The effect of the REACT strategy on students’ achievements with regard to solubility equilibrium: using chemistry in contexts
Tuğçe
Günter

Bulent Ecevit University, Ahmet Erdogan Vocational School of Health Services, Department of Medical Laboratory and Techniques, Kozlu, Zonguldak, Turkey. E-mail: tugcegunter85@gmail.com
First published on 18th July 2018
Abstract
The objective of this research was to investigate the effect of a context-based approach (CBA) ‘Relating, Experiencing, Applying, Cooperating, Transferring’ (REACT) strategy used in relation to the topic of solubility equilibrium in the laboratory chemistry course on students’ achievement at associate level in a health-related program. In this regard, two contexts related to the topic of solubility equilibrium were developed and applied. The study had pre-test post-test with a control group research design and the participants consisted of sophomore students studying in the Medical Laboratory Techniques (MLT) and the Pharmacy Services (PS) Programs of the Ahmet Erdogan Vocational School of Health Services at Bulent Ecevit University (N = 96). The students enrolled in the MLT program were randomly assigned as the experimental group (n = 47) and the students in the PS program were randomly assigned as the control group (n = 49). The experimental group was taught solubility equilibrium by a CBA REACT strategy, whereas the control group was taught the relevant topic by conventional teaching. The ‘Equilibrium of Solubility Achievement Test (ESAT)’ and ‘Structured Interview Form’ were used as data collection tools in the research. The results of content analysis of ESAT post-test showed that the frequency of answers in the sound understanding category was higher for the experimental group compared to the control group students. In addition, the results of Mann–Whitney U and Wilcoxon tests of the ESAT indicated that post-test scores were higher in both groups compared to pre-test scores and the increase was higher in the experimental group compared to the control group. The content analysis results of structured interview form and semi-structured interviews showed that the students expressed positive views concerning the instruction and the qualities of the contexts in general. In this research, it was concluded that the CBA REACT strategy used in relation to the topic of solubility equilibrium in the laboratory chemistry course improved students’ sound understanding and achievement and helped them develop positive views regarding the instruction and the quality of the contexts.
Introduction
‘Solubility Equilibrium’, a common topic in chemistry courses at high school, associate, and undergraduate levels, is a topic which students may encounter in their everyday lives and find interesting. In particular, a student enrolled in an associate-level health program at university is required to know various events related to solubility equilibrium which will be necessary in various real life tasks such as the preparation of serums or injections used in the hospital, the determination of drug dosages or the preparation of mercury amalgam, which is a solid solution used in dental fillings. In addition, it is a difficult topic to learn due to its abstract nature and heavy mathematical calculations (Schmidt, 1991; Taylor and Coll, 1997; Hawkes, 1998; Raviolo and Alexander, 2001; Onder and Geban, 2006; Purwati and Dwisuyanti, 2014; Orwat et al., 2017; Rahmi et al., 2017). Hence, it was considered that teaching solubility equilibrium using contexts related to everyday life in an associate-level chemistry course in a health-related university program might improve student achievement and allow students to consider the topic from different angles.The context-based approach (CBA) is based on constructivism and utilizes situations where learners can associate previously learned knowledge with newly acquired knowledge. For this reason, CBA is used to allow learners to associate their scientific knowledge with events which they may encounter in everyday life (Bennett, 2003; Bennett and Lubben, 2006; Gilbert, 2006; Bennett et al., 2007; King, 2007; Kortland, 2010; Vos et al., 2011; Ultay, 2017). The difference between CBA and other constructivism-based approaches and active learning methods (the problem-based learning approach (PBL) and case-based learning method (CBL)) is that the scenarios are not complicated and present the content very clearly (DeJong, 2006; Ultay, 2017). In this sense, it is quite important that contexts used for CBA involve topics and applications which students can associate with their own cultures, everyday lives, families and friends in order to achieve efficient learning. Contexts are presented as problem-solving case studies (Potter and Overton, 2006). Students cooperatively research, discuss and develop possible solutions in small groups, thereby improving their own learning (Eilks and Byers, 2010; Vogelzang and Admiraal, 2017). Context-based chemistry education, in particular, is used to establish relations between real life and the scientific content of the chemistry courses (Ultay and Calık, 2012). There are various studies in the literature where CBA has been efficiently used to teach high school and undergraduate-level chemistry topics.
For example, King and Ritchie (2013) examined the effect of intra-classroom interactions on student learning in a context-based chemistry course at high-school level. The researchers developed and applied a real life-based context involving the examination of the water quality in a local creek. The study results showed that intra-group interactions and cooperative working allowed students to relate real life problems to chemistry topics. Cigdemoglu (2012) explored the effect of CBA through 5E learning cycle model used in the topic of ‘chemical reactions and energy’ at high-school level on students’ understanding, achievement and chemical literacy. The author concluded that CBA 5E learning cycle model improved students’ understanding, achievement and chemical literacy. Additionally, it was noted that the approach also improved students’ intrinsic motivation and their interest in the course. Mandler et al. (2012) developed and applied a context about drinking water quality and the greenhouse effect in an undergraduate-level analytical chemistry course. The researchers demonstrated that the context allowed students to develop an awareness of environmental issues and improved their conceptualizations. Schwartz-Bloom et al. (2011) developed and applied a context related to pharmacology topics including acid–bases, polarity, oxidation–reduction, enzymes, the structure and functions of cells, and the circulatory system in order to teach students chemistry and biology. It was found that the context improved students’ conceptual development. Demircioglu et al. (2009) examined the effectiveness of CBA in the topic of the ‘periodic table’ in a high-school level chemistry course. The researchers showed that the contexts allowed students to better understand concepts related to the periodic table and develop positive attitudes toward the chemistry course. Teichert et al. (2008) investigated the effects of contexts related to conductivity and the boiling points of aqueous solutions on students’ ideas about what happens at a molecular level in aqueous solutions. The researchers concluded that the contexts improved students’ ideas. Bulte et al. (2006) developed activities about ‘water quality’ at high-school level that were based on real events in the form of contexts according to the ‘need to know’ principle. The researchers suggested that such activities could ensure meaningful learning for students. Mihok et al. (2006) developed contexts related to water chemistry involving topics such as acid–base balance, kinetics, thermodynamics, chromatographic analysis and spectroscopy in undergraduate-level general chemistry course. The study results demonstrated that contexts encouraged students to associate chemistry topics with everyday life. Belt et al. (2005) investigated the effect of CBA used in an undergraduate-level physico-chemistry course on students’ understanding of thermodynamics, kinetics and electrochemistry. The researchers found that students gained experience in cooperative working and problem-solving skills.
As can be seen in the literature, studies show that CBA improves students’ meaningful learning, achievement, conceptual understanding, cooperative working skills, problem-solving skills, interest in the course, comprehension and intrinsic motivation, and helps them develop more positive attitudes toward the chemistry course.
The REACT strategy is one of the application strategies used in CBA, and consists of five stages: Relating, Experiencing, Applying, Cooperating and Transferring (Crawford, 2001). The ‘Relating’ stage is where new contexts are presented for learners to associate existing information with new information. The ‘Experiencing’ stage involves activities which allow learners to concretize abstract concepts. The ‘Applying’ stage motivates learners to solve problems. In the ‘Cooperating’ stage, learners work in cooperative groups and share ideas to solve problems. In the ‘Transferring’ stage, which is the last stage of the REACT strategy, learners use their acquired information in new case studies (Gilbert et al., 2011). In the literature, there are also numerous studies investigating the effect of CBA REACT strategy on students’ learning and conceptual understanding concerning various chemistry topics.
For example, Karslı and Yigit (2017) investigated the effect of the CBA REACT strategy on high school students’ alternative conceptions and conceptual understanding of the ‘alkenes’ topic. As a result of the study, the researchers showed that the REACT strategy was effective in reducing students’ misconceptions, and that students retained the newly learned concepts in their long-term memory for a month following the application. Ultay and Calık (2016) investigated the effect of CBA on prospective science teachers’ attitudes toward concepts in the ‘acids and bases’ topic and the chemistry course. They used different teaching designs (the REACT strategy, 5E learning model and traditional learning). The researchers found that the CBA REACT strategy, in particular, helped students learn new topics and retain newly acquired information in their long-term memory.
The purpose and importance of the study
As seen in the studies using CBA and CBA REACT strategy, although there are studies in the literature where solubility equilibrium is taught using CBL and laboratory applications in high school and undergraduate level chemistry courses (Friedfeld et al., 1998; Cacciatore et al., 2008; Cam and Geban, 2013; Hazen and Cleary, 2014; Bellova et al., 2016), the number of studies where the topics of solutions, solubility, and solubility equilibrium are taught using everyday life contexts and CBA is limited (Ultay et al., 2015; Blonder et al., 2016).In addition, there is no study in the literature where CBA REACT strategy is used at associate level in a health-related program. Thus, the purpose of this study was to investigate the effect of the CBA REACT strategy used in relation to the topic of solubility equilibrium in the laboratory chemistry course on students’ achievement at associate level in a health-related program.
The problem statement of this study is, “What is the effect of the context-based approach REACT strategy applied in relation to the topic of solubility equilibrium in the laboratory chemistry course on the achievement of students enrolled in the Medical Laboratory Techniques (MLT) Program and the Pharmacy Services (PS) Program of the Ahmet Erdogan Vocational School of Health Services, Bulent Ecevit University?”. The sub-problem statements of this study are as follows:
• “Is there any significant difference between the experimental and control groups in terms of achievement in the equilibrium of solubility topic in the laboratory chemistry course?”
• “Is there any significant difference between the pre-test and post-test achievement scores of the experimental group in the equilibrium of solubility topic in the laboratory chemistry course?”
• “Is there any significant difference between the pre-test and post-test achievement scores of the control group in the equilibrium of solubility topic in the laboratory chemistry course?”
• “What are the views of the experimental group students about the contexts in terms of their quality, relevance to everyday life and suitability for the topic?”
• “Is there any significant difference between the experimental and control groups concerning the views obtained from semi-structured interviews?”
Method
The participants
A pre-test post-test controlled quasi-experimental research design was employed in the study (Dugard and Todman, 2006; Kırıkkaya and Bozkurt, 2012) and the participants consisted of sophomore students enrolled in the Medical Laboratory Techniques (MLT) Program (n = 47) and the Pharmacy Services (PS) Program (n = 49) of the Ahmet Erdogan Vocational School of Health Services, Bulent Ecevit University during the Fall Semester of the 2017–2018 academic year (N = 96). These are associate's degree level programs in which students acquire 5th level vocational and academic qualifications. The main purpose of these programs is to train the students as health professionals who have basic theoretical and practical information related to pharmaceutical services and medical laboratories. The students gained theoretical knowledge about solution preparation, solubility, the molar solubility of a substance which is poorly soluble in water, and concentration types in their freshman year, and put their theoretical knowledge into practice in the laboratory environment.The students from both programs had similar scores in their freshman year mid-term and final exams related to the relevant topics. Thus, the application related to solubility equilibrium was carried out in the second-year MLT 225 Laboratory Chemistry course for the Medical Laboratory Techniques program, and the second-year PS 207 Pharmacy Services Application Laboratory I course for the Pharmacy Services program. The content of both courses involved chemistry topics. The purpose of the application was to teach the students solubility equilibrium within the scope of the laboratory chemistry course.
The students enrolled in the MLT program were randomly assigned as the experimental group, while the students enrolled in the PS program were assigned as the control group. As the students from both programs had similar scores in their freshmen year exams related to the topics of solution preparation, solubility, the molar solubility of a substance which is poorly soluble in water, and concentration types, this selection was made randomly. In this research, the students were not randomly assigned to these groups; instead already the relevant programs were used (Fraenkel and Wallen, 2006). In the study, the names of the relevant programs (MLT and PS) were written on two pieces of papers and put into a box. Then a piece was drawn and assigned to the experimental group and the other program was assigned to the control group by an independent researcher.
The experimental group learned the solubility equilibrium topic with the CBA REACT strategy, whereas the control group learned the topic in a manner appropriate to conventional teaching. Written informed consent was collected from the students prior to the application. The students were explained that participation in the study was not obligatory, they were free to quit the study at any time, and there was no risk of being exposed to health-threatening chemicals during the study process.
Data collection tools
The open-ended questions in the ESAT test were the questions which were asked in the mid-term and final exams of Laboratory Chemistry courses to the students studying at the MLT and PS Programs in Bulent Ecevit University in previous years. In addition, the relevant test was applied to second grade students studying at the MLT and PS Programs during the Spring Semester (N = 128) who were taught the equilibrium of solubility topic in their previous years as a pilot test. In the data analysis, the right responses of students (including accurate or partial information) were scored as ‘1’ and the false responses (including misconception, partial information with misconception, empty or unrelated responses) were scored as ‘0’. Therefore, the discriminatory (r) and difficulty indices (p) of the ESAT test items were analyzed. As a conclusion, it was calculated that the mean item discriminatory index was 0.92 and the mean item difficulty index (p) was 0.51. The reliability coefficient of the test (Kuder–Richardson 20) was found to be 0.921. In the light of these data, it was concluded that the reliability of ESAT test was high and it had medium difficulty and very good discrimination power.
Data analysis
The open-ended questions in ESAT were analysed using the content analysis method. The answers given by the students to the open-ended questions were categorized and scored separately by two experts specializing in analytical chemistry. The categories included Sound Understanding (SU), Partial Understanding (PU), Specific Misconception with Partial Understanding (SMPU), Specific Misconception (SM), and Incomprehension/Unanswered (UA) (Karslı and Yigit, 2015; Gunter and Kılınc Alpat, 2017). Accurate and scientific answers given by the students to the open-ended questions were assigned to the SU category; answers indicating partial understanding were assigned to the PU category; answers showing partial understanding with misconceptions were assigned to the SMPU; answers showing complete misconceptions were assigned to the SM category; answers indicating incomprehension, or blank answers, were assigned to the UA category. Answers in the SU category were scored as 4 points; answers in the PU category were scored as 3 points; answers in the SMPU category were scored as 2 points; answers in the SM category were scored as 1 point; and answers in the UA category were scored as 0 points. Therefore, the minimum and maximum scores obtained from the ESAT test were 0.00 and 40.00, respectively. The average agreement percentage between the two analytical chemistry experts regarding the classification and scoring was calculated to be 0.88. The statistical analysis of the scores obtained by the students in the ESAT was performed with the SPSS 19.0 software package. The normal distribution of the test scores was evaluated with the Shapiro Wilk test (Akbulut, 2011; Buyukozturk, 2011; Secer, 2015). The Wilcoxon test was employed to reveal whether or not there was a significant difference within the groups in terms of pre-test and post-test scores. The Mann Whitney U test was employed to determine whether or not there was a significant difference between the groups in terms of pre-test and post-test scores (Pearson and Hartley, 1958; Pallant, 2001; Buyukozturk, 2011). In all statistical comparisons, results were accepted to be statistically significant for p < 0.05.Structured and semi-structured interviews were analyzed by two analytical chemistry experts using the content analysis separately and the average percentage of agreement between the experts was calculated. Therefore, the first stage of content analysis was that coding the data obtained from the structured and semi-structured interviews. These codes were collected under certain themes and then a code list was formed independently by the two analytical chemistry experts. After forming the code list, the average percentage was calculated by the formula P = Na × 100/Na + Nd (Na = the amount of agreement; Nd = the amount of disagreement; P = the percentage of agreement) (Robson, 2015). The average percentage of agreement was found to be 0.80 and 0.85 for structured and semi-structured interviews, respectively. These data were expressed by frequency and percentage.
In addition, 20 students were randomly selected from both groups for the semi-structured interviews and these random selections from both groups were made according to the students’ scores in the ESAT post-test. Firstly, the mean scores, minimum and maximum scores in the post-test were determined and secondly the ranges of scores were distributed to the three groups in accordance with the students whose scores were low, medium and high for both groups. This selection was made according to stratified rational sampling technique. And then, each student from both groups whose scores were low, medium and high was selected in accordance with the simple random sampling technique by using random numbers table (Suresh, 2011; Christensen et al., 2015).
For the experimental group students’ mean score in the post-test was found to be 35.6170 and when the post-test scores were examined, it was determined that the minimum and maximum scores of students were 26.00 and 40.00, respectively. Therefore, eight students whose scores were between 26.00 and 34.00; six students whose scores were between 35.00 and 36.00 and six students whose scores were between 37.00 and 40.00, were randomly selected.
For the control group, the students’ mean score in the same post-test was found to be 25.8776. For the post-test scores, it was determined that the minimum and maximum scores of the control group students were 1.00 and 39.00, respectively. Therefore, eight students whose scores were between 1.00 and 24.00; six students whose scores were between 25.00 and 27.00 and six students whose scores were between 28.00 and 39.00, were also randomly selected for the semi-structured interviews.
Creation of contexts
Two contexts were developed after receiving opinions from two experts specializing in analytical chemistry and reviewing the relevant literature (Meyer, 1925; Skoog et al., 1996; Gunduz, 2008; Tez, 2010a, 2010b, 2017; Wexler, 2015 ; Bellova et al., 2016; Nakiboglu, 2016) (see Appendix 4). The context ‘Archaeological Excavations in Egypt’ was based on Candy the Archaeologist and her colleagues finding samples of kohl in excavations in the Faiyum Region of Ancient Egypt, and discovering that the main component of the kohl is galena as a result of laboratory analysis. The context ‘Pale-faced Roman Women’ was based on Candy the Archaeologist finding high concentrations of lead in the remains of Roman corpses in her previous archaeological excavations, and learning from History of Science Professor Hawkins that Roman women used to use powdered cerussite to make their faces look pale. The content of the contexts included the same sub-topics with ESAT.Implementation of CBA REACT strategy
The experimental group students were given information about the CBA REACT strategy, the application process, the roles of the tutor and the students, and it was explained that participation in the study was not obligatory, they were free to quit the study at any time and there was no risk of them being exposed to health-threatening chemicals during the study process, after which their written informed consent was received. Then, the ESAT was applied to the students as a pre-test. Following the pre-test, the students were randomly assigned to eight groups of five and one group of seven. This random assignment was made in accordance with the experimental group students’ scores obtained from the ESAT pre-test. Since the students had similar scores in the pre-test, the students were distributed to nine groups by block randomization method. When this process was done, random numbers between 1 and 9 were generated with Minitab 16.0 package program and each student was placed in the corresponding block (Suresh, 2011; Buyukozturk et al., 2016).In the ‘Relating’ stage of the REACT strategy, the contexts were handed out, and the students in the groups were asked comment about them.
In the ‘Experiencing’ stage, the tutor reminded them about experiments related to solution preparation, solubility, the molar solubility of a substance which is poorly soluble in water and concentration types (how to calculate calcium hydroxide solution's (Ca(OH)2) molar solubility, how to prepare copper(II) sulphate (CuSO4) solution, how to prepare acetic acid (CH3COOH) solution) that the students had performed previously to refresh their memories.
In the ‘Applying’ stage, the tutor asked the students to solve the problems given in the contexts with their group mates.
In the ‘Cooperating’ stage, the tutor gave the students research topics, and asked them to prepare them for the next class. The research topics included:
• How to calculate the solubility product of a substance which is poorly soluble in water (Group I),
• How to calculate the solubility of a substance which is poorly soluble in water based on its solubility product (Group II),
• The effects of ionic strength on the solubility of a substance which is poorly soluble in water (Group III and IV),
• The effects of hydrogen ion on the solubility of a substance which is poorly soluble in water (Group V),
• The effects of hydrolyzing on the solubility of a substance which is poorly soluble in water (Group VI and VII),
• The metabolism of lead in body, its toxic effect, its minimum lethal dose and the effects of using lead in water transmission pipes (Group VIII and IX).
After their research, each group presented their research findings in the intra-classroom discussion environment under the guidance of the tutor and answered questions in the contexts.
In the ‘Transferring’ stage, the tutor asked each group about the effects of physical and chemical environmental conditions on the formation and sedimentation of travertine.
After the application, the ESAT was applied as post-test. The Structured Interview Form was also applied to reveal the views of the students about the quality of the scenarios. The application was carried out for 11 course hours. At the end of the application, semi-structured interviews were held with 20 randomly selected students to reveal their views about the instruction and group work.
In addition, throughout the application, the tutor played multiple roles as a ‘facilitator’, an ‘observer’, a ‘guide’ and an ‘evaluator’, facilitating students’ collaborative work and their research, encouraging them to contribute to the learning process and enabling them to be actively involved in the discussion environment.
Implementation of conventional teaching
Prior to the application, the control group students were given information about the application and their informed consent was received. Then the ESAT was applied to the students as a pre-test. Conventional teaching is not a ‘tutorial process’, since the instructor transmits his/her expert knowledge to the students and assumes a position of authority. Therefore, the students were taught how to calculate the solubility equilibrium, solubility, the solubility product of a substance which is poorly soluble in water, the effects of hydrogen ion and ionic strength on the solubility, and the effects of anion hydrolysis on the solubility using the lecture-based conventional question–answer method. Then the ESAT was applied as post-test. Following the ESAT, semi-structured interviews were held with 20 randomly selected students to reveal their views about the instruction. The conventional application was carried out for the same length of time as the CBA application (11 course hours) and by the same researcher. The researcher especially took into account the change of role from a facilitator in CBA application to a knowledge transformer in conventional teaching.Results and discussion
Results concerning the contexts
The results concerning the contexts were given in Table 1 according to the stages of REACT strategy:| Stages | ‘Archaeological Excavations in Egypt’ | ‘Pale-faced Roman Women’ |
|---|---|---|
| Relating | Students were given the relevant contexts and they were asked to read and comment. | |
| This context was based on Candy the Archaeologist and her colleagues finding samples of kohl during their excavation in Egypt. After some analyses carried out on the samples, it was found that the main component of the kohl was galena. | The context titled ‘Pale-faced Roman Women’ was based on Candy the Archaeologist finding high concentrations of lead in remains of Roman corpses in her previous archaeological excavations, and learning from History of Science Professor Hawkins that Roman women used to use powdered cerussite, lead carbonate [2PbCO3·Pb(OH)2], to make their faces look pale, and that lead was used for cosmetics and water transmission in the Roman Empire. | |
| Experiencing | The students were reminded about experiments concerning solution preparation, solubility, the molar solubility of a substance which is poorly soluble in water and concentration types that they had performed previously. | |
| Applying | The experimental group students were asked to calculate the solubility of galena (lead(II) sulphide ‘PbS’), the main component of kohl when applied to skin, and the solubility of galena in water and salt water when the kohl is removed using water or salt water. Additionally, they were asked to calculate the solubility of galena in water assuming no external effects. | The students were asked to calculate cerussite's solubility when applied to the skin, cerussite's solubility when removed using water and salt water, and cerussite's solubility without an external effect. Additionally, the students researched how water transmission by lead pipes would cause lead poisoning. |
| Cooperating | Each group were given research topics and were asked to prepare them for the next class. After their research, each group presented their research findings in discussion environment under the guidance of the tutor and answered questions in the contexts. | |
| The solubility of PbS on skin | The solubility of PbCO3 on skin | |
| As a result of their research, the students found the skin's pH value to be approximately 5.5, the concentration of the hydrogen ion (H+) to be 3.16 × 10−6 M, the constant of solubility product of PbS (Ksp) to be 3.4 × 10−28, and the resulting hydrogen sulphide (H2S) to be Ka1 = 9.6 × 10−8 and Ka2 = 1.3 × 10−14, and correctly calculated the solubility of PbS on skin as shown below: | First of all, the students calculated the hydroxyl (OH−) ion concentration in the environment and the hydrogen (H+) concentration when cerussite is applied to the skin based on the constant of concentration product of lead(II) oxide (PbO): | |
| PbS(s) ⇌ Pb2+(aq) + S2−(aq) Ksp = [Pb2+][S2−] = 3.4 × 10−28 | PbO(s) + H2O ⇌ Pb(aq)2+ + 2OH(aq)− Ksp = 8.1 × 10−19 (yellow lead) | |
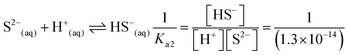
|
K sp = [Pb2+][OH−]2 = s × (2s)2 = 4s3 = 8.1 × 10−19 | |
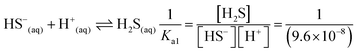
|
s = 5.87 × 10−7 M, [OH−] = 1.18 × 10−6 M | |
| The students achieved the following formula for mass balance: | Then, the students subtracted the concentration of hydroxyl ion (1.18 × 10−6 M) from the concentration of hydrogen ion (3.16 × 10−6), which they had found based on the skin's pH, and calculated the concentration of [H+] ion remaining in the environment as 1.982 × 10−6 M. | |
| [Pb2+] = [S2−] + [HS−] + [H2S] | Then the students correctly calculated the solubility of lead(II) carbonate in the environment where the concentration of [H+] ion is 1.982 × 10−6 M: | |
| As the first solution path, the students wrote the mass balance formula in terms of [S2−] based on the S2− ion's decomposition percentage: | PbCO3(s) ⇌ Pb2+(aq) + CO32−(aq) Ksp = 7.4 × 10−14 | |
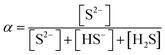
|
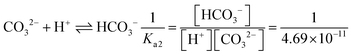
|
|
|
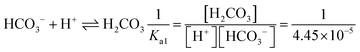
|
|
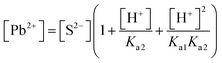
|
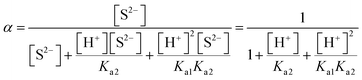
In the [Pb2+] = [CO32−] + [HCO3−] + [H2CO3] mass balance, the students neglected the [H2CO3] concentration as well as [CO32−] and [HCO3−] concentrations, obtained the [Pb2+] = [H2CO3] equation, and multiplied the constants in bicarbonate ion and carbonic acid formation equations. In this way, the students correctly found what to write in place of the [CO32−] ion concentration: | |
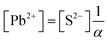
|
||
| K sp = [Pb2+][S2−] = s × sα = s2α |
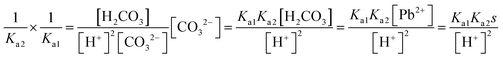
|
|
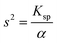
|
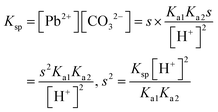
|
|
| Based on this, the students calculated the α value taking [H+] ion's concentration as 3.16 × 10−6, and found the solubility to be: |
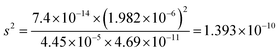
|
|
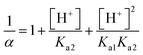
|
s = 1.180 × 10−5 M | |
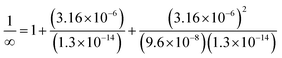
|
From this, the students calculated PbCO3's solubility in grams as 6.306 × 10−6 grams using molarity and the mole number formula, and correctly stated that it would not cause a toxic effect: | |
| α = 1.213 × 10−10 |
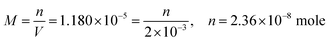
|
|
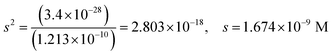
|
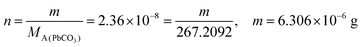
|
|
| As the second solution path, the students calculated the solubility of PbS based on negligible aspects of mass balance as a result of their research. The students wrote the mass balance as [Pb2+] = [H2S] by neglecting the [H2S] concentration as well as [S2−] and [HS−] concentrations. In this way, the students multiplied the acidity constants in ionization of H2S in water: | ||
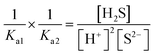
|
||
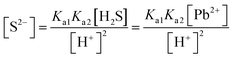
|
||
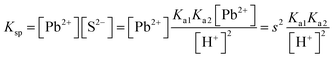
|
||
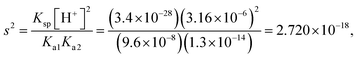
|
||
| s = 1.649 × 10−9 M | ||
| The students generally preferred the second path to calculate the effect of hydrogen ion on the solubility of PbS. Then they calculated the amount of lead in 2 mL mixture: | ||
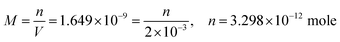
|
||
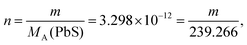
|
||
| m = 7.89 × 10−10 g | ||
| As a result of their research, the students found the acute lethal dose of inorganic water-soluble lead substances to be 20 grams, and the minimum lethal dose to be 10 grams for a 70 kg person. Accordingly, the students correctly concluded that the soluble amount of PbS in solution (7.89 × 10−10 grams) with a pH value of 5.5 would not cause a toxic effect. | ||
| The effect of anion hydrolysis on solubility | ||
| The students correctly calculated the anion hydrolysis formula of PbS when the kohl is removed with water as follows: | Similarly, the students calculated the solubility of cerussite when removed with water. Based on the hydrogen ion concentration (1.982 × 10−6 M), which they had calculated based on the concentration product of PbO, the students found the hydroxyl ion concentration to be 5.05 × 10−9 M. Then the students correctly found the cerussite's solubility in water considering the anion hydrolysis: | |
| As the first solution path; | ||
| PbS(s) ⇌ Pb(aq)2+ + S(aq)2− Ksp = [Pb2+][S2−] = 3.4 × 10−28 | ||
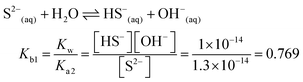
|
||
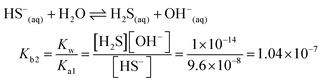
|
PbCO3(s) ⇌ Pb2+(aq) + CO32−(aq) Ksp = 7.4 × 10−14 | |
| [Pb2+] = [S2−] + [HS−] + [H2S] |
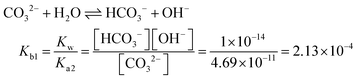
|
|
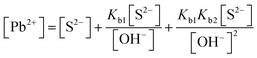
|
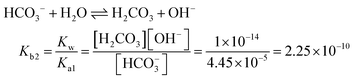
|
|
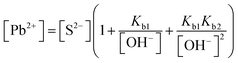
|
In the [Pb2+] = [CO32−] + [HCO3−] + [H2CO3] mass balance, the students neglected the [H2CO3] concentration as well as [CO32−] and [HCO3−] concentrations: | |
| From this, the students ignored the skin's pH and continued the calculation by taking [OH−] concentration as 1 × 10−7 M: |
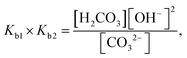
|
|
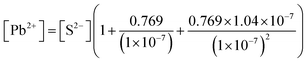
|
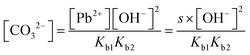
|
|
[Pb2+] = [S2−] × 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 687 687![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601 601 |
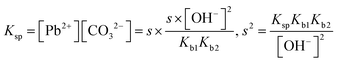
|
|
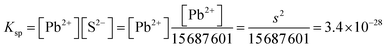
|
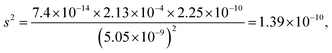
|
|
| s 2 = 5.33 × 10−21, s = 7.30 × 10−11 M | s = 1.18 × 10−5 M | |
| As the second solution path; | Using molarity and the mole number formulas, the students found the solubility to be 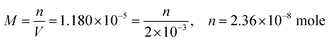 , , 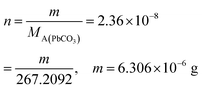 , and correctly stated that it would not cause a toxic effect. , and correctly stated that it would not cause a toxic effect. |
|
| PbS(s) ⇌ Pb2+(aq) + S2−(aq) Ksp = [Pb2+][S2−] = 3.4 × 10−28 | ||
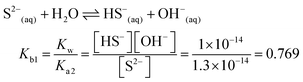
|
||
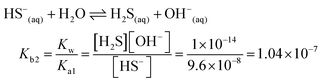
|
||
| The students rewrote the mass balance as [Pb2+] = [H2S] by neglecting the [H2S] concentration as well as [S2−] and [HS−] concentrations in [Pb2+] = [S2−] + [HS−] + [H2S] mass balance. In this way, the students multiplied the alkalinity constants in ionization of S2− in water: | ||
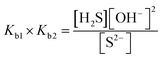
|
||
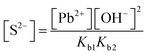
|
||
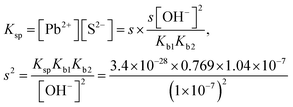
|
||
| s 2 = 2.719 × 10−21, s = 5.215 × 10−11 M | ||
| The students generally preferred the second path to calculate the effect of hydrolyzing on the solubility of PbS. Then they calculated the amount of lead in 2 mL mixture: | ||
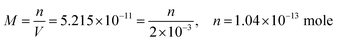
|
||
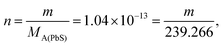
|
||
| m = 2.495 × 10−11 g | ||
| Accordingly, the students correctly concluded that the soluble amount of PbS in water (2.495 × 10−11 grams) would not cause a toxic effect. | ||
| The effect of ionic strength and activity coefficient on solubility | ||
| The students correctly calculated PbS’ solubility when the kohl is removed with salt water (0.03 M NaCl solution) using the ionic strength and activity coefficient formulas as follows: | The students correctly calculated PbCO3's solubility when the cerussite is removed with salt water (0.05 M NaCl solution) using the ionic strength and activity coefficient formulas as follows: | |
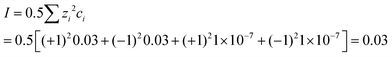
|
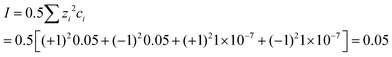
|
|
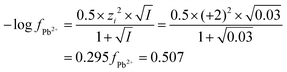
|
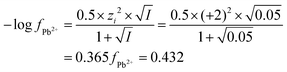
|
|
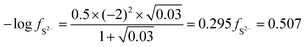
|
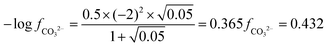
|
|
| K sp = aPb2+ × aS2− = fPb2+[Pb2 +] × fS2−[S2−] | K sp = aPb2+ × aCO32− = fPb2+[Pb2+] × fCO32−[CO32−] | |
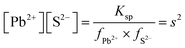
|
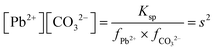
|
|
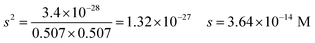
|
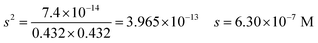
|
|
| From this, the students calculated PbS’ solubility in grams as 1.740 × 10−14 grams using molarity and the mole number formula, and correctly stated that it would not cause a toxic effect: | From this, the students calculated PbCO3's solubility in grams as 3.365 × 10−7 grams using molarity and the mole number formula, and correctly stated that it would not cause a toxic effect: | |
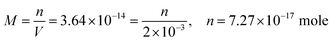
|
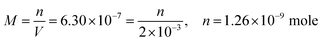
|
|
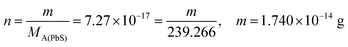
|
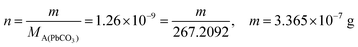
|
|
| Solubility without an external effect | ||
| Finally, the students correctly calculated the solubility of PbS, the main component of galena, in water without an external effect using the constant of solubility product formula: | The students correctly calculated the solubility of cerussite without an external effect using the constant of solubility product: | |
| PbS(s) ⇌ Pb2+(aq) + S2−(aq) Ksp = [Pb2+][S2−] = s2 = 3.4 × 10−28 | K sp = [Pb2+] × [CO32−] = s2 = 7.4 × 10−14, s = 2.72 × 10−7 M | |
| s = 1.84 × 10−14 M | Additionally, the students found as a result of their research that lead pipes were not suitable for water transmission. This is because oxygen and carbon dioxide gases dissolved in water solve the lead to a certain degree, which causes a contamination with lead powder, albeit in low levels. There should not be any lead compounds in drinking water since they are harmful to human health. | |
| Transferring | In addition, the students stated that travertine is formed as a result of deposition of calcium ion in water as calcium carbonate due to the reduced solubility of carbon dioxide in underground water induced by environmental conditions (Heimann and Sass, 1989; Polat, 2011), which indicates that they were able to present newly acquired information in new cases. | |
| Categories | SUa | PUb | SMPUc | SMd | UAe | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Questions | ff | %g | ff | %g | ff | %g | ff | %g | ff | %g |
| a SU = sound understanding. b PU = partial understanding. c SMPU = specific misconception with partial understanding. d SM = specific misconception. e UA = incomprehension/unanswered. f f = frequency. g % = percentage. | ||||||||||
| Experimental group (n = 47) | ||||||||||
| 1 | 0 | 0.0 | 10 | 21.3 | 0 | 0.0 | 0 | 0.0 | 37 | 78.7 |
| 2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| 4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| 5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| 6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| 7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| 8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| 9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| 10 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 47 | 100.0 |
| Total | 0 | 0.0 | 10 | 2.1 | 0 | 0.0 | 0 | 0.0 | 460 | 97.9 |
| Control group (n = 49) | ||||||||||
| 1 | 0 | 0.0 | 5 | 10.2 | 0 | 0.0 | 6 | 12.2 | 38 | 77.6 |
| 2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| 4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| 5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| 6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| 7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| 8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| 9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| 10 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 49 | 100.0 |
| Total | 0 | 0.0 | 5 | 1.0 | 0 | 0.0 | 6 | 1.2 | 479 | 97.8 |
Additionally, ESAT pre-test content analysis showed that ten students in the experimental group and five students in the control group gave answers in the PU category, whereas six students in the control group gave answers in the SM category to the first question. Considering the number of answers in the PU category given by students in both groups to the first question, it was seen that the students were able to perform mole number and molarity calculations based on the solubility of a poorly soluble substance; they were not able to find the solubility product using the solubility product balance formula. Considering the answers in the SM category given by the control group students, it was observed that the students wrote mole number and molarity formulas incorrectly.
The results of the content analysis applied to the ESAT post-test answers of the experimental and control group students as frequency and percentage (Table 3).
| Categories | SUa | PUb | SMPUc | SMd | UAe | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Questions | ff | %g | ff | %g | ff | %g | ff | %g | ff | %g |
| a SU = sound understanding. b PU = partial understanding. c SMPU = specific misconception with partial understanding. d SM = specific misconception. e UA = incomprehension/unanswered. f f = frequency. g % = percentage. | ||||||||||
| Experimental group (n = 47) | ||||||||||
| 1 | 19 | 40.4 | 10 | 21.3 | 2 | 4.3 | 7 | 14.9 | 9 | 19.1 |
| 2 | 34 | 72.3 | 8 | 17.0 | 0 | 0.0 | 2 | 4.3 | 3 | 6.4 |
| 3 | 32 | 68.0 | 10 | 21.3 | 2 | 4.3 | 0 | 0.0 | 3 | 6.4 |
| 4 | 33 | 70.2 | 0 | 0.00 | 7 | 14.9 | 6 | 12.75 | 1 | 2.15 |
| 5 | 33 | 70.2 | 6 | 12.75 | 2 | 4.3 | 6 | 12.75 | 0 | 0.00 |
| 6 | 42 | 89.4 | 5 | 10.6 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 7 | 43 | 91.5 | 0 | 0.00 | 4 | 8.5 | 0 | 0.00 | 0 | 0.00 |
| 8 | 46 | 97.9 | 0 | 0.00 | 1 | 2.1 | 0 | 0.00 | 0 | 0.00 |
| 9 | 45 | 95.8 | 0 | 0.00 | 2 | 4.2 | 0 | 0.00 | 0 | 0.00 |
| 10 | 47 | 100.0 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Total | 374 | 79.6 | 39 | 8.3 | 20 | 4.3 | 21 | 4.5 | 16 | 3.3 |
| Control group (n = 49) | ||||||||||
| 1 | 29 | 59.2 | 8 | 16.3 | 6 | 12.2 | 2 | 4.1 | 4 | 8.2 |
| 2 | 45 | 91.8 | 2 | 4.1 | 0 | 0.0 | 0 | 0.0 | 2 | 4.1 |
| 3 | 22 | 44.9 | 12 | 24.5 | 2 | 4.1 | 1 | 2.0 | 12 | 24.5 |
| 4 | 15 | 30.6 | 1 | 2.0 | 14 | 28.6 | 13 | 26.6 | 6 | 12.2 |
| 5 | 18 | 36.7 | 11 | 22.4 | 4 | 8.2 | 6 | 12.2 | 10 | 20.5 |
| 6 | 39 | 79.6 | 0 | 0.0 | 0 | 0.0 | 2 | 4.1 | 8 | 16.3 |
| 7 | 20 | 40.8 | 10 | 20.4 | 2 | 4.1 | 0 | 0.00 | 17 | 34.7 |
| 8 | 12 | 24.5 | 2 | 4.1 | 13 | 26.5 | 8 | 16.3 | 14 | 28.6 |
| 9 | 15 | 30.6 | 6 | 12.2 | 3 | 6.2 | 8 | 16.3 | 17 | 34.7 |
| 10 | 7 | 14.3 | 33 | 67.4 | 0 | 0.0 | 0 | 0.0 | 9 | 18.3 |
| Total | 222 | 45.3 | 85 | 17.4 | 44 | 9.0 | 40 | 8.2 | 99 | 20.1 |
According to ESAT post-test content analysis, the frequency of answers in the SU category was higher for the experimental group students (f: 374, 79.6%) than for the control group students (f: 222, 45.3%), whereas the frequency of answers in other categories (PU, SMPU, SM, UA) was lower compared to the control group. This finding indicates that the CBA REACT strategy improved students’ sound understanding of the topic of solubility equilibrium. The majority of the experimental group students had a higher frequency of answers in the SU category than in the other categories (PU, SMPU, SM, UA) for all questions, whereas the majority of the control group students gave answers in the SU category to questions 1–7, answers in the UA category to questions 8 and 9, and answers in the PU category to question 10. This finding indicates that the control group students did not understand the effects of the hydrogen ion and anion hydrolysis on the solubility of a poorly soluble substance, and partially understood the metabolism of lead in human body, its toxic effect, and its minimum lethal dose.
In the ESAT post-test, it was observed that the frequency of answers in the SMPU and SM categories was lower in the experimental group (SMPU, f: 20, 4.3%; SM, f: 21, 4.5%) than in the control group (SMPU, f: 44, 9.0%; SM, f: 40, 8.2%). This finding indicates that the CBA REACT strategy reduced students’ misconceptions about the topic of solubility equilibrium. According to the ESAT post-test content analysis, some students from both groups gave answers in the SMPU category to questions 1, 3, 4, 5, 7, and 9. A review of the answers in this category showed that mole number calculations were correct, but there were mistakes in molarity calculations in the first question. In the third and the seventh questions, the students correctly calculated the ionic strength and activity coefficient; however, they did not square the activity coefficient in the Ksp equation. In the fourth and eight questions regarding the effects of the hydrogen ion on solubility, the students correctly wrote the 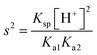 formula; however, they either did not square the hydrogen ion concentration in calculations or incorrectly calculated the hydrogen ion from the given pH value. In the fifth and ninth questions regarding the anion hydrolysis, the students once again correctly wrote the
formula; however, they either did not square the hydrogen ion concentration in calculations or incorrectly calculated the hydrogen ion from the given pH value. In the fifth and ninth questions regarding the anion hydrolysis, the students once again correctly wrote the 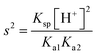 formula; however, they either did not square the hydrogen ion concentration in calculations or did not take the hydroxyl ion concentration as 1 × 10−7 M, but used the hydrogen ion concentration which they had calculated from the pH value given in the previous question.
formula; however, they either did not square the hydrogen ion concentration in calculations or did not take the hydroxyl ion concentration as 1 × 10−7 M, but used the hydrogen ion concentration which they had calculated from the pH value given in the previous question.
In the ESAT post-test, the experimental group gave answers in the SM category to questions 1–2 and 4–5, whereas the control group gave answers in the SM category to questions 1, 3–6 and 8–9. In the first question, both the experimental group and the control group students confused the molarity formula with the mole number formula, and incorrectly expressed molarity as 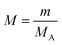 .
.
The experimental group students incorrectly used the effect of the hydrogen ion on the solubility of sulfur in water in the second question, incorrectly used the anion hydrolysis in the fourth question regarding the effect of the hydrogen ion, and incorrectly used the effect of the hydrogen ion in the fifth question regarding anion hydrolysis. The control group students wrote the formulas for anion hydrolysis, ion effect, and hydrogen ion effect incorrectly in questions 3–6 and 8–9, or confused the aforementioned formulas with each other. Some studies in the literature support the fact that students have misconceptions regarding the molar solubility of a substance which is poorly soluble in water, the constant of solubility product, and the effects of ionic strength, hydrolysis and pH value on solubility (Schmidt, 1991; Onder and Geban, 2006; Orwat et al., 2017; Rahmi et al., 2017).
| ESAT | Experimental group (n = 47) | Control group (n = 49) | ||
|---|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | U | p | |
| Pre-test | 0.00 (0–0) | 0.00 (0–0) | 1135 | 0.867 |
| Post-test | 37.00 (33–39) | 27.00 (20.5–32) | 374.5 | <0.001 |
| Groups | Pre-test | Post-test | z | p |
|---|---|---|---|---|
| Experimental | 0.00 (0–0) | 37.00 (33–39) | −5.977 | <0.001 |
| Control | 0.00 (0–0) | 27.00 (20.5–32) | −6.095 | <0.001 |
The data in Table 4 shows that there was no statistically significant difference between the ESAT pre-test scores of the experimental group and the control group students (U = 1135, p > 0.05); however, there was a significant difference between ESAT post-test scores of the groups in favor of the experimental group (U = 374.5, p < 0.05). The results of the Wilcoxon test given in Table 5 shows that both the experimental group and the control group had a statistically significant change in their ESAT pre-test and post-test scores (z = −5.997, p < 0.001; z = −6.095, p < 0.001, respectively). In light of these findings, it was concluded that post-test scores were higher in both groups compared to pre-test scores; however, the increase was higher in the experimental group than in the control group. This finding indicates that the CBA REACT strategy improved students’ achievement regarding the topic of solubility equilibrium.
Although there are numerous studies in the literature reporting that CBA and the CBA REACT strategy improve students’ conceptual understanding, achievement and learning in various chemistry topics at high school and undergraduate level (Cigdemoglu, 2012; Ultay and Calık, 2016; Karslı and Yigit, 2017), there is no study on the effect of the CBA REACT strategy on students’ achievement in the topic of solutions and solubility equilibrium at associate level.
However, there are studies in the literature on the effect of CBA and the CBA REACT strategy on students’ views and conceptual understanding of the relevant topics.
For example, Blonder et al. (2016) developed a course based on a context related to everyday life titled ‘The Story of Lead’ at high school level and attempted to find out the views of teachers and students about the application. The context developed for their study was based on Romans’ use of lead for water pipes, tea pots and wine cups, which was thought to be one of the reasons behind the fall of the Roman Empire. The purpose was to create a discussion environment in which students could assess the use of lead from ethical, social and environmental standpoints using this historical context. The result of the study showed that teachers gained a more critical outlook on the world, while students participated more actively.
Ultay et al. (2015) investigated the effect of the REACT strategy on students’ understanding of solution chemistry at undergraduate level. The researchers found that the REACT strategy was effective in reducing students’ misconceptions in relation to solution chemistry.
| Positive views | Negative views | ||||
|---|---|---|---|---|---|
| Main theme-1: ‘Archaeological Excavations in Egypt’ | f | % | Main theme-1: ‘Archaeological Excavations in Egypt’ | f | % |
| Sub-themes: | 130 | 99.2 | Sub-themes: | 1 | 0.8 |
| Completely positive | 34 | 26.2 | Difficulty in using calculator | 1 | 100.0 |
| Understandable/informative | 32 | 24.6 | |||
| Relevant to everyday life | 20 | 15.4 | |||
| Catchy | 17 | 13.1 | |||
| Interesting | 14 | 10.8 | |||
| Research | 13 | 9.9 | |||
| Main theme-2: suitability for the topic | f | % | Main theme-2: suitability for the topic | f | % |
| Sub-themes: | 51 | 100.0 | Sub-themes: | 0 | 0.00 |
| Suitable | 45 | 88.2 | None | 0 | 0.00 |
| Understandable/informative | 6 | 11.8 | |||
| Main theme-3: relevance to everyday life | f | % | Main theme-3: relevance to everyday life | f | % |
| Sub-themes: | 46 | 93.9 | Sub-themes: | 3 | 6.1 |
| Relevant | 43 | 93.5 | Irrelevant | 3 | 100.0 |
| Understandable/informative | 3 | 6.5 | |||
| Main theme-4: sufficiency | f | % | Main theme-4: sufficiency | f | % |
| Sub-themes: | 46 | 97.9 | Sub-themes: | 1 | 2.1 |
| Sufficient | 46 | 100.0 | Insufficient | 1 | 100.0 |
| Main theme-5: ‘Pale-faced Roman Women’ | f | % | Main theme-5: ‘Pale-faced Roman Women’ | f | % |
| Sub-themes: | 110 | 100.0 | Sub-themes: | 0 | 0.00 |
| Completely positive | 40 | 36.4 | None | 0 | 0.00 |
| Understandable/informative | 22 | 20.0 | |||
| Relevant to everyday life | 14 | 12.7 | |||
| Research | 12 | 10.9 | |||
| Interesting | 12 | 10.9 | |||
| Group work | 6 | 5.5 | |||
| Developing a different perspective | 4 | 3.6 | |||
| Main theme-6: suitability for the topic | f | % | Main theme-6: suitability for the topic | f | % |
| Sub-themes: | 53 | 100.0 | Sub-themes: | 0 | 0.00 |
| Suitable | 47 | 88.7 | None | 0 | 0.00 |
| Understandable/informative | 6 | 11.3 | |||
| Main theme-7: relevance to everyday life | f | % | Main theme-7: relevance to everyday life | f | % |
| Sub-themes: | 48 | 94.1 | Sub-themes: | 3 | 5.9 |
| Relevant | 44 | 91.7 | Irrelevant | 3 | 100.0 |
| Catchy | 4 | 8.3 | |||
| Main theme-8: sufficiency | f | % | Main theme-8: sufficiency | f | % |
| Sub-themes: | 45 | 95.8 | Sub-themes: | 2 | 4.2 |
| Sufficient | 45 | 100.0 | Insufficient | 2 | 100.0 |
| Total (positive views) | 529 | 98.1 | Total (negative views) | 10 | 1.9 |
As shown in Table 6, the majority of the students found the contexts to be completely positive, memorable, understandable, informative, suitable for the topic, interesting and relevant to everyday life, and expressed positive views (f: 529, 98.1%). There are studies in the literature which report that scenarios improve students’ interest in the course, enhance their enthusiasm and satisfaction, and allow them to associate chemistry topics with everyday life (Kegley et al., 1996; Mihok et al., 2006; Potter and Overton, 2006; Cigdemoglu, 2012; Mandler et al., 2012; Karslı and Yigit, 2017).
Only 10 students stated that they found it difficult to use the calculator, found the contexts to be inadequate, could not relate the topic with everyday life, and expressed negative views. Additionally, the majority of the students stated that the contexts were adequate when asked about how the scenarios could be improved. A few students suggested that additional examples could be included in the scenarios:
“The context titled ‘Archaeological Excavations in Egypt’ was adequate. Since it is one of the most suitable contexts for the solubility equilibrium that I can think of, I don’t think it would be possible to improve it any further. It was a perfect fit.” (EG-S6)
“We learned the toxic effect of galena in the context titled ‘Archaeological Excavations in Egypt’, so it was adequate.” (EG-S14)
“The ‘Pale-faced Roman Women’ could be improved with different scenarios.” (EG-S15)
“The context titled ‘Archaeological Excavations in Egypt’ could be improved with a few additional examples.” (EG-S36)
The majority of the students answered the question, “What sort of material would you design for the solubility equilibrium topic?” by stating that they would design context-based material similar to that used in this research. Additionally, eight students suggested designing different materials such as animations, presentations, visual elements and models:
“If I were you, I would create animations about the topic of solubility equilibrium.” (EG-S9) (EG-S22) (EG-S25) (EG-S26)
“I would add informative presentations to the scenarios.” (EG-S11) (EG-S35)
“I would improve the visual aspect.” (EG-S16)
“A model or something similar could be used for visualization.” (EG-S19)
When asked the question, “What sort of context would you develop for the solubility equilibrium topic?”, the majority of the students replied that they would use the same contexts. Two students suggested that they would use caves and ‘fairy chimneys’ (hoodoos found in Cappadocia, Turkey) as scenarios:
“We could use a context about caves. It could be about the effect of water dripping in the cave on the skin.” (EG-S28)
“I would develop a context about ‘fairy chimneys’.” (EG-S30)
| For the experimental group | |||||
|---|---|---|---|---|---|
| Total (positive views) | Total (negative views) | ||||
| Main theme-1: CBA REACT strategy | f | % | Main theme-1: CBA REACT strategy | f | % |
| Sub-themes: | 82 | 95.4 | Sub-themes: | 4 | 4.6 |
| Completely positive | 20 | 24.4 | Calculations are challenging | 4 | 100.0 |
| Understandable/informative | 20 | 24.4 | |||
| Reinforcing/catchy | 13 | 15.9 | |||
| Fun | 11 | 13.4 | |||
| Educator's role | 11 | 13.4 | |||
| Interesting | 2 | 2.4 | |||
| Active participation | 2 | 2.4 | |||
| Communication | 2 | 2.4 | |||
| Planned/scheduled studying | 1 | 1.3 | |||
| Main theme-2: group work | f | % | Main theme-2: group work | f | % |
| Sub-themes: | 56 | 88.9 | Sub-themes: | 7 | 11.1 |
| Completely positive | 18 | 32.1 | Irresponsible friends | 3 | 42.9 |
| Information exchange | 10 | 17.9 | Communication gap | 3 | 42.9 |
| Understandable/informative | 10 | 17.9 | Individual work is better | 1 | 14.2 |
| Research | 9 | 16.05 | |||
| Discussion environment | 9 | 16.05 | |||
| Total (positive views) | 138 | 92.6 | Total (negative views) | 11 | 7.4 |
| For the control group | |||||
|---|---|---|---|---|---|
| Total (positive views) | Total (negative views) | ||||
| Main theme-1: conventional teaching | f | % | Main theme-1: conventional teaching | f | % |
| Sub-themes: | 44 | 100.0 | Sub-themes: | 0 | 0.00 |
| Completely positive | 20 | 45.5 | None | 0 | 0.00 |
| Understandable/informative | 12 | 27.25 | |||
| Teacher's role | 12 | 27.25 | |||
| Total (positive views) | 44 | 100.0 | Total (negative views) | 0 | 0.00 |
As shown in Table 7, the experimental group (f: 82) had more positive views than the control group (f: 44) regarding the instruction used in the course, whereas the majority of the experimental group students had more positive views (f: 56) than negative views (f: 11).
In general, the majority of the experimental group found the CBA REACT strategy to be memorable, understandable, interesting and fun, and thought that the group work allowed for the exchange of information, that it guided their research and provided an environment for discussion, and they expressed positive views. These findings are supported by some studies in the literature (Belt et al., 2005; Cigdemoglu, 2012; King and Ritchie, 2013).
For example, Potter and Overton (2006) used a problem/context-based approach in a course on energy metabolism for undergraduate chemistry students. The results of the study showed that students were very satisfied with the course, found the scenario to be interesting and expressed positive views, stating that this style of presentation helped their learning.
Kegley et al. (1996) developed contexts based on an environmental problem involving drinking water quality, the use of lead in local parks and recreational areas, pesticides in fruits and vegetables, and hair dyes, and applied a CBA to allow students to associate chemistry topics with everyday life. The researchers reported that such applications might improve students’ critical thinking skills and allow them to associate chemistry topics with everyday life. The negative views expressed by the experimental group students included difficulties with calculations, communication issues in group work and their friends being irresponsible. All control group students agreed on conventional teaching's being understandable and informative and on the important role of the instructor in education, and expressed positive views. It was seen that all the control group students had positive opinions about conventional teaching. In accordance with these findings, although student-centered learning approaches can lead to some increased frustration among students, it was observed that they can be effective for managing students’ affect in the classroom and can produce more robust outcomes.
Some examples from the views of the students regarding the course instruction and group work can be seen below:
“The way that the course was carried out was very positive for me, we solved each problem together, which helped me understand them, and the course was enjoyable. There were no negative aspects.” (EG-S1)
“I am pleased with the way the course was carried out. In my opinion, 100% of liking and understanding a course is down to the role of tutor. I like how you lecture and manage the class. So I try my best to understand the topics that you lecture about. You and your lecture style have a big role in this.” (EG-S6)
“It allowed us to obtain detailed information about the course topic, and study in a planned and scheduled manner. It was very positive in terms of enhancing our knowledge. There were no negative aspects.” (EG-S7)
“The course was quite enjoyable thanks to both our tutor and our communication in the classroom. To me, there were no negative aspects. Everything was understandable and informative.” (EG-S8)
“As someone who does not like chemistry at all, I believe I have made great progress in chemistry and understand it thanks to this course.” (EG-S9)
“The course was a lot of fun. However, I had difficulties with the calculations.” (EG-S10)
“Our knowledge has become more permanent. We improved what we had learned with questions. The contexts made the course quite interesting. There were no negative aspects.” (EG-S11)
“Initially, I did not understand the type of teaching used in the course, but then I understood it very well. Initially, I found it to be confusing, but then I understood the topic better with the contexts and questions.” (EG-S13)
“I do not think there were negative aspects. I believe the scenarios and questions helped us improve our knowledge of the topic.” (EG-S15)
“The positive aspects of the course were that we participated more and that the course was fun. There were no negative aspects.” (EG-S20)
“First of all, learning theoretical information from you, the tutor, allowed us to learn better. There were no negative aspects.” (CG-S1)
“The instruction was very positive. I do not think there were any negative aspects.” (CG-S5)
“It allowed us to learn theoretical information better.” (CG-S10)
“The course instruction was very good. You explained everything to us. We learned easily.” (CG-S18)
“My classmates could explain some parts that I didn’t understand, there was an environment in which we could have discussions, and we were able to share knowledge.” (EG-S1)
“Working with my group mates made the information more understandable.” (EG-S5)
“The negative aspect was that there were irresponsible people in the group. They tried to take advantage of someone else's work without trying to understand the topic first.” (EG-S10)
“As a positive, we learned together with our group mates. The negative was we couldn’t even speak to some of the people in the group.” (EG-S15)
“I think individual work would be better.” (EG-S18)
Conclusions and implications
The purpose of this study was to investigate the effect of a CBA REACT strategy used in relation to the topic of solubility equilibrium in the laboratory chemistry course on students’ achievement at associate level in a health-related program. According to the ESAT post-test content analysis, the frequency of answers in the SU category was higher for the experimental group students than for the control group students, whereas the frequency of answers in the PU, SMPU, SM, and UA categories was lower compared to the control group. This finding indicates that the CBA REACT strategy improved students’ sound understanding of the topic of solubility equilibrium. According to the results of the Mann–Whitney U and Wilcoxon tests performed to determine whether there were statistically significant differences within and between the groups in terms of ESAT pre-test and post-test scores, there was no difference between the pre-test scores of the experimental group and the control group, whereas there was a significant difference between the post-test scores of the groups in favor of the experimental group, and both groups had higher post-test scores compared to their pre-test scores. In light of these findings, it was concluded that the CBA REACT strategy improved students’ achievement regarding the topic of solubility equilibrium.It was also found in this study that some students had misconceptions regarding the molar solubility of a substance which is poorly soluble in water, the constant of solubility products, and the effects of ionic strength, hydrolysis and pH value on solubility.
In addition, the structured-interview form applied to the experimental group and the semi-structured interviews held with students randomly selected from both groups showed that the majority of the students found the contexts and the CBA REACT strategy to be memorable, understandable, interesting, fun and relevant to everyday life, and thought that group work helped information to be shared in an environment that allowed for discussion.
In summary, it was found in this study that the CBA REACT strategy used in relation to the topic of solubility equilibrium in the laboratory chemistry course improved students’ sound understanding and achievement and helped them develop positive views regarding the instruction and the quality of the contexts.
Since there is no study on the effect of a CBA REACT strategy used in relation to the topic of solubility equilibrium in a laboratory chemistry course on students’ achievement at associate level in a health-related program, it is considered that this study may be important as the first in the literature and will pave the way for future research. This research also adds some new insights into how students understand calculations with regard to solubility equilibrium.
Although this research was conducted here for the first time, there was a limitation in that no other independent variables were controlled due to the application of pre- and post-test with a control group research design. In addition, this study was centered on students’ understanding of the quantitative (symbolic/mathematical level) component of solubility equilibrium, so future studies could use the data obtained on this topic for translating between various types of representations (symbolic, submicroscopic, and macroscopic).
Conflicts of interest
There are no conflicts to declare.Appendix 1. Equilibrium of solubility achievement test (ESAT)
(1) Since PbI2 has a solubility of 70.1 mg in 100 mL water at 25 °C, please calculate the solubility product (Pb: 207.2; I: 126.9045 g mol−1)(2) Please calculate the solubility of PbS in water (for PbS; Ksp = 3.4 × 10−28)
(3) Please calculate the solubility of PbS in 0.03 M NaCl solution (for PbS; Ksp = 3.4 × 10−28)
(4) Please calculate the solubility of PbS in a pH = 5.5 environment (for H2S; Ka1 = 9.6 × 10−8; Ka2 = 1.3 × 10−14; for PbS Ksp = 3.4 × 10−28)
(5) Please calculate the solubility of PbS in water considering hydrolysis (for H2S; Ka1 = 9.6 × 10−8; Ka2 = 1.3 × 10−14; for PbS Ksp = 3.4 × 10−28)
(6) Please calculate the solubility of PbCO3 in water (PbCO3 for Ksp = 7.4 × 10−14)
(7) Please calculate the solubility of PbCO3 in 0.05 M NaCl solution (PbCO3 for Ksp = 7.4 × 10−14)
(8) Please calculate the solubility of PbCO3 in a pH = 5.5 environment (for H2CO3Ka1 = 4.45 × 10−5; Ka2 = 4.69 × 10−11; PbCO3 for Ksp = 7.4 × 10−14)
(9) Please calculate the solubility of PbCO3 in water considering hydrolysis (H2CO3Ka1 = 4.45 × 10−5; Ka2 = 4.69 × 10−11; PbCO3 for Ksp = 7.4 × 10−14)
(10) In your opinion, what sort of metabolism does lead have in human body and does it have toxic effects? If you think it does, what are these toxic effects?
Ek 1. Çözünürlük Dengesi Başarı Testi†
(1) PbI2’nin 25 °C’de 100 mL sudaki çözünürlüğü 70.1 mg olduğuna göre çözünürlük çarpımını hesaplayınız (Pb: 207.2; I: 126.9045 g mol−1)(2) PbS katısının sudaki çözünürlüğünü hesaplayınız (PbS için; Ksp = 3.4 × 10−28)
(3) PbS katısının 0.03 M NaCl çözeltisindeki çözünürlüğünü hesaplayınız (PbS için; Ksp = 3.4 × 10−28)
(4) PbS katısının pH = 5.5 olan bir ortamdaki çözünürlüğünü hesaplayınız (H2S için; Ka1 = 9.6 × 10−8; Ka2 = 1.3 × 10−14; PbS için Ksp = 3.4 × 10−28)
(5) PbS katısının sudaki çözünürlüğünü hidrolizlenmeyi göz önünde bulundurarak hesaplayınız (H2S için; Ka1 = 9.6 × 10−8; Ka2 = 1.3 × 10−14; PbS için Ksp = 3.4 × 10−28)
(6) PbCO3 katısının sudaki çözünürlüğünü hesaplayınız (PbCO3 için Ksp = 7.4 × 10−14)
(7) PbCO3 katısının 0.05 M NaCl çözeltisi içindeki çözünürlüğünü hesaplayınız (PbCO3 için Ksp = 7.4 × 10−14)
(8) PbCO3 katısının pH = 5.5 olan bir ortamdaki çözünürlüğünü hesaplayınız (H2CO3 için Ka1 = 4.45 × 10−5; Ka2 = 4.69 × 10−11; PbCO3 için Ksp = 7.4 × 10−14)
(9) PbCO3 katısının sudaki çözünürlüğünü hidrolizlenmeyi göz önünde bulundurarak hesaplayınız (H2CO3 için Ka1 = 4.45 × 10−5; Ka2 = 4.69 × 10−11; PbCO3 için Ksp = 7.4 × 10−14)
(10) Sizce kurşunun vücut içindeki metabolizması nasıldır ve toksik etkileri var mıdır? Toksik etkileri varsa nelerdir?
Appendix 2. Structured interview form
(1) What are positive and negative aspects of the context titled ‘Archaeological Excavations in Egypt’?(2) Is the context titled ‘Archaeological Excavations in Egypt’ suitable for the solubility equilibrium topic?
(3) Is the context titled ‘Archaeological Excavations in Egypt’ relevant to everyday life?
(4) Is the context titled ‘Archaeological Excavations in Egypt’ adequate? In your opinion, how can it be improved?
(5) What are positive and negative aspects of the context titled ‘Pale-faced Roman Women’?
(6) Is the context titled ‘Pale-faced Roman Women’ suitable for the solubility equilibrium topic?
(7) Is the context titled ‘Pale-faced Roman Women’ relevant to everyday life?
(8) Is the context titled ‘Pale-faced Roman Women’ adequate? In your opinion, how can it be improved?
(9) What sort of material would you design for the solubility equilibrium topic?
(10) What sort of context would you develop for the solubility equilibrium topic?
Ek 2. Yapılandırılmış Görüşme Formu†
(1) ‘Mısır’da Arkeolojik Kazılar’ durum çalışmasının olumlu ve olumsuz yönleri nelerdir?(2) ‘Mısır’da Arkeolojik Kazılar’ durum çalışması çözünürlük dengesi konusu kapsamına uygun mudur?
(3) ‘Mısır’da Arkeolojik Kazılar’ durum çalışması günlük hayatla ilişkili midir?
(4) ‘Mısır’da Arkeolojik Kazılar’ durum çalışması yeterli midir? Sizce nasıl geliştirilebilir?
(5) ‘Solgun Yüzlü Romalı Kadınlar’ durum çalışmasının olumlu ve olumsuz yönleri nelerdir?
(6) ‘Solgun Yüzlü Romalı Kadınlar’ durum çalışması çözünürlük dengesi konusu kapsamına uygun mudur?
(7) ‘Solgun Yüzlü Romalı Kadınlar’ durum çalışması günlük hayatla ilişkili midir?
(8) ‘Solgun Yüzlü Romalı Kadınlar’ durum çalışması yeterli midir? Sizce nasıl geliştirilebilir?
(9) Siz çözünürlük dengesi konusuna yönelik nasıl bir materyal tasarlardınız?
(10) Siz çözünürlük dengesi konusuna yönelik nasıl bir durum çalışması hazırlardınız?
Appendix 3. Semi-structured interviews
For the experimental group;(1) What are the positive and negative aspects of teaching the course in this manner?
(2) What are the positive and negative aspects of group work?
For the control group;
(1) What are the positive and negative aspects of teaching the course in this manner?
Ek 3. Yarı-yapılandırılmış Görüşmeler†
Deney Grubu İçin;(1) Sizce dersin bu şekilde işleyişinin olumlu ve olumsuz yönleri nelerdir?
(2) Grup çalışmasının olumlu ve olumsuz yönleri nelerdir?
Kontrol Grubu İçin;
(1) Sizce dersin bu şekilde işleyişinin olumlu ve olumsuz yönleri nelerdir?
Appendix 4. Contexts
Context-1
Candy and her teammates found galena mostly in excavations near the Red Sea. She concluded that women and men in Ancient Egypt used to dye the area around their eyes with powdered galena, which has a grey-blackish color. They used this coloring pigment by mixing it with resin, salt and specific oils. Candy had found high amounts of lead in the bones of Ancient Roman remains in some of her previous excavations. Candy shared her findings with Professor Hawkins, a History of Science Professor, and tried to learn about galena and its use in Ancient Egypt. Professor Hawkins mentioned that galena was used in the treatment of eye disorders in that period, and various sources had different ideas about the use of this material. He noted that some scientists believed that galena had been used as kohl for thousands of years, suggesting it was not toxic and did not elevate the lead level in blood, while some scientists believed that lead was a poisonous component.
Let us assume that you prepared a 2 mL mixture consisting of 1.5 mL of galena, salt, resin, and oils, and applied this mixture on your skin.
(a) Considering the solubility of galena on skin, can you share your ideas about whether or not it causes a toxic effect and, if you have any, your evidence for this with Candy and Professor Hawkins?
(b) You tried to remove the kohl using water. Considering the solubility of galena in water, can you share your ideas about whether or not it causes a toxic effect and, if you have any, your evidence for this with Candy and Professor Hawkins? (Note: You can only consider anion hydrolysis.)
(c) You tried to remove the kohl using salt water (0.03 M NaCl solution). Please calculate the solubility of galena in salt water. (Pb: 207.2; S: 32.066 g mol−1)
(d) Assuming no external effects, calculate the solubility of galena in water.
Context-2
(a) Considering the solubility of cerussite applied to the skin in powdered form, can you share your ideas about whether or not it causes a toxic effect and, if you have any, your evidence for this with Candy and Professor Hawkins? (Note: Consider PbO(k) as yellow lead.)
(b) You tried to remove the cerussite using water. Considering the solubility of cerussite in water, can you share your ideas about whether or not it causes a toxic effect and, if you have any, your evidence for this with Candy and Professor Hawkins? (Note: You can only consider anion hydrolysis.)
(c) You tried to remove the cerussite using salt water. Please calculate the solubility of cerussite in 0.05 M NaCl solution. (Pb: 207.2; C: 12.011; O: 15.9994 g mol−1).
(d) Assuming no external effects, calculate the solubility of cerussite in water.
(e) In your opinion, how could water transmission through lead pipes cause lead poisoning? Please explain.
Ek 4. Durumlar†
Durum-1
Candy ve ekibi galena cevherine çoğunlukla Kızıldeniz civarında yaptığı kazılarda rastlamıştır. Demek ki Eski Mısırlı kadınlar ve erkekler göz çevrelerindeki bölgeyi gri-siyahımsı renkteki galena cevheri tozlarıyla boyuyorlardı. Bu renk verici pigmenti reçine ve tuz eşliğinde bazı sıvı yağlarla karıştırarak kullanıyorlardı. Candy daha önce yaptığı arkeolojik kazılarda da Romalı ölülerin kemiklerinde yüksek oranda kurşuna rastlamıştı. Bunun üzerine Candy Bilim Tarihi Profesörü Prof. Dr Hawkins ile bulgularını paylaşmış ve o dönemde galena cevheriyle ilgili bilgiler öğrenmeye çalışmıştır. Prof. Dr Hawkins o dönemde galenanın göz hastalıkları tedavisinde kullanıldığını ve çeşitli kaynaklarda bu cevherin kullanımına yönelik farklı düşünceler olduğundan bahsetmiştir. Bazı bilim adamları, galenanın sürme olarak binlerce yıldan beri gözlere uygulanmasından dolayı toksik olmadığını ve kandaki kurşun düzeyini arttırmadığını düşünürken; bazı bilim adamları ise kurşunun zehirli bir bileşen olduğunu düşündüklerini belirtmiştir.
Diyelim ki, 1.5 mL'si galena cevheri ile tuz, reçine ve sıvı yağlardan oluşan 2 mL’lik bir karışım hazırladınız ve bu karışımı sürme olarak cilde uyguladınız.
(a) Galenanın cilt üzerindeki çözünürlüğünü göz önünde bulundurarak cevherin toksik etki yaratıp yaratmadığına dair düşüncelerinizi varsa ispatlarınızı arkeolog Candy ve Prof. Dr Hawkins ile paylaşır mısınız?
(b) Sürmeyi suyla çıkarmaya çalıştınız. Galenanın sudaki çözünürlüğünü göz önünde bulundurarak cevherin toksik etki yaratıp yaratmadığına dair düşüncelerinizi varsa ispatlarınızı arkeolog Candy ve Prof. Dr Hawkins ile paylaşır mısınız? (Not: Sadece anyon hidrolizlenmesini düşünebilirsiniz)
(c) Sürmeyi tuzlu suyla çıkarmaya çalıştınız (0.03 M NaCl çözeltisi). Galena cevherinin tuzlu sudaki çözünürlüğünü hesaplayınız. (Pb: 207.2; S: 32.066 g mol−1).
(d) Dışarıdan hiçbir etki olmadığını varsayarak galenanın sudaki çözünürlüğünü hesaplayınız.
Durum-2
(a) Sizce cilde toz halinde uygulanan üstübecin cilt üzerindeki çözünürlüğünü göz önünde bulundurarak toksik olup olmadığına dair düşüncelerinizi ve ispatlarınızı arkeolog Candy ve Prof. Dr Hawkins ile paylaşır mısınız? (Not: PbO(k) katısını sarı kurşun olarak düşününüz)
(b) Üstübeci suyla çıkarmaya çalıştınız. Üstübecin sudaki çözünürlüğünü göz önünde bulundurarak toksik olup olmadığına dair düşüncelerinizi ve ispatlarınızı arkeolog Candy ve Prof. Dr Hawkins ile paylaşır mısınız? (Not: Sadece anyon hidrolizlenmesini düşünebilirsiniz.)
(c) Üstübeci tuzlu suyla çıkarmaya çalıştınız. Üstübecin 0.05 M NaCl çözeltisindeki çözünürlüğünü hesaplayınız. (Pb: 207.2; C: 12.011; O: 15.9994 g mol−1)
(d) Dışarıdan hiçbir etki olmadığını varsayarak üstübecin sudaki çözünürlüğünü hesaplayınız.
(e) Sizce su sevkiyatının kurşun borular aracılığıyla yapılmasının kurşun zehirlenmesine nasıl yol açabileceğini açıklayınız.
Acknowledgements
The author wishes to thank all students who participated the study and Ahmet Erdogan Vocational School of Health Services for supporting this research. The test items, interview questions, contextual materials in this research have been translated to English by taking help from an editing service.References
- Akbulut Y., (2011), SPSS applications in social sciences, İstanbul: İdeal Yayıncılık.
- Bellova R., Melichercikova D. and Tomcik P., (2016), Calculation of conditional equilibrium in serial multiple precipitation of metal sulfides with hydrogen sulfide stream generated from sodium sulfide: a didactic tool for chemistry teaching, Quim. Nova, 39(6), 765–769.
- Belt S. T., Leisvik M. J., Hyde A. J. and Overton T. L., (2005), Using a context-based approach to undergraduate chemistry teaching: a case study for introductory physical chemistry, Chem. Educ. Res. Pract., 6(3), 166–179.
- Bennett J., (2003), Teaching and learning science, London: Bookcraft.
- Bennett J. and Lubben F., (2006), Context-based chemistry: the salters approach, Int. J. Sci. Educ., 28(9), 999–1015.
- Bennett J., Lubben F., and Hogarth S., (2007), Bringing science to life: a synthesis of the research evidence on the effects of context-based and STS approaches to science teaching, Sci. Educ., 91, 347–370.
- Blonder R., Zemler E. and Rosenfeld S., (2016), The history of lead: a context for learning about responsible research and innovation (RRI) in the chemistry classroom, Chem. Educ. Res. Pract., 17, 1145–1155.
- Bulte A. M. W., Westbroek H. B., de Jong O. and Pilot A., (2006), A research approach to designing chemistry education using authentic practices as contexts, Int. J. Sci. Educ., 28(9), 1063–1086.
- Buyukozturk S., (2011), Manual of data analysis for social sciences, Ankara: PegemAkademiYayıncılık.
- Buyukozturk S., Cakmak E. B., Akgun O. E., Karadeniz S. and Demirel F., (2016), Scientific research methods, Ankara: PegemAkademiYayıncılık.
- Cacciatore K. L., Amado J., Evans J. J. and Sevian H., (2008), Connecting solubility, equilibrium, and periodicity in a green inquiry experiment for the general chemistry laboratory, J. Chem. Educ., 85(2), 251–253.
- Cam A. and Geban O., (2013), Effectiveness of case-based learning instruction on students’ understanding of solubility equilibrium concepts, Hacettepe Univ. J. Educ., 44, 97–108.
- Christensen L. B., Johnson R. B. and Turner L. A., (2015), Research methods: Design and Analysis, Ankara: Anı Yayıncılık.
- 14Cigdemoglu C., (2012), Effectiveness of context-based approach through 5E learning cycle model on students’ understanding of chemical reactions and Energy concepts, and their motivation to learn chemistry, Doctoral Dissertation, Middle East Technical University.
- Crawford M. L., (2001), Teaching contextually: Research, rationale, and techniques for improving student motivation and achievement in mathematics and science, Waco, TX: CCI Publishing.
- De Jong O., (2006), Context-based chemical education: How to improve it?, Retrieved 05.11.2011, from http://old.iupac.org/publications/cei/vol8/0801xDeJong.pdf.
- Demircioglu H., Demircioglu G. and Calık M., (2009), Investigating the effectiveness of storylines embedded within a context-based approach: the case for the periodic table, Chem. Educ. Res. Pract., 10, 241–249.
- Dugard P. and Todman J., (2006), Analysis of pre-test-post-test control group designs in educational research, Int. J. Exp. Educ. Psychol., 15(2), 181–198.
- Eilks I. and Byers B., (2010), The need for innovative methods of teaching and learning chemistry in higher education – reflections from a project of the European Chemistry Thematic Network, Chem. Educ. Res. Pract., 11(4), 233–240.
- Fraenkel J. R. and Wallen N. E., (2006). How to design and evaluate research in education, New York: The McGraw-Hill.
- Friedfeld S. J., He S. and Tomson M. B., (1998), The temperature and ionic strength dependence of the solubility product constant of ferrous phosphonate, Langmuir, 14(3), 3698–3703.
- Gilbert J. K., (2006), On the nature of ‘‘context’’ in chemical education, Int. J. Sci. Educ., 28(9), 957–976.
- Gilbert J. K., Bulte A. M. W. and Pilot A., (2011), Concept development and transfer in context-based science education, Int. J. Sci. Educ., 33(6), 817–837.
- Gunduz T., (2008). Qualitative analysis textbook, Ankara: Gazi Kitabevi.
- Gunter T. and Kılınc Alpat S., (2017), The effects of problem-based learning (PBL) on the academic achievement of students studying ‘Electrochemistry’, Chem. Educ. Res. Pract., 18(1), 78–98.
- Hawkes S. J., (1998), What should we teach beginners about solubility and solubility products?, J. Chem. Educ., 75, 1179–1181.
- Hazen J. L. and Cleary D. A., (2014), Yielding unexpected results: precipitation of Ba3(PO4)2 and implications for teaching solubility principles in the general chemistry curriculum, J. Chem. Educ., 91(8), 1261–1263.
- Heimann A. and Sass E. (1989), Travertines in the Northern Hula Valley, Israel, Sedimentology, 36(1), 95–108.
- Karslı F. and Yigit M., (2015), Effect of context-based learning approach on 12 grade students’ conceptual understanding about alkanes, Inonu Univ. J. Fac. Educ., 16(1), 43–61.
- Karslı F. and Yigit M., (2017), Effectiveness of the REACT Strategy on 12th Grade Students’ Understanding of the Alkenes Concept, Res. Sci. Technol. Educ., 35(3), 274–291.
- Kegley S., Stacy A. M. and Carroll M. K., (1996), Environmental chemistry in the general chemistry laboratory, Part I: a context-based approach to teaching chemistry, Chem. Educ., 1(4), 1–14.
- Kırıkkaya E. B. and Bozkurt E., (2012), The effect of prepared activities by using the newspapers in science course on students’ academic achievement, Educ. Sci., 37(165), 65–80.
- King D. T., (2007), Teacher beliefs and constraints in implementing a context based approach in chemistry, Teach. Sci.: J. Aust. Sci. Teach. Assoc., 53(1), 14–18.
- King D. T. and Ritchie S. M., (2013), Academic success in context-based chemistry: demonstrating fluid transitions between concepts and context, Int. J. Sci. Educ., 35(7), 1159–1182.
- Kortland J., (2010), Scientific literacy and context-based science curricula: exploring the didactical friction between context and science knowledge, Paper presented at the GDCP Conference, Potsdam, Germany.
- Mandler D., Mamlok-Naaman R., Blonder R., Yayon M. and Hofstein A., (2012), High-school chemistry teaching through environmentally oriented curricula, Chem. Educ. Res. Pract., 13, 80–92.
- Meyer R., (1925), Chemie in Natur und Kultur, VerlagvonFriedr, Vieweg and Sohn (Eds), Braunschweig.
- Mihok M., Keiser J. T., Bortiatynski J. M. and Mallouk T. E., (2006), An environmentally focused general chemistry laboratory, J. Chem. Educ., 83(2), 250–252.
- Nakiboglu C., (2016), General Chemistry 3: Analytical Chemistry, Ankara: Anı Yayıncılık.
- Onder İ. and Geban Ö., (2006), The effect of conceptual change texts oriented instruction on students’ understanding of the solubility equilibrium concept, Hacettepe Univ. J. Educ., 30, 166–173.
- Orwat K., Bernard P. and Migdal-Mikuli A., (2017), Alternative conceptions of common salt hydrolysis among upper-secondary-school students, J. Balt. Sci. Educ., 16(1), 64–76.
- Pallant J., (2001), SPSS survival manual, Maideenhead, PA: Open University Press.
- Pearson E. S. and Hartley H. O., (1958), Biometrika tables for statistician, Cambridge University Press, New York.
- Polat S., (2011), The formation, spread area and protection of travertine in Turkey, Marmara Geogr. J., 23, 389–428.
- Potter N. M. and Overton T. L., (2006), Chemistry in sport: context-based e-learning in chemistry, Chem. Educ. Res. Pract., 7(3), 195–202.
- Purwati Y. and Dwisuyanti D. R., (2014), The influence of guided inquiry learning method with Macromedia flash toward student's achievement in the solubility and solubility product topic, J. Pendidikan Kim., 6(2), 32–37.
- Rahmi C., Wiji S. K. and Mulyani S., (2017), Students’ mental models on the solubility product concept, AIP Conf. Proc., 1848(1), DOI:10.1063/1.4983933.
- Raviolo A. and Alexander J., (2001), Assessing students' conceptual understanding of solubility equilibrium, J. Chem. Educ., 78(5), 629–631.
- Robson C., (2015), Scientific Research Methods Real World Research, 1st edn, Ankara: Anı Yayıncılık.
- Schmidt H., (1991), A label as a hidden persuader: chemists’ neutralization concept, Int. J. Sci. Educ., 13(4), 459–471.
- Schwartz-Bloom R. D., Halpin M. J. and Reiter J. P., (2011), Teaching high school chemistry in the context of pharmacology helps both teachers and students learn, J. Chem. Educ., 88, 744–750.
- Secer İ., (2015), Psychological test development and adaptation process: The Applications of SPSS ve LISREL, Ankara: Anı Yayıncılık.
- Skoog D. A., Holler F. J. and West D. M., (1996), Fundamentals of analytical chemistry, 7th edn, Orlanda, Florida, USA: Saunders College Publications.
- Suresh K. P., (2011), An overview of randomization techniques: an unbiased assessment of outcome in clinical research, J. Human Reprod. Sci., 4(1), 8–11.
- Taylor N. and Coll R., (1997), The use of analogy in the teaching of solubility to pre-service primary teachers, Aust. Sci. Teach. J., 43, 58–64.
- Teichert M. A., Tien L. D., Anthony S. and Rickey D., (2008), Effects of context on students’ molecular-level ideas, Int. J. Sci. Educ., 30(8), 1095–1114.
- Tez Z., (2010a), The magic world of medicine and perfume-History of pharmacy, fine stinks and cosmetics, İstanbul: Hayykitap.
- Tez Z., (2010b), The mysterious history of medicine-“Healing” in the coexistence of symbols, majors and rituals, İstanbul: Hayykitap.
- Tez Z., (2017), The mysterious backyard of Chemistry: Alchemy, İstanbul: Hayykitap.
- Ultay E., (2017), Examination of context-based problem-solving abilities of pre-service physics teachers, J. Balt. Sci. Educ., 16(1), 113–122.
- Ultay N. and Calık, M., (2012), A thematic review of studies into the effectiveness of context-based chemistry curricula, J. Sci. Educ. Technol., 21, 686–701.
- Ultay N. and Calık M., (2016), A comparison of different teaching designs of ‘acids and bases’ subject, Eurasia J. Math., Sci. Technol. Educ., 12(1), 57–86.
- Ultay N., Durukan Ü. G. and Ultay E., (2015), Evaluation of the effectiveness of conceptual change texts in the REACT strategy, Chem. Educ. Res. Pract., 16, 22–38.
- Vogelzang J. and Admiraal W. F., (2017), Classroom action research on formative assessment in a context-based chemistry course, Educ. Action Res., 25(1), 155–166.
- Vos M. A. J., Taconis R., Jochems W. M. G. and Pilot A., (2011), Classroom implementation of context based chemistry education by teachers: the relation between experiences of teachers and the design of materials, Int. J. Sci. Educ., 33(10), 1407–1432.
- Wexler P., (2015), History of toxicology and environmental in antiquity, Vol. II, Amsterdam: Elsevier, Academic Press.
Footnote |
| † The instruments and contexts used in this research are the original Turkish language instruments and contexts. |
| This journal is © The Royal Society of Chemistry 2018 |
