Postsecondary chemistry curricula and universal design for learning: planning for variations in learners’ abilities, needs, and interests
Erin
Scanlon
 a,
Tamra
Legron-Rodriguez
b,
Jillian
Schreffler
c,
Elijah
Ibadlit
a,
Eleazar
Vasquez
c and
Jacquelyn J.
Chini
a,
Tamra
Legron-Rodriguez
b,
Jillian
Schreffler
c,
Elijah
Ibadlit
a,
Eleazar
Vasquez
c and
Jacquelyn J.
Chini
 *a
*a
aPhysics Department, University of Central Florida, USA. E-mail: jchini@ucf.edu
bChemistry Department, University of Central Florida, USA
cChild, Family, and Community Sciences Department, University of Central Florida, USA
First published on 2nd July 2018
Abstract
Federal legislation requires equitable access to education for all students at all levels, including in the postsecondary setting. While there have been a few studies in the chemistry education research literature base focused on how to support students with specific disabilities, this work seems to exist as a separate stream of research without direct impact on curriculum development and the overall community. This study focused on investigating how well three sets of general chemistry curricular materials support variations in students’ abilities, interests, and needs. To accomplish this, we compared the curricular materials with the Universal Design for Learning (UDL) framework, which describes steps to account for variations in ability among learners during curriculum development. The UDL framework is organized into three guidelines (multiple means of representation, action and expression, and engagement), further delineated by nine principles and thirty-one finer-grained checkpoints for designing courses. We looked for examples of enactment of the UDL checkpoints in a representative sample of activities. Across all three sets of curricular materials, only four of the thirty-one checkpoints were enacted in at least 75% of the activities, indicating high enactment. On the other hand, eleven of the checkpoints were enacted in less than 25% of the activities, indicating low enactment. Overall, there is much room for improvement in consistently providing support for learner variation within these general chemistry curricular materials. We argue that some of the burden of making curricular materials supportive of all students lies with curriculum developers and provide recommendations for improving support and accessibility.
1 Introduction
The chemistry education community has focused on using knowledge of how students learn chemistry to develop research-based teaching practices and materials. These practices typically involve student-centered, active learning (Cole, 2015), which has been shown to support student learning (Freeman et al., 2014). However, some sub-groups of learners have not been fully incorporated in the development of and research on such teaching practices and materials. For example, a 2013 editorial in the Journal of Chemical Education calls attention to the dearth of studies that investigate similarities, differences, and impacts of interventions on sub-groups of learners, specifically by race and ethnicity, sex, and major (Towns, 2013). The 2012 Discipline-Based Education Report calls attention to additional populations, such as students with disabilities and students for whom English is a second language (Singer et al., 2012). In fact, a number of chemistry education researchers have explored learning by students with diagnoses such as physical disabilities (Miner et al., 2001), learning disabilities (Compton et al., 2012; King-Sears et al., 2017), hearing impairments (Pagano et al., 2015; Pagano, 2017), and visual impairments (Minkara et al., 2015; Nepomuceno et al., 2016; Vitoriano et al., 2016). However, this work seems to exist as a separate stream of research without direct impact on curriculum development and the overall community.We argue for the use of alternative frameworks for considering variation among learners: Universal Design for Learning (UDL) and ability profiles. UDL provides a framework for developing flexible teaching strategies and materials that support diverse learning needs (CAST, 2011). “Ability profile” is a useful way to consider learner variation across multiple dimensions such as physical, cognitive, metacognitive, motivational, and affective (Azevedo, 2015; Thorius and Santamaria Graff, 2018). Some students, such as dominant language learners and those with diagnosed disabilities, may have uniquely large variations compared with their peers. More generally, all students vary across the multiple dimensions of ability, interest, and need. By using UDL to design curricular materials, support can be provided to all students.
A common misconception is that “UDL is just good teaching” (Edyburn, 2010, p. 38). Following this belief, one might expect that well-designed, research-based teaching strategies and materials will naturally enact many UDL strategies. Edyburn calls for researchers to “find ways to define and measure implementation of UDL in order to discern when it is being implemented and when it is not” (Edyburn, 2010, p. 38). We selected three sets of high quality chemistry learning materials to explore the extent to which their curricular materials enact strategies aligned with the UDL framework. In all cases, the curriculum developers focused on important aspects of student learning and did not express an intentional focus on UDL. Thus, this analysis serves as a test of the misconception that “UDL is just good teaching” and not of the overall strength of any of the selected curricula.
We use the case of students with disabilities to illustrate the need to provide embedded options and support so that all learners, who vary in abilities, needs, and interests, can engage with our curricular materials. Federal law mandates equitable access to postsecondary education for students with disabilities. Recent lawsuits highlight examples of inaccessible pedagogies and curricular materials that the reader likely finds familiar, such as classroom response systems (Zou, 2011) and online homework (Dudley v. Miami University). While the law only requires access options when a barrier is present for a specific student, some universities and instructors may decide to require fully accessible courses regardless of enrollment; for example, universities are discussing how to work together to vet technologies for accessibility (McKenzie, 2017). Additionally, in a recent settlement the U.S. Department of Justice has required a university to make all materials accessible, specifically requiring “the University will only purchase, develop or use technology and instructional materials that allow persons who are blind or who have other vision disabilities the equal opportunity to access, use, and avail themselves of such technology or instructional materials in as full, equal, and independent a manner as persons without disabilities” (Louisiana Tech University v. United States of America, 2013). If we want all students to participate in the scientific community, we must provide access in our courses in a manner that communicates that each student is an anticipated and welcome participant in our community.
1.1 Students with disabilities in postsecondary education
Enrollment of students with disabilities in postsecondary education has been increasing rapidly in recent decades. The percentage of students with disabilities participating in any type of postsecondary education (e.g., 4-year college, 2-year college or community college, and vocational, business, or technical school) increased from 26% in 1995 to 46% in 2005 (Newman et al., 2010). This has shifted the representation of students with disabilities in postsecondary education from 6% in 1995 to 11% in 2011 (Riccobono et al., 1997; Snyder and Dillow, 2015).In the United States, legislative mandates require that students with disabilities have equal access to postsecondary education. The reauthorized Individuals with Disabilities Education Improvement Act of 2004 requires secondary institutions to prepare students with disabilities for further education. Provisions in the Higher Education Opportunities Act of 2008 (PL 110-3145) further expanded postsecondary education opportunities for students with disabilities by developing and improving postsecondary programs and extending federal financial aid opportunities for students with disabilities to attend postsecondary institutions (Council for Exceptional Children, 2008).
Evidence suggests many students with disabilities in postsecondary education have difficulty completing their programs. Data from National Longitudinal Transition Study-2 (NLTS-2) showed students with disabilities had lower rates (34%) of four-year college completion than their nondisabled counterparts (51%; Newman et al., 2011). The completion rates of any postsecondary education by students with disabilities is more than 10% lower (41%) than for students without disabilities (52%; Newman et al., 2011).
Students with disabilities face unique challenges when transitioning to postsecondary education, as they are responsible for requesting and managing accommodations by self-disclosure (Finn et al., 2008; Newman and Madaus, 2015). A recent study found only 24% of college students who previously received special education services in high school also disclosed their disabilities to postsecondary schools (Cortiella and Horowitz, 2014). Students may not disclose their disability for a variety of reasons such as fearing disability-specific negative stereotypes, judgement by peers, or increased academic anxiety (Trammell, 2009), or may struggle to produce the documentation required to access services at the postsecondary level (Cortiella and Horowitz, 2014). Students with disabilities are frequently underprepared in self-advocacy and lack the compensation strategies (e.g., self-regulation and study skills) necessary to deal with large STEM courses which require more organizational, goal-setting, and executive function skills (Parker and Boutelle, 2009). Many students with disabilities may also lack effective communication skills to interact with professors or disability service staff in order to acquire or use accommodations to support their learning needs (Bae, 2007).
1.2 Faculty preparation to support students with disabilities
Although faculty interactions play a pivotal role in the success of students with disabilities, many instructors lack an understanding of the needs of students with disabilities, of the daily operations of disability services offices, and of inclusive instructional strategies to enhance students’ success (Vasek, 2005; Burgstahler and Moore, 2009; Behling and Linder, 2017). Most faculty members may only be aware of disability services through the accommodation notifications they receive from their institution's disabilities services office regarding a specific student. It is often not until faculty struggle with the accommodation needs of a student that faculty contact university disability services for guidance.Faculty attitudes are one of the major impediments to success in higher education for students with disabilities (Rao, 2004). Lack of knowledge about disabilities or of inclusive teaching strategies may unduly influence faculty perceptions and result in stereotyping or fear of lowering academic quality standards. In particular, faculty have been found to have more negative attitudes toward psychiatric and attention (e.g., attention deficit disorder or attention-deficit/hyperactivity disorder) disabilities than physical disabilities, which may be the result of less understanding of these specific disabilities (Hindes and Mather, 2007). Lombardi (2010) reported that when faculty have a greater knowledge about disabilities, they are more likely to hold positive attitudes toward students with disabilities in four-year colleges and universities.
Investigation of faculty knowledge has focused mostly on knowledge of legal requirements pertaining to students with disabilities in higher education, and some studies suggest faculty in higher education have limited knowledge of disability laws (Vasek, 2005; Vogel et al., 2008). Studies examining the areas needed for professional development at higher education institutions have routinely suggested faculty and staff need more opportunities to gain knowledge about disability and the best ways to create a more inclusive institutional environment. Similarly, researchers have found faculty give high ratings to the importance of program content aimed at increasing knowledge of the needs of students with disabilities and education on disability law and accommodations (Debrand and Salzberg, 2005), as well as topics on universal design instructional techniques (Cook et al., 2007, 2009). However, results from Behling and Linder (2017) provide evidence that faculty members find it difficult or unnecessary to attend informational trainings related to access or to the specific needs of students with disabilities. Moreover, the results suggest faculty members tend to think anything beyond providing basic accommodations to students is not their job (Behling and Linder, 2017).
The consensus of the literature suggests programs designed to improve attitudes towards students with disabilities should increase knowledge of laws, student needs, and resources available, and should give practical ideas and applications for accommodative teaching strategies, such as universal design (Wynants and Dennis, 2017). This literature also suggests that curriculum developers should not rely on individual faculty to adapt curricula to support students with disabilities.
1.3 Universal design for learning: framework and guidelines
Universal Design for Learning is a framework for designing and delivering flexible approaches to teaching and learning that address student diversity within the classroom context (CAST, 2011). By proactively planning for flexibility using instructional design concepts, pedagogical knowledge, and instructional technology, learning and teaching are made accessible for all students. The underlying principles of UDL provide developers and teachers with guidelines for designing and implementing instruction in a flexible manner, meeting the varying needs of learners (Rose et al., 2005), while improving the learning process for all students (He, 2014; Katz and Sokal, 2016; Navarro et al., 2016). The framework of UDL is described by three principles which can be summarized as multiple means of representing knowledge, multiple means for students to demonstrate their understanding, and multiple means of engaging students. These principles are underpinned by nine guidelines and thirty-one checkpoints, as outlined in Table 1.| Principle | Guideline | Checkpoint | Description |
|---|---|---|---|
| Provide multiple means of representation | Provide options for perception | 1.1 | Offer ways of customizing the display of information |
| 1.2 | Offer alternatives for auditory information | ||
| 1.3 | Offer alternatives for visual information | ||
| Provide options for language, mathematical expressions, and symbols | 2.1 | Clarify vocabulary and symbols | |
| 2.2 | Clarify syntax and structure | ||
| 2.3 | Support decoding of text, mathematical notation, and symbols | ||
| 2.4 | Promote understanding across languages | ||
| 2.5 | Illustrate through multiple media | ||
| Provide options for comprehension | 3.1 | Activate or supply background knowledge | |
| 3.2 | Highlight patterns, critical features, big ideas, and relationships | ||
| 3.3 | Guide information processing, visualization, and manipulation | ||
| 3.4 | Maximize transfer and generalization | ||
| Provide multiple means of action and expression | Provide options for physical action | 4.1 | Vary the methods for response and navigation |
| 4.2 | Optimize access to tools and assistive technologies | ||
| Provide options for expression and communication | 5.1 | Use multiple media for communication | |
| 5.2 | Use multiple tools for construction and composition | ||
| 5.3 | Build fluencies with graduated levels of support for practice and performance | ||
| Provide options for executive functions | 6.1 | Guide appropriate goal-setting | |
| 6.2 | Support planning and strategy development | ||
| 6.3 | Facilitate managing information and resources | ||
| 6.4 | Enhance capacity for monitoring progress | ||
| Provide multiple means of engagement | Provide options for recruiting interest | 7.1 | Optimize individual choice and autonomy |
| 7.2 | Optimize relevance, value, and authenticity | ||
| 7.3 | Minimize threats and distractions | ||
| Provide options for sustaining effort and persistence | 8.1 | Heighten salience of goals and objectives | |
| 8.2 | Vary demands and resources to optimize challenge | ||
| 8.3 | Foster collaboration and community | ||
| 8.4 | Increase mastery-oriented feedback | ||
| Provide options for self-regulation | 9.1 | Promote expectations and beliefs that optimize motivation | |
| 9.2 | Facilitate personal coping skills and strategies | ||
| 9.3 | Develop self-assessment and reflection | ||
1.4 Purpose
To summarize, the literature shows that students with disabilities are enrolling in postsecondary education at an increasing rate, that students with disabilities may not register with the disability services office at their institution, and that faculty may not know how or be willing to provide accommodations to students with disabilities in their courses. All students vary across multiple dimensions in the skills, needs, and interests they bring to learning. The challenges faced by students with disabilities are also faced to varying degrees by their classmates. Universal Design for Learning provides a way for curriculum developers to proactively prepare to support all students.The purpose of this study was to determine how well general chemistry curricular materials support students with varying abilities, needs, and interests through the lens of UDL. We did not examine the aspects of the curricular materials that are the hallmarks and focal points as intended by their developers. For example, the Chemistry, Life, the Universe, and Everything (CLUE; Cooper and Klymkowsky, 2017) curriculum presents the chemistry topics around four central themes in a radically different manner than more traditional texts. This is independent of the accessibility of the curricular materials which was the focus of this study. We selected three sets of curricular materials and looked for examples of the UDL checkpoints in a representative set of activities. This investigation was intended to determine the state of general chemistry courses’ support of the variations in learners’ abilities, needs, and interests as well as to make suggestions for future curricula. The research questions guiding this inquiry were:
(1) How do general chemistry curricula support the diversity of learners as measured by their level of enactment of Universal Design for Learning checkpoints?
(2) How can general chemistry curricula be modified to better support all students (and thereby enact more of the Universal Design for Learning checkpoints)?
2 Methodology
We selected three sets of curricular materials that were developed for general chemistry courses: Chemistry, Life, the Universe, and Everything (CLUE; Cooper and Klymkowsky, 2017); Mastering Chemistry (Mastering-SP; Mastering Chemistry, 2017) with an atoms-first textbook (specifically, Structure and Properties; Tro, 2018); and Chemistry: A Guided Inquiry (POGIL-CGI; Moog and Farrell, 2017). We selected these curricula because they were reformed and research-based curricula (e.g., POGIL-CGI and CLUE are discussed as exemplars of research-informed curricula in Cole, 2015). The Pearson Mastering platform is commonly used in introductory chemistry courses and the Structure and Properties book is among the most popular general chemistry texts selected to accompany the Mastering platform (personal communication, 10/17). None of the authors had previously used the selected curricula, so we contacted the developers and others close to the curriculum to gain access and familiarity with the curricula.2.1 Curricular materials
Below is a description of each set of curricular materials.Research on the implementation of the CLUE curriculum has shown that students enrolled in CLUE show “marked improvements” in drawing Lewis structures and in their ability to decode the information in the Lewis structures, as compared with a statistically equivalent group of students in a traditional chemistry class (Cooper et al., 2012). In a longitudinal comparison, students enrolled in a CLUE course made connections between structure and properties earlier than a matched cohort of students in a traditional chemistry class (Underwood et al., 2016). Also, students enrolled in a CLUE course more quickly understood the concept of inter-molecular forces and maintained this correct understanding longer than their non-CLUE peers (Williams et al., 2015). Overall, the research indicates that when enrolled in a CLUE course, students are more likely to understand traditionally difficult general chemistry topics and to maintain their understanding into future courses.
Little research has been published to date regarding the implementation of an atoms-first text compared to a traditional chemistry text. Esterling and Bartels (2013) found instructors need to gain experience with the atoms-first approach and may initially find fewer students passing the first and second quarters of the general chemistry series. With one year of instructor experience, the researchers found the passing rate had “more than reversed” in the first quarter, but that challenges persisted in the second quarter.
Mastering Chemistry is an online homework system with hundreds of activities, including quantitative problems, conceptual questions, and tutorials. Mastering Chemistry is designed around three learning principles: support learning via scaffolding; support knowledge retention; and customize content through adaptivity (Mastering Chemistry, 2017).
Eichler and Peeples (2013) found that students who used the Mastering Chemistry online homework system for an entire semester of general chemistry had an 11 point increase in their final exam scores when compared to a similar cohort of students who did not use an online homework system. In another study, 85% of health science students indicated that Mastering Chemistry was a useful tool in improving their understanding and 77% of the same students reported that the timely feedback provided by the system was helpful (Wahab and Thomas, 2015).
The POGIL pedagogy has been widely implemented in secondary and post-secondary settings and across many STEM disciplines, including chemistry, biochemistry, biology, computer science, engineering, and calculus. The effectiveness of POGIL has been assessed at a range of institutions of different class sizes and different courses. Moog et al. (2006) state that implementation of POGIL:
“generally results in lower student attrition from the courses using POGIL than courses using traditional methods (with attrition being considered earning a grade of “D” or “F” or withdrawing from the course), along with student content mastery that is at least as high or higher than that for students in comparable traditional sections” (p. 46).
Research has also demonstrated that inquiry-based instruction in chemistry has many advantages including improved Drop–Fail–Withdraw (DFW) rates (Farrell et al., 1999), improved test performance (Lewis and Lewis, 2005), and increased student confidence (Schroeder and Greenbowe, 2008).
2.2 Sampling of curricular components
Since our purpose was to explore how the postsecondary chemistry education community was supporting variation among chemistry learners, we selected curricular materials with varying curricular components to develop a fuller picture of the support provided by these various components. Each set of curricular materials is composed of curricular components such as homework problems, lecture slides, and textbooks. The types of curricular components included in our sample are shown in Fig. 1; percentages indicate how much of each curricular component was included in our sample. For example, 38 out of 62 (61%) of the POGIL-CGI textbook sections were included in our analysis. The percentages listed vary within a curriculum because not all of the curricular sections included each type of curricular component.The Chemistry: Guided Inquiry (CGI) book is composed of 60 activities for students to work through. The activities include a description of a chemical model, warm-up questions, critical thinking questions, problems, and exercises. This is the only curricular component in the POGIL-CGI curricular materials and was included in our sample.
Mastering-SP includes the atoms-first Chemistry: Structure and Properties textbook and the Mastering online homework system items. In addition to the text, the Chemistry: Structure and Properties textbook includes text, figures, solved example problems, and key concept videos to present information. The Mastering online homework system includes items that can be assigned to students, disaggregated by textbook section. The items come in multiple forms: tutorials, in which new information is presented for students as they answer questions; activities, which are like tutorials but with new information presented in video format; test bank questions, which are exam-style questions; reading questions, which are multiple choice questions focusing on content directly presented in the textbook; and end-of-chapter questions. All of these curricular components, except the test bank questions, were included in our analysis. We excluded test bank questions from our analysis because these are typically intended to assess learning instead of helping students learn.
CLUE includes a textbook, in-class worksheets, recitation assignments, lecture slides, and mock exams. The textbook has a traditional layout, but the information presented is markedly different than a traditional chemistry book (see the description of the CLUE curriculum is Section 2.1.1). The textbook also includes “questions to answer”, “questions to ponder”, and “questions for later” for students to work through. The in-class worksheets and recitation questions are intended to be used during class time and provide practice problems for students. The recitation questions are used in the traditional recitation part of the course and the worksheets are used during regular class time. The lecture slides are written to be used with a classroom response system (i.e., there are imbedded questions for students to respond to). The test bank includes at least three exams for each of the two semesters of the course with multiple choice and short answer questions in each. All of these curricular components, except the mock exam questions, were included in our analysis.
2.3 Chemistry topics selected
Although all of the sets of curricular materials cover general chemistry content, the three selected curricula are each structured differently: CLUE is composed of 9 chapters with 54 sections; Mastering-SP is composed of 22 chapters with 200 sections; and POGIL-CGI is composed of 11 chapters and 60 activities. For consistency across curricula, the content-level delineations of the curricula will be called sections. Thus, the curricular materials were also sampled based on the chemistry topics to ensure that common general chemistry themes and topics were consistently included in our sample. We sampled common introductory chemistry topics, including: atomic structure, molecular structure and intermolecular forces, stoichiometry, gases, acids and bases, thermodynamics, equilibrium, kinetics, and oxidation–reduction. See Appendix A for a complete list of the themes and corresponding topics. Sections from the three curricula that cover these topics were included in our sample in order to ensure appropriate representation. After this sampling, 33 sections of CLUE, 51 sections of Mastering-SP, and 38 activities of POGIL-CGI were selected for analysis.2.4 Data analysis
We conducted a qualitative investigation of the enactment of the UDL checkpoints in the selected general chemistry curricular materials. Each individual UDL checkpoint was compared with each curricular component in each curricular section included in the sample; thus, the unit of analysis for the study was the curricular section. After the comparison process, the number of sections within a curricular component that had at least one example of enactment was documented. The analysis was conducted at the section level for each component of each set of curricular materials.We used the UDL Guidelines 2.0 Full-Text Representation (CAST, 2011) as the basis for our UDL checkpoint operationalizations. This document provides detailed descriptions of each principle, guideline and checkpoint, including a definition and examples of how the checkpoints could be implemented in a classroom, and includes an overall description of the concepts behind UDL. For example, the implementation examples for checkpoint 2.1 (Clarify vocabulary and symbols) include pre-teaching vocabulary and symbols, highlighting how complex terms are composed of simpler components, and embedding support for understanding vocabulary and symbols in text through means such as hyperlinks to dictionaries. A full list of the operationalizations for each checkpoint are included in Appendix D.
Some of the UDL checkpoints focus on aspects of how a classroom is run that would not appear in a written text. For example, we would not expect UDL checkpoint 7.3 (Minimize threats and distractions) to appear in the student version of the written activities because it focuses on classroom culture rather than tasks the students are asked to complete. We made no assumptions about how the curricula were implemented in a classroom setting unless the curricular materials (including instructor guides) specifically described details about how it should be implemented. Therefore, the selected curricula could be implemented in a classroom in a way that enacts more of the UDL framework than is shown here. In our analysis, we track the checkpoints most directly affected by this limitation; see the Sources of Low Enactment section for more information.
2.5 Reliability
We investigated the reliability of the data analysis to ensure our results reflect the actual prevalence of enactment of the UDL checkpoints in the curricular materials. There were two primary coders for this analysis: a chemistry education researcher (T. L. R.) and a physics education researcher (E. S.). A third researcher, an education graduate student (J. S.), served as a secondary rater for comparison. The coders were selected to enhance the validity of the study by including both chemistry and UDL experts in the data analysis process. In order to train all raters on the UDL checkpoint operationalizations the three raters read the UDL Guidelines 2.0 Full-Text Representation (CAST, 2011), coded at least one instance for each checkpoint, coded an activity individually, and then came together to discuss. After all three coders were trained on the UDL checkpoints and their operationalizations, each was assigned a set of sections of curricular materials (which include the curricular components listed in Fig. 1) to analyze. The assignments were made such that the chemistry and physics coders nearly evenly split the sections and that the education coder examined at least 10% of sections in common with the other two raters.The three raters then analyzed their assigned sections independently. Table 2 shows the number and percent of total textbook sections analyzed by each of the three raters. There were four possibilities for overlap in coding as shown under the Analyzed by Multiple Raters heading. At least 50% of sections in each curriculum were coded by two or more raters, and there was a minimum of 29% overlap between the physics and chemistry raters (including sections coded by all three raters). At least 13% of all textbook sections included were analyzed by all three raters.
| Total | Analyzed by one rater | Analyzed by multiple raters | |||||
|---|---|---|---|---|---|---|---|
| Chemist only | Physicist only | Chemist and physicist | Chemist and education | Physicist and education | All raters | ||
| CLUE | 33 | 6 (18%) | 7 (21%) | 0 (0%) | 5 (15%) | 5 (15%) | 10 (30%) |
| Mastering-SP | 51 | 7 (14%) | 7 (14%) | 14 (27%) | 8 (16%) | 8 (16%) | 7 (14%) |
| POGIL-CGI | 38 | 9 (24%) | 10 (26%) | 6 (16%) | 4 (11%) | 4 (11%) | 5 (13%) |
After the independent coding, the three raters met to discuss their findings. The discussion process was completed one curriculum at a time and the co-coded sections were discussed one UDL checkpoint at a time. When disagreements arose, the reasoning behind the raters' unalignment/alignment were discussed as related to the UDL implementation guide and examples. Typically, the disagreements were resolved by refining operationalizations of the UDL checkpoint due to unique features of each curriculum. If the checkpoint operationalization refinement affected the previously completed analysis, the refinements were implemented in the previous sections in order to maintain consistency.
After discussion, most instances were agreed upon by all three raters. During the discussion process, disciplinary differences were addressed and operationalizations were refined to account for the specifics of each curricula. The physics coder's responses were included in the analysis for the few instances where disagreement persisted after discussion. Table 3 shows the results of the inter-rater reliability process as described by Gwet's AC1 metric (Gwet, 2002).
| UDL principle | Before discussion | After discussion | ||||
|---|---|---|---|---|---|---|
| Edu. & Phys. | Edu. & Chem. | Chem. & Phys. | Edu. & Phys. | Edu. & Chem. | Chem. & Phys. | |
| † Indicates at least substantial agreement between raters. | ||||||
| 1 | 0.64† | 0.63† | 0.61† | 0.97† | 0.99† | 0.96† |
| 2 | 0.69† | 0.55 | 0.54 | 1† | 1† | 0.99† |
| 3 | 0.44 | 0.23 | 0.69† | 0.99† | 0.94† | 0.99† |
| 4 | 0.73† | 0.76† | 1† | 1† | 1† | 1† |
| 5 | 0.41 | 0.48 | 0.80† | 1† | 0.97† | 0.99† |
| 6 | 0.68† | 0.42 | 0.47 | 0.97† | 0.96† | 0.98† |
| 7 | 0.80† | 0.88† | 0.90† | 0.99† | 1† | 1† |
| 8 | 0.64† | 0.88† | 0.90† | 1† | 1† | 0.99† |
| 9 | 1† | 1† | 1† | 1† | 1† | 1 |
Unlike other kappa statistics, Gwet's AC1 metric does not depend on the prevalence of the trait under study, so it is more stable in cases when a code is observed very frequently or very infrequently. Gwet's AC1 ranges from 0 (meaning no agreement) to 1 (meaning perfect agreement). AC1 values between 0.61 and 0.8 indicate substantial agreement, and values greater than 0.81 indicate almost perfect agreement (Gwet, 2014). Before discussion, principles 1, 4, 7, 8, and 9 had at minimum substantial agreements between all three raters while principles 2, 3, 5, and 6 did not reach substantial agreement between all three raters. All principles after discussion had Gwet's AC1 values greater than 0.81, indicating almost perfect agreement (Gwet, 2014). Thus, we find substantial evidence to support the reliability of the data analysis after discussion.
3 Findings
We disaggregated our findings of enactment of the UDL checkpoints in each set of curricular materials by curricular component. These findings are summarized in Table 4. In this section, we discuss major findings across all three sets of curricular components.No quantitative measures of the extent of enactment of UDL currently exist in the literature base, so we operationalized three levels of enactment for this study. We define “high enactment” as when a minimum of 75% of at least one curricular component enacted a UDL checkpoint. For example, we identified examples of checkpoint 2.1 in more than 75% of the textbook sections and lecture slide sets analyzed for the CLUE curriculum, but not in the recitation and worksheet activities. Since the curricular components work together to support student learning, we classify this type of enactment as “high.” Four checkpoints (1.1, 2.1, 2.5, and 3.2) showed consistently high enactment across all three sets of curricular materials, as shown in Table 4. Similarly, we define “some enactment” as when at least 25% of at least one curricular component aligned with the UDL checkpoint. Checkpoints 1.3, 3.4, 6.2, and 6.3 consistently showed some to high enactment across the three sets of curricular materials. Finally, we define “low enactment” as when less than 25% of all curricular components aligned with the UDL checkpoint. Eleven checkpoints (2.3, 2.4, 4.2, 5.2, 7.1, 7.3, 8.1, 8.2, 9.1, 9.2, and 9.3) showed low enactment across all three sets of curricular materials. Checkpoints 1.2, 2.2, 3.1, 3.3, 5.1, 7.2, and 8.3 showed mixed enactment across the sets of curricular materials. Additionally, Mastering-SP showed uniquely high enactment of checkpoints 4.1, 5.3, 6.1, 6.4, and 8.4. These detailed findings are listed in Tables 6, 7, and 8 in Appendix B.
| Enactment | UDL checkpoint |
|---|---|
| The Low-IV column indicates low enactment due to implementation variability. * Indicates uniquely high in Mastering-SP. | |
| High | 1.1 Offer ways of customizing the display of information |
| 2.1 Clarify vocabulary and symbols | |
| 2.5 Illustrate through multiple media | |
| 3.2 Highlight patterns, critical features, big ideas, and relationships | |
| Some | 1.3 Offer alternatives for visual information |
| 3.4 Maximize transfer and generalization | |
| 6.2 Support planning and strategy development | |
| 6.3 Facilitate managing information and resources | |
| Low | 2.3 Support decoding of text, mathematical notation, and symbols |
| 2.4 Promote understanding across languages | |
| 7.1 Optimize individual choice and autonomy | |
| 8.1 Heighten salience of goals and objectives | |
| 8.2 Vary demands and resources to optimize challenge | |
| 9.1 Promote expectations and beliefs that optimize motivation | |
| 9.3 Develop self-assessment and reflection | |
| Low-IV | 4.2 Optimize access to tools and assistive technologies |
| 5.2 Use multiple tools for construction and composition | |
| 7.3 Minimize threats and distractions | |
| 9.2 Facilitate personal coping skills and strategies | |
| Mixed | 1.2 Offer alternatives for auditory information |
| 2.2 Clarify syntax and structure | |
| 3.1 Activate or supply background knowledge | |
| 3.3 Guide information processing, visualization, and manipulation | |
| 4.1 Vary the methods for response and navigation* | |
| 5.1 Use multiple media for communication | |
| 5.3 Build fluencies with graduated levels of support for practice and performance* | |
| 6.1 Guide appropriate goal-setting* | |
| 6.4 Enhance capacity for monitoring progress* | |
| 7.2 Optimize relevance, value, and authenticity | |
| 8.3 Foster collaboration and community | |
| 8.4 Increase mastery-oriented feedback* | |
4 Discussion
Here, we describe ways that the curricula did or could enact selected UDL checkpoints. While some checkpoints are not discussed, an example for each is provided in Appendix C.4.1 High enactment
In this section, we discuss the UDL checkpoints that had consistently high enactment across all three sets of curricular materials.Solely providing curricula in a digital format does not guarantee the curricula will be accessible because not all digital formats are equally accessible. In the present study, we have not analyzed the specific accessibility features of the digital formats. Guidance on creating accessible digital content is provided by the Web Content Accessibility Guidelines (https://www.w3.org/WAI/intro/wcag).
“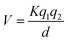 . The potential energy (V) of two stationary charged particles is given by the equation above, where q1 and q2 are the charges on the particles (e.g., −1 for an electron), d is the distance between the particles, and k is a positive-valued proportionality constant.” (POGIL, p. 16)
. The potential energy (V) of two stationary charged particles is given by the equation above, where q1 and q2 are the charges on the particles (e.g., −1 for an electron), d is the distance between the particles, and k is a positive-valued proportionality constant.” (POGIL, p. 16)
In this excerpt all of the variables were defined, supporting students who have not yet mastered these concepts. Learners coming from different cultural and lexical backgrounds and with non-dominant native languages can have particular difficulty accessing information when words and symbols are not clearly defined.
“Atoms are neither created nor destroyed when chemical reactions take place. Therefore, the number of atoms of each element must be identical on the reactant (left) and product (right) sides of a balanced chemical reaction. Such a chemical equation is said to be atom balanced.” (POGIL, p. 171)
In this excerpt, the key vocabulary was bolded, drawing students’ attention to the term and indicating its importance. While experts can read through a text and easily identify the important features and relationships, novices typically struggle with this task (CAST, 2011). Adding support and scaffolding to assist students with the identification of the key features and relationships will especially benefit students with less background knowledge.
4.2 Some enactment
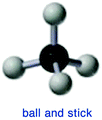
In this section, UDL checkpoints that had at least some enactment will be discussed. We defined a lenient threshold (a minimum of 25% of the sections within at least one curricular component demonstrating the UDL checkpoint) since the curricula were not designed to align with the UDL framework.“The ball-and-stick model of methane shows the central carbon (black ball) attached to four hydrogens (white balls) by sticks that represent the bonds between the atoms. Although this model is probably the easiest to visualize, it is misleading because it could give the impression that bonds are like sticks holding the atoms together. It also does not represent either the actual volume occupied by the molecule or its electrostatic surface features.” (CLUE, pp. 81–82)†
This example shows a description of the image in the text, offering an alternative for the visual information. Students with difficulties interpreting diagrams or those who are visually impaired can be disproportionately affected by the presentation of information solely in a visual format.
The curricular materials could have improved enactment of this checkpoint by providing links between material learned in class and new situations as well as by assisting students with linking previously learned material with new content. Future curricula should both incorporate multiple opportunities for students to review and practice what they have learned in class, and link course material with new content and with new situations to allow for more robust generalization and transfer.
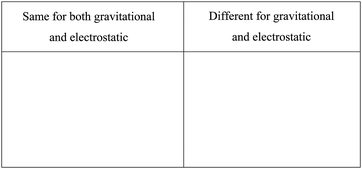
“For gravitational and electrostatic forces: Discuss what aspects are the same for each force and what are different. Be sure to indicate how the forces are different. (CLUE, Recitation 2, p. 1)”
The recitation problem provides a table to assist students in categorizing their information, reducing the executive function load on students.
4.3 Low enactment
One common theme is that the multiple means of engagement guideline has low enactment across all three sets of curricular materials. In fact, checkpoints 7.1 (Optimize individual choice and autonomy), 8.1 (Heighten salience of goals and objectives), 8.2 (Vary demands and resources to optimize challenge), 9.1 (Promote expectations and beliefs that optimize motivation), and 9.3 (Develop self-assessment and reflection) were not enacted at all in any of the materials we analyzed. Overall, variations in the ways in which students are engaged in the learning process are not encouraged and/or allowed. The curricula do not attend to students' affect and variations in interest, motivation, self-regulation, and perceived challenge. (See the Suggestions for Modifications and Future Curricula section for more information about how to address these issues in a classroom setting.)We identified two discrete reasons that the UDL checkpoints had low enactment in the curricular materials: (1) low enactment that occurred because curricular materials did not best serve all students and (2) low enactment due to implementation variability, or variation in how instructors could implement the curricula. For example, in order to enact UDL checkpoint 7.3 (Minimize threats and distractions) an instructor would need to create a welcoming classroom environment that promotes inclusivity, involve all students in whole class discussions, or vary the level of sensory stimulation. These types of classroom practices were not articulated in the written components of the curricula which were examined in this study. When such practices are not addressed in the written instructional materials (e.g., instructor guides), individual instructors are left to identify and address these areas or to tacitly not address them, leaving room for variation across implementations. Returning to our example, checkpoint 7.3 (Minimize threats and distractions) manifests in the classroom culture. We encourage curriculum developers to include information related to these checkpoints in instructor guides to support instructors, as discussed in the Limitations section. We classified checkpoints 4.2 (Optimize access to tools and assistive technologies), 5.2 (Use multiple tools for construction and composition), 7.3 (Minimize threats and distractions), and 9.2 (Facilitate personal coping skills and strategies) as subject to implementation variability and expect that these checkpoints would not typically be addressed in the written curricular components.
4.4 Mixed enactment
There were a few UDL checkpoints that had mixed enactment, which means that the enactment was not consistent across all three sets of curricula. Checkpoints 1.2 (Offer alternatives for auditory information), 3.1 (Activate or supply background knowledge), and 7.2 (Optimize relevance, value, and authenticity) had at least some enactment in the CLUE and Mastering-SP sets of curricular materials. Checkpoint 5.1 (Use of multiple media for communication) had at least some enactment in the CLUE and POGIL-CGI sets of curricular materials. Checkpoint 2.2 (Clarify syntax and structure), 3.3 (Guide information processing, visualization, and manipulation), and 8.3 (Foster collaboration and community) had at least some enactment of the Mastering-SP and POGIL-CGI sets of curricular materials. See Appendix C for examples of enactment of these checkpoints.It may seem surprising that checkpoints 3.1 (Activate or supply background knowledge) and 8.3 (Foster collaboration and community) only had mixed enactment across the sets of curricular materials. Checkpoint 3.1 was only sparsely enacted in the POGIL book because it did not regularly link concepts to previously learned material, use advanced organizers, bridge concepts with analysis, or make explicit cross-curricular connections. However, the Mastering-SP online homework end-of-chapter items enacted checkpoint 3.1 by, for example, referencing the textbook section in which the question's content was covered. Similarly, the CLUE textbook would refer back to previously covered material through statements such as “Recall in the previous chapter…” These strategies allow the students to more easily assimilate the material into their existing knowledge structures and can particularly help lower barriers to success for learners who lack background knowledge or understanding of the relevance of the background knowledge they possess.
Checkpoint 8.3 (Foster collaboration and community) had mixed alignment because the POGIL-CGI and Mastering-SP curricular materials explicitly state that students should work in groups as they work through the given activities. The CLUE curricular materials do not explicitly require group work and thus did not enact this checkpoint. We only examined the written portions of the curricular materials and did not make assumptions about how the curricular materials would be implemented in the classroom. Thus, it is possible that a CLUE classroom would align with this checkpoint, but analyzing classrooms was beyond the scope of this study.
Although the POGIL-CGI and Mastering-SP curricular materials explicitly promoted group work, they did not discuss how this should manifest in the classroom. In the UDL guidelines, checkpoint 8.3 describes group work that is well structured to support students. Structuring group work can help students who have difficulties with social interactions or with disabilities that affect their ability to interact with others (e.g., autism spectrum disorder, social communication disorder).
4.5 Uniquely high enactment
The Mastering-SP curricular materials consistently enacted some of the UDL checkpoints that were rarely or never enacted in the other two sets of curricular materials. Specifically, Mastering-SP has uniquely high enactment of checkpoints 4.1 (Vary the methods for response and navigation), 5.3 (Build fluencies with graduated levels of support for practice and performance), 6.1 (Guide appropriate goal-setting), 6.4 (Enhance capacity for monitoring progress), and 8.4 (Increase mastery-oriented feedback). In the Mastering-SP curricular materials, we identified enactment of these principles in 100% of the sections for several material types, while we identified enactment of these principles in less than 5% of the sections in our samples from CLUE and POGIL-CGI.“Use molecular shape to determine polarity of a molecule.” (Tro, 2018)
By modeling for learners how to set appropriate goals, we can encourage and scaffold their goal-setting abilities.
“According to the collision model, why does increasing the temperature increase the rate of a reaction?
(A) Increasing the temperature increases the number of collisions that can occur with enough energy for the reaction to occur.
(B) Increasing the temperature causes more of the collisions to occur with the correct particle orientation for the reaction to occur.
(C) Increasing the temperature causes more particles to occupy the same amount of space which therefore increases the reaction rate.” (Conceptual Connection 14.1)
If a student chooses answer B the following feedback is given:
“Incorrect; Try Again. Increasing the temperature does not change the orientation of the collisions. You may want to review page 586.” (Conceptual Connection 14.1)
This feedback is not only timely, but it is also “substantive and informative rather than comparative or competitive” (CAST, 2011). Giving mastery-oriented feedback to students is a more productive form of assessment and can be “particularly important for learners whose disabilities have been interpreted, by either themselves or their caregivers, as permanently constraining and fixed” (p. 32). Mastery-oriented feedback not only benefits students with disabilities but benefits all students.
5 Suggestions for modifications and future curricula
In this section, suggestions for modifications to curricula and for future curricula will be made such that the curricula align with the UDL guidelines and better support the range of learners.5.1 Add diagrams with text
Visual alternatives for key information should be provided in chemistry curricula to support students who have difficulties with deciphering and understanding textual information. All three of the curricula aligned with checkpoint 2.5 (Illustrate through multiple media) and therefore provide examples for future curricula. When more diagrams accompany the text, more opportunities are present for students to understand the material. However, pictures should also be accompanied by text descriptions such that students with difficulties in understanding and interpreting visual information or with visual impairments can also have equitable access to the information. This would allow the curriculum to align with checkpoint 1.3 (Offer alternative for visual information). Curricular accessibility is enhanced by providing different presentations of the material so that students can choose the methods that maximize their strengths.5.2 Promote understanding across languages
We did not find examples of enactment of checkpoint 2.4 (Promote understanding across languages). Accessibility of the digital components of curricula would be enhanced for non-dominant language speakers through the simple addition of a link to key terms in non-dominant language dictionaries. The Mastering-SP curriculum does provide links to definitions of key terms, but only in the dominant language (in this case English). Providing definitions of discipline-specific key terms makes the curricula more accessible for students who have difficulties with the dominant language or who are more fluent in a different language. Thus, future chemistry curricula should not only be available digitally (thereby also aligning with checkpoint 1.1 – Offer ways of customizing the display of information), but should also provide access to key, discipline-specific vocabulary in multiple languages.5.3 Add multiple means of engagement
Curriculum developers should take into account student affect when designing chemistry curricula. Motivating students to meaningfully engage with the curricula as well as sustaining their interest and persistence over the course of a semester is just as important as presenting the content to students. However, across all three curricula, we observed few instances of enactment of the checkpoints related to this principle. Future curricula could address this by providing students options of the level of challenge, types of rewards, or tools used for assignments; making the course content personalized to students’ cultures, lives, or personal interests; creating a welcoming and accepting classroom culture; and emphasizing process and improvement in achieving goals, encouraging and building coping skills.6 Limitations
One limitation to our analysis is that it was based solely on the written curricular materials. We did not observe the classes in which the curricular materials were implemented nor did we talk with instructors about their accessibility practices. Typically, instructors alter their curricular materials as students with varying needs enroll in their courses in order to comply with their school's disability services office and support the students’ learning. These accommodations were not captured in our analysis. While this limits the statements we can make about the accessibility of the entire class, looking at the curricular materials provides a perspective on how much emphasis we as a community place on ensuring equitable access for our students. We argue that useful accommodations should be documented as part of the instructor guide for curricula to support faculty in creating accessible classes.Another limitation is that the UDL framework does not cover all of the accommodation needs for all students who could conceivably enroll in a general chemistry course. Each student is unique and has a different ability profile. The UDL framework provides a starting point to create more accessible and accommodating classrooms for all students. However, it would be challenging to enact all of the UDL checkpoints at once.
A final possible limitation is that each set of curricular materials was composed of different components (e.g., homework questions, textbook, lecture slides), which does not allow for comparative analysis. However, our purpose in this study was not to compare general chemistry curricula to one another, but rather to map the current landscape of accessibility of general chemistry curricular materials.
7 Implications
Overall, there is much room for improvement in consistently providing support for learner variation within our curricular materials. Specific attention is needed to provide multiple means for recruiting and sustaining student engagement. However, we note that Mastering-SP had uniquely high enactment of several UDL checkpoints. This curriculum is developed by a for-profit corporation that has specifically focused on accessibility, as evidenced in the accessibility guidelines that were developed for the Mastering platform (found here: http://wps.pearsoned.com/accessibility/115/29601/7577872.cw/). On one hand, we note that this focus on accessibility led to more accessible curricular materials. On the other hand, we note that, as a for-profit company, the curriculum developers for Mastering-SP had the resources to work with accessibility experts.We argue that some of the burden for providing all students with equal access to the curriculum falls on the curriculum developers. While a campus disability services office provides many accommodations, those are only available to students who can navigate the requirements to access these accommodations and who self-identify. Creating more inclusive curricula benefits all students. Research indicates that many postsecondary STEM faculty do not have the knowledge and skills to make the necessary accommodations to support all student needs. Also, it does not make sense for individual faculty to repeatedly make the same accommodations or for students to lose access to high quality curricula because of its inaccessibility. Edyburn (2010) states:
“A fundamental question that has yet to be addressed is whether or not the demands of daily instruction will allow teachers to function effectively as instructional designers. That is, are teachers the principal stakeholders as they design and deliver instruction in accordance with UDL principles? Or, is UDL a task for developers who make instructional products?” (p. 37)
We argue effort should continue to support faculty, staff, and administrators to become knowledgeable of and fluent in ways to accommodate learner variation in skills, interests and needs. At the same time, accessibility options and implementation guidelines should be built into high quality curricular materials to support faculty in this process. Thus, we also call on funding agencies to encourage curriculum developers to make their curricula accessible and to provide the resources needed to partner with accessibility experts.
Ultimately, who we are prepared to teach communicates who we expect to participate in the chemistry community. Instructors engage in a balancing act when they select a curriculum, considering possible conflicting factors such as content coverage, instructional design, and cost to their students. We argue that accessibility is another dimension that we must consider and suggest the UDL framework as a useful tool for curriculum developers to make progress towards enhanced accessibility.
8 Future work
Further investigation is required in order to better understand the digital components of the curricula. Even if curricular components are available digitally, there are some digital formats that are more accessible than others. There is an online website accessibility checker called the Web Accessibility Evaluation Tool (WAVE) freely available to scan websites and other digital documents for multiple facets of accessibility including compatibility with screen reader software, visual accessibility, and structural elements of the website that aid in accessibility. Future work should investigate the digital components of curricula to determine how well the existing digital curricular components are serving students.Helpful resources
For more information about the Universal Design for Learning framework see http://www.cast.org or http://www.udlcenter.org.Conflicts of interest
There are no conflicts to declare.Appendix A: Common general chemistry topics included in our sample
Table 5.| Theme | Topic |
|---|---|
| Atomic structure | Atomic numbers, atomic mass, atomic structure, atomic orbitals, electron configurations, Coulomb's law, light and the electromagnetic spectrum, wave-particle nature of matter |
| Molecular structure and intermolecular forces | Covalent bonding, ionic bonding, metallic bonding, dipole moments, electronegativity, intermolecular forces, Lewis structures, molecular shapes, orbital hybridization, phase diagrams |
| Stoichiometry | Mole concept, empirical formulas, writing and balancing chemical equations, limiting reagents, molarity |
| Gases | Ideal gas law, mixtures of gases, partial pressures |
| Acids and bases | Acids and bases, acid and base dissociation, pH, buffers |
| Thermodynamics | Enthalpy of atom combinations, enthalpy changes of chemical reactions, entropy changes in chemical reactions |
| Equilibrium | Equilibrium constants, reaction quotient, solubility product constants |
| Kinetics | Rates of chemical reactions, integrated rate law, effect of temperature on reaction rates, effect of temperature on rate constants |
| Oxidation–reduction | Redox reactions, oxidation numbers, electrochemical cells |
| Miscellaneous | Radioactivity, organic chemistry, coordination compounds |
Appendix B: percent alignment data for each curricula disaggregated by curricular component
The tables below show the percent alignment for each curricular component with each of the UDL checkpoints. The N listed in the column headers refer to the number of each type of curricular component that were analyzed in this study. N varies across curricular component in a single curriculum because not all sections included each type of curricular component. For example, for CLUE, we analyzed 33 total sections, all of which had a textbook section. But only 10 had recitations, 20 had worksheets, and 24 had lecture slides provided (Tables 6–8).| UDL checkpoints | Textbook (N = 33) | Recitation (N = 10) | Worksheet (N = 20) | Lecture slides (N = 24) | All (N = 33) | Enactment |
|---|---|---|---|---|---|---|
| Bolding indicates at least 75% of the curricular material aligns with UDL checkpoint. In the last column, H represents high enactment, S represents some enactment, and L represents low enactment. | ||||||
| 1.1 | 100 | 100 | 100 | 100 | 100 | H |
| 1.2 | 15.2 | 0 | 0 | 38 | 30 | S |
| 1.3 | 39.4 | 10 | 0 | 21 | 48 | S |
| 2.1 | 75.8 | 0 | 0 | 83 | 76 | H |
| 2.2 | 21.2 | 0 | 0 | 13 | 24 | L |
| 2.3 | 9.1 | 0 | 0 | 0 | 9.1 | L |
| 2.4 | 0 | 0 | 0 | 0 | 0 | L |
| 2.5 | 78.8 | 20 | 0 | 100 | 97 | H |
| 3.1 | 48.5 | 20 | 5 | 46 | 39 | S |
| 3.2 | 24.2 | 40 | 20 | 75 | 61 | H |
| 3.3 | 6.1 | 0 | 0 | 4.2 | 9.1 | L |
| 3.4 | 54.5 | 0 | 0 | 0 | 55 | S |
| 4.1 | 0 | 0 | 0 | 0 | 0 | L |
| 4.2 | 0 | 0 | 0 | 0 | 0 | L |
| 5.1 | 45.5 | 70 | 85 | 8.3 | 70 | H |
| 5.2 | 0 | 0 | 5 | 0 | 3 | L |
| 5.3 | 0 | 0 | 0 | 4.2 | 3 | L |
| 6.1 | 0 | 0 | 0 | 0 | 0 | L |
| 6.2 | 6.1 | 30 | 20 | 8.3 | 24 | S |
| 6.3 | 0 | 50 | 20 | 0 | 21 | S |
| 6.4 | 0 | 0 | 0 | 0 | 0 | L |
| 7.1 | 0 | 0 | 0 | 0 | 0 | L |
| 7.2 | 12.1 | 0 | 0 | 38 | 30 | S |
| 7.3 | 0 | 0 | 0 | 0 | 0 | L |
| 8.1 | 0 | 0 | 0 | 0 | 0 | L |
| 8.2 | 0 | 0 | 0 | 0 | 0 | L |
| 8.3 | 9.1 | 0 | 0 | 4.2 | 12 | L |
| 8.4 | 0 | 0 | 0 | 0 | 0 | L |
| 9.1 | 0 | 0 | 0 | 0 | 0 | L |
| 9.2 | 0 | 0 | 0 | 0 | 0 | L |
| 9.3 | 0 | 0 | 0 | 0 | 0 | L |
| UDL checkpoints | Book (N = 38) | Enactment |
|---|---|---|
| Bolding indicates at least 75% of the curricular material aligns with UDL checkpoint. In the last column, H represents high enactment, S represents some enactment, and L represents low enactment. | ||
| 1.1 | 100 | H |
| 1.2 | 0 | L |
| 1.3 | 67.9 | S |
| 2.1 | 84.2 | H |
| 2.2 | 34.2 | S |
| 2.3 | 10.5 | L |
| 2.4 | 0 | L |
| 2.5 | 84.2 | H |
| 3.1 | 23.7 | L |
| 3.2 | 86.8 | H |
| 3.3 | 100 | H |
| 3.4 | 78.9 | H |
| 4.1 | 0 | L |
| 4.2 | 0 | L |
| 5.1 | 55.3 | S |
| 5.2 | 2.6 | L |
| 5.3 | 0 | L |
| 6.1 | 0 | L |
| 6.2 | 94.7 | H |
| 6.3 | 52.6 | S |
| 6.4 | 0 | L |
| 7.1 | 0 | L |
| 7.2 | 15.8 | L |
| 7.3 | 0 | L |
| 8.1 | 0 | L |
| 8.2 | 0 | L |
| 8.3 | 100 | H |
| 8.4 | 0 | L |
| 9.1 | 0 | L |
| 9.2 | 0 | L |
| 9.3 | 0 | L |
| UDL checkpoints | Textbook (N = 51) | Tutorial (N = 47) | Activity (N = 37) | Reading (N = 49) | EOC (N = 51) | All (N = 51) | Enactment |
|---|---|---|---|---|---|---|---|
| Bolding indicates at least 75% of the curricular material aligns with UDL checkpoint. In the last column, H represents high enactment, S represents some enactment, and L represents low enactment. | |||||||
| 1.1 | 100 | 100 | 100 | 100 | 100 | 100 | H |
| 1.2 | 68.6 | 12.8 | 100 | 0 | 0 | 74.5 | H |
| 1.3 | 80.4 | 0 | 0 | 0 | 0 | 80.4 | H |
| 2.1 | 94.1 | 0 | 0 | 0 | 0 | 94.1 | H |
| 2.2 | 58.8 | 0 | 0 | 0 | 0 | 58.8 | S |
| 2.3 | 19.6 | 0 | 0 | 0 | 0 | 19.6 | L |
| 2.4 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 2.5 | 100 | 0 | 0 | 0 | 0 | 100 | H |
| 3.1 | 0 | 0 | 0 | 0 | 96.1 | 96.1 | H |
| 3.2 | 100 | 57.4 | 0 | 0 | 0 | 100 | H |
| 3.3 | 0 | 76.6 | 0 | 0 | 78.4 | 90.2 | H |
| 3.4 | 100 | 100 | 100 | 100 | 100 | 100 | H |
| 4.1 | 0 | 100 | 100 | 100 | 100 | 100 | H |
| 4.2 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 5.1 | 0 | 12.8 | 0 | 0 | 19.6 | 29.4 | L |
| 5.2 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 5.3 | 0 | 100 | 100 | 100 | 100 | 100 | H |
| 6.1 | 100 | 100 | 100 | 100 | 100 | 100 | H |
| 6.2 | 78.4 | 0 | 0 | 0 | 39.2 | 84.3 | S |
| 6.3 | 0 | 70.2 | 10.8 | 0 | 82.4 | 86.3 | H |
| 6.4 | 100 | 0 | 0 | 0 | 0 | 100 | H |
| 7.1 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 7.2 | 25.5 | 14.9 | 2.7 | 6.1 | 41.2 | 47.1 | S |
| 7.3 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 8.1 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 8.2 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 8.3 | 0 | 0 | 0 | 0 | 51 | 51 | S |
| 8.4 | 0 | 100 | 100 | 100 | 100 | 100 | H |
| 9.1 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 9.2 | 0 | 0 | 0 | 0 | 0 | 0 | L |
| 9.3 | 0 | 0 | 0 | 0 | 0 | 0 | L |
Appendix C: Examples of UDL checkpoint enactment in the chemistry curricula
Table 9.| Checkpoint | Curricular enactment examples | |||
|---|---|---|---|---|
| 1.1 | Provide digital copies to allow for customization. | PDF and online documents | ||
| 1.2 | Provide ASL translation, written copies of verbal instructions, etc. | Videos included in e-Text provided closed captioning. (Mastering-SP) | ||
| 1.3 | Provide text descriptions for all salient features of diagrams and other visual information. | “Diamond is the name given to one of the naturally occurring forms (known as allotropes) of pure C; the other allotropes of carbon are graphite, graphene, and various fullerenes (↓), which we will return to later.” (CLUE, p. 71) | ||
|
||||
| The figure is reproduced from CLUE (2016) and was published under the Creative Commons Attribution-Non Commercial-Share A Like 4.0 International License. | ||||
| 2.1 | Define vocabulary and symbols. | “A catalyst is a substance that is neither produced nor consumed in a chemical reaction, yet causes the rate of the reaction to be increased without changing the temperature.” (POGIL-CGI, Chem Activity 59) | ||
| 2.2 | Define/clarify structure of equations and relationships. | “You probably recall that “like charges repel and unlike charges attract”, and that this interaction, which is known as a Coulombic interaction, depends on the sizes and signs of the charges, and is inversely proportional to the square of the distance between them (this interaction can be modeled by the equation: Fα(q1q2)/r (Coulomb's Law), where q1 and q2 are the charges on the particles and r is the distance between them). (CLUE, p. 28) | ||
| 2.3 | Support the decoding of text and symbols to assist with comprehension. | “In an orbital diagram, the direction of the arrow (pointing up or pointing down) represents the orientation of the electron's spin. Recall from Section 2.5 that the orientation of the electron's spin is quantized, with only two possibilities: spin up 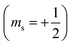 and spin down and spin down 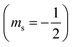 . In an orbital diagram, we represent . In an orbital diagram, we represent 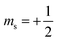 with a half-arrow pointing up (↿) and with a half-arrow pointing up (↿) and 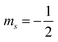 with a half-arrow pointing down (⇂). (Mastering, e-Text Section 3.3) with a half-arrow pointing down (⇂). (Mastering, e-Text Section 3.3) |
||
| 2.4 | Link to non-dominant language dictionaries, include definitions in non-dominant languages, etc. | See Appendix D | ||
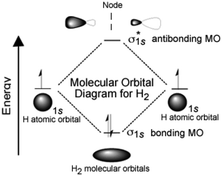
| 2.5 | Use diagrams or graphs to explain/clarify key concepts. | “If the interaction is destructive, there is no stabilizing interaction. In the case of hydrogen each atom has a single (1s) orbital occupied by a single electron. As the atoms approach one another these 1s atomic orbitals interact to form two possible MOs: a lower energy, constructive or bonding MO, and a higher energy, destructive or anti-bonding MO. Notice that the bonding MO, a so-called σ1s (sigma) orbital, has electron density (that is a high probability that the electrons would be found there if we looked) between the two hydrogen nuclei. In the anti-bonding MO, known as σ1s*, the electrons are mostly not between the nuclei.” (CLUE, p. 67) | ||
|
||||
| The figure is reproduced from CLUE (2016) and was published under the Creative Commons Attribution-Non Commercial-Share A Like 4.0 International License. | ||||
| 3.1 | Remind students of previously covered concepts or equations or referring to previous activities. | “In photosynthesis, plants form glucose (C6H12O6) and oxygen from carbon dioxide and water. | ||
| You may want to reference (pages 820–826) Section 18.8 while completing this problem. | ||||
| Write a balanced equation for photosynthesis.” (Mastering-SP, Exercise 18.60) | ||||
| 3.2 | Emphasize key concepts with formatting, such as italics, bolding, or font changes. | “Atoms are neither created nor destroyed when chemical reactions take place. Therefore, the number of atoms of each element must be identical on the reactant (left) and product (right) sides of a balanced chemical reaction. Such a chemical equation is said to be atom balanced.” (POGIL-CGI, Chem Activity 27) | ||
| 3.3 | Problem are chunked into smaller pieces that are sequentially released. | “Part A. What is Coulomb's law? | ||
•  |
||||
• ![[E with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0045_20d1.gif) = −∇φ = −∇φ |
||||
• 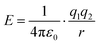 |
||||
• 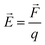 |
||||
| Part B. This question will be shown after you complete previous question(s).” | ||||
| (Mastering-SP, Exercise 3.8) | ||||
| 3.4 | Allow opportunities for review and synthesis of physics topics or generalize physics concepts to new situations. | Exercises and problems at end of textbook sections allow for review and practice. | ||
| 4.1 | Allow students to respond to questions in formats other than written format. | Mastering exercises are specifically designed to be compatible with alternative keyboards. (http://help.pearsoncmg.com/mastering/instructor/standalone/Topics/accessible_content_finding.htm?cshid=accessible_content) | ||
| 4.2 | Provide assistive technologies for students such as alternative keyboards. | See Appendix D | ||
| 5.1 | Request students to show their understanding through a myriad of media, such as text and speech. | “In a certain hypothetical experiment, the initial concentration of the reactant R is 1.00 mol L−1, and its rate constant is 0.0150 mol L−1 s−1. It follows a zero-order reaction mechanism for the consumption of reactant R. Plot the graph of concentration versus time. Consider the time intervals as 0, 10, 20, 30, 40, and 50 s.” (Mastering-SP, exercise ± Zero-Order Reactions) | ||
| 5.2 | Provide supports, such as calculators, graphing paper, and speech-to-text software. for students to construct or compose their responses. | “Use a molecular modeling set to make the following molecules: CH4; NH3; H2O. (In many modeling kits: carbon is black; oxygen is red; nitrogen is blue; hydrogen is white. Nonbinding electrons are not represented in these models).” (POGIL-CGI, pg. 110) | ||
| 5.3 | Provide differentiated feedback that is customized to the individual learners. | Mastering gives individualized feedback based on reason for incorrect answers. | ||
| For example, “Incorrect; Try again. Check your signs.” (Mastering-SP) | ||||
| 6.1 | Provide guides and checks to support and scaffold goal-setting. | Learning outcomes for each exercise are available for students to access. | ||
| 6.2 | Prompt students to stop and show/explain work. | “What would you predict would be the relative boiling points of methanol (CH3OH), dimethyl ether (CH3OCH3) and ethane (CH3CH3)? Explain your answer, being sure to use the ideas of forces and energy.” (CLUE, 1st semester recitation week 12) | ||
| 6.3 | Provide templates for data collection and organization of information. | “Complete the following table:” | ||
| Element | n for the valence shell | IE1 (MJ mol−1) | ||
| N | ||||
| Ar | ||||
| (POGIL-CGI, ChemActivity 62) | ||||
| 6.4 | Ask questions to guide self-monitoring and reflection. | The Mastering-SP textbook provides self-assessment problems at the end of each section. | ||
| 7.1 | Provide design activities where students choose aspects, such as level of challenge, materials, and timing of completion of tasks. Also, provide choice in which components of activities must be completed. | See Appendix D | ||
| 7.2 | Choose activities that optimize the relevance of curriculum to students’ lives and invite personal response to content and activities. | “What does pH mean to you?” (Clue PowerPoint 7-5) | ||
| 7.3 | Create an accepting and supportive classroom environment for all students. | See Appendix D | ||
| 8.1 | List goals and objectives for each activity. | See Appendix D | ||
| 8.2 | Provide alternatives for tools and scaffolds required to complete activities or vary the standards for acceptable performance. | See Appendix D | ||
| 8.3 | Create groups with clear roles and responsibilities. | Teamwork is one of the process skills emphasized throughout the POGIL-CGI book. | ||
| 8.4 | Provide and encourage mastery-oriented feedback. | Mastering gives prompt, substantive, and informative feedback to students as they complete exercises. | ||
| 9.1 | Give activities that promote self-reflection and setting of personal goals. | See Appendix D | ||
| 9.2 | Provide models and feedback for managing frustration. | See Appendix D | ||
| 9.3 | Promote monitoring of behaviors or provide feedback about emotional reactivity. | See Appendix D | ||
Appendix D: Universal design for learning checkpoint operationalizations
The table below presents the UDL checkpoints and examples of implementation from the UDL Guidelines 2.0 (CAST, 2011). The UDL implementation examples and descriptions were used as the operationalizations of the UDL checkpoints in this study (Table 10).| UDL description | UDL checkpoint | Operationalization examples |
|---|---|---|
| Offer ways of customizing the display of information | 1.1 | • Display information in a flexible format so that the following perceptual features can be varied: |
| • The size of text, images, graphs, tables, or other visual content | ||
| • The contrast between background and text or image | ||
| • The color used for information or emphasis | ||
| • The volume or rate of speech or sound | ||
| • The speed or timing of video, animation, sound, simulations, etc. | ||
| • The layout of visual or other elements | ||
| • The font used for print materials | ||
| Offer alternatives for auditory information | 1.2 | • Use text equivalents in the form of captions or automated speech-to-text (voice recognition) for spoken language |
| • Provide visual diagrams, charts, notations of music or sound | ||
| • Provide written transcripts for videos or auditory clips | ||
| • Provide American Sign Language (ASL) for spoken English | ||
| • Use visual analogues to represent emphasis and prosody (e.g., emoticons, symbols, or images) | ||
| • Provide visual or tactile (e.g., vibrations) equivalents for sound effects or alerts | ||
| • Provide visual and/or emotional description for musical interpretation | ||
| Offer alternatives for visual information | 1.3 | • Provide descriptions (text or spoken) for all images, graphics, video, or animations |
| • Use touch equivalents (tactile graphics or objects of reference) for key visuals that represent concepts | ||
| • Provide physical objects and spatial models to convey perspective or interaction | ||
| • Provide auditory cues for key concepts and transitions in visual information | ||
| • Follow accessibility standards (NIMAS, DAISY, etc.) when creating digital text | ||
| • Allow for a competent aide, partner, or “intervener” to read text aloud | ||
| • Provide access to text-to-speech software | ||
| Clarify vocabulary and symbols | 2.1 | • Pre-teach vocabulary and symbols, especially in ways that promote connection to the learners’ experience and prior knowledge |
| • Provide graphic symbols with alternative text descriptions | ||
| • Highlight how complex terms, expressions, or equations are composed of simpler words or symbols | ||
| • Embed support for vocabulary and symbols within the text (e.g., hyperlinks or footnotes to definitions, explanations, illustrations, previous coverage, translations) | ||
| • Embed support for unfamiliar references within the text (e.g., domain specific notation, lesser known properties and theorems, idioms, academic language, figurative language, mathematical language, jargon, archaic language, colloquialism, and dialect) | ||
| Clarify syntax and structure | 2.2 | • Clarify unfamiliar syntax (in language or in math formulas) or underlying structure (in diagrams, graphs, illustrations, extended expositions or narratives) through alternatives that: |
| • Highlight structural relations or make them more explicit | ||
| • Make connections to previously learned structures | ||
| • Make relationships between elements explicit (e.g., highlighting the transition words in an essay, links between ideas in a concept map, etc.) | ||
| Support decoding of text, mathematical notation, and symbols | 2.3 | • Allow the use of text-to-speech |
| • Use automatic voicing with digital mathematical notation (Math ML) | ||
| • Use digital text with an accompanying human voice recording (e.g., Daisy Talking Books) | ||
| • Allow for flexibility and easy access to multiple representations of notation where appropriate (e.g., formulas, word problems, graphs) | ||
| • Offer clarification of notation through lists of key terms | ||
| Promote understanding across languages | 2.4 | • Make all key information in the dominant language (e.g., English) also available in first languages (e.g., Spanish) for learners with limited-English proficiency and in ASL for learners who are deaf |
| • Link key vocabulary words to definitions and pronunciations in both dominant and heritage languages | ||
| • Define domain-specific vocabulary (e.g., “map key” in social studies) using both domain-specific and common terms | ||
| • Provide electronic translation tools or links to multilingual glossaries on the web | ||
| • Embed visual, non-linguistic supports for vocabulary clarification (pictures, videos, etc.) | ||
| Illustrate through multiple media | 2.5 | • Present key concepts in one form of symbolic representation (e.g., an expository text or a math equation) with an alternative form (e.g., an illustration, dance/movement, diagram, table, model, video, comic strip, storyboard, photograph, animation, physical or virtual manipulative) |
| • Make explicit links between information provided in texts and any accompanying representation of that information in illustrations, equations, charts, or diagrams | ||
| Activate or supply background knowledge | 3.1 | • Anchor instruction by linking to and activating relevant prior knowledge (e.g., using visual imagery, concept anchoring, or concept mastery routines) |
| • Use advanced organizers (e.g., KWL methods, concept maps) | ||
| • Pre-teach critical prerequisite concepts through demonstration or models | ||
| • Bridge concepts with relevant analogies and metaphors | ||
| • Make explicit cross-curricular connections (e.g., teaching literacy strategies in the social studies classroom) | ||
| Highlight patterns, critical features, big ideas, and relationships | 3.2 | • Highlight or emphasize key elements in text, graphics, diagrams, formulas |
| • Use outlines, graphic organizers, unit organizer routines, concept organizer routines, and concept mastery routines to emphasize key ideas and relationships | ||
| • Use multiple examples and non-examples to emphasize critical features | ||
| • Use cues and prompts to draw attention to critical features | ||
| • Highlight previously learned skills that can be used to solve unfamiliar problems | ||
| Guide information processing, visualization, and manipulation | 3.3 | • Give explicit prompts for each step in a sequential process |
| • Provide options for organizational methods and approaches (tables and algorithms for processing mathematical operations) | ||
| • Provide interactive models that guide exploration and new understandings | ||
| • Introduce graduated scaffolds that support information processing strategies | ||
| • Provide multiple entry points to a lesson and optional pathways through content (e.g., exploring big ideas through dramatic works, arts and literature, film and media) | ||
| • “Chunk” information into smaller elements | ||
| • Progressively release information (e.g., sequential highlighting) | ||
| • Remove unnecessary distractions unless they are essential to the instructional goal | ||
| Maximize transfer and generalization | 3.4 | • Provide checklists, organizers, sticky notes, electronic reminders |
| • Prompt the use of mnemonic strategies and devices (e.g., visual imagery, paraphrasing strategies, method of loci, etc.) | ||
| • Incorporate explicit opportunities for review and practice | ||
| • Provide templates, graphic organizers, concept maps to support note-taking | ||
| • Provide scaffolds that connect new information to prior knowledge (e.g., word webs, half-full concept maps) | ||
| • Embed new ideas in familiar ideas and contexts (e.g., use of analogy, metaphor, drama, music, film, etc.) | ||
| • Provide explicit, supported opportunities to generalize learning to new situations (e.g., different types of problems that can be solved with linear equations, using physics principles to build a playground) | ||
| • Offer opportunities over time to revisit key ideas and linkages between ideas | ||
| Vary the methods for response and navigation | 4.1 | • Provide alternatives in the requirements for rate, timing, speed, and range of motor action required to interact with instructional materials, physical manipulatives, and technologies |
| • Provide alternatives for physically responding or indicating selections (e.g., alternatives to marking with pen and pencil, alternatives to mouse control) | ||
| • Provide alternatives for physically interacting with materials by hand, voice, single switch, joystick, keyboard, or adapted keyboard | ||
| Optimize access to tools and assistive technologies | 4.2 | • Provide alternate keyboard commands for mouse action |
| • Build switch and scanning options for increased independent access and keyboard alternatives | ||
| • Provide access to alternative keyboards | ||
| • Customize overlays for touch screens and keyboards | ||
| • Select software that works seamlessly with keyboard alternatives and alt keys | ||
| Use multiple media for communication | 5.1 | • Compose in multiple media such as text, speech, drawing, illustration, design, film, music, dance/movement, visual art, sculpture or video |
| • Use physical manipulatives (e.g., blocks, 3D models, base-ten blocks) | ||
| • Use social media and interactive web tools (e.g., discussion forums, chats, web design, annotation tools, storyboards, comic strips, animation presentations) | ||
| • Compose in multiple media such as text, speech, drawing, illustration, comics, storyboards, design, film, music, visual art, sculpture, or video | ||
| • Solve problems using a variety of strategies | ||
| Use multiple tools for construction and composition | 5.2 | • Provide spellcheckers, grammar checkers, word prediction software |
| • Provide text-to-speech software (voice recognition), human dictation, recording | ||
| • Provide calculators, graphing calculators, geometric sketchpads, or pre-formatted graph paper | ||
| • Provide sentence starters or sentence strips | ||
| • Use story webs, outlining tools, or concept mapping tools | ||
| • Provide Computer-Aided-Design (CAD), music notation (writing) software, or mathematical notation software | ||
| • Provide virtual or concrete mathematics manipulatives (e.g., base-10 blocks, algebra blocks) | ||
| • Use web applications (e.g., wikis, animation, presentation) | ||
| Build fluencies with graduated levels of support for practice and performance | 5.3 | • Provide differentiated models to emulate (i.e. models that demonstrate the same outcomes but use differing approaches, strategies, skills, etc.) |
| • Provide differentiated mentors (i.e., teachers/tutors who use different approaches to motivate, guide, feedback or inform) | ||
| • Provide scaffolds that can be gradually released with increasing independence and skills (e.g., embedded into digital reading and writing software) | ||
| • Provide differentiated feedback (e.g., feedback that is accessible because it can be customized to individual learners) | ||
| • Provide multiple examples of novel solutions to authentic problems | ||
| Guide appropriate goal-setting | 6.1 | • Provide prompts and scaffolds to estimate effort, resources, and difficulty |
| • Provide models or examples of the process and product of goal-setting | ||
| • Provide guides and checklists for scaffolding goal-setting | ||
| • Post goals, objectives, and schedules in an obvious place | ||
| Support planning and strategy development | 6.2 | • Embed prompts to “stop and think” before acting as well as adequate space |
| • Embed prompts to “show and explain your work” (e.g., portfolio review, art critiques) | ||
| • Provide checklists and project planning templates for understanding the problem, setting up prioritization, sequences, and schedules of steps | ||
| • Embed coaches or mentors that model think-alouds of the process | ||
| • Provide guides for breaking long-term goals into reachable short-term objectives | ||
| Facilitate managing information and resources | 6.3 | • Provide graphic organizers and templates for data collection and organizing information |
| • Embed prompts for categorizing and systematizing | ||
| • Provide checklists and guides for note-taking | ||
| Enhance capacity for monitoring progress | 6.4 | • Ask questions to guide self-monitoring and reflection |
| • Show representations of progress (e.g., before and after photos, graphs and charts showing progress over time, process portfolios) | ||
| • Prompt learners to identify the type of feedback or advice that they are seeking | ||
| • Use templates that guide self-reflection on quality and completeness | ||
| • Provide differentiated models of self-assessment strategies (e.g., role-playing, video reviews, peer feedback) | ||
| • Use of assessment checklists, scoring rubrics, and multiple examples of annotated student work/performance examples | ||
| Optimize individual choice and autonomy | 7.1 | • Provide learners with as much discretion and autonomy as possible by providing choices in such things as: |
| • The level of perceived challenge | ||
| • The type of rewards or recognition available | ||
| • The context or content used for practicing and assessing skills | ||
| • The tools used for information gathering or production | ||
| • The color, design, or graphics of layouts, etc. | ||
| • The sequence or timing for completion of subcomponents of tasks | ||
| • Allow learners to participate in the design of classroom activities and academic tasks | ||
| • Involve learners, where and whenever possible, in setting their own personal academic and behavioral goals | ||
| Optimize relevance, value, and authenticity | 7.2 | • Vary activities and sources of information so that they can be: |
| • Personalized and contextualized to learners’ lives | ||
| • Culturally relevant and responsive | ||
| • Socially relevant | ||
| • Age and ability appropriate | ||
| • Appropriate for different racial, cultural, ethnic, and gender groups | ||
| • Design activities so that learning outcomes are authentic, communicate to real audiences, and reflect a purpose that is clear to the participants | ||
| • Provide tasks that allow for active participation, exploration and experimentation | ||
| • Invite personal response, evaluation and self-reflection to content and activities | ||
| • Include activities that foster the use of imagination to solve novel and relevant problems, or make sense of complex ideas in creative ways | ||
| Minimize threats and distractions | 7.3 | • Create an accepting and supportive classroom climate |
| • Vary the level of novelty or risk | ||
| • Charts, calendars, schedules, visible timers, cues, etc. that can increase the predictability of daily activities and transitions | ||
| • Creation of class routines | ||
| • Alerts and previews that can help learners anticipate and prepare for changes in activities, schedules, and novel events | ||
| • Options that can, in contrast to the above, maximize the unexpected, surprising, or novel in highly routinized activities | ||
| • Vary the level of sensory stimulation | ||
| • Variation in the presence of background noise or visual stimulation, noise buffers, number of features or items presented at a time | ||
| • Variation in pace of work, length of work sessions, availability of breaks or time-outs, or timing or sequence of activities | ||
| • Vary the social demands required for learning or performance, the perceived level of support and protection and the requirements for public display and evaluation | ||
| • Involve all participants in whole class discussions | ||
| Heighten salience of goals and objectives | 8.1 | • Prompt or require learners to explicitly formulate or restate goal |
| • Display the goal in multiple ways | ||
| • Encourage division of long-term goals into short-term objectives | ||
| • Demonstrate the use of hand-held or computer-based scheduling tools | ||
| • Use prompts or scaffolds for visualizing desired outcome | ||
| • Engage learners in assessment discussions of what constitutes excellence and generate relevant examples that connect to their cultural background and interests | ||
| Vary demands and resources to optimize challenge | 8.2 | • Differentiate the degree of difficulty or complexity within which core activities can be completed |
| • Provide alternatives in the permissible tools and scaffolds | ||
| • Vary the degrees of freedom for acceptable performance | ||
| • Emphasize process, effort, improvement in meeting standards as alternatives to external evaluation and competition | ||
| Foster collaboration and community | 8.3 | • Create cooperative learning groups with clear goals, roles, and responsibilities |
| • Create school-wide programs of positive behavior support with differentiated objectives and supports | ||
| • Provide prompts that guide learners in when and how to ask peers and/or teachers for help | ||
| • Encourage and support opportunities for peer interactions and supports (e.g., peer-tutors) | ||
| • Construct communities of learners engaged in common interests or activities | ||
| • Create expectations for group work (e.g., rubrics, norms, etc.) | ||
| Increase mastery-oriented feedback | 8.4 | • Provide feedback that encourages perseverance, focuses on development of efficacy and self-awareness, and encourages the use of specific supports and strategies in the face of challenge |
| • Provide feedback that emphasizes effort, improvement, and achieving a standard rather than on relative performance | ||
| • Provide feedback that is frequent, timely, and specific | ||
| • Provide feedback that is substantive and informative rather than comparative or competitive | ||
| • Provide feedback that models how to incorporate evaluation, including identifying patterns of errors and wrong answers, into positive strategies for future success | ||
| Promote expectations and beliefs that optimize motivation | 9.1 | • Provide prompts, reminders, guides, rubrics, checklists that focus on: |
| • Self-regulatory goals like reducing the frequency of aggressive outbursts in response to frustration | ||
| • Increasing the length of on-task orientation in the face of distractions | ||
| • Elevating the frequency of self-reflection and self-reinforcements | ||
| • Provide coaches, mentors, or agents that model the process of setting personally appropriate goals that take into account both strengths and weaknesses | ||
| • Support activities that encourage self-reflection and identification of personal goals | ||
| Facilitate personal coping skills and strategies | 9.2 | • Provide differentiated models, scaffolds and feedback for: |
| • Managing frustration | ||
| • Seeking external emotional support | ||
| • Developing internal controls and coping skills | ||
| • Appropriately handling subject specific phobias and judgments of “natural” aptitude (e.g., “how can I improve on the areas I am struggling in?” rather than “I am not good at math”) | ||
| • Use real life situations or simulations to demonstrate coping skills | ||
| Develop self-assessment and reflection | 9.3 | • Offer devices, aids, or charts to assist individuals in learning to collect, chart and display data from their own behavior for the purpose of monitoring changes in those behaviors |
| • Use activities that include a means by which learners get feedback and have access to alternative scaffolds (e.g., charts, templates, feedback displays) that support understanding progress in a manner that is understandable and timely | ||
Acknowledgements
This work is supported in part by NSF Grant No. DUE 1612009. The authors thank Richard Moog for his fruitful feedback and discussions.References
- Azevedo R., (2015), Defining and measuring engagement and learning in science: conceptual, theoretical, methodological, and analytical issues, Educ. Psychol., 50(1), 84–94.
- Bae S. J., (2007), Self-determination and academic achievement of individuals with disabilities in postsecondary education: a meta-analysis, Unpublished doctoral dissertation, Lawrence: University of Kansas.
- Behling K. and Linder K. E., (2017), Collaborations between centers for teaching and learning and offices of disability services: current partnerships and perceived challenges, J. Postsecond. Educ. Disabil., 30(1), 5–15.
- Burgstahler S. and Moore E., (2009), Making student services welcoming and accessible through accommodations and universal design, J. Postsecond. Educ. Disabil., 21, 155–174.
- CAST, (2011), Universal Design for Learning Guidelines version 2.0, Wakefield, MA: CAST.
- CLUE, (2016), http://clue.chemistry.msu.edu, accessed 24/10/17.
- Cole R., (2015), Using chemistry education research to inform teaching strategies and design of instructional materials, in García-Martínez J. and Serrano-Torregrosa E. (ed.), Chemistry education: best practices, opportunities and trends, Weinheim: Wiley-VCH, pp. 141–180.
- Compton D. L., Fuchs L. S., Fuchs D., Lambert W. and Hamlett C., (2012), The cognitive and academic profiles of reading and mathematics learning disabilities, J. Learn. Disabil.45(1), 79–95.
- Cook L., Hennessey M. L., Cook B. G. and Rumrill P. D., (2007), The views of university faculty members and service providers regarding the increased enrollment of students with learning disabilities, Learn. Disabil.: Multidisciplin. J., 14, 205–216.
- Cook L., Rumrill P. D. and Tankersley M., (2009), Priorities and understanding of faculty members regarding college students with disabilities, Int. J. Teach. Learn. High. Educ., 21, 84–96.
- Cooper M. M. and Klymkowsky M. W., (2017), Chemistry, Life, the Universe, & Everything, http://clue.chemistry.msu.edu, accessed 24/10/17.
- Cooper M. M., Underwood S. M., Hilley C. Z. and Klymkowsky M., (2012), Development and Assessment of a Molecular Structure and Properties Learning Progression, J. Chem. Educ., 89, 1351–1357.
- Cortiella C. and Horowitz S. H., (2014), The state of learning disabilities: facts, trends and emerging issues, New York, NY: National Center for Learning Disabilities.
- Council for Exceptional Children, (2008), Higher Education Act reauthorization: summary of selected provisions for individuals with exceptionalities and the professionals who work on their behalf.
- Debrand C. C. and Salzberg C. L., (2005), A validated curriculum to provide training to faculty regarding students with disabilities in higher education, J. Postsecond. Educ. Disabil., 18, 49–62.
- Dudley A. v. Miami University and David C. Hodge, (2016), Case No.: 1:14-cv-38, retrieved from https://nfb.org/images/nfb/documents/pdf/miami%20teach.pdf.
- Edyburn D. L., (2010), Would you recognize universal design for learning if you saw it? Ten propositions for new directions for the second decade of UDL, Learn. Disabil. Quart., 33(1), 33–41.
- Eichler J. F. and Peeples J., (2013), Online homework put to the test: a report on the impact of two online learning systems on student performance in general chemistry, J. Chem. Educ., 90(9), 1137–1143.
- Esterling K. M. and Bartels L., (2013), Atoms-First Curriculum: A Comparison of Student Success in General Chemistry, J. Chem. Educ., 90, 1433–1436.
- Farrell J. J., Moog R. S. and Spencer J. N., (1999) A Guided Inquiry General Chemistry Course, J. Chem. Educ., 76, 570–574.
- Finn D., Getzel E. E. and McManus S., (2008), Adapting the self-determined learning model for instruction of college students with disabilities, Career Dev. Except. Individ., 31, 85–93.
- Freeman S., Eddy S., McDonough M., Smith M., Okoroafor N., Jordt H. and Wenderoth M. P, (2014), Active learning increases student performance in science, engineering, and mathematics, Proc. Natl. Acad. Sci. U. S. A., 111(23), 8410–8415.
- Gwet K., (2002), Statistical Methods for Inter-Rater Reliability Assessment Series 2, retrieved from http://www.agreestat.com/research_papers/inter_rater_reliability_dependency.pdf.
- Gwet K., (2014), Handbook of Inter-Rater Reliability: The Definitive Guide to Measuring the Extent of Agreement Among Raters, 4th edn, Gaithersburg: Advanced Analytics.
- He Y., (2014), Universal Design for Learning in an Online Teacher Education Course: Enhancing Learners’ Confidence to Teach Online, MERLOT J. Online Learn. Teach., 10(2): 283–297.
- Hindes Y. and Mather J., (2007), Inclusive education at the post-secondary level: attitudes of students and professors, Except. Educ. Canada, 17, 107–128.
- Individuals with Disabilities Education Improvement Act, (2004), 20 U.S.C. § 614 et seq.
- Katz J. and Sokal L., (2016), Universal Design for Learning as a Bridge to Inclusion: A Qualitative Report of Student Voices, Int. J. Whole Sch., 12(2), 37–63.
- King-Sears M. E., Evmenoa A. S. and Johnson T. M., (2017), Using technology for accessible chemistry homework for high school students with and without learning disabilities, Learn. Disabil. Res. Pract.32(2), 121–131.
- Lewis S. E. and Lewis J. E., (2005), Departing from Lectures: An Evaluation of a Peer-Led Guided Inquiry Alternative, J. Chem. Educ., 82, 135–139.
- Lombardi A., (2010), Measuring faculty attitudes and perceptions toward disability at a four-year university: a validity study, [Dissertation] retrieved from https://eric.ed.gov/?id=ED516823.
- Louisiana Tech University v. United States of America, (2013), Case No.: 204-33-116, retrieved from https://www.ada.gov/louisiana-tech.htm#ex1.
- Mastering Chemistry, (2017), http://www.pearsonmylabandmastering.com/northamerica/masteringchemistry, accessed 31/10/17.
- McKenzie L., (2017), An IT accessibility watchdog? Inside Hig. Educ., retrieved from https://www.insidehighered.com/news/2017/11/15/universities-mull-creation-it-accessibility-group-review-vendor-products.
- Miner D. L., Nieman R., Swanson A. and Woods M. (ed.), (2001), Teaching chemistry to students with disabilities: a manual for high schools, colleges, and graduate programs, 4th edn, Washington, DC: American Chemical Society.
- Minkara M. S., Weaver M. N., Gorske J., Bowers C. R. and Merz K. M., (2015), Implementation of protocols to enable doctoral training in physics and computational chemistry of a blind graduate students, J. Chem. Educ., 92(8), 1280–1283.
- Moog R. S. and Farrell J. J., (2017), Chemistry: A Guided Inquiry, New Jersey: Wiley.
- Moog R. S., Creegan F. J., Hanson D. M., Spencer J. N. and Straumanis A. R., (2006), Process-oriented guided inquiry learning: POGIL and the POGIL project, Metrop. Univ. Int. Forum, 17, 41–52.
- Navarro S. B., Zeveras P., Gesa R. F. and Sampson D. G., (2016), Developing Teachers’ Competencies for Designing Inclusive Learning Experiences, Educ. Technol. Soc., 19(1), 17–27.
- Nepomuceno G. M., Decker D. M., Shaw J. D., Boyes L., Tantillo D. J. and Wedler H. B., (2016), The value of safety and practicality: recommendations for training disabled students in the sciences with a focus on blind and visually impaired students in chemistry laboratories, J. Chem. Health Saf., 23(1), 5–11.
- Newman L. A. and Madaus J. W., (2015), An analysis of factors related to receipt of accommodations and services by postsecondary students with disabilities, Remedial Spec. Educ., 36, 208–219 DOI:10.1177/0741932515572912.
- Newman L. A., Wagner M., Cameto R., Knokey A. M. and Shaver D., (2010), Comparisons across time of the outcomes of youth with disabilities up to 4 years after high school. A report of findings from the National Longitudinal Transition Study-2 (NLTS2), Menlo Park, CA: SRI International.
- Newman L. A., Wagner M., Knokey A. M., Marder C., Nagle K., Shaver D. and Wei X., (2011), The post-high school outcomes of young adults with disabilities up to 8 years after high school. A report from the National Longitudinal Transition Study-2 (NLTS2) (NCSER 2011-3005), Menlo Park, CA: SRI International.
- Pagano T., (2017), Making education and careers in chemistry accessible and successful for deaf/heard-of-hearing students, in Nelson D. J. and Cheng H. N. (ed.), Diversity in the scientific community volume 2: perspectives and exemplary programs, Washington, DC: American Chemical Society, pp. 125–132.
- Pagano T., Ross A. and Smith S. B., (2015), Undergraduate research involving deaf and hard-of-hearing students in interdisciplinary science project, Educ. Sci., 5, 146–165.
- Parker D. R. and Boutelle K., (2009), Executive function coaching for college students with learning disabilities and ADHD: a new approach for fostering self-determination, Learn. Disabil. Res. Pract., 24, 204–215.
- POGIL, (2017), http://pogil.org, accessed 20/12/17.
- Rao S., (2004), Faculty attitudes and students with disabilities in higher education: a literature review, Coll. Stud. J., 38, 191–198.
- Riccobono J. A., Whitmore R. W., Gabel T. J., Traccarella M. A., Pratt D. J., Berkner L. K. and Malizio A. G., (1997), National Postsecondary Student Aid Study, 1995–96 (NPSAS: 96) Methodology Report (NCES 98-073), Washington, DC: U.S. Department of Education, National Center for Education Statistics.
- Rose D. H., Meyer A. and Hitchcock C., (2005), The Universally Designed Classroom: Accessible Curriculum and Digital Technologies, Boston, MA: Harvard Education Press.
- Schroeder J. D. and Greenbowe T. J., (2008), Implementing POGIL in the lecture and the Science Writing Heuristic in the laboratory—student perceptions and performance in undergraduate organic chemistry, J. Chem. Educ. Res. Pract., 9, 149–156.
- Singer S. R., Nielson N. R. and Schweingruber H. A. (ed.), (2012), Discipline-based education research: understanding and improving learning in undergraduate science and engineering, Washington, DC: The National Academics Press.
- Snyder T. D. and Dillow S. A., (2015), Digest of education statistics 2013 (NCES 2015-011), Washington, DC: U.S. Department of Education, National Center for Education Statistics, Institute of Education Sciences.
- Thorius K. A. K. and Santamaría Graff C., (2018), Extending Peer-Assisted Learning Strategies for Racially, Linguistically, and Ability Diverse Learners, Interv. Sch. Clin., 53(3), 163–170.
- Towns M. H., (2013), New guidelines for chemistry education research manuscripts and future directions of the field, J. Chem. Educ., 90(9), 1107–1108.
- Trammell J., (2009), Postsecondary students and disability stigma: development of the postsecondary student survey of disability-related stigma (PSSDS), Journal of Postsecondary Education and Disability, 22(2), 106–116.
- Tro N. J., (2018), Chemistry: Structure and Properties, New Jersey, Pearson Education.
- Underwood S. M., Reyes-Gastelum D. and Cooper M. M., (2016), When do students recognize relationships between molecular structure and properties? A longitudinal comparison of the impact of traditional and transformed curricula, J. Chem. Educ. Res. Pract., 17, 365–380.
- Vasek D., (2005), Assessing the knowledge base of faculty at a private, four-year institution, Coll. Stud. J., 39, 307–315.
- Vitoriano F. A., Teles V. L. G., Rizzatti I. M. and Pesssoa de Lima R. C., (2016), Promoting inclusive chemistry teaching by developing an accessible thermometer for students with visual disabilities, J. Chem. Educ., 93(12), 2046–2051.
- Vogel S. A., Holt J. K., Sligar S. and Leake E., (2008), Assessment of campus climate to enhance student success, J. Postsecond. Educ. Disabil., 21, 15–31.
- Wahab R. A. and Thomas, S. P., (2015), Students experiences of an online learning tool at college of health sciences, Proceedings of the Fifth International Conference on e-Learning, pp. 603–607.
- Williams L. C., Underwood S. M., Klymkowsky M. W. and Cooper M. M., (2015), Are noncovalent interactions an achilles heel in chemistry education? A comparison of instructional approaches, J. Chem. Educ., 92(12), 1979–1987.
- Wynants S. A. and Dennis J. M., (2017), Embracing diversity and accessibility: a mixed methods study of the impact of an online disability awareness program, J. Postsecond. Educ. Disabil., 30(1), 33–48.
- Zou J. J., (2011), Blind Florida State U. students sue over e-learning systems, Chronicle of Higher Education, retrieved from https://www.chronicle.com/blogs/wiredcampus/blind-florida-state-u-students-sue-over-e-learning-systems.
Footnote |
| † The figure is reproduced from CLUE (2016) and was published under the Creative Commons Attribution-NonCommerical-Share A Like 4.0 International License. |
| This journal is © The Royal Society of Chemistry 2018 |