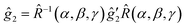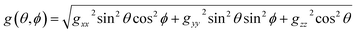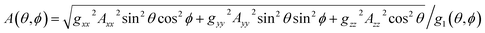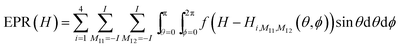Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diastereoisomers of L-proline-linked trityl-nitroxide biradicals: synthesis and effect of chiral configurations on exchange interactions†
Weixiang
Zhai
a,
Yalan
Feng
a,
Huiqiang
Liu
a,
Antal
Rockenbauer
 *b,
Deni
Mance
c,
Shaoyong
Li
a,
Yuguang
Song
*a,
Marc
Baldus
*b,
Deni
Mance
c,
Shaoyong
Li
a,
Yuguang
Song
*a,
Marc
Baldus
 c and
Yangping
Liu
c and
Yangping
Liu
 *a
*a
aTianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics, School of Pharmacy, Tianjin Medical University, Tianjin 300070, P. R. China. E-mail: liuyangping@tmu.edu.cn; songyuguang@tmu.edu.cn
bInstitute of Materials and Environmental Chemistry, Hungarian Academy of Sciences, Department of Physics, Budapest University of Technology and Economics, Budafoki ut 8, 1111 Budapest, Hungary. E-mail: rockenbauer.antal@ttk.mta.hu
cNMR Spectroscopy, Bijvoet Center for Biomolecular Research, Utrecht University, 3584 CH Utrecht, The Netherlands
First published on 5th April 2018
Abstract
The exchange (J) interaction of organic biradicals is a crucial factor controlling their physiochemical properties and potential applications and can be modulated by changing the nature of the linker. In the present work, we for the first time demonstrate the effect of chiral configurations of radical parts on the J values of trityl-nitroxide (TN) biradicals. Four diastereoisomers (TNT1, TNT2, TNL1 and TNL2) of TN biradicals were synthesized and purified by the conjugation of a racemic (R/S) nitroxide with the racemic (M/P) trityl radical viaL-proline. The absolute configurations of these diastereoisomers were assigned by comparing experimental and calculated electronic circular dichroism (ECD) spectra as (M, S, S) for TNT1, (P, S, S) for TNT2, (M, S, R) for TNL1 and (P, S, R) for TNL2. Electron paramagnetic resonance (EPR) results showed that the configuration of the nitroxide part instead of the trityl part is dominant in controlling the exchange interactions and the order of the J values at room temperature is TNT1 (252 G) > TNT2 (127 G) ≫ TNL2 (33 G) > TNL1 (14 G). Moreover, the J values of TNL1/TNL2 with the S configuration in the nitroxide part vary with temperature and the polarity of solvents due to their flexible linker, whereas the J values of TNT1/TNT2 are almost insensitive to these two factors due to the rigidity of their linkers. The distinct exchange interactions between TNT1,2 and TNL1,2 in the frozen state led to strongly different high-field dynamic nuclear polarization (DNP) enhancements with ε = 7 for TNT1,2 and 40 for TNL1,2 under 800 MHz DNP conditions.
Introduction
Exchange-coupled biradicals have recently attracted tremendous interest owing to their unique physiochemical properties and potential applications in high-frequency dynamic nuclear polarization (DNP),1,2 molecule-based magnetism,3–5 and molecular charge transfer6–8 as well as molecular sensing.9,10 A key point in the design of new biradicals is to control the magnitude and sign of the spin–spin exchange interaction that determines their physiochemical properties and potential applications. The intramolecular exchange interaction of biradicals strongly depends on the number of chemical bonds in the linkage between the two spins, the linker conformation, and the σ/π contributions to the bonding as well as the environment (e.g., temperature and solvent). This exchange interaction can be through-bond and/or through-space, and its value varies by many orders of magnitude due to different linkers between the two radical moieties.11,12 In general, for the biradicals with π-conjugated backbones, the exchange interaction is mediated more effectively via through-bond mechanisms. The large exchange interactions have been achieved by enhancing the degree of π-orbital overlap between the spacer and two radical moieties through a conformational constraint to enforce their coplanarity.13–16 Using donor–bridge–acceptor biradical systems, the dependence of magnetic exchange interaction on the degree of π-orbital overlap and torsional rotations between different parts has been well demonstrated.17–19 Comparatively, the exchange interactions in the biradicals with nonconjugated spacers are much weaker and can be through-bond and/or through-space, depending on the flexibility of the spacers. Recently, weakly coupled nitroxide biradicals have attracted intense attention as high-field DNP polarizing agents which boost the sensitivity of solid-state nuclear magnetic resonance spectroscopy.20–27 Nonconjugated linkers were applied in these biradicals and the rigidity and conformation of the linkers were well tailored in order to optimize the through-bond exchange and dipolar interactions and simultaneously realize the matching conditions of the EPR frequency. In addition, the supramolecular interaction with host molecules has been an effective approach to modulate the exchange interaction in nitroxide biradicals.28–31 Despite these extensive studies, to the best of our knowledge, there is no related study on the chiral effect of the linker and radical moieties on the exchange interaction of biradicals.In the past few years, we have developed trityl-nitroxide (TN) biradicals which combine two different radical properties into unique molecules.32–36 Similar to other biradicals, the exchange interaction of TN biradicals is a key factor for their applications. We have fine-tuned the exchange interactions of TN biradicals by structural modification of the linkers37 as well as by supramolecular interaction with cyclodextrins.38 The largest DNP enhancement at a high magnetic field (18.8 T) has been achieved by using these TN biradicals as polarizing agents,35,36 in part due to the lack of nuclear depolarization.39 In the present work, we utilize the chiral effect of the linker and two radical parts to modulate the spin–spin exchange interaction of TN biradicals. The trityl radical has a propeller configuration which affords the right-handed (P) and left-handed (M) helices. Due to the large steric bulk of three aryl groups, the interconversion between the two enantiomeric helices is slow and they are separable at room temperature.40,41 Thus, the conjugation of the trityl radical CT-03 with the racemic mixture of the nitroxide APO through L-proline leads to four enantiomerically pure diastereoisomers (TNT1, TNT2, TNL1 and TNL2, Scheme 1) which were separated by reversed-phase (TNT1 and TNT2) or chiral (TNL1 and TNL2) HPLC. The configurations of these diastereoisomers were determined by the comparison of experimental and calculated electronic circular dichroism (ECD) spectra. The effect of the configurations of the trityl and nitroxide radicals on the exchange interactions was investigated by EPR at different temperatures and solvents. Moreover, the DNP properties of TNT1,2 and TNL1,2 were also studied at a high field (18.8 T).
Results and discussion
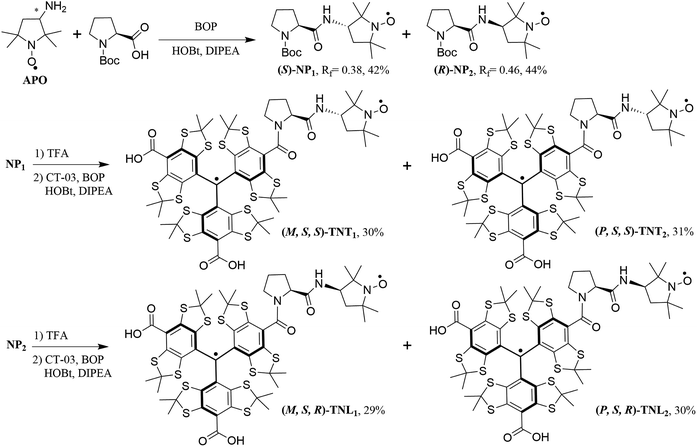
Synthesis of the four enantiomerically pure diastereoisomers
Scheme 1 shows the synthetic procedure of the four biradical diastereoisomers. The racemic mixture of the nitroxide APO was firstly coupled with N-Boc-L-proline to afford the nitroxide diastereoisomers NP1 (yield, 42%) and NP2 (yield, 44%) which were easily separated using column chromatography on silica gel. Then NP1 and NP2 underwent deprotection by TFA, followed by conjugation with the trityl radical CT-03 to give the biradicals TNT1,2 and TNL1,2, respectively. TNT1,2 displayed two close peaks (11.34 and 11.58 min) with an area ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on reversed-phase high performance liquid chromatography (RP-HPLC, Fig. S1†) due to the P/M propeller configurations of the trityl part.40,41 Therefore, it is possible to separate the two enantiomerically pure diastereoisomers of TNT1,2 by semipreparative RP-HPLC and ∼2 mg of both diastereoisomers (TNT1 and TNT2) were obtained. In contrast, only one peak was observed in the RP-HPLC chromatogram of TNL1,2 under the same conditions (Fig. S1†). Attempts to separate the two diastereoisomers of TNL1,2 by optimizing the RP-HPLC conditions failed. In addition, attempts to obtain the pure diastereoisomers of TNL1,2 through their esterification using chiral agents such as S-(−)-1,1′-binaphthyl-2,2′-diol were unsuccessful. As such, the separation of TNL1 and TNL2 was achieved by analytical HPLC with a chiral stationary phase (Fig. S3†) and this separation procedure was repeated ∼50 times to afford ∼0.4 mg of TNL1 and ∼0.4 mg of TNL2. The amounts obtained for the four diastereoisomers are sufficient for subsequent ECD and EPR experiments.
1 on reversed-phase high performance liquid chromatography (RP-HPLC, Fig. S1†) due to the P/M propeller configurations of the trityl part.40,41 Therefore, it is possible to separate the two enantiomerically pure diastereoisomers of TNT1,2 by semipreparative RP-HPLC and ∼2 mg of both diastereoisomers (TNT1 and TNT2) were obtained. In contrast, only one peak was observed in the RP-HPLC chromatogram of TNL1,2 under the same conditions (Fig. S1†). Attempts to separate the two diastereoisomers of TNL1,2 by optimizing the RP-HPLC conditions failed. In addition, attempts to obtain the pure diastereoisomers of TNL1,2 through their esterification using chiral agents such as S-(−)-1,1′-binaphthyl-2,2′-diol were unsuccessful. As such, the separation of TNL1 and TNL2 was achieved by analytical HPLC with a chiral stationary phase (Fig. S3†) and this separation procedure was repeated ∼50 times to afford ∼0.4 mg of TNL1 and ∼0.4 mg of TNL2. The amounts obtained for the four diastereoisomers are sufficient for subsequent ECD and EPR experiments.
Configuration of the four biradical diastereoisomers
In order to determine the absolute configurations of the four biradical diastereoisomers, the experimental electronic circular dichroism (ECD) spectra of NP1/NP2 and the four diastereoisomers were recorded in methanol. Meanwhile, the theoretical ECD spectra of NP1, TNT2 and TNL2 were also calculated using quantum chemical methods. All calculated ECD spectra with the TD-DFT method show an acceptable fitting with the corresponding experimental spectra in terms of the spectral pattern and sign of the bands with respect to the wavelength. As illustrated in Fig. 1A, NP1 exhibits a relatively strong ECD signal with a negative Cotton effect at 217 nm and a broad signal with a positive Cotton effect at 420 nm. Good agreement between the experimental and calculated ECD spectral patterns of NP1 confirms the S absolute configuration of the nitroxide part. Accordingly, the absolute configuration of the nitroxide part of NP2 is assigned as R.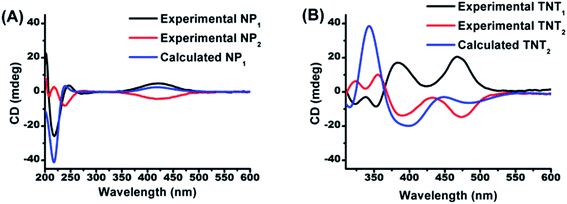 | ||
| Fig. 1 Comparison of the calculated ECD spectrum of (A) NP1 with the experimental ones of NP1 and NP2 and (B) of TNT2 with the experimental ones of TNT1 and TNT2. | ||
Based on the absolute configuration of the nitroxide part, the absolute configurations of the four biradical diastereoisomers were further determined. The ECD spectrum of TNT1 is characteristic of the trityl radical with two strong positive Cotton effects at 384 nm and 467 nm and two relatively weak negative Cotton effects at 324 and 352 nm.42 Interestingly, the ECD spectra of TNT1 and TNT2 are mirror images most likely because the ECD signals of the nitroxide part in these biradical diastereoisomers are very weak and negligible as compared to the signals of the trityl part. Using TD-DFT calculations, the absolute configurations of TNT1 and TNT2 were assigned as (M, S, S) and (P, S, S), respectively. Likewise, the configurations of TNL1 and TNL2 were assigned as (M, S, R) and (P, S, R), respectively (Fig. S10†).
Room-temperature EPR studies
Fig. 2 shows the EPR spectra of the four diastereoisomers in aqueous solutions at room temperature. Both TNT1 and TNT2 in phosphate buffer (PB, 20 mM, pH 7.4) exhibit well-resolved triplets with line separations of ∼8.2 G, about half the 14N hyperfine splitting (αN, 16.0 G) of APO. This spectral feature is characteristic of strong intramolecular exchange interaction between the two spins.37 Of note is that this EPR triplet pattern was only observed for the directly linked TN biradicals in previous studies.32–34,37 Therefore, the strong exchange interactions for TNT1 and TNT2 are unexpected given that there are multiple σ bonds (proline linker) between the two radical moieties. In contrast to TNT1,2, TNL1,2 had much more complicated and less symmetric EPR spectra with an intense central line surrounded by multiple weak peaks (TNL1) or one broad peak (TNL2) at both sides. The broad EPR peak of TNL2 at the left side indicates that it has a wide conformational distribution possibly due to the relatively high flexibility of the linker.37 Since the anisotropic hyperfine and dipolar interactions of TN biradicals are averaged out in the liquid state, the exchange interactions (J) can be estimated from their EPR spectra in aqueous solution. Using the well-established simulation program,43 the J values of TNL1 and TNL2 at room temperature are estimated to be 14 G and 32 G (Table 1), respectively. As expected, TNT1 (252 G) and TNT2 (128 G) have much higher J values than TNT1,2. Interestingly, the β and γ relaxation parameters in the line width formula of the nitrogen hyperfine pattern obtained for TNL1,2 are larger than those of TNT1,2, indicating that the molecular rotation of TNL1,2 is slower than that of TNT1,2. The slow molecular rotation further demonstrates that TNL1,2 has loose geometries where the effective rotational radius is rather large and the two radical moieties are far away from each other. This spatial separation of the two radical moieties in TNL1,2 may further explain why they have small J values as compared to TNT1,2. Taken together, the much stronger exchange interaction of TNT1,2 than TNL1,2 suggests that the R/S configurations of the nitroxide part play more important roles in modulating their exchange interactions than the M/P helices of the trityl part. This is unsurprising since the molecular structure of the trityl radical has high symmetry. Thus, it can also be deduced that the relative geometric conformation between the trityl part and the proline linker is similar for TNT1/TNT2 or TNL1/TNL2. To verify this hypothesis, the four diastereoisomers of TN biradicals were reduced with ascorbate to the corresponding trityl monoradicals and their EPR spectra were recorded. As shown in Fig. S5,† the four trityl monoradicals had almost identical and partially overlapped triplet signals under anaerobic conditions due to the hyperfine splitting (αN, 0.18 G) of the nitrogen from the proline linker. The identical αN value confirms that the relative geometric conformation between the trityl part and the proline linker is the same for the four monoradicals and the M/P helices of the trityl part have a minor effect on their exchange interactions.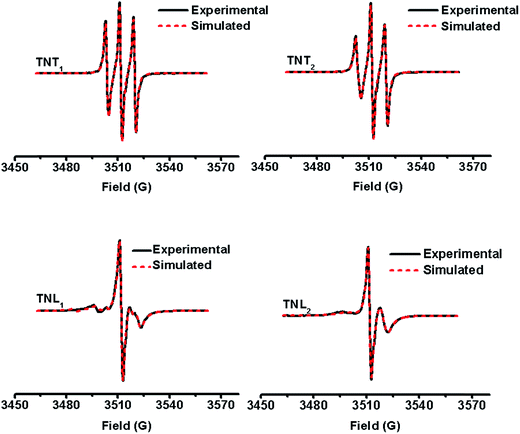 | ||
| Fig. 2 Experimental (black solid line) and simulated (red dotted line) EPR spectra of TNT1, TNT2, TNL1 and TNL2 in phosphate buffer (20 mM, pH 7.4) at room temperature. | ||
| Biradical | J (RT) | J (LT) | D |
|---|---|---|---|
| TNT1 | 252 | 196 | 9 |
| TNT2 | 127 | 172 | 11 |
| TNL1 | 14 | 5.4 | 8 |
| TNL2 | 33 | 6.3 | 8 |
Effect of temperature on the exchange interactions
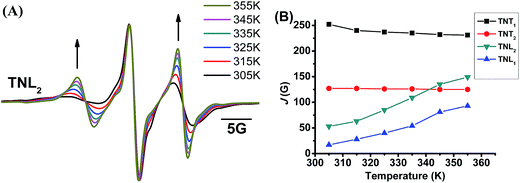
The through-space exchange interaction of the biradicals with flexible linkers can be enhanced at high temperature due to the rapid rotation of the chemical bonds in the linkers which increases the collisional frequencies of the two radical parts. As mentioned above, TNL2 has a flexible linker as evidenced by its broad EPR low-field peak. To further investigate the effect of the linker flexibility of the four diastereoisomers on their exchange interactions, their EPR spectra were recorded at high temperatures in the range of 305 K to 355 K. As shown in Fig. 3A, the EPR spectra of TNL2 exhibited outstanding changes with temperature with two relatively stronger peaks at both sides than the central peak at high temperature. Similar results were also observed for TNL1. However, the EPR spectra of the two diastereoisomers TNT1,2 at 355 K did not exhibit a significant change except for slightly narrow linewidths (Fig. S8†). Spectral simulation revealed that the J values of TNL1 and TNL2 increased with temperature from 17 G (305 K) to 93 G (355 K) and 53 G (305 K) to 149 G (355 K), respectively. The positive response of J values with temperature for TNL1,2 can be explained by their flexible linkers which allow for fast rotation of the chemical bonds in the linker, increase the collisional frequencies of the two radical moieties and further enhance the through-space exchange interactions.44 In contrast, the EPR spectra of TNT1,2 had no noticeable changes at different temperatures and their J values kept constant throughout the temperature range studied, implying that these two biradical diastereoisomers have rigid linkers (Fig. S8†). Therefore, the configuration of the nitroxide part plays a critical role in modulating the flexibility of the linkers and further the dependence of the exchange interactions on temperature.Solvent effect on the exchange interactions
Solvents have an important influence on the exchange interaction of biradicals by changing the population of the conformations or collisional frequencies of their two radical parts. Thus, the solvent effect on the exchange interactions of the four biradical diastereoisomers was also investigated in seven solvents (PB, MeOH, DMF, acetonitrile, acetone, THF and dioxane) at room temperature. EPR spectra were recorded (Fig. S9†) under anaerobic conditions where the potential effect of oxygen concentration on the EPR linewidth can be eliminated. These four diastereoisomers, in general, have relatively low magnitudes of exchange interactions in polar solvents (Table 2). For example, the J values of the two diastereoisomers of TNL1,2 exhibited a negative correlation with the solvent polarities. As for TNT1,2, strong exchange interactions were consistently observed with no marked difference in all organic solvents. Considering the large J values of the four diastereoisomers in viscous dioxane (1.54), enhanced exchange interactions in organic solvents most likely arises from the conformational conversion instead of the increased intramolecular collisional frequencies between the two radical parts. Since TNT1,2 have very rigid linkers, their conformations are strongly solidified and little dependence of their exchange interactions on solvents is expected.| Biradical | PB | DMF | MeOH | ACN | DMK | THF | Diox |
|---|---|---|---|---|---|---|---|
| a PB, phosphate buffer. DMF, N,N-dimethylformamide. MeOH, methanol. ACN, acetonitrile. DMK, acetone. THF, tetrahydrofuran. Diox, 1,4-dioxane. | |||||||
| TNT1 | 252 | 301 | 342 | 322 | 310 | 313 | 373 |
| TNT2 | 127 | 303 | 308 | 356 | 378 | 380 | 383 |
| TNL1 | 14 | 58 | 58 | 209 | 227 | 281 | 351 |
| TNL2 | 33 | 110 | 101 | 233 | 276 | 282 | 293 |
Solid-state EPR studies and spectral simulation
It has been demonstrated that the magnitudes of both dipolar and exchange interactions are very critical for the applications of biradicals in DNP which is carried out at low temperature. For this reason, the estimation of these two parameters from the frozen-solution spectra was attempted. Fig. 4 shows the EPR spectra of the four diastereoisomeric biradicals in a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v) glycerol/H2O glass-forming solution at low temperature (∼220 K). At the first glimpse, the two diastereoisomers of TNT1,2 have strong exchange interactions as reflected from small overall separations between the outermost lines (i.e., 52 G for TNT1 and 51 G for TNT2) in their EPR spectra which are much smaller than 2Azz (∼70 G) of the nitroxide radical. Comparatively, the exchange interactions of the two diastereoisomers of TNL1,2 are identical and quite weak since there is a large overall separation of 73 G between the outermost lines in their EPR spectra. In order to reliably determine D and J values from the frozen solution spectra, especially when the J coupling is smaller than the dipolar and hyperfine interactions, we modified our previous EPR simulation program (ROKI/EPR)37 by taking into account the anisotropic dipolar interaction besides g and hyperfine tensors. Note that this new program (called ROKI/DNP) enables the precise measurement of D and J values even determining the sign of J. Furthermore, it can also determine two polar angles which define the orientation of the linker in the principal direction of the nitroxide moiety. If the spectra are recorded at a high resonance frequency, the three Euler angles can be estimated giving the relative orientation of the principal axes of g and hyperfine tensors between the nitroxide and the trityl moieties (see more details in the Experimental section). As shown in Table 1, the order of the J values for the four biradical diastereoisomers in the frozen state is TNT1 (196 G) ∼ TNT2 (172 G) ≫ TNL2 (6.3 G) ∼ TNL1 (5.4 G). This trend is similar to the results obtained at room temperature. As mentioned above, the flexible linkers in TNL1,2 allow for the spatial proximity of the two radical parts and their exchange interactions are mostly based on a though-space mechanism above room temperature. In the frozen state, their molecular conformations were immobilized and their through-space exchange interactions were inhibited. Thus, the very weak exchange interactions observed for TNL1,2 in the frozen state are most likely due to the through-bond mechanism. The close J values of TNL1 and TNL2 indicate that they have similar preferential conformation(s) at low temperature. The similar conformations of TNL1,2 in the frozen state were further confirmed by their identical dipolar interactions (D = 8 G) which indicate the same distances between the two spins in TNL1,2. Comparatively, the exchange interactions of TNT1,2 did not significantly vary with temperature and had similar J values at ∼220 K and room temperature. These temperature-insensitive exchange interactions are due to the rigid linkers in TNT1,2 which prevent the rotation of the chemical bonds in the linker and solidify their molecular conformations.
40 (v/v) glycerol/H2O glass-forming solution at low temperature (∼220 K). At the first glimpse, the two diastereoisomers of TNT1,2 have strong exchange interactions as reflected from small overall separations between the outermost lines (i.e., 52 G for TNT1 and 51 G for TNT2) in their EPR spectra which are much smaller than 2Azz (∼70 G) of the nitroxide radical. Comparatively, the exchange interactions of the two diastereoisomers of TNL1,2 are identical and quite weak since there is a large overall separation of 73 G between the outermost lines in their EPR spectra. In order to reliably determine D and J values from the frozen solution spectra, especially when the J coupling is smaller than the dipolar and hyperfine interactions, we modified our previous EPR simulation program (ROKI/EPR)37 by taking into account the anisotropic dipolar interaction besides g and hyperfine tensors. Note that this new program (called ROKI/DNP) enables the precise measurement of D and J values even determining the sign of J. Furthermore, it can also determine two polar angles which define the orientation of the linker in the principal direction of the nitroxide moiety. If the spectra are recorded at a high resonance frequency, the three Euler angles can be estimated giving the relative orientation of the principal axes of g and hyperfine tensors between the nitroxide and the trityl moieties (see more details in the Experimental section). As shown in Table 1, the order of the J values for the four biradical diastereoisomers in the frozen state is TNT1 (196 G) ∼ TNT2 (172 G) ≫ TNL2 (6.3 G) ∼ TNL1 (5.4 G). This trend is similar to the results obtained at room temperature. As mentioned above, the flexible linkers in TNL1,2 allow for the spatial proximity of the two radical parts and their exchange interactions are mostly based on a though-space mechanism above room temperature. In the frozen state, their molecular conformations were immobilized and their through-space exchange interactions were inhibited. Thus, the very weak exchange interactions observed for TNL1,2 in the frozen state are most likely due to the through-bond mechanism. The close J values of TNL1 and TNL2 indicate that they have similar preferential conformation(s) at low temperature. The similar conformations of TNL1,2 in the frozen state were further confirmed by their identical dipolar interactions (D = 8 G) which indicate the same distances between the two spins in TNL1,2. Comparatively, the exchange interactions of TNT1,2 did not significantly vary with temperature and had similar J values at ∼220 K and room temperature. These temperature-insensitive exchange interactions are due to the rigid linkers in TNT1,2 which prevent the rotation of the chemical bonds in the linker and solidify their molecular conformations.
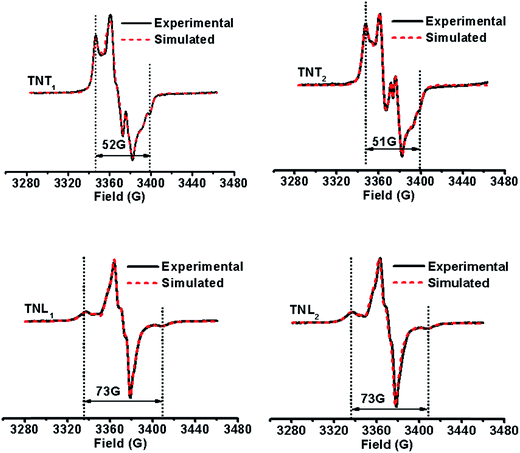 | ||
| Fig. 4 Experimental (black solid line) and simulated (red dotted line) EPR spectra of TNT1, TNT2, TNL1 and TNL2 in glycerol/water (v/v, 60/40) at low temperatures (∼220 K). | ||
Dynamic nuclear polarization studies
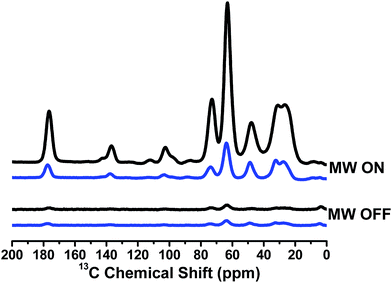
Since TN biradicals are very promising DNP polarizing agents especially at a high field,35,36 the DNP performances of TNT1,2 and TNL1,2 were also evaluated on an 800 MHz/527 GHz DNP/MAS solid-state NMR system.45 The enhancements of 13C NMR signals of 13C–15N proline were measured using TNT1,2 and TNL1,2 as polarizing agents. As shown in Fig. 5, TNL1,2 exhibits an over 40-fold signal enhancement, whereas TNT1,2 has only a 7-fold enhancement. The distinct enhancements imply that the nitroxide chirality and thus the amplitude of the exchange interactions significantly influence the DNP properties of TNT1,2 and TNL1,2. Our present study experimentally verified the adverse effect of the too strong exchange interactions of TNT1,2 on its DNP enhancement although a similar result was theoretically suggested in a previous study.46 In addition, the exchange interaction may affect the electron spin relaxation of biradicals47 that has been shown to be a crucial factor controlling the DNP properties of nitroxide biradicals.48,49 Thus, the effect of the exchange interaction is multiple. Detailed theoretical simulations may quantitatively describe the effect of the exchange interactions on the DNP properties of TN biradicals.39,50 On the other hand, the 40-fold signal enhancement of TNL1,2 is somewhat lower than that previously seen for TEMTriPol-1 (ε = 65 for 13C-urea) which differs from TNL1,2 only by the linker and has an exchange coupling of 17 G determined by the new simulation program, which was reported to be 26 G in a previous paper.36 Since TNL1,2 (J ∼ 6 G) has weaker exchange interaction than TEMTriPol-1 (J = 17 G), an optimal J value for TN biradicals may exist that is large enough for highly efficient polarization transfer but is so small as not to interfere with the frequency matching required for DNP.51Conclusions
We synthesized four enantiomerically pure diastereoisomers (TNT1, TNT2, TNL1 and TNL2) of TN biradicals with different chiral configurations in the nitroxide and trityl parts. The chiral configuration of the nitroxide part plays important roles in modulating the exchange interactions of these diastereoisomers and their dependence on temperature and solvents due to the different flexibilities of the linkers. The diastereoisomeric mixture TNL1,2 with a small and similar J value (∼6 G) has a much higher DNP enhancement (ε = 40) than TNT1,2 (J > 190 G, ε = 7), accounting for the adverse effect of too strong exchange interactions on the DNP enhancement.36 This study demonstrates that the chiral configuration of the radical part is an effective factor modulating the exchange interaction of TN biradicals and this strategy can be used to develop new biradicals with improved DNP properties.Experimental section
General information
All reactions were carried out under an argon atmosphere. Dichloromethane (CH2Cl2) was redistilled with CaH2 and dimethylformamide (DMF) were passed through a column of molecular sieves. Boc-L-proline, 1-hydroxybenzotriazole (HOBt), (benzotriazol-1-yloxy)tris(dimethylamino)phosphoniumhexafluoro-phosphate (BOP), N,N-diisopropylethyl-amine (DIPEA), 2,2,5,5-tetramethyl-3-amino-pyrrolidine-1-oxyl free radical (APO) and ascorbic acid were purchased and used without purification. CT-03 was prepared according to a previously reported method.52 Thin layer chromatography was performed on 0.25 mm silica gel plates. Flash column chromatography was employed using silica gel with a 200–300 mesh. Thin layer chromatography plates were visualized by exposure to UV light. High-resolution mass spectrometry was carried out in methanol employing electrospray ionization (ESI) methods. High resolution mass spectrometry (HRMS) analyses were performed on a LCMS-IT-TOF Shimadzu liquid chromatograph mass spectrometer. EPR measurements were carried out on a Bruker EMX-plus X-band spectrometer. ECD spectra were recorded on a Jasco J715 spectropolarimeter (Jasco Corporation, Tokyo, Japan). Analytical HPLC was carried out on an Agilent 1100 equipped with a G1315B DAD detector and G1311A pump. Semipreparative HPLC was carried out on an SSI 1500 equipped with a UV/vis detector and versa pump.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as an eluent to give two diastereoisomers NP1 (41 mg, 42% yield) as a yellow oil and NP2 (43 mg, 44% yield) as a yellow solid. NP1, MS (ESI, [M + H]+, m/z): 355.2 (measured), 355.2 (calculated); ([M + Na]+, m/z): 377.2 (measured), 377.2 (calculated). EPR: αN = 15.7 G in MeOH/H2O (v/v, 1
2) as an eluent to give two diastereoisomers NP1 (41 mg, 42% yield) as a yellow oil and NP2 (43 mg, 44% yield) as a yellow solid. NP1, MS (ESI, [M + H]+, m/z): 355.2 (measured), 355.2 (calculated); ([M + Na]+, m/z): 377.2 (measured), 377.2 (calculated). EPR: αN = 15.7 G in MeOH/H2O (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and 14.5 G in CH2Cl2. NP2, MS (ESI, [M + H]+, m/z): 355.2 (measured), 355.2 (calculated); (ESI, [M + Na]+, m/z): 377.3 (measured), 377.2 (calculated). EPR: αN = 15.7 G in MeOH/H2O (v/v, 1/2) and 14.5 G in CH2Cl2.
2) and 14.5 G in CH2Cl2. NP2, MS (ESI, [M + H]+, m/z): 355.2 (measured), 355.2 (calculated); (ESI, [M + Na]+, m/z): 377.3 (measured), 377.2 (calculated). EPR: αN = 15.7 G in MeOH/H2O (v/v, 1/2) and 14.5 G in CH2Cl2.
Reduction of the four biradical diastereoisomers by ascorbic acid
Each diastereoisomer (50 μM) was mixed with ascorbic acid (2 mM) in PBS (20 mM, pH 7.4). After the reaction was completed, the resulting solution was transferred into a capillary tube and EPR spectra were recorded using the following EPR parameters: microwave power, 0.5 mW; modulation amplitude, 0.03 G.EPR spectroscopy
EPR measurements were carried out on a Bruker EMX-plus X-band spectrometer at room temperature (298 K) or low temperature (∼220 K). General instrumental settings were as follows: modulation frequency, 100 kHz; microwave power, 10 mW; and modulation amplitude, 1 G for room temperature and 2 G for low temperature. Measurements were performed in 50 μL capillary tubes. Spectral simulation was performed by using the program developed by Professor Rockenbauer.43 In this study, the exchange, dipolar and hyperfine couplings are given in Gauss units which can be converted into cm−1 by multiplying with g × 4.6686 × 10−5, where g is the respective Zeeman factor. EPR measurements under anaerobic conditions were carried out using a gas-permeable Teflon tube (i.d. = 0.8 mm). Briefly, the sample solution was transferred to the tube which was then sealed at both ends. The sealed sample was placed inside a quartz EPR tube with open ends. Argon gas was allowed to bleed into the EPR tube, and then an EPR spectrum was recorded.EPR simulation
The EPR spectra are computed by using a perturbation solution of the biradical spin Hamiltonian:HSH = ![[H with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0048_20d1.gif) ĝ1 ĝ1![[S with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0053_20d1.gif) 1μB + 1μB + ![[H with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0048_20d1.gif) ĝ2 ĝ2![[S with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0053_20d1.gif) 2μB + J 2μB + J![[S with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0053_20d1.gif) 1 1![[S with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0053_20d1.gif) 2 + D′′(2Sz1Sz2 − Sx1Sx2 − Sy1Sy2) + 2 + D′′(2Sz1Sz2 − Sx1Sx2 − Sy1Sy2) + ![[S with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0053_20d1.gif) 1Â1 1Â1![[I with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0049_20d1.gif) 1 + 1 + ![[S with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0053_20d1.gif) 2Â2 2Â2![[I with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0049_20d1.gif) 2 2 |
The computations are carried out in three different frames:
(1) The (x, y, z) reference frame is chosen as the principal axis of the ĝ1 Zeeman and Â1 hyperfine tensor of the nitroxide moiety. The orientation of the magnetic field is given by the θ and ϕ polar angles and defined in the above frame.
Hx = H0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ, Hy = H0 ϕ, Hy = H0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ, Hz = H0 ϕ, Hz = H0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
(2) The slightly anisotropic ĝ2 tensor and a small Â2 hyperfine tensor of the trityl are defined in the (x′, y′, z′) frame. The relative orientation of the two frames is characterized by the three Euler angles α, β and γ:
The same transformation defines the relationship of the hyperfine tensors Â1 and Â2.
(3) The dipolar interaction D′′ is defined in a third frame, where the z′′ axis shows the direction of the linker and its orientation is given by two polar angles ξ and ζ. A perturbation procedure is applied in the case when the Zeeman term is large compared to the exchange, dipolar and hyperfine interactions. Then in the Zeeman and hyperfine terms, only the z component of the electron and nuclear spin remains:
| HZ = H0g1(θ1, ϕ1)μBSz1 + H0g2(θ2, ϕ2)μBSz2 |
| HHF = A1(θ1, ϕ1)Sz1Iz1 + A2(θ2, ϕ2)Sz2Iz2 |
Here the g-factor is
The angular dependence of the hyperfine constant is
The angle dependence of the dipolar interaction is given as
D = D′′/2(1 − 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos2 cos2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) δ) |
The δ angle between the magnetic field and the z′′ axis can be expressed by the polar angles ξ and ζ:
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = sin δ = sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ξ ξ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ ϕ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ζ + sin ζ + sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ξ ξ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ ϕ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ζ + cos ζ + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ξ ξ |
The biradicals have four energy levels that can be assigned to the S = 1 triplet and S = 0 singlet state. Both the exchange and dipolar terms can mix the MS = 0 states of the S = 1 triplet and S = 0 singlet:
| HExch + HDip = Sz1Sz2(2D + J) + 1/2(S+1S−2 + S−1S+2)(J − D) |
The diagonal elements in the |S, MS〉 basis are
| 〈1, 1|HExch + HDip|1, 1〉 = 〈1, −1|HExch + HDip|1, −1〉 = J/4 + D/2 |
| 〈1, 0|HExch + HDip|1, 0〉 = J/4 − D |
| 〈0, 0|HExch + HDip|0, 0〉 = −3J/4 |
The Zeeman and hyperfine interactions have only diagonal elements in the |S, MS, MI1, MI2〉 basis if MS = 1 or −1:
| 〈1, 1|HZ + HHF|1, 1〉 = −〈1, −1|HZ + HHF|1, −1〉 = 1/2[(g1 + g2)μBH0 + A1MI1 + A2MI2] |
For the MS = 0 state, these interactions give zero diagonal elements, but in this case the off-diagonal elements can be non-zero:
| 〈1, 0|HZ + HHF|0, 0〉 = 1/2[(g1 − g2)μBH0 + A1MI1 − A2MI2] |
Since the |1, 1〉 and |1, −1〉 triplet states are well separated from the central states, the eigenvalues can be obtained directly from the diagonal elements. We introduce the indices i = 1 and 4 for the highest |1, 1〉 and lowest |1, −1〉 states, respectively. Furthermore i = 2 and 3 denote the two central states with MS = 0.
| E1,MI1,MI2 = −E4,MI1,MI2 = J/4 + D/2 + 1/2(g1 + g2)μBH0 + 1/2(A1MI1 + A2MI2) |
The mixing of |1, 0〉 and |0, 0〉 states can be obtained by the solution of the secular equation:
| |2〉 = p1|1, 0〉 + p2|0, 0〉 |
| |3〉 = p2|1, 0〉 − p1|0, 0〉 |
The mixing coefficients can be expressed by the factor R:
| R2 = (J − D)2 + ((g1 − g2)μBH0 + A1MI1 − A2MI2)2 |
| p12 = 1/2|R + J − D|/R, p22 = 1/2|R − J + D|/R |
It is worth noting that the transition probability and energy depend on the relative sign of D and J, which offers a possibility to decide from the frozen solution EPR spectra whether the Heisenberg exchange is ferro- or anti-ferromagnetic. Then, the energy expressions for the central states are
| Ei,MI1,MI2 = −J/4 − D/2 ± R/2, i = 2, 3. |
Due to the triplet–singlet mixing, four transitions can take place in the whole MI1, MI2 subspace. The two resonances have the transition probability
| W12 = W24 = |〈1|S+|2〉|2 = 2p12 |
The respective frequency is
| ℏω12 or 24 = 1/2(g1 + g2)μBH0 + 1/2(A1MI1 + A2MI2) ± (J/2 + D − R/2). |
The above resonances can be called “allowed”, since their intensities are larger than 1.0 and the line separations are smaller than J. The other pair of resonances has the transition probability
| W13 = W34 = |〈1|S+|3〉|2 = 2p22 |
This transition can be considered as “forbidden” due to the smaller transition probability: 2p22 < 1.0 and the frequency spread can be extended in a broad range comparable with the value of J:
| ℏω13 or 34 = 1/2(g1 + g2)μBH0 + 1/2(A1MI1 + A2MI2) ± (J/2 + D + R/2) |
For a fixed ωEPR microwave frequency the above relationships allow the determination of the corresponding four resonance fields Hi,MI1,MI2(θ, ϕ), where the i index denotes the four transitions.
The ROKI/DNP program can use either Lorentzian or Gaussian, or mixed line shapes f(H) and the powder pattern can be obtained by integrating over the angles θ and ϕ:
All parameters can be optimized to achieve the best fit of the experimental spectra; the method of optimization is the same as that described in the ROKI/EPR program.43 Due to the large number of optimized parameters ambiguity can occur. This problem can be reduced by recording the frozen solution spectra at different resonance frequencies. In X band spectra the determination of the three Euler angles is rather problematic due to the small g anisotropy of the trityl moiety. The polar angles, however, are more reliable since the nitroxide part has a large Zeeman and hyperfine anisotropy.
DNP/ssNMR spectroscopy of the diastereoisomeric mixtures TNT1,2 and TNL1,2
DNP experiments were performed on frozen solutions of 10 mM biradical in d8-glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O
D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 60
H2O 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v/v with 0.25 M U–13C–15N proline. DNP experiments at 800 MHz were performed on a Bruker BioSpin 527 GHz solid-state NMR DNP spectrometer. This spectrometer is equipped with a Bruker 800 WB/RS Plus magnet with a sweep coil, an Avance III NMR console, and a low-temperature 3.2 mm triple-resonance DNP MAS NMR probe.45 A gyrotron microwave source emits microwaves at a frequency of 527.043 GHz. In the DNP experiments the nuclear polarization is measured through the spectrum of 13C–15N proline, which is observed via13C–1H cross-polarization (CP). A CP spin-locking field of 50 kHz is applied on 13C, while the spin-locking field on 1H is ramped from 36 to 45 kHz. The contact time was set to 2 ms. During the acquisition SPINAL-64 decoupling53 was used at 83 kHz. Each CP acquisition was preceded by a presaturation sequence consisting of 300 90° 1H pulses 20 μs apart at an RF power of 100 kHz, followed by a period of polarization build-up, which was set to 1.26T1 for optimal sensitivity. Each spectrum was acquired with a 4-step phase cycle and acquired three times to confirm stability and reproducibility. The MAS frequency was set to 8 kHz and the sample temperature was kept at 103 K, unless noted otherwise. The enhancement, ε, was obtained from comparison of the 13C signal amplitude with and without microwaves. T1 (microwaves off) and TB (microwaves on) were measured via a saturation recovery experiment. T2 was determined from the decay of the echo intensity during a rotor-synchronized Hahn echo sequence. To obtain the CE DNP enhancement field profiles the magnetic field is swept and at each field position the nuclear polarization is measured via the cross-polarization experiment as described above.
10 v/v/v with 0.25 M U–13C–15N proline. DNP experiments at 800 MHz were performed on a Bruker BioSpin 527 GHz solid-state NMR DNP spectrometer. This spectrometer is equipped with a Bruker 800 WB/RS Plus magnet with a sweep coil, an Avance III NMR console, and a low-temperature 3.2 mm triple-resonance DNP MAS NMR probe.45 A gyrotron microwave source emits microwaves at a frequency of 527.043 GHz. In the DNP experiments the nuclear polarization is measured through the spectrum of 13C–15N proline, which is observed via13C–1H cross-polarization (CP). A CP spin-locking field of 50 kHz is applied on 13C, while the spin-locking field on 1H is ramped from 36 to 45 kHz. The contact time was set to 2 ms. During the acquisition SPINAL-64 decoupling53 was used at 83 kHz. Each CP acquisition was preceded by a presaturation sequence consisting of 300 90° 1H pulses 20 μs apart at an RF power of 100 kHz, followed by a period of polarization build-up, which was set to 1.26T1 for optimal sensitivity. Each spectrum was acquired with a 4-step phase cycle and acquired three times to confirm stability and reproducibility. The MAS frequency was set to 8 kHz and the sample temperature was kept at 103 K, unless noted otherwise. The enhancement, ε, was obtained from comparison of the 13C signal amplitude with and without microwaves. T1 (microwaves off) and TB (microwaves on) were measured via a saturation recovery experiment. T2 was determined from the decay of the echo intensity during a rotor-synchronized Hahn echo sequence. To obtain the CE DNP enhancement field profiles the magnetic field is swept and at each field position the nuclear polarization is measured via the cross-polarization experiment as described above.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (No. 21572161 and 31500684), the Science & Technology Projects of Tianjin (No. 15JCZDJC32300 and 15JCYBJC23700) and the Hungarian National Research, Development and Innovation Office (NKFTH) Grant (K119442 to A. R.). The DNP experiments were funded in part by the Netherlands Organization for Scientific Research (NWO) (No. 700.26.121 and 700.10.443 to M. B.).Notes and references
- Q. Z. Ni, E. Daviso, T. V. Can, E. Markhasin, S. K. Jawla, T. M. Swager, R. J. Temkin, J. Herzfeld and R. G. Griffin, Acc. Chem. Res., 2013, 46, 1933–1941 CrossRef CAS PubMed.
- A. J. Rossini, A. Zagdoun, M. Lelli, A. Lesage, C. Coperet and L. Emsley, Acc. Chem. Res., 2013, 46, 1942–1951 CrossRef CAS PubMed.
- H. Iwamura and N. Koga, Acc. Chem. Res., 1993, 26, 346–351 CrossRef CAS.
- A. Rajca, Chem. Rev., 1994, 94, 871–893 CrossRef CAS.
- M. Abe, Chem. Rev., 2013, 113, 7011–7088 CrossRef CAS PubMed.
- E. A. Weiss, M. J. Ahrens, L. E. Sinks, A. V. Gusev, M. A. Ratner and M. R. Wasielewski, J. Am. Chem. Soc., 2004, 126, 5577–5584 CrossRef CAS PubMed.
- A. B. Ricks, K. E. Brown, M. Wenninger, S. D. Karlen, Y. A. Berlin, D. T. Co and M. R. Wasielewski, J. Am. Chem. Soc., 2012, 134, 4581–4588 CrossRef CAS PubMed.
- M. A. Harris, A. K. Mishra, R. M. Young, K. E. Brown, M. R. Wasielewski and F. D. Lewis, J. Am. Chem. Soc., 2016, 138, 5491–5494 CrossRef CAS PubMed.
- G. I. Roshchupkina, A. A. Bobko, A. Bratasz, V. A. Reznikov, P. Kuppusamy and V. V. Khramtsov, Free Radicals Biol. Med., 2008, 45, 312–320 CrossRef CAS PubMed.
- E. A. Legenzov, S. J. Sims, N. D. Dirda, G. M. Rosen and J. P. Kao, Biochemistry, 2015, 54, 6973–6982 CrossRef CAS PubMed.
- D. A. Shultz, R. M. Fico, H. Lee, J. W. Kampf, K. Kirschbaum, A. A. Pinkerton and P. D. Boyle, J. Am. Chem. Soc., 2003, 125, 15426–15432 CrossRef CAS PubMed.
- D. A. Shultz, A. K. Boal and G. T. Farmer, J. Am. Chem. Soc., 1997, 119, 3846–3847 CrossRef CAS.
- A. Rajca, A. Olankitwanit and S. Rajca, J. Am. Chem. Soc., 2011, 133, 4750–4753 CrossRef CAS PubMed.
- A. Olankitwanit, M. Pink, S. Rajca and A. Rajca, J. Am. Chem. Soc., 2014, 136, 14277–14288 CrossRef CAS PubMed.
- N. M. Gallagher, A. Olankitwanit and A. Rajca, J. Org. Chem., 2015, 80, 1291–1298 CrossRef CAS PubMed.
- N. M. Gallagher, J. J. Bauer, M. Pink, S. Rajca and A. Rajca, J. Am. Chem. Soc., 2016, 138, 9377–9380 CrossRef CAS PubMed.
- M. L. Kirk, D. A. Shultz, E. C. Depperman, D. Habel-Rodriguez and R. D. Schmidt, J. Am. Chem. Soc., 2012, 134, 7812–7819 CrossRef CAS PubMed.
- M. L. Kirk, D. A. Shultz, D. E. Stasiw, D. Habel-Rodriguez, B. Stein and P. D. Boyle, J. Am. Chem. Soc., 2013, 135, 14713–14725 CrossRef CAS PubMed.
- D. E. Stasiw, J. Zhang, G. Wang, R. Dangi, B. W. Stein, D. A. Shultz, M. L. Kirk, L. Wojtas and R. D. Sommer, J. Am. Chem. Soc., 2015, 137, 9222–9225 CrossRef CAS PubMed.
- C. Song, K. N. Hu, C. G. Joo, T. M. Swager and R. G. Griffin, J. Am. Chem. Soc., 2006, 128, 11385–11390 CrossRef CAS PubMed.
- C. Ysacco, E. Rizzato, M. A. Virolleaud, H. Karoui, A. Rockenbauer, M. F. Le, D. Siri, O. Ouari, R. G. Griffin and P. Tordo, Phys. Chem. Chem. Phys., 2010, 12, 5841–5845 RSC.
- M. K. Kiesewetter, B. Corzilius, A. A. Smith, R. G. Griffin and T. M. Swager, J. Am. Chem. Soc., 2012, 134, 4537–4540 CrossRef CAS PubMed.
- E. L. Dane, B. Corzilius, E. Rizzato, P. Stocker, T. Maly, A. A. Smith, R. G. Griffin, O. Ouari, P. Tordo and T. M. Swagert, J. Org. Chem., 2012, 77, 1789–1797 CrossRef CAS PubMed.
- C. Sauvee, M. Rosay, G. Casano, F. Aussenac, R. T. Weber, O. Ouari and P. Tordo, Angew. Chem., Int. Ed., 2013, 52, 10858–10861 CrossRef CAS PubMed.
- A. P. Jagtap, M. A. Geiger, D. Stoppler, M. Orwick-Rydmark, H. Oschkinat and S. T. Sigurdsson, Chem. Commun., 2016, 52, 7020–7023 RSC.
- D. J. Kubicki, G. Casano, M. Schwarzwalder, S. Abel, C. Sauvee, K. Ganesan, M. Yulikov, A. J. Rossini, G. Jeschke, C. Coperet, A. Lesage, P. Tordo, O. Ouari and L. Emsley, Chem. Sci., 2015, 7, 550–558 RSC.
- C. Sauvee, G. Casano, S. Abel, A. Rockenbauer, D. Akhmetzyanov, H. Karoui, D. Siri, F. Aussenac, W. Maas and R. T. Weber, Chem.–Eur. J., 2016, 22, 5598–5606 CrossRef CAS PubMed.
- J. Y. Chen, N. Jayaraj, S. Jockusch, M. F. Ottaviani, V. Ramamurthy and N. J. Turro, J. Am. Chem. Soc., 2008, 130, 7206–7207 CrossRef CAS PubMed.
- S. Yi, B. Captain, M. F. Ottaviani and A. E. Kaifer, Langmuir, 2011, 27, 5624–5632 CrossRef CAS PubMed.
- C. Casati, P. Franchi, R. Pievo, E. Mezzina and M. Lucarini, J. Am. Chem. Soc., 2012, 134, 19108–19117 CrossRef CAS PubMed.
- F. Romano, R. Manoni, P. Franchi, E. Mezzina and M. Lucarini, Chem.–Eur. J., 2015, 21, 2775–2779 CrossRef CAS PubMed.
- Y. P. Liu, F. A. Villamena, A. Rockenbauer and J. L. Zweier, Chem. Commun., 2010, 46, 628–630 RSC.
- Y. P. Liu, F. A. Villamena, Y. G. Song, J. Sun, A. Rockenbauer and J. L. Zweier, J. Org. Chem., 2010, 75, 7796–7802 CrossRef CAS PubMed.
- Y. P. Liu, Y. G. Song, A. Rockenbauer, J. Sun, C. Hemann, F. A. Villamena and J. L. Zweier, J. Org. Chem., 2011, 76, 3853–3860 CrossRef CAS PubMed.
- K. N. Hu, V. S. Bajaj, M. Rosay and R. G. Griffin, J. Chem. Phys., 2007, 126, 44512 CrossRef PubMed.
- G. Mathies, M. A. Caporin, V. K. Michaelis, Y. P. Liu, K. N. Hu, D. Mance, J. L. Zweier, M. Rosay, M. Baldus and R. G. Griffin, Angew. Chem., Int. Ed., 2015, 54, 11770–11774 CrossRef CAS PubMed.
- Y. P. Liu, F. A. Villamena, A. Rockenbauer, Y. G. Song and J. L. Zweier, J. Am. Chem. Soc., 2013, 135, 2350–2356 CrossRef CAS PubMed.
- X. L. Tan, Y. G. Song, H. Q. Liu, Q. W. Zhong, A. Rockenbauer, F. A. Villamena, J. L. Zweier and Y. P. Liu, Org. Biomol. Chem., 2016, 14, 1694–1701 CAS.
- F. Mentink-Vigier, G. Mathies, Y. P. Liu, A. L. Barra, M. A. Caporini, D. Lee, S. Hediger, R. G. Griffin and G. D. Paepe, Chem. Sci., 2017, 8, 8150–8163 RSC.
- B. Driesschaert, R. Robiette, F. Lucaccioni, B. Gallez and J. Marchand-Brynaert, Chem. Commun., 2011, 47, 4793–4795 RSC.
- V. M. Tormyshev, A. M. Genaev, G. E. Salnikov, O. Y. Rogozhnikova, T. I. Troitskaya, D. V. Trukhin, V. I. Mamatyuk, D. S. Fadeev and H. J. Halpern, Eur. J. Org. Chem., 2012, 3, 623–629 CrossRef PubMed.
- J. Suk, V. R. Naidu, X. Liu, M. S. Lah and K. S. Jeong, J. Am. Chem. Soc., 2011, 133, 13938–13941 CrossRef CAS PubMed.
- A. Rockenbauer and L. Korecz, Appl. Magn. Reson., 1996, 10, 29–43 CrossRef CAS.
- S. Sankarapandi, M. Sukumar, P. Balaram and P. T. Manoharan, Biochem. Biophys. Res. Commun., 1995, 213, 439–446 CrossRef CAS PubMed.
- E. J. Koers, E. A. W. van der Cruijsen, M. Rosay, M. Weingarth, A. Prokofyev, C. Sauvee, O. Ouari, J. van der Zwan, O. Pongs, P. Tordo, W. E. Maas and M. Baldus, J. Biomol. NMR, 2014, 60, 157–168 CrossRef CAS PubMed.
- K. N. Hu, G. T. Debelouchina, A. A. Smith and R. G. Griffin, J. Chem. Phys., 2011, 134, 125105 CrossRef PubMed.
- H. Sato, V. Kathirvelu, G. Spagnol, S. Rajca, A. Rajca, S. S. Eaton and G. R. Eaton, J. Phys. Chem. B, 2008, 112, 2818–2828 CrossRef CAS PubMed.
- A. Zagdoun, G. Casano, O. Ouari, M. Schwarzwalder, A. J. Rossini, F. Aussenac, M. Yulikov, G. Jeschke, C. Coperet, A. Lesage, P. Tordo and L. Emsley, J. Am. Chem. Soc., 2013, 135, 12790–12797 CrossRef CAS PubMed.
- D. J. Kubicki, G. Casano, M. Schwarzwalder, S. Abel, C. Sauvee, K. Ganesan, M. Yulikov, A. J. Rossini, G. Jeschke, C. Coperet, A. Lesage, P. Tordo, O. Ouari and L. Emsley, Chem. Sci., 2016, 7, 550–558 RSC.
- D. Mance, P. Gast, M. Huber, M. Baldus and K. L. Ivanov, J. Chem. Phys., 2015, 142, 234201 CrossRef PubMed.
- K. N. Hu, C. Song, H. H. Yu, T. M. Swager and R. G. Griffin, J. Chem. Phys., 2008, 128, 052302 CrossRef PubMed.
- Y. P. Liu, F. A. Villamena, J. Sun, Y. Xu, I. Dhimitruka and J. L. Zweier, J. Org. Chem., 2008, 73, 1490–1497 CrossRef CAS PubMed.
- B. M. Fung, A. K. Khitrin and K. Ermolaev, J. Magn. Reson., 2000, 142, 97–101 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc00969d |
| This journal is © The Royal Society of Chemistry 2018 |