Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Phosphine-catalyzed [5+1] annulation of δ-sulfonamido-substituted enones with N-sulfonylimines: a facile synthesis of tetrahydropyridines†
Leijie
Zhou
,
Chunhao
Yuan
,
Yuan
Zeng
,
Honglei
Liu
,
Chang
Wang
,
Xing
Gao
,
Qijun
Wang
,
Cheng
Zhang
 and
Hongchao
Guo
and
Hongchao
Guo
 *
*
Department of Applied Chemistry, China Agricultural University, Beijing 100193, China. E-mail: hchguo@cau.edu.cn
First published on 5th January 2018
Abstract
Phosphine-catalyzed [5+1] annulation of δ-sulfonamido-substituted enones with N-sulfonylimines for the synthesis of 1,2,3,6-tetrahydropyridines is developed. The reaction proceeds smoothly under mild reaction conditions to give the annulation products in moderate to excellent yields. Mechanistic exploration of this new annulation shows that the δ-sulfonamido-substituted enone and the N-sulfonylimine serve as C5 and C1 synthons to furnish the annulation, respectively. Using chiral phosphine as the catalyst, an asymmetric variant of the model reaction gave the chiral product in up to 73% ee.
Introduction
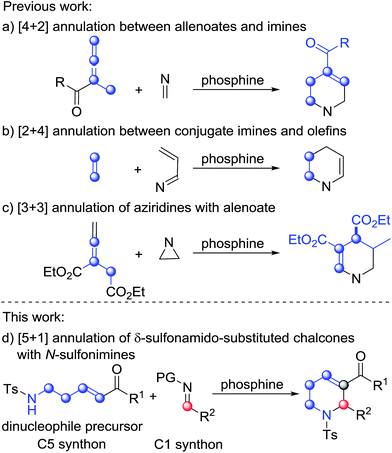
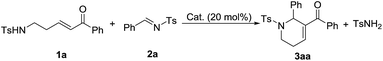
Phosphine-catalyzed annulation reactions are powerful synthetic tools to construct carbo- and heterocycles.1 Since the pioneering work of Lu on phosphine catalysis,2 many types of phosphine-promoted annulation reaction such as [1+n],3 [2+n],4 [3+n],5 and [4+n]6 annulations have been developed. In these reactions, the reactive intermediates from nucleophilic addition of phosphine to activated allenes, Morita–Baylis–Hillman carbonates, activated alkynes, etc., namely phosphorus ylides, serve as one-, two-, three-, or four-membered synthons when reacting with a variety of electrophilic coupling partners. Despite the fact that extremely diverse annulation reactions have been developed in the past two decades,1 the development of phosphine catalysis is reaching its limit since phosphine catalysis is stuck with a single activation mode. Therefore, exploration of new activation modes and synthons is very significant. Generally, phosphorus ylides work as equal to or less than four-membered synthons in phosphine catalysis. Examples with phosphorus ylides as greater than or equal to five-membered synthons, which could probably be used for synthesis of six-membered or medium-ring cyclic compounds, have not been reported.Functionalized tetrahydropyridines are important structural motifs of numerous biologically active natural products and synthetic pharmaceuticals, and their synthesis has attracted much attention.7 In the area of phosphine catalysis, several attractive strategies involving phosphine-catalyzed annulation reactions have been established for the synthesis of functionalized tetrahydropyridines. In 2003, Kwon described PBu3-catalyzed [4+2] annulation of imines with allenes as a facile pathway to access functionalized tetrahydropyridines (Scheme 1a).8 Two years later, through the use of a bulky tert-butyl-substituted binaphthyl-based chiral phosphine as the catalyst, Fu accomplished asymmetric versions of the above [4+2] reactions with excellent enantioselectivities.9 After the work of Kwon and Fu, Shi,10a Marinetti10b and Zhao10c made great contributions to the development of this classic [4+2] annulation reaction, and the reaction was also utilized by Kwon as a key step in the synthesis of natural products.11 In 2012, Loh and Zhong reported the phosphine-catalyzed asymmetric [2+4] annulation of olefins with conjugate imines, which provided an alternative approach to the synthesis of enantioenriched tetrahydropyridines (Scheme 1b).12 After the work, Chi,13a Shi,13b Wu13c and Zhang13d enriched this reaction by introducing intramolecular modes or other types of catalysts. The [3+3] annulation mode is another typical way to synthesize functionalized tetrahydropyridines. In 2009, Kwon developed the first phosphine-promoted [3+3] annulation of aziridines with allenoates to afford highly functionalized tetrahydropyridines under mild conditions,14 broadening the synthetic strategies of tetrahydropyridines by utilizing different types of building block (Scheme 1c). Herein, as the initial attempt of our exploration of new five-membered linear synthons for the [5+n] annulation reaction, we report a phosphine-catalyzed [5+1] annulation reaction of δ-sulfonamido-substituted enones with N-sulfonylimines (Scheme 1d). To the best of our knowledge, this is the first phosphine-catalyzed [5+1] annulation with a phosphorus ylide as a five-membered synthon.
Results and discussion
At the outset of our experiment, the reaction between δ-sulfonamido-substituted enone 1a and N-sulfonylimine 2a was chosen as the model reaction, and various Lewis bases such as phosphines and amines were examined as the catalyst (Table 1). PPh3 (20 mol%) did not show any catalytic activity, and no annulation product was observed after the reaction mixture was stirred at rt for 72 h (Table 1, entry 1). Under otherwise identical conditions, the [5+1] annulation product 3aa was obtained in 20% yield when MePPh2 was employed as the catalyst (entry 2). It seems that more nucleophilic phosphines were beneficial to the reaction. With the use of Me2PPh as the catalyst, the reaction worked at rt for 36 h to give the product 3aa in 75% yield (entry 3). Compared with Me2PPh, more nucleophilic Bu3P displayed much better catalytic activity, greatly shortening the reaction time to 3 h to afford the product 3aa in 95% yield (entry 4). Lowering the catalyst loading to 10 mol% still resulted in the product in 90% yield, albeit requiring a reaction time of 28 h (entry 5). However, when the catalyst loading was lowered to 5 mol%, the yield of 3aa was greatly decreased to 15% (entry 6). With the use of organic amines such as Et3N, DMAP and DABCO instead of phosphines as the catalyst, no annulation product was observed even when the reaction time was prolonged to 72 h under otherwise identical conditions (entries 7–9). A stronger Lewis base DBU displayed certain catalytic activity, promoting the reaction to afford the annulation product 3aa in 20% yield (entry 10).| Entry | Cat. | t (h) | Yieldb (%) |
|---|---|---|---|
| a Unless otherwise stated, all reactions were carried out with 1a (0.1 mmol), 2a (0.15 mmol), and catalyst (0.02 mmol) in CH2Cl2 (1 mL) at rt. b Isolated yield. c 10 mol% PBu3 was used. d 5 mol% PBu3 was used. e No reaction. | |||
| 1 | Ph3P | 72 | NRe |
| 2 | MePPh2 | 72 | 20 |
| 3 | Me2PPh | 36 | 75 |
| 4 | Bu3P | 3 | 95 |
| 5c | Bu3P | 28 | 90 |
| 6d | Bu3P | 72 | 15 |
| 7 | Et3N | 72 | NR |
| 8 | DMAP | 72 | NR |
| 9 | DABCO | 72 | NR |
| 10 | DBU | 72 | 20 |
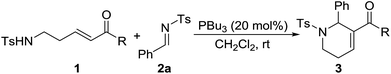
After the optimal conditions were determined, various N-sulfonylimines with different substituents were carefully investigated (Table 2). The results indicated that imines with either electron-deficient or electron-rich substituents on the benzene ring are suitable substrates, and the corresponding 1,2,3,6-tetrahydropyridine derivatives were obtained with usually good to high yields. However, the position of the substituent on the benzene ring had a remarkable influence on the reaction. For example, the 2-Cl, 2-Br, and 2-Me substituted aryl imines led to lower yields of the products compared with their 3- or 4-substituted counterparts (entry 5 vs. entries 6–7, entry 8 vs. entries 9–10, entry 11 vs. entries 12–13). The 2-thiophenyl and 2-naphthyl imines were also compatible substrates under the optimal reaction conditions and the corresponding products were obtained in excellent yields (entries 16–17). The N-Boc-3-indole derived imine 2r also underwent the reaction, providing the product 3ar in 72% yield (entry 18). Unfortunately, the alkyl N-sulfonylimine 2s did not perform the reaction and no desired product was observed (entry 19). The structure of the [5+1] annulation product was unambiguously determined through X-ray crystallographic analysis of the product 3aq.15
| Entry | R | t (h) | 3 | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise stated, all reactions were carried out with 1a (0.2 mmol), 2 (0.3 mmol), and PBu3 (0.04 mmol) in CH2Cl2 (2 mL) at rt. b Isolated yield. c No reaction. | ||||
| 1 | Ph (2a) | 3 | 3aa | 95 |
| 2 | 2-FC6H4 (2b) | 2 | 3ab | 96 |
| 3 | 3-FC6H4 (2c) | 6 | 3ac | 85 |
| 4 | 4-FC6H4 (2d) | 2 | 3ad | 93 |
| 5 | 2-ClC6H4 (2e) | 12 | 3ae | 75 |
| 6 | 3-ClC6H4 (2f) | 9 | 3af | 82 |
| 7 | 4-ClC6H4 (2g) | 1 | 3ag | 85 |
| 8 | 2-BrC6H4 (2h) | 16 | 3ah | 68 |
| 9 | 3-BrC6H4 (2i) | 6 | 3ai | 81 |
| 10 | 4-BrC6H4 (2j) | 2 | 3aj | 84 |
| 11 | 2-MeC6H4 (2k) | 28 | 3ak | 41 |
| 12 | 3-MeC6H4 (2l) | 48 | 3al | 79 |
| 13 | 4-MeC6H4 (2m) | 10 | 3am | 95 |
| 14 | 3-MeOC6H4 (2n) | 8 | 3an | 90 |
| 15 | 4-MeOC6H4 (2o) | 6 | 3ao | 98 |
| 16 | 2-Thiophenyl (2p) | 0.75 | 3ap | 95 |
| 17 | 2-Naphthyl (2q) | 2 | 3aq | 98 |
| 18 | N-Boc-3-indole (2r) | 24 | 3ar | 72 |
| 19 | Et (2s) | 72 | 3as | NRc |
As shown in Table 3, a series of functionalized ketones with variations of the R group were examined under the optimal reaction conditions. The results showed that no matter what the electronic properties or the substitution positions of the substituents, such as F-, Cl-, Br-, Me-, MeO-, and –NO2 substituted enones at the benzene ring, the reactions proceeded smoothly to afford the desired 1,2,3,6-tetrahydropyridine derivatives with good to excellent yields (entries 1–12). However, the MeO and NO2 substituted substrates required longer times to finish the reaction (entries 10–12). In addition, the 2-thiophenyl and 2-naphthyl modified enones underwent the [5+1] annulation reaction to produce the corresponding products in excellent yields (entries 13–14). To our delight, aliphatic enone 1s underwent this reaction to afford the desired product 3sa in 73% yield (entry 15).
| Entry | R | t (h) | 3 | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise stated, all reactions were carried out with 1 (0.2 mmol), 2a (0.3 mmol), and PBu3 (0.04 mmol) in CH2Cl2 (2 mL) at rt. b Isolated yield. | ||||
| 1 | 2-FC6H4 (1b) | 4 | 3ba | 97 |
| 2 | 4-FC6H4 (1c) | 3 | 3ca | 90 |
| 3 | 2-ClC6H4 (1d) | 2 | 3da | 90 |
| 4 | 3-ClC6H4 (1e) | 3 | 3ea | 91 |
| 5 | 4-ClC6H4 (1f) | 3 | 3fa | 84 |
| 6 | 3-BrC6H4 (1g) | 5 | 3ga | 98 |
| 7 | 4-BrC6H4 (1h) | 5 | 3ha | 87 |
| 8 | 3,4-Cl2C6H3 (1i) | 1.5 | 3ia | 90 |
| 9 | 4-MeC6H4 (1j) | 5 | 3ja | 91 |
| 10 | 2-MeOC6H4 (1k) | 12 | 3ka | 98 |
| 11 | 3-MeOC6H4 (1l) | 12 | 3la | 98 |
| 12 | 4-NO2C6H4 (1m) | 36 | 3ma | 75 |
| 13 | 2-Thiophenyl (1n) | 12 | 3na | 93 |
| 14 | 2-Naphthyl (1o) | 3 | 3oa | 88 |
| 15 | Me (1s) | 16 | 3sa | 73 |
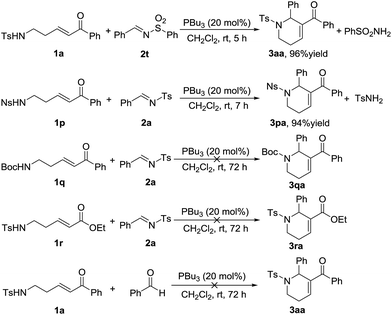
Following the substrate scope evaluation, we attempted to figure out how the reaction works. As a result, several control experiments were carried out under the optimal reaction conditions (Scheme 2). In the presence of PBu3, treatment of enone 1a with N-sulfonylimine 2t afforded the annulation product 3aa in 96% yield together with benzenesulfonamide. In contrast, treatment of enone 1p with imine 2a afforded the annulation product 3pa in 94% yield together with p-toluenesulfonamide. By comparison, δ-Boc-amido-substituted enones did not undergo annulation in the presence of phosphine. Meanwhile, when the benzoyl group was replaced by an ester group, the reaction did not work either. Therefore, the acidity of the substituted amine group at the δ-position of the ketone probably has a remarkable impact on the reaction process, and so does the benzoyl group. Replacement of the imine substrate with benzaldehyde did not yield the desired annulation product (Scheme 2). These results demonstrated that the amino-group in the annulation product comes from the enone substrate.
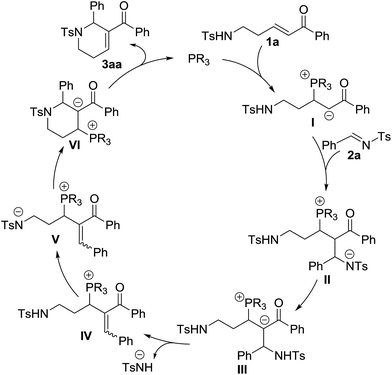
On the basis of the results obtained, a plausible mechanism was proposed (Scheme 3).1 Nucleophilic addition of the phosphine catalyst to enone 1a produces a zwitterionic intermediate I, which then performs another nucleophilic addition to imine 2a to afford the intermediate II. The intermediate III generated from the intermediate IIvia an intramolecular proton transfer eliminates a 4-methylbenzenesulfonamide anion to produce the intermediate IV. Through the abstraction of a proton, the intermediate IV is transformed into the intermediate V. Subsequent intramolecular nucleophilic addition furnishes annulation to form the intermediate VI, which regenerates the phosphine catalyst to give the final annulation product 3aa.
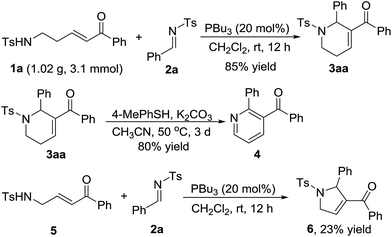
As indicated in Scheme 4, a gram-scale preparation of the product 3aa was carried out. 1.02 g of enone 1a (3.1 mmol) reacted with N-sulfonylimine 2a (1.22 g, 4.7 mmol) under the optimal reaction conditions to give 1,2,3,6-tetrahydropyridine derivative 3aa in 85% yield. Treatment of 3aa with 4-methylbenzenethiol and K2CO3 in air provided a good yield of the pyridine derivative 4via dehydrogenation aromatization. Further exploration on the variety of the sulfonamido-substituted enone indicated that enone 5, which is a homologue of enone 1a, could work as a C4 synthon to perform the [4+1] annulation reaction to give 2,5-dihydro-1H-pyrrole derivative 6 in 23% yield (Scheme 4).
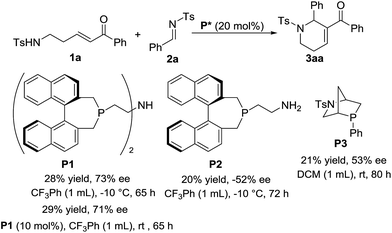
As shown in Scheme 5, the asymmetric version of the reaction was also investigated. To our delight, with the use of chiral phosphine P1 as the catalyst and CF3Ph as the solvent, the [5+1] annulation of enone 1a with N-sulfonylimine 2a worked at −10 °C for 65 h to give chiral product 3aa in 28% yield with up to 73% ee. When we decreased the amount of P1 to 10 mol%, the reaction worked at rt to give the product in 29% yield and 71% ee, which is similar to the result from the reaction using 20 mol% of the catalyst at low temperature. With the use of chiral phosphines P2 or P3 as the catalyst, moderate enantiomeric excesses were obtained. Unfortunately, a variety of attempts to improve enantioselectivity failed.
Conclusions
In summary, we have developed a phosphine-catalyzed [5+1] annulation of δ-sulfonamido-substituted enones with N-sulfonimines to prepare tetrahydropyridines with good to excellent yields. The reaction has broad substrate scope for both enones and N-sulfonimines. A plausible mechanism was proposed according to the results of control experiments. In addition, the reaction on the gram-scale worked well and further transformation of the product provided the pyridine derivative. The asymmetric version of the model [5+1] annulation reaction was also investigated, and up to 73% ee was achieved.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the NSFC (21372256 and 21572264) and the opening foundation of the Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University (No. 2014GDGP0103).Notes and references
- For selected reviews, see: (a) A. Marinetti and A. Voituriez, Synlett, 2010, 174 CrossRef CAS; (b) S. Wang, X. Han, F. Zhong, Y. Wang and Y. Lu, Synlett, 2011, 2766 CAS; (c) Q. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (d) Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (e) Y. Wei and M. Shi, Chem. Rev., 2013, 113, 6659 CrossRef CAS PubMed; (f) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (g) Y. Xiao, Z. Sun, H. Guo and O. Kwon, Beilstein J. Org. Chem., 2014, 10, 2089 CrossRef PubMed; (h) P. Xie and Y. Huang, Org. Biomol. Chem., 2015, 13, 8578 RSC; (i) Y. Xiao, H. Guo and O. Kwon, Aldrichimica Acta, 2016, 49, 3 CAS; (j) T. Wang, X. Han, F. Zhong, W. Yao and Y. Lu, Acc. Chem. Res., 2016, 49, 1369 CrossRef CAS PubMed; (k) W. Li and J. Zhang, Chem. Soc. Rev., 2016, 45, 1657 RSC; (l) Y. Wei and M. Shi, Org. Chem. Front., 2017, 4, 1876 RSC; (m) H. Li and Y. Lu, Asian J. Org. Chem., 2017, 6, 1130 CrossRef CAS.
- C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS.
- For selected examples, see: (a) J. Szeto, V. Sriramurthy and O. Kwon, Org. Lett., 2011, 13, 5420 CrossRef CAS PubMed; (b) X. Han, W. Yao, T. Wang, Y. R. Tan, Z. Yan, J. Kwiatkowski and Y. Lu, Angew. Chem., Int. Ed., 2014, 53, 5643 CrossRef CAS PubMed; (c) Z. Gao, C. Wang, C. Yuan, L. Zhou, Y. Xiao and H. Guo, Chem. Commun., 2015, 51, 12653 RSC.
- For selected examples, see: (a) X. Meng, Y. Huang, H. Zhao, P. Xie, J. Ma and R. Chen, Org. Lett., 2009, 11, 991 CrossRef CAS PubMed; (b) R. Na, C. Jing, Q. Xu, H. Jiang, X. Wu, J. Shi, J. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard, H. Guo and O. Kwon, J. Am. Chem. Soc., 2011, 133, 13337 CrossRef CAS PubMed; (c) J. Zheng, Y. Huang and Z. Li, Org. Lett., 2013, 15, 5064 CrossRef CAS PubMed; (d) W. Yao, X. Dou and Y. Lu, J. Am. Chem. Soc., 2015, 137, 54 CrossRef CAS PubMed; (e) H. Ni, W. Yao, A. Waheed, N. Ullah and Y. Lu, Org. Lett., 2016, 18, 2138 CrossRef CAS PubMed.
- For selected examples, see: (a) A. Voituriez, A. Panossian, N. Fleury-Brégeot, P. Retailleau and A. Marinetti, J. Am. Chem. Soc., 2008, 130, 14030 CrossRef CAS PubMed; (b) H. Xiao, Z. Chai, C. Zheng, Y. Yang, W. Liu, J. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 4467 CrossRef CAS PubMed; (c) Y. Fujiwara and G. C. Fu, J. Am. Chem. Soc., 2011, 133, 12293 CrossRef CAS PubMed; (d) X. Han, Y. Wang, F. Zhong and Y. Lu, J. Am. Chem. Soc., 2011, 133, 1726 CrossRef CAS PubMed; (e) F. Zhong, X. Han, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2011, 50, 7837 CrossRef CAS PubMed; (f) X. Han, F. Zhong, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 767 CrossRef CAS PubMed; (g) X. Zhang and M. Shi, ACS Catal., 2013, 3, 507 CrossRef CAS; (h) C. E. Henry, Q. Xu, Y. C. Fan, T. J. Martin, L. Belding, T. Dudding and O. Kwon, J. Am. Chem. Soc., 2014, 136, 11890 CrossRef CAS PubMed; (i) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316 CrossRef CAS PubMed; (j) S. Y. Lee, Y. Fujiwara, A. Nishiguchi, M. Kalek and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 4587 CrossRef CAS PubMed; (k) M. Gicquel, Y. Zhang, P. Aillard, P. Retailleau, A. Voituriez and A. Marinetti, Angew. Chem., Int. Ed., 2015, 54, 5470 CrossRef CAS PubMed; (l) Y. Gu, P. Hu, C. Ni and X. Tong, J. Am. Chem. Soc., 2015, 137, 6400 CrossRef CAS PubMed; (m) D. Wang, G.-P. Wang, Y.-L. Sun, S.-F. Zhu, Y. Wei, Q.-L. Zhou and M. Shi, Chem. Sci., 2015, 6, 7319 RSC; (n) X. Han, W.-L. Chan, W. Yao, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2016, 55, 6492 CrossRef CAS PubMed; (o) M. G. Sankar, M. Garcia-Castro, C. Golz, C. Strohmann and K. Kumar, Angew. Chem., Int. Ed., 2016, 55, 9709 CrossRef CAS PubMed; (p) E. Li, H. Jin, P. Jia, X. Dong and Y. Huang, Angew. Chem., Int. Ed., 2016, 55, 11591 CrossRef CAS PubMed; (q) G. Zhan, M. L. Shi, Q. He, W.-J. Lin, Q. Ouyang, W. Du and Y.-C. Chen, Angew. Chem., Int. Ed., 2016, 55, 2147 CrossRef CAS PubMed; (r) W. Zhou, H. Wang, M. Tao, C. Zhu, T. Lin and J. Zhang, Chem. Sci., 2017, 8, 4660 RSC; (s) W. Yao, Z. Yu, S. Wen, H. Ni, N. Ullah, Y. Lan and Y. Lu, Chem. Sci., 2017, 8, 5196 RSC; (t) H. Ni, Z. Yu, W. Yao, Y. Lan, N. Ullah and Y. Lu, Chem. Sci., 2017, 8, 5699 RSC.
- For selected examples, see: (a) Y. S. Tran and O. Kwon, J. Am. Chem. Soc., 2007, 129, 12632 CrossRef CAS PubMed; (b) S. Castellano, H. D. G. Fiji, S. S. Kinderman, M. Watanabe, P. de Leon, F. Tamanoi and O. Kwon, J. Am. Chem. Soc., 2007, 129, 5843 CrossRef CAS PubMed; (c) Q. Zhang, L. Yang and X. Tong, J. Am. Chem. Soc., 2010, 132, 2550 CrossRef CAS PubMed; (d) F. Zhong, X. Han, Y. Wang and Y. Lu, Chem. Sci., 2012, 3, 1231 RSC; (e) S. Kramer and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 3803 CrossRef CAS PubMed; (f) Y. Gu, P. Hu, C. Ni and X. Tong, J. Am. Chem. Soc., 2015, 137, 6400 CrossRef CAS PubMed; (g) H. Liu, Y. Liu, C. Yuan, G.-P. Wang, S. F. Zhu, Y. Wu, B. Wang, Z. Sun, Y. Xiao, Q.-L. Zhou and H. Guo, Org. Lett., 2016, 18, 1302 CrossRef CAS PubMed; (h) H. Ni, X. Tang, W. Zheng, W. Yao, N. Ullah and Y. Lu, Angew. Chem., Int. Ed., 2017, 56, 14222 CrossRef CAS PubMed.
- (a) D. O’Hagan, Nat. Prod. Rep., 2000, 17, 435 RSC; (b) J. P. Michael, Nat. Prod. Rep., 2004, 21, 625 RSC; (c) W. Maison, in Highlights in Bioorganic Chemistry: Pipecolic acid derivatives, Wiley-VCH, Weinheim, 2004, p. 18 Search PubMed.
- X. F. Zhu, J. Lan and O. Kwon, J. Am. Chem. Soc., 2003, 125, 4716 CrossRef CAS PubMed.
- R. P. Wurz and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 12234 CrossRef CAS PubMed.
- (a) G. Zhao and M. Shi, Org. Biomol. Chem., 2005, 3, 3686 RSC; (b) A. Panossian, N. Fleury-Brégeot and A. Marinetti, Eur. J. Org. Chem., 2008, 3826 CrossRef CAS; (c) H. Xiao, Z. Chai, H. Wang, X. Wang, D. Cao, W. Liu, Y. Lu, Y. Yang and G. Zhao, Chem.–Eur. J., 2011, 17, 10562 CrossRef CAS PubMed.
- (a) Y. S. Tran and O. Kwon, Org. Lett., 2005, 7, 4289 CrossRef CAS PubMed; (b) D. Cruz, Z. Wang, J. Kibbie, R. Modlin and O. Kwon, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6769 CrossRef CAS PubMed; (c) R. A. Villa, Q. Xu and O. Kwon, Org. Lett., 2012, 14, 4634 CrossRef CAS PubMed; (d) G. A. Barcan, A. Patel, K. N. Houk and O. Kwon, Org. Lett., 2012, 14, 5388 CrossRef CAS PubMed.
- (a) Z. Shi, Q. Tong, W. W. D. Leong and G. Zhong, Chem.–Eur. J., 2012, 18, 9802 CrossRef CAS PubMed; (b) Z. Shi, P. Yu, T. P. Loh and G. Zhong, Angew. Chem., Int. Ed., 2012, 51, 7825 CrossRef CAS PubMed.
- (a) Z. Jin, R. Yang, Y. Du, B. Tiwari, R. Ganguly and Y. R. Chi, Org. Lett., 2012, 14, 3226 CrossRef CAS PubMed; (b) X. N. Zhang, G. Q. Chen, X. Dong, Y. Wei and M. Shi, Adv. Synth. Catal., 2013, 355, 3351 CrossRef CAS; (c) G. Wang, R. Rexiti, F. Sha and X. Y. Wu, Tetrahedron, 2015, 71, 4255 CrossRef CAS; (d) H. Wang, W. Zhou, M. Tao, A. Hu and J. Zhang, Org. Lett., 2017, 19, 1710 CrossRef CAS PubMed.
- H. Guo, Q. Xu and O. Kwon, J. Am. Chem. Soc., 2009, 131, 6318 CrossRef CAS PubMed.
- Crystallographic data for 3aq have been deposited with the Cambridge Crystallographic Data Centre as deposition number CCDC 1575011.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1575011. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc04515h |
| This journal is © The Royal Society of Chemistry 2018 |