. Xie, B. Pan and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 1195 CrossRef PubMed; (b) X. Ai, C. J. H. Ho, J. Aw, A. B. E. Attia, J. Mu, Y. Wang, X. Wang, Y. Wang, X. Liu, H. Chen, M. Gao, X. Chen, E. K. L. Yeow, G. Liu, M. Olivo and B. Xing, Nat. Commun., 2016, 7, 10432 CrossRef PubMed; (c) A. Chatterjee and K. Biswas, Angew. Chem., Int. Ed., 2015, 54, 5623 CrossRef PubMed; (d) J. H. Han, S. Lee and J. Cheon, Chem. Soc. Rev., 2013, 42, 2581 RSC; (e) J. Yu, J. Li, W. Zhang and H. Chang, Chem. Sci., 2015, 6, 6705 RSC.
- (a) S. Jeong, D. Yoo, J.-t. Jang, M. Kim and J. Cheon, J. Am. Chem. Soc., 2012, 134, 18233 CrossRef PubMed; (b) W. Yang, L. Zhang, J. Xie, X. Zhang, Q. Liu, T. Yao, S. Wei, Q. Zhang and Y. Xie, Angew. Chem., Int. Ed., 2016, 55, 6716 CrossRef PubMed; (c) K. Ramasamy, H. Sims, W. H. Butler and A. Gupta, J. Am. Chem. Soc., 2014, 136, 1587 CrossRef PubMed; (d) A. H. M. Leung, S. D. Pike, A. J. Clancy, H. C. Yau, W. J. Lee, K. L. Orchard, M. S. P. Shaffer and C. K. Williams, Chem. Sci., 2018, 9, 2135 RSC.
- (a) P. J. Sideris, U. G. Nielsen, Z. Gan and C. P. Grey, Science, 2008, 321, 113 CrossRef PubMed; (b) S.-J. Choi, G. E. Choi, J.-M. Oh, Y.-J. Oh, M.-C. Park and J.-H. Choy, J. Mater. Chem., 2010, 20, 9463 RSC; (c) R. Tian, S. Zhang, M. Li, Y. Zhou, B. Lu, D. Yan, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2015, 25, 5006 CrossRef; (d) W. Liu, S. Xu, S. Guan, R. Liang, M. Wei, D. G. Evans and X. Duan, Adv. Mater., 2018, 30, 1704376 CrossRef PubMed.
- (a) Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124 CrossRef PubMed; (b) R. Liang, R. Tian, L. Ma, L. Zhang, Y. Hu, J. Wang, M. Wei, D. Yan, D. G. Evans and X. Duan, Adv. Funct. Mater., 2014, 24, 3144 CrossRef; (c) Z. Gu, J. J. Atherton and Z. P. Xu, Chem. Commun., 2015, 51, 3024 RSC; (d) J.-M. Oh, M. Park, S.-T. Kim, J.-Y. Jung, Y.-G. Kang and J.-H. Choy, J. Phys. Chem. Solids, 2006, 67, 1024 CrossRef.
- (a) L. Li, W. Gu, J. Chen, W. Chen and Z. P. Xu, Biomaterials, 2014, 35, 3331 CrossRef PubMed; (b) D.-H. Park, J.-E. Kim, J.-M. Oh, Y.-G. Shul and J.-H. Choy, J. Am. Chem. Soc., 2010, 132, 16735 CrossRef PubMed.
- (a) R. Tian, D. Yan, C. Li, S. Xu, R. Liang, L. Guo, M. Wei, D. G. Evans and X. Duan, Nanoscale, 2016, 8, 9815 RSC; (b) B. Li, Z. Gu, N. Kurniawan, W. Chen and Z. P. Xu, Adv. Mater., 2017, 29, 1700370 Search PubMed.
- (a) R. Gao and D. Yan, Chem. Sci., 2017, 8, 590 RSC; (b) Y. Zhao, B. Li, Q. Wang, W. Gao, C. J. Wang, M. Wei, D. G. Evans, X. Duan and D. O'Hare, Chem. Sci., 2014, 5, 951 RSC; (c) X. Mei, S. Xu, T. Hu, L. Peng, R. Gao, R. Liang, M. Wei, D. G. Evans and X. Duan, Nano Res., 2018, 11, 195 CrossRef; (d) Z. Li, M. Shao, H. An, Z. Wang, S. Xu, M. Wei, D. G. Evans and X. Duan, Chem. Sci., 2015, 6, 6624 RSC.
- (a) H. Wang, H. Xie, J. Wu, X. Wei, L. Zhou, X. Xu and S. Zheng, Angew. Chem., Int. Ed., 2014, 126, 11716 CrossRef; (b) J. Wang, J. A. Kaplan, Y. L. Colson and M. W. Grinstaff, Angew. Chem., Int. Ed., 2016, 55, 2796 CrossRef PubMed; (c) Y. Shen, E. Jin, B. Zhang, C. J. Murphy, M. Sui, J. Zhao, J. Wang, J. Tang, M. Fan, E. Van Kirk and W. J. Murdoch, J. Am. Chem. Soc., 2010, 132, 4259 CrossRef PubMed; (d) H. Wang, H. Xie, J. Wang, J. Wu, X. Ma, L. Li, X. Wei, Q. Ling, P. Song, L. Zhou, X. Xu and S. Zheng, Adv. Funct. Mater., 2015, 25, 4956 CrossRef; (e) K. Cai, J. Yen, Q. Yin, Y. Liu, Z. Song, S. Lezmi, Y. Zhang, X. Yang, W. G. Helferich and J. Cheng, Biomater. Sci., 2015, 3, 1061 RSC; (f) Y. Li, K. Xiao, J. Luo, J. Lee, S. Pan and K. S. Lam, J. Controlled Release, 2010, 144, 314 CrossRef PubMed; (g) T. Liu, C. Wang, X. Gu, H. Gong, L. Cheng, X. Shi, L. Feng, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 3433 CrossRef PubMed; (h) H. Wang, J. Chen, C. Xu, L. Shi, M. Tayier, J. Zhou, J. Zhang, J. Wu, Z. Ye, T. Fang and W. Han, Theranostics, 2017, 7, 3638 CrossRef PubMed; (i) H. Wang, Z. Lu, L. Wang, T. Guo, J. Wu, J. Wan, L. Zhou, H. Li, Z. Li, D. Jiang, P. Song, H. Xie, L. Zhou, X. Xu and S. Zheng, Cancer Res., 2017, 77, 6963 CrossRef PubMed; (j) Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 130, 10876 CrossRef PubMed.
- (a) F. Song and X. Hu, Nat. Commun., 2014, 5, 4477 CrossRef PubMed; (b) D. Yan, J. Lu, J. Ma, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2011, 123, 746 CrossRef.
- Y. Li, X. Hu, D. Ding, Y. Zou, Y. Xu, X. Wang, Y. Zhang, L. Chen, Z. Chen and W. Tan, Nat. Commun., 2017, 8, 15653 CrossRef PubMed.
- D. L. Longo, P. Z. Sun, L. Consolino, F. C. Michelotti, F. Uggeri and S. Aime, J. Am. Chem. Soc., 2014, 136, 14333 CrossRef PubMed.
- P. Huang, X. Qian, Y. Chen, L. Yu, H. Lin, L. Wang, Y. Zhu and J. Shi, J. Am. Chem. Soc., 2017, 139, 1275 CrossRef PubMed.
- M. Grossi, M. Morgunova, S. Cheung, D. Scholz, E. Conroy, M. Terrile, A. Panarella, J. C. Simpson, W. M. Gallagher and D. F. O'Shea, Nat. Commun., 2016, 7, 10855 CrossRef PubMed.
- F. Liu, X. He, H. Chen, J. Zhang, H. Zhang and Z. Wang, Nat. Commun., 2015, 6, 8003 CrossRef PubMed.
- W. Malin, D. Cecilia, S. Takashi, M. Jelena, B. Ninib, F. M. Ole, L. Rolf, K. Per, Z. G. Peter and J. I. John, Int. J. Cancer, 2013, 132, 1516 CrossRef PubMed.
- J. Shi, L. Wang, J. Gao, Y. Liu, J. Zhang, R. Ma, R. Liu and Z. Zhang, Biomaterials, 2014, 35, 5771 CrossRef PubMed.
- G. Yang, L. Xu, Y. Chao, J. Xu, X. Sun, Y. Wu, R. Peng and Z. Liu, Nat. Commun., 2017, 8, 902 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc01288a |
| This journal is © The Royal Society of Chemistry 2018 |