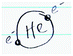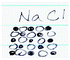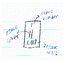An examination of preservice elementary teachers’ representations about chemistry in an intertextuality- and modeling-based course
Minjung
Ryu
 *a,
Jocelyn Elizabeth
Nardo
b and
Meng Yang Matthew
Wu
b
*a,
Jocelyn Elizabeth
Nardo
b and
Meng Yang Matthew
Wu
b
aDeparment of Curriculum and Instruction, Department of Chemistry, Purdue University, USA. E-mail: mryu@purdue.edu
bDepartment of Chemistry, Purdue University, USA
First published on 20th March 2018
Abstract
The chemistry education aspect of elementary teacher education faces a unique set of challenges. On one hand, preservice and in-service elementary teachers tend to not like chemistry and have negative feelings toward chemistry. On the other hand, learning chemistry requires reasoning about natural phenomena from the submicroscopic perspective that deals with the properties and behaviors of unobservable particles. The present study addresses these challenges in chemistry education for preservice elementary teachers (PSETs) by designing a chemistry curriculum that improves the relevance of chemistry learning to students via intertextuality and modeling practices. An analysis of chemistry representations that PSETs generated before and after taking the designed chemistry course demonstrates that they initially perceived chemistry as vivid chemical changes occurring in lab spaces or a discipline related to atoms while failing to provide connections between the chemical reactions and atoms. After taking the course, many students came to see doing chemistry as epistemic practices that construct submicroscopic explanations for observable phenomena and its relevance to everyday lives such as food, car emissions, and their local surroundings. They also came to recognize various epistemic roles that people play in doing chemistry. We provide important implications for engaging PSETs in chemical reasoning and designing chemistry curricula that are more approachable and build on learners’ knowledge resources.
Introduction
Chemistry is often depicted as a subject that is hard to learn and alienates many learners. Often-cited reasons for challenges in chemistry education include the particulate and kinetic natures of chemistry that are inherently abstract (Nakhleh, 1992) and curricular and instructional materials that are poorly designed or do not incorporate useful learning resources (Habraken, 1996; Horton, 2007). In addition to the nature of the discipline itself and curricular materials, poorly trained teachers can be another factor which may impede students’ chemistry learning (Arzi and White, 2008). As early as elementary school, teachers play an important role in establishing attitudes and foundational content knowledge of young learners (Konstantopoulos, 2011). Early learning of science in elementary schools may further impact the learning and motivation to learn in successive grade levels.Unfortunately, research has suggested that many preservice elementary teachers (PSETs) are not adequately trained to teach science. It has been documented that PSETs generally lack science content knowledge (Rice, 2005; Nowicki et al., 2013) and do not enjoy science (Fones et al., 1999; Siegel and Ranney, 2003). Moreover, Bursal and Paznokas (2006) showed that PSETs have more negative feelings toward chemistry and physics, than biology and earth and environmental sciences, and lower confidence in teaching them. Many PSETs attribute the low content knowledge in and negative attitudes toward science to their experiences in K-12 science classes, describing them as boring, too long, and unpleasant which made them feel dumb, fearful, and doubtful (Tosun, 2000; Hechter, 2011). Having negative feelings toward science has been shown to affect the ways in which PSETs learn and eventually convey those cognitions to their future students (Siegel and Ranney, 2003). While it is important to improve K-12 science education, we also recognize the importance of providing positive learning experiences in undergraduate science courses that potentially improve PSETs’ pre-existing perceptions about, attitudes toward, and relationship with science (Kelly, 2000; Appleton, 2003; de Boer et al., 2016).
Highlighting and teaching the relevance of chemistry can be an effective means to enhance chemistry learning experiences (Gonsalves et al., 2013). Stuckey et al. (2013) argued that science can be made relevant to students by “preparing students for potential careers in science and engineering” (interest); facilitating students in “understanding scientific phenomena and coping with the challenges in a learner's life” (meaningfulness); and helping “students [become] effective future citizens in the society in which they live” (worth, p. 8). Providing relevant contexts for chemistry learning allows learners to develop a sense of ownership of what they learn since the content would resonate with students’ present and anticipated interests, engendering commitment to learning the material and enhancing the overall classroom experience (Gilbert, 2006; Holbrook and Rannikmae, 2007). This approach is consistent with the National Research Council's (2012) recommendation that science educators should encourage students to not only learn content knowledge, but also apply content knowledge to address authentic science and engineering problems since learners would responsibly contribute to societal decision-making processes.
The literature suggests that learning chemistry content within relevant contexts, rather than as enclosed and isolated chemistry facts, can change preservice teachers’ attitudes and perceptions of science and help them revisit how they will present chemistry content to future students. For example, inquiry labs in which PSETs design and conduct their own experiments and communicate results to peers (Bencze, 2010) and curricula that situate scientific inquiry in familiar phenomena (Raborn and Daniel, 1999; Bergman and Morphew, 2015) were found to positively impact PSETs’ attitudes toward science learning and teaching. Sendur et al. (2017) showed that after taking the History and Philosophy of Chemistry course, preservice chemistry teachers expanded perceptions about chemistry and chemists to incorporate their impacts on other disciplines, daily lives, and improvement of the society and human lives. They also noted that prospective teachers were able to make more creative connections between their daily activities and the microscopic behaviors that compose those everyday activities. Curricula that change attitudes and perceptions about the relevance of chemistry and chemists may impact PSETs’ future pedagogies from more teacher-centered to student-centered approaches (Ucar, 2012).
We situate the current study within this large body of literature that aims to enhance preservice teachers’ experiences about science and science learning by enhancing chemistry's relevance to learners. The first author of this paper drew on ideas of intertextuality and modeling in designing instructional materials and activities. While we recognize the potential for enhancing the relevance of chemistry by employing intertextuality and modeling as key principles of learning practices, this approach has not been widely applied to chemistry education for PSETs. Throughout a semester, the course aims to engage PSETs in scientific sense-making practices, rather than rote memorization and problem solving, and provide relevant examples from everyday lives and social environmental issues, rather than presenting abstract and isolated facts. As reflexive educators (Feucht et al., 2017), we examine how this curricular approach influenced PSETs’ ideas about chemistry. In the following section, we theoretically discuss our instructional approach to a general chemistry course for PSETs, followed by research design and findings. Based on the findings, we will suggest implications for designing a chemistry course for PSETs and, further, non-chemistry major students that facilitate their appreciation of chemistry and engagement in epistemic practices of chemistry.
Theoretical framework
To encourage PSETs to realize the relevance of chemistry, we draw on ideas of intertextuality and modeling. Intertextuality refers to the process of sense-making across multiple contexts wherein multiple familiar and unfamiliar texts are juxtaposed and linkages are constructed between them (Lemke, 1990; Pappas et al., 2003). Here, text is defined not just as a piece of writing, but broadly as the functional language which can be spoken or written using various types of symbolic systems, including equations, diagrams, and music scores (Halliday and Hasan, 1985). Drawing from Bahktin's (1981) theories of dialogism, intertextuality focuses on discourses in which actors in the learning environment utilize their own reasoning to engage, respond, and reorganize their understandings. Intertextuality thus is a discursive collaboration in which learners connect their experiences and knowledge, potentially resulting in new meanings and creative, inquiry-oriented epistemologies. Intertextual connections enhance sense-making by presenting science as a continuously merged social practice comprised of human endeavors and values within everyday life (Moje et al., 2001).Many chemistry educators and scholars have developed curricula that connect chemistry to various contexts, such as history, culture, food science, and global issues, to promote students’ engagement and greater awareness of how chemistry is relevant to human lives. In these curricula, for instance, chemistry content was situated in the history of explosives and paintings made by a local Spanish company (Pinto and Garrido-Escudero, 2016), an examination of consumable oils in margarine and butter (Avargil et al., 2012), and the investigation of chemical ideas in global catastrophes, climate change, and HIV/AIDS (Middlecamp et al., 2006). While these curricula have been demonstrated to promote students’ content learning and/or interest and engagement in chemistry, potential intertextual connections were provided by instructors rather than constructed collaboratively and dialogically by students and teachers. Although students may be actively engaged in constructing intertextual connections of their own, by not foregrounding students’ experiences, these studies do not provide evidence as to how students realize their own idiosyncratic relevance while learning science.
Building on the idea of intertextuality as a sense-making process, we are interested in how learners construct and recognize intertextual connections between abstract chemistry ideas and phenomena outside of the chemistry classroom. In this regard, we conceptualize intertextuality as a sense-making process in which students juxtapose and link experiences within and outside of formal chemistry learning spaces, such as classrooms, laboratories, and textbooks. For example, Varelas et al. (2006) viewed intertextuality as a privileging space that offers opportunities for learners to explore complex chemistry ideas. In learning about evaporation, boiling, and condensation, elementary students and teachers dialogically constructed connections between abstract chemistry texts and familiar texts, such as the mirror fogging up following a hot shower, rising bubbles in water when making tea, or the sweating on a cookie sheet. Bricker and Bell (2014) similarly explore how a youth marshalled her interests in perfumery and everyday moments to learn science and identify herself with science. In a study with PSETs, Gunning and Moore Mensah (2011) asked them to create photo albums that captured science within their local surroundings. This task raised PSETs’ awareness that science was present all around them (e.g., the streets of New York or a student's apartment) and encouraged them to make connections between their experiences within and outside the classroom. This approach that foregrounds student-generated intertextuality can create a learning environment that may better ease students such as PSETs into science content.
As a way to encourage students to agentively make intertextual connections, we utilize modeling practices. Models are tools that facilitate learners’ reasoning, explanation, inquiry, and generation of new knowledge (Nersessian, 2008; Schwarz et al., 2009; Cheng and Brown, 2015). In particular, Passmore et al. (2014) argue that models are contextually determined by their usage and partial manifestations of given occurrences. Thus, modeling is the process of scientific sense-making, rather than a learning goal itself, and students are positioned as epistemic agents that connect between models and phenomena and make sense of the represented relationship. Students are the locus of sense-making as they produce models in order to achieve more epistemologically-oriented goals, such as understanding structure–function relationships or causal mechanisms (Gouvea and Passmore, 2017).
In chemistry, learners use representations in modeling practices to visualize the unseen, describe complex relationships, and surmount spatial and temporal obstacles (Bussey and Orgill, 2015). Chemical representations entail macroscopic, submicroscopic, and symbolic components of knowledge (Johnstone, 1991). Thus, understanding each component and making appropriate transitions among these three to provide causal explanations for observable phenomena is considered an important goal of learning chemistry (Hoffman et al., 2003; Gilbert and Treagust, 2009). However, as Passmore et al. (2014) argued, modeling practices should go beyond the usage of representations. Modeling should incorporate learners’ epistemic practices of generating, evaluating, and revising content knowledge to describe how they perceive chemistry (Oh and Oh, 2011; Berland et al., 2016; Krell et al., 2017). For instance, understanding of water's phase change is more than learning its representation symbolically and submicroscopically, but should and can be facilitated through modeling practices that relate the observable phenomenon's chemistry-specific symbols and molecular behaviors of water.
We note that these two frameworks, intertextuality and modeling, position students as sense-makers who construct their own linkages between texts and develop and manipulate models as tools for learning (Knuuttila, 2011). In an instructional approach that draws on these frameworks, learners not only advance their content knowledge but also are empowered as epistemic agents (Gonsalves et al., 2013). In addition, these frameworks encourage researchers to move beyond seeking to replace PSETs’ misconceptions with correct ideas or demonstrating inadequacies in their prior knowledge. Aligned with this perspective, we view PSETs as learners whose knowledge is valuable and can be leveraged to construct chemistry knowledge and models rather than simply replacing with correct ones. We seek to engage chemistry learners in various modeling practices that are open-ended and epistemically-oriented in order to explicitly facilitate learners’ own connections between chemistry phenomena and aspects of chemistry beyond the walls of chemistry classrooms.
While we believe that this approach has benefits for all students, it is particularly beneficial for PSETs for three reasons. First, as discussed earlier, many studies have found that PSETs often have negative attitudes of and perceptions toward learning chemistry. Modeling practices that allow learners to make their own and creative intertextual linkages may empower PSETs to better see themselves as capable individuals who can learn and do chemistry (Sjöström and Talanquer, 2014). Second, emphasizing student agencies may encourage PSETs to view their future students as active knowledge constructors as well and, in turn, utilize young learners’ wealth of knowledge for new learning and scientific literacy development (Varelas et al., 2006). Third, Next Generation Science Standards (NGSS, NGSS Lead States, 2013) identify modeling as a key component of scientific and engineering practices that K-12 students need to master. By inviting PSETs to modeling practices in an intertextually-focused, college-level content course, we aim to induct them into this newly agreed upon standard of science education where they may apply these pedagogical approaches to their future teaching.
Curricular design
The target chemistry course Fundamentals of Chemistry is a two-credit hour, one semester-long introductory chemistry course. The research site is a chemistry class at a large public Midwestern university in the United States that is designed specifically for PSETs. The course entails two mandatory components: lab and lecture. Students meet for lab once a week for two hours and 50 minutes in which they investigate and experience the ideas that would later be covered in the lecture. A fifty-minute lecture follows each week's lab, in which students revisit the experiments conducted in the lab, formulate relevant chemistry concepts, and relate concepts to new examples. Here, we summarize how we designed the course to realize the theoretical premises discussed above.Encouraging intertextual connections
Each week's class topic was situated in everyday examples that are familiar to many college students and socioscientific issues. The course introduced a range of chemistry ideas (e.g., density, electronegativity, polarity, intermolecular forces) in relation to the properties of water, perhaps the most familiar and important compound. A number of examples drawn from cooking and weather phenomena were shown to students, and students were encouraged to bring examples from their own lives. Other topics were situated in socioscientific issues, such as climate change, acid rain, environmental impacts of plastics, and food and nutrition. In addition, most lab activities were designed to use typical household materials that can be bought in a grocery store, and adaptable to elementary school science classes. For example, in a lab that deals with acid and base chemistry, students test the pH of household substances like lemon juice, vinegar, bleach, and Alka Seltzer using a red cabbage indicator that they have made in the lab. The results from this lab were then used to situate the socioscientific issue of acid rain. Students conduct research about how acid rain affects different countries and present their findings in the form of a poster presentation. Moreover, students were assigned to read a pre-selected article each week and write a short reflection paper in which they summarized the article and connected to the course learning and their experiences. Twelve articles out of a total of 14 were selected from ChemMatters (published by the American Chemical Society) that explains chemical concepts with familiar examples or historical events.Promoting modeling practices
Sevian and Talanquer (2014) identified crosscutting disciplinary concepts of chemistry such as chemical identities, structure–property relationships, chemical causalities, chemical mechanisms, chemical control, and benefits–costs–risks. Out of these six crosscutting concepts, the focal course highlighted chemical identities, structure–property relationships, and chemical causalities through modeling practices. For instance, in each lecture and lab, students were prompted to collaboratively explain observable phenomena by describing or sketching the behavior of submicroscopic particles and revise their sketches after learning more chemistry ideas (for a similar approach, see Cooper et al., 2017). Sample questions were, “Explain why water forms a dome-shaped droplet, as opposed to alcohol that forms a flatter droplet, using submicroscopic behaviors of water and alcohol molecules”, and “Explain why soap water forms bubbles with a sketch of submicroscopic views of bubbles.” In some modeling practices, students generated models to predict phenomena, such as “What do you think happens to a balloon when you put it in the freezer? Explain your prediction using particle behavior.”We note that modeling practices in the focal course were designed to prepare PSETs for future teaching in elementary schools in order to make the chemistry course relevant to students’ future career (Stuckey et al., 2013). First, our modeling practices adopted elementary science education standards for modeling practices in NGSS (NGSS Lead States, 2013), such as collaboratively developing and revising models and using models to describe and/or predict phenomena. In addition, since a highly-specialized language of chemistry (e.g., chemical formulae and equations) is not necessary for teaching in elementary schools, such a formality was not highlighted in the course. Rather, students were allowed to use any representation that best conveyed their ideas. Students mostly used circles to represent submicroscopic particles without specifying their molecular identity or even used chemically-incorrect representations (e.g., N2 for N2). Students were not graded for correctness in their chemical representations; instead, they received feedback regarding their ideas and representations.
Research design
To evaluate the effectiveness of our approach and draw implications for future instructions, we examined how students’ understandings of what chemistry is has changed. As a means to gain insights into it, we used what Nersessian (2009) called an “external representation,” a visual and verbal presentation of how learners perceive and think about phenomena in science. Specifically, we ask the following two research questions:• How do PSETs’ understandings of what chemistry is change after taking a chemistry course designed to promote modeling practices and intertextuality?
• What intertextual connections do PSETs make in their representations after taking the chemistry course, as defined as connections between experiences within and outside of the formal chemistry learning space?
To answer these questions, we collected and analyzed students’ representations of chemistry at the beginning and end of the semester (pre- and post-representations, respectively). An answer to these questions provides not only information regarding the effectiveness of our pedagogical approach but also deeper insight into what intertextual connections PSETs make and how they generate these connections. This insight further informs us how students agentively made chemistry relevant to themselves and what intertextual connections can and should be further foregrounded in chemistry teaching practices.
Participants
Study participants were students enrolled in the Fundamentals of Chemistry course, which was taught by the same instructor (i.e., the first author of this paper). We collected data throughout the three semesters from three separate cohorts (Spring 2016, Fall 2016, and Spring 2017; n = 43, 59, 42, respectively; total 144). These students were primarily PSETs in their sophomore and junior years (ages 19–21 years), while a few students majored in other programs, such as early childhood education and business. The participants enrolled were predominantly White and female (approximately 95%). This study was approved by the Institutional Review Board. All participants’ identifiable information was deleted from the data.Data collection and analysis
Data of this study were derived from students’ responses to the question What is chemistry? Students were encouraged, but not required, to answer this question by both writing and sketching. We administered this question on the first day of the class and in the final exam for each cohort. While responses to this question were not graded with a grading rubric, those who responded in the final exam were awarded extra credit (less than 0.5% of the total grade). Because of the voluntary nature of the task and absences in the class, not all students responded to both pre- and post-questions. Out of a total of 144 enrolled students, 120 answered both the pre- and post-question and were analyzed for this study.As instructed, students responded to the question both verbally and visually. We coded verbal and visual representations separately. The coding process was both deductive and inductive. It was deductive in that we first coded visual representations based on Johnstone's (1991) three representational components (macroscopic, submicroscopic, and symbolic). Then, within these three first-tier codes we inductively coded the data based on repeating patterns and compared with other codes to further divide or collapse them (Corbin and Strauss, 1990). We coded verbal representations similarly in an inductive and iterative manner but without coding them by Johnstone's three representational components. We generated a codebook early in the analysis process and revised it throughout the progression of the collaborative coding. Table 1 summarizes our final codebook. All three authors coded Spring 16 data and discussed any disagreements to achieve interrater reliability, serving as calibration. Once the consensus had been reached, a set of two authors coded the Fall 16 and Spring 17 data sets and achieved 98.2 percent agreement.
| Verbal Representation | |||||
|---|---|---|---|---|---|
| Verbal-reaction | Phrases indicating a chemical reaction, change, and mixing | “Mixing and interacting with each other to make substances” | Verbal-macro–Submicro Relation | Transitioning between macroscopic and submicroscopic phenomena | “Chemistry is explaining what we observe submicroscopically” |
| Environmental Issue | Phrases indicating environmental issues | “Global warming”, “acid rain” | Mechanistic Explanation | Description of causal explanations for observable phenomena | “Testing & retesting to explain phenomena” |
After the coding, we quantitatively analyzed the coded data by counting and comparing the frequencies of the codes in pre- and post-representation data. To determine whether there is a statistically significant difference in the frequencies between pre- and post-representation data, we conducted a McNemar's test for each code using STATA. To complement the quantitative analysis, we analyzed the data qualitatively. Specifically, we selected three codes, Everyday Object, Human Being, and Macro–Submicro Relation, because these codes provided the most descriptive and diverse student responses and demonstrated statistically significant changes between pre- and post-representations. These changes also aligned with the overarching goals of the course. To capture rich details regarding specific changes individuals made between pre- and post-representations, we will show three selected students’ responses whose differences between pre- and post-representations were most notable.
Findings
Representations of chemistry and changes in the representations
As Table 1 shows, four codes emerged for Verbal Representations, verbal-Reaction, Environmental Issue, verbal-Macro–Submicro Relation, and Mechanistic Explanation. We note that students’ statements that imply a causal and mechanistic explanation was coded as Mechanistic Explanation even when it was not explicitly stated (e.g., “testing and retesting to explain phenomena”) because such statements indicate a beginning of mechanistic reasoning or a mechanistic reasoning that students could not fully articulate in this representation task. Visual Representation was first coded into three representation components, Macroscopic, Submicroscopic, and Symbolic. Within the Visual Representation-Macroscopic code, four codes emerged (Lab Equipment, Everyday Object, Human Being, and visual-Reaction); within the Visual Representation-Submicroscopic code, another set of four codes emerged (Individual Particle, Multiple Particles, visual-Macro–Submicro Relation, and Random Motion). The Visual Representation-Symbolic code was set as Chemical Symbol and was not further coded because we did not identify any meaningful patterns. These codes were derived given the aims of the course which were to promote students’ intertexual connections and modeling practices in the context of chemistry. For example, Everyday Object denotes objects that are not typically found within the chemistry lab (e.g., cars, houses, kitchens, foods, trees, etc.), whereas Lab Equipment includes glassware and other standard lab equipment that students used to conduct their experiments. Students’ drawing of Everyday Objects indicates that they make intertextual connections between chemistry and objects that are more familiar and relevant to their lives. In addition, all codes under the Visual Representation-Submicroscopic code illustrate modeling practices in the context of chemistry.Fig. 1–3 show the percent frequency of each code (i.e., the percentage of students who were coded with each code out of a total of 120 students) in pre- and post-representations from all three semesters. Here, we highlight several important findings with respect to the frequency of each code and differences between pre- and post-representations. First, many students brought the idea that chemistry is to create reactions that are initiated by mixing substances or heating them, resulting in dynamic and observable changes (e.g., gas formation and explosions) in lab settings. This was shown in both verbal and visual portions in the pre-representation. In pre-verbal-representations (Fig. 1), 78 students (65%) were coded for verbal-Reaction, while in pre-visual-representations (Fig. 2), 59 (49%) drew Lab Equipment and 38 (32%) made a depiction that suggested reactions (visual-Reaction). After taking the course, however, many students started to represent chemistry differently. Only 25 students (21%) included verbal-Reaction in their post-representations, a statistically significant drop from their pre-representations (χ2(1, n = 120) = 39.56, p < 0.001), although the other two codes Lab Equipment and visual-Reaction remained consistent, as shown by 66 students (55%) and 29 (24%) respectively. Instead, in the post-representation, more students associated chemistry with objects more familiar to them: 79 students (66%) included Everyday Object, such as foods, kitchens, and houses (χ2(1, n = 120) = 73.05, p < 0.001), and 51 (43%) included Human Being (χ2(1, n = 120) = 43.32, p < 0.001) in the visual representations. Both changes were statistically significant.
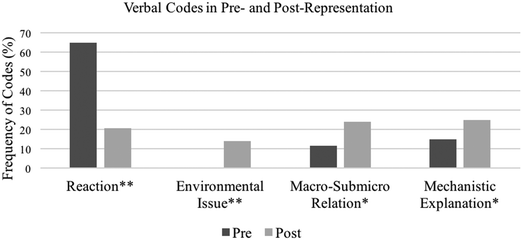 | ||
| Fig. 1 A pre- and post-comparison of Verbal codes. * denotes that the difference is statistically significant at p < 0.05 and ** at p < 0.001. | ||
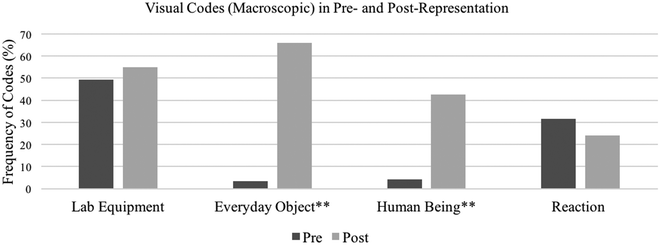 | ||
| Fig. 2 A pre- and post-comparison of Visual-Macroscopic codes. * denotes that the difference is statistically significant at p < 0.05 and ** at p < 0.001. | ||
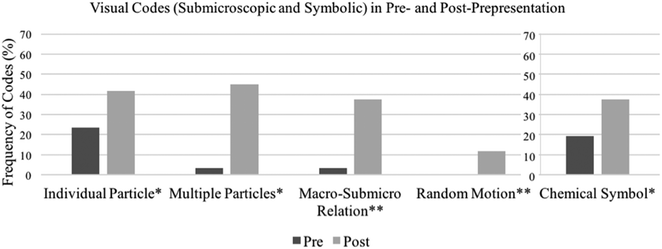 | ||
| Fig. 3 A pre- and post-comparison of Visual-Submicroscopic and Visual-Symbolic codes. * denotes that the difference is statistically significant at p < 0.05 and ** at p < 0.001. | ||
Second, before taking the course, few students described chemistry as having to do with submicroscopic entities (Fig. 3). Initially, less than 25% of the students drew Individual Particle (n = 28, 23%) and Multiple Particles (n = 4, 3%) and recognized Macro–Submicro Relation in verbal representations (n = 14, 12%) and in visual representations (n = 4, 3%). After taking the course, frequencies of these codes increased statistically significantly. Fifty (42%) students drew Individual Particle (χ2(1, n = 120) = 10.08, p < 0.05), 54 (45%) drew Multiple Particles (χ2(1, n = 120) = 48.08, p < 0.001), and 45 (38%) drew visual-Macro–Submicro Relation (χ2(1, n = 120) = 37.36, p < 0.001), although the increase in the verbal-Macro–Submicro Relation was not statistically significant.
Finally, we summarize findings regarding Mechanistic Explanation, Random Motion, and Chemical Symbol codes. Initially, 18 (15%) students had incorporated Mechanistic Explanation (Fig. 1), and none had included instances of Random Motion (Fig. 3). In the post-representations, 30 (25%) students incorporated Mechanistic Explanation (χ2(1, n = 120) = 4.5, p < 0.05) and 14 (12%) had included Random Motion (χ2(1, n = 120) = 14.00, p < 0.001). While these changes are statistically significant, the frequencies are still low in the post-representations. For the Visual Representation-Symbolic portion (Fig. 3), 23 (19%) students included Chemical Symbol in their pre-representations; 45 (38%) included Chemical Symbol in their post-representations. The change was statistically significant (χ2(1, n = 120) = 10.52, p < 0.05).
Qualitative analysis of changes in the representations
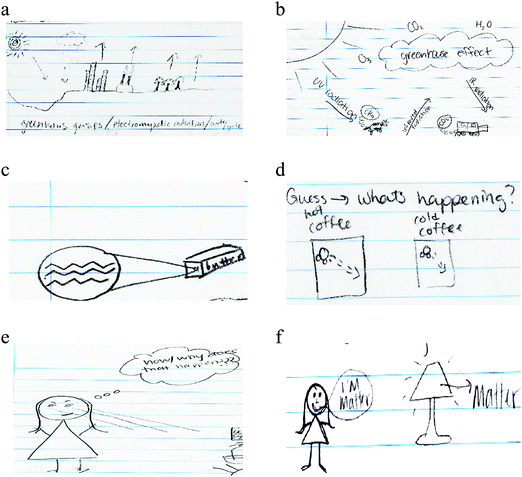
In order to provide deeper insight into students’ representations of chemistry, we discuss details shown in Everyday Object, visual-Macro–Submicro Relation, and Human Being codes that demonstrated the most noticeable changes between pre- and post-representations.Everyday Object that students depicted in the post-representations included outdoor activities (e.g., camping by a fire, strolling in a park), food (e.g., coffee, pizza, butter, and candy; Fig. 4c and d), and environmental implications (e.g., car exhaust, methane emissions from cows, and plastic recycling; Fig. 4a and b). We noted that some everyday objects, but not all, were mentioned in class (e.g., coffee, butter, car exhaust). These illustrative features may have been a result of students appropriating content from the course, thereby applying these concepts in ways that were meaningful and relevant to them. More importantly, many visual-Macro–Submicro Relation were situated in these everyday instances (Fig. 4c and d). Often using callout lines or magnifying glasses, students represented what submicroscopic entities of everyday objects would look like. While some students’ representations were simply a connection between an object and its molecule (e.g., butter and a butter molecule; Fig. 4c), other students demonstrated a submicroscopic explanation for observable phenomena. For instance, a student drew three states of matter and connected to behaviors of molecules, and another student drew hot and cold coffee and connected to differences in random motions of molecules (Fig. 4d).
As mentioned in the previous section, the frequencies of Human Being statistically significantly increased in the post-representations. In the pre-representations, Human Being was rarely shown as a part of chemistry. When humans were included in pre-representations, they were lab technicians manipulating glassware, typically at a lab bench. However, in the post-representation, not only did we observe a statistically significant increase in Human Being but also the variety of roles of human beings that students depicted. For instance, Human Being was drawn as a Knowledge Constructor, portrayed as a person who asks questions and thinks about observed phenomena; Subject of Chemistry, situated parallel to other subjects of chemistry study; Observer, depicted as looking at, feeling, and interacting with natural phenomena (Fig. 4e and f). These various roles of human beings in the representations suggested that students started to see diverse ways in which they can build relationships with chemistry, rather than work at a lab bench or become passive receivers of canonical chemistry knowledge.
Changes in individual students’ representations
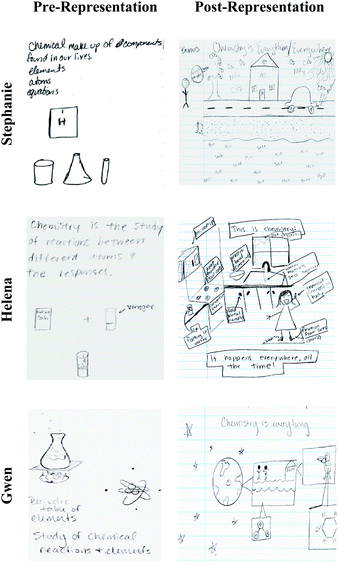
In this section, we described how the changes discussed above occurred within individuals. To that end, we chose and analyzed pre- and post-representations of three students, Stephanie, Helena, and Gwen (Fig. 5). We selected these three individuals because they demonstrated dramatic changes between the pre- and post-representations in Everyday Object, visual-Macro–Submicro Relation, and Human Being. Stephanie and Helena were enrolled in Fall 2016, whereas Gwen was in the Spring 17 semester. Helena and Gwen were juniors majoring in elementary education major, while Stephanie was a sophomore in a special education/elementary education double major program at the time of our data collection. Helena received A as her final grade, and Gwen and Stephanie received B.Stephanie: In the pre-representation task, Stephanie wrote, “chemical makeup of compounds found in our lives. Elements, atoms, equations.” Additionally, she drew the hydrogen symbol as depicted on the periodic table and laboratory glassware (i.e. a beaker, a flask, and a test tube). It appeared as if she emphasized the verbal description of chemistry, “elements, atoms” by adding a visual representation of the chemical symbol of hydrogen. Yet, no clear verbal connection was suggested between the hydrogen symbol and laboratory glassware, and these two were even spatially separated.
Her post-representation showed a human contemplating while situated next to a tree, a house, a beach, and a car along a road. It appeared as if Stephanie was depicting a scene salient to her either in her real life or in imagination. The scene of a tree, house, beach, and car was juxtaposed with several chemistry symbols. Perhaps, Stephanie tried to convey a story regarding carbon dioxide in car exhaust and its environmental impacts. Within the thought bubble was written “Why? Why does?”, indicating that the human being is a Knowledge Constructor who actively tries to make sense of natural phenomena by asking questions and pursuing answers. We noted that macroscopic depictions of everyday objects and chemical symbols were juxtaposed and inter-blended. This spatial proximity suggested her own ways of using various representational tools to construct a story and close interconnection between chemistry and everyday lives. In addition, she included the idea of photosynthesis, which was not a topic of the focal chemistry course, but was heavily addressed in her core biology courses. This indicated that Stephanie made intertextual connections to her biology course as well. On top of these visual representations, she also wrote, “chemistry is everything/everywhere.” This verbal representation supported our interpretation that Stephanie recognized how chemistry was closely related to everyday lives and other subjects like biology.
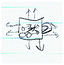
Helena: In the pre-representation, Helena stated, “Chemistry is the study of reactions between different atoms and the responses.” Her drawing that depicts the bubbling reaction between baking soda and vinegar provides a concrete example of this statement. Although she mentioned “atoms” in the verbal representation of chemistry, it was not clear whether she meant the submicroscopic entities or used the term as a generic description of materials.
In the post-representation as well, it was not evident that she made a connection between observable phenomena and submicroscopic particles. However, her representation depicted chemistry topics discussed in the course contextualized within a typical scene of kitchen. In her sketch of a kitchen, she marked “ice floating in water,” “heat transfer from coils,” “plastic bowl (recycled)”, and “friction from socks (static).” She also wrote, “water molecules stick together” next to water coming out of a faucet. Helena further added a human being to her kitchen scene. Here, the role of the human was a Subject of Chemistry as she became the locus in which various chemical phenomena (i.e. static electricity and biochemical reactions) took place. Helena wrote, “It happens everywhere, all the time!”, which attested to our interpretation of her viewing chemistry occurring both within and around human bodies. By appending an everyday kitchen scenario with chemistry content learned throughout the course, Helena presented a contextualized perspective of chemistry that is intertextually connected in a variety of aspects salient to her.
Gwen: Gwen's pre-representation depicted a flask atop a mesh wire and a flame which was presumably heating up a solution. On the other side of the worksheet, she drew a Bohr model of an atom. She wrote, “periodic table of elements, study of chemical reactions and elements.” Similar to Helena's and Stephanie's pre-representations, her visual representation seemed to be a reiteration of what she wrote—a representation of “chemical reactions” on the upper left side and “elements in the periodic table” on the right side of the paper. Yet, no clear connection was provided between elements and chemical reactions. It appeared as if chemistry consisted of two different, separable threads: elements and chemical reactions.
However, her post-representation showed a transition of scale from a planet to individual human beings to molecules and their connectedness. Her planet was made up of places in which we live, which was made up of individual people, environments in which we reside, and the food we consume. Here, people, surroundings, and foods were made up of different kinds of molecules. For instance, Gwen drew a body of water with a zoom-in depiction of its bent molecular structure. While it was difficult to interpret what molecule Gwen drew on the right side of the paper, we inferred that she may have wanted to convey that human beings were made up of molecules that come from foods that we eat. She may also be conveying hints of the molecular structure of glucose that makes up the ingested food as shown by her drawing of a hexagon within the person's stomach. In this post-representation, chemistry was not depicted as a set of discrete facts (e.g., elements, chemical reactions) as in the pre-representation, but was presented as a scaled submicro–macro connection to various aspects of our lives including living and nonliving things.
Overall, Stephanie, Helena, and Gwen represented chemistry in a more traditional (e.g., glassware, reactions, atoms, and features from the periodic table) setting, but after taking the course they represented chemistry in a more everyday life context (e.g., kitchens, foods, and car emissions), in which humans played a salient role. The different roles that students depicted for humans (e.g., Knowledge Constructor and Subject of Chemistry) may suggest the various ways that students can engage with chemistry and their personal interpretations of chemistry (i.e., how they made sense of chemistry within their own lives). Moreover, Stephanie's, Helena's, and Gwen's examples demonstrate that after taking the course students made more connections between different bits of chemistry (e.g., macroscopic and submicroscopic phenomena, school-chemistry knowledge and everyday lives), whereas their ideas about chemistry were represented as a list of unconnected concepts in the pre-representation (e.g., elements, atoms, chemical reactions, the periodic table). These changes in the three students’ representations illustrate the trends observed in the quantitative analysis of changes all student participants.
Discussion
After taking the focal chemistry course, PSETs’ responses to What is chemistry? have transformed. Instances of verbal- and visual-Reaction decreased, while Everyday Object, verbal- and visual-Macro–Submicro Relation, and Human Being increased. Many students came to view chemistry more holistically and as epistemic practices, encompassing familiar scenes in everyday lives, submicroscopic features as an explanation for macroscopic worlds, and the different roles people adopt in chemistry. These findings suggest that students came to value and connect their relevant experiences in constructing chemistry knowledge rather than parroting the chemistry concepts that they had experienced in the lab and lecture. Furthermore, students at times represented chemistry as a process of investigation where human beings are sensemakers of chemistry phenomena, rather than simply conducting laboratory experiments. They also may have depicted themselves as those human beings who try to construct chemistry knowledge in their post-representations.Mahaffy (2006) argued for promoting the “humanistic” aspect in chemistry learning, such as the blending of content, processes, and representations in the authentic contexts of the cultures of people who produce, use, and understand chemistry. Humanizing chemistry may illustrate the importance in acknowledging students’ everyday contexts to better foster scientific literacy. Our findings show that many students in the focal chemistry class personalized chemistry concepts by situating human beings within the content or depicting everyday aspects as artifacts of chemistry phenomena. For example, Stephanie and Helena show that chemistry is a human endeavor whereby people serve as conduits to interpret and make sense of the world around them. Moreover, Gwen made connections between food and its chemical composition, illustrating that chemistry can be a lens with which to view relevant aspects of everyday life. If educators continue to present chemistry within a non-relevant, institutionalized vacuum, this may constrain students’ abilities to understand chemistry in the greater sociocultural constellations of their lives (Talanquer, 2013).
Furthermore, our findings suggest that the designed course facilitated students’ linking between macroscopic and submicroscopic features of chemical phenomena. Research has shown that chemistry students face challenges in transitioning from macroscopic features of chemical phenomena to submicroscopic features (e.g., Gilbert and Treagust, 2009). PSETs, who have been reported as lacking in content knowledge (e.g., Nowicki et al., 2013), may experience similar, or more severe, difficulties in doing so. Our analysis showed that after taking the focal course many PSETs integrated the macroscopic and submicroscopic components of chemistry. Furthermore, these macroscopic–submicroscopic connections were situated within the contexts of everyday scenes. In the learning process of the focal chemistry course, PSETs were asked to express chemistry-specific knowledge through their sketches of phase changes, air composition, and food constituents. Our finding underscores that having a course which foregrounds intertextuality and modeling can improve understandings about the particulate nature of chemistry.
Limitations
We recognize a few limitations in our teaching and research approach. First, despite the aforementioned success of the focal course, not many students depicted mechanistic explanations or random motions of particles as part of chemistry. Although providing mechanistic explanations for observed phenomena and random motions of particles were highlighted throughout the course, students might not have found them as salient features of chemistry. Alternatively, it is possible that the prompt in pre- and post-representation questions could not adequately solicit their ideas about mechanistic explanations and random motions. That is, although our representation data certainly provide insight into PSETs’ understanding about what chemistry is, representation data alone may not provide the full picture of their understanding. Second, our data collection methods do not consist of assessments that would have been able to measure students’ understanding of chemistry concepts. Third, although our ultimate goal of the course was to broaden PSETs’ ideas toward chemistry teaching, we did not collect data to measure it directly and, thus, cannot make a claim regarding how the observed change affects their future teaching in elementary classrooms. Finally, there was no control group that did not learn our curriculum to which we could compare learning outcomes. This design limits our ability to make a causal claim regarding the effectiveness of our curricular design.To improve the curricula and research methods in the future, we can modify the course activities to incorporate more learning activities in which students are exposed to random motions of particles through the use of visualizations and generate representations of random motions on their own. Instructors can then promote students to mechanistically explain how and why particles move in a way that references macroscopic properties and have them reflect on these sense-making practices. A future study can reevaluate the efficacy of the modified curriculum and examine how it affects students’ understandings of chemistry and their approach to teaching chemistry by evaluating their performances on a content assessment and/or through interviews.
Implications & conclusion
Based on our findings and limitations, we suggest several pedagogical and research implications. First, we focus on PSETs’ experiences and knowledge that were familiar to them and that they found to be relevant to chemistry because learning environments that utilize diverse resources of students can facilitate their learning (Bergman and Morphew, 2015). In this regard, everyday scenes that students used in making intertextual connections offer insight into designing a course and deciding what everyday examples can be more foregrounded to make chemistry ideas more salient to students. Among our students, foods were frequently drawn in the post-representation. They also frequently mentioned environmental concerns. The frequent appearance of environmental concerns in the representation indicates that PSETs may care about environmental issues and want to know more about them. We note that learning about environmental issues indeed is an important goal of science education and civic engagement (Middlecamp et al., 2006). Chemistry courses should more actively utilize these examples (e.g., foods, environments) to provide applicability of and accessibility to chemistry and encourage students to find and share examples from their own lives.Second, we recognize the importance of offering diverse ways to interact with chemistry and natural phenomena. As our analysis showed, PSETs did recognize a few different ways that human beings can interact with chemistry: Lab Technician, Knowledge Constructor, Observer, and Subject of Chemistry. Students should be encouraged to make close observations by fully utilizing all five senses, to reason about what is going on at the submicroscopic scale for which humans cannot use five senses, and to imagine processes occurring at the submicroscopic scale. To promote students’ agentive chemical reasoning, learning environments should also allow space for students to ponder and bear uncertainty when they are not sure about the correctness of their ideas. In these environments, learners may worry less about the scientific correctness of their ideas and more fully engage in multiple different ways of interacting with chemistry building on their rich resources of knowledge and reasoning skills.
Finally, modeling practices implemented consistently and repeatedly throughout the course may facilitate students’ chemical and scientific reasoning. The NGSS (NGSS Lead States, 2013) defines Developing and Using Models as a key science and engineering practice for K-12 science classrooms. However, modeling in accordance with the NGSS vision can still be interpreted as models that are descriptive in nature and goals of learning as opposed to processes that are epistemically purposeful and enhance learning (Gouvea and Passmore, 2017). We present modeling as the process of student-centered sense-making and constructing connections between abstract chemistry ideas to their implications and applications. In these practices, instructors should highlight that the purpose of modeling practices is not memorizing and copying correct textbook-representations, but recording their observations, reasoning about what is important to draw from their observation onto the paper and why, and verbally and visually articulating ideas about unseen submicroscopic phenomena. This approach can further empower students as epistemic agents and encourage them to become more aware of their reasoning as they construct and manipulate models as tools to fulfill their particular epistemic aims (Knuuttila, 2011).
From a research perspective, we argue that research and teaching practices should position PSETs as epistemic agents who can engage in meaningful scientific reasoning and recognize science and chemistry in their lives. Expanding the notion of intertextuality, we recommend examining whether, when, and how PSETs recognize chemistry in their daily lives outside of chemistry classes, and draw on those intertextual connections into chemistry classes. Innovative research methods, such as photovoice (Wang and Burris, 1997), may be a useful tool for conducting this line of research. In addition, to complement limitations of the current study, future research should be conducted to examine the impact of our current curricular approach on their attitude toward learning chemistry and other science disciplines. Studies should also ask whether PSETs who learn chemistry through this approach become less reluctant to teach chemistry and how they incorporate perspectives of chemistry into future pedagogies (Baran and Correia, 2009).
We argue that although our study was conducted with PSETs, principles of learning environment design discussed in this paper can be used in other chemistry courses, especially those for non-chemistry majors. Learning practices used in the focal course (e.g., dialogically making intertextual connections and promoting modeling practices in relevant contexts) may serve as a platform upon which learners may view themselves as active sense-makers who interact with natural phenomena and ask questions about them. Questions relevant to application of chemistry to everyday lives, such as “what are the best ways to clean greasy kitchen cabinets and why?” and “how do bathbombs work?”, or topics relevant to current events, such as drinking water contamination or hydraulic fracking, can be good vehicles for introducing chemistry ideas. Learning chemistry may be more challenging to non-majors for whom introductory level chemistry classes may be their last exposure to chemistry. We argue that educators should recalibrate goals of college chemistry education, define carefully what the most important knowledge, practices, and reasoning for non-chemistry majors are, and design chemistry courses accordingly.
We set out to understand how PSETs’ views of chemistry have changed as a result of taking a chemistry content course that foregrounds intertextuality and modeling practices to enhance the relevance of chemistry to the learners. While the prior literature has shown that PSETs lack scientific content knowledge and, in turn, avoid science teaching or teach it poorly, our analysis presents an alternative story: PSETs are capable epistemic agents who can model chemistry content with intertextual connections within and beyond the classroom. Forwarding students’ agencies as active constructors of knowledge promotes their ownership of their learning as well. Empowering PSETs can hopefully encourage communication of these attitudes to their future students and teach them to be able to reason about science.
Conflicts of interest
There are no conflicts to declare.References
- Appleton K., (2003), How do beginning primary school teachers cope with science? Toward an understanding of science teaching practice, Res. Sci. Educ., 33(1), 1–25.
- Arzi H. J. and White R. T., (2008), Change in teachers' knowledge of subject matter: a 17-year longitudinal study, Sci. Educ., 92, 221–251.
- Avargil S., Herscovitz O. and Dori Y. J., (2012), Teaching Thinking Skills in Context-Based Learning: Teachers’ Challenges and Assessment Knowledge, J. Sci. Educ. Technol., 21, 207–225.
- Bahktin M. M., (1981), in Emerson C. and Holquist M., (Trans.), Holquist M. (ed.), The dialogic imagination: four essays, Austin: University of Texas Press.
- Baran E. and Correia A. P., (2009), Student-led facilitation strategies in online discussions, Distance Educ., 30(3), 339–361.
- Bencze J. L., (2010), Promoting student-led science and technology projects in elementary teacher education: entry into core pedagogical practices through technological design, Int. J. Technol. Des. Ed., 20(1), 43–62.
- Bergman D. J. and Morphew J., (2015), Effects of a science content course on elementary preservice teachers' self-efficacy of teaching science, J. Coll. Sci. Teach., 44(3), 73–81.
- Berland L. K., Schwarz C. V., Krist C., Kenyon L., Lo A. S. and Reiser B. J., (2016), Epistemologies in Practice: Making Scientific Practices Meaningful for Students, J. Res. Sci. Teach., 53(7), 1082–1112.
- Bricker L. A. and Bell P., (2014), “What Comes to Mind When You Think of Science? The Perfumery!”: Documenting Science-Related Cultural Learning Pathways Across Contexts and Timescales, J. Sci. Res. Teach., 51(3), 260–285.
- Bursal M. and Paznokas L., (2006), Mathematics anxiety and preservice elementary teachers' confidence to teach mathematics and science, Sch. Sci. Math., 106(4), 173–180.
- Bussey T. J. and Orgill M., (2015), What do biochemistry students pay attention to in external representations of protein translation? The case of the Shine–Dalgarno sequence, Chem. Educ. Res. Pract., 16(4), 714–730.
- Cheng M. F. and Brown D. E., (2015), The role of scientific modeling criteria in advancing students' explanatory ideas of magnetism, J. Res. Sci. Teach., 52(8), 1053–1081.
- Cooper M. M., Stieff M. and DeSutter D., (2017), Sketching the Invisible to Predict the Visible: From Drawing to Modeling in Chemistry, Top. Cogn. Sci., 3–21.
- Corbin J. and Strauss A., (1990), Grounded theory research: Procedures, canons and evaluative criteria, Qual. Sociol., 13(1), 418–427.
- de Boer E., Janssen F. J. and van Driel J. H., (2016), Using an attribution support tool to enhance the teacher efficacy of student science teachers. J. Sci. Teach. Educ., 27(3), 303–324.
- Feucht F. C., Lunn Brownlee J. and Schraw, G., (2017), Moving beyond reflection: reflexivity and epistemic cognition in teaching and teacher education, Educ. Psychol., 52(4), 234–241.
- Fones S. W., Wagner J. R. and Caldwell E. R., (1999), Promoting attitude adjustments in science for preservice elementary teachers, J. Coll. Sci. Teach., 28(4), 231–236.
- Gilbert J. K. (2006), On the nature of “context” in chemical education, Int. J. Sci. Educ., 28(9), 957–976.
- Gilbert J. K. and Treagust D. F., (2009), Multiple representations in chemical education, Dordrecht: Springer.
- Gonsalves A., Rahm, J. and Carvalho A., (2013), “We Could Think of Things That Could Be Science”: Girls’ Re-Figuring of Science in an Out-of-School-Time-Club, J. Sci. Res. Teach., 50(9), 1068–1097.
- Gouvea J. and Passmore C., (2017), ‘Models of’ versus “Models for’, Sci. Educ., 26, 49–63.
- Gunning A. M. and Moore Mensah F., (2011), Preservice Elementary Teachers’ Development of Self-Efficacy and Confidence to Teach Science: A Case Study, J. Sci. Teac. Educ., 22, 171–185.
- Habraken C. L., (1996), Perceptions of chemistry: Why is the common perception of chemistry, the most visual of sciences, so distorted? J. Sci. Educ. Technol., 5(3), 193–201.
- Halliday M. A. and Hasan R., (1985), Language, text and context, Victoria: Deakin University.
- Hechter R. P., (2011), Changes in preservice elementary teachers’ personal science teaching efficacy and science teaching outcome expectancies: the influence of context, J. Sci. Teacher Educ., 22(2), 187–202.
- Hoffman J. L., Wu H. K., Krajcik J. S. and Soloway E., (2003), The nature of middle school learners' science content understandings with the use of on-line resources, J. Res. Sci. Teach., 40(3), 323–346.
- Holbrook J. and Rannikmae M., (2007), The nature of science education for enhancing scientific literacy, Int. J. Sci. Educ., 29(11), 1347–1362.
- Horton W. S., (2007), The influence of partner-specific memory associations on language production: evidence from picture naming, Lang. Cognit. Proc., 22(7), 1114–1139.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Kelly S. N., (2000), Preservice music education student fears of the internship and initial inservice teaching experience, Contributions to Music Education, 27(1), 41–50.
- Knuuttila T., (2011), Modeling and representing: an artefactual approach to model-based representation, Stud. Hist. Philos. Sci., 42, 262–271.
- Konstantopoulos S., (2011), Fixed effects and variance components estimation in three-level meta-analysis, Res. Syn. Meth., 2, 61–76.
- Krell M., Walzer C., Hergert S. and Krüger D., (2017), Development and Application of a Category System to Describe Pre-Service Science Teachers’ Activities in the Process of Scientific Modeling, Res. Sci. Educ., 1–27.
- Lemke J. L., (1990), Talking science: Language, learning, and values, Norwood, NJ: Ablex Publishing Corporation.
- Mahaffy P., (2006), Moving chemistry education into 3D; a tetrahedral metaphor for understanding chemistry. Union Carbide Award for Chemical Education, J. Chem. Educ., 83(1), 49–55.
- Middlecamp C. H., Jordan T., Shachter A. M., Oates K. K. and Lottridge S., (2006), Chemistry, Society, and Civic Engagement (Part 1): The SENCER project, J. Chem. Educ., 83(9), 1301–1307.
- Moje E. B., Collazo T., Carrillo R. and Marx R. W., (2001), “Maezistro, What is ‘Quality’?”: Language, Literacy, and Discourse in Project-Based Science, J. Res. Sci. Teach., 38(4), 469–498.
- Nakhleh M. B., (1992), Why some students don't learn chemistry: chemical misconceptions, J. Chem. Educ., 69(3), 191–196.
- National Research Council, (2012), A framework for K-12 science education: practices, crosscutting concepts, and core ideas, National Academies Press.
- Nersessian N. J., (2008), in Vosniadou S. (ed.), International Handbook of Research on Conceptual Change, London: Routledge, ch. 15, pp. 391–416.
- Nersessian N. J., (2009), Conceptual change: creativity, cognition, and culture, in Meheus J. and Nickles T. (ed.), Models of discovery and creativity, Springer Dordrecht, pp. 127–166.
- NGSS Lead States, (2013), Next Generation Science Standards: For States, By States, Washington, DC: The National Academies Press.
- Nowicki B. L., Sullivan-Watts B., Shim M. K., Young B. and Pockalny R., (2013), Factors Influencing Science Content Accuracy in Elementary Inquiry Science Lessons, Res. Sci. Educ., 43(3), 1135–1154.
- Oh P. S. and Oh S. J., (2011), What Teachers of Science Need to Know about Models: An overview, Int. J. Sci. Educ., 33(8), 1109–1130.
- Pappas C. C., Varelas M., Barry A. and Rife A., (2003), Dialogic inquiry around information texts: the role of intertextuality in constructing scientific understandings in urban primary classrooms, Linguist. Educ., 13(4), 435–482.
- Passmore C., Gouvea J. S. and Giere R., (2014), in Matthews M. R. (ed.), International Handbook of Research in History, Philosophy and Science Teaching, Dordrecht: Springer, ch. 36, pp. 1171–1202.
- Pinto G. and Garrido-Escudero A., (2016), Chemistry and Explosives: An Approach to the Topic through an Artistic and Historical Contribution Made by a Spanish Global Explosives Supplier, J. Chem. Educ., 93(1), 103–110.
- Raborn D. T. and Daniel M. J., (1999), Oobleck: a scientific encounter of the special education kind, Teaching Exceptional Children, 31(6), 32–40.
- Rice D. C., (2005), I didn't know oxygen could boil! What preservice and inservice elementary teachers' answers to ‘simple’ science questions reveals about their subject matter knowledge, Int. J. Sci. Educ., 27(9), 1059–1082.
- Schwarz C. V., Reiser B. J., Davis E. A., Kenyon L., Achér A., Fortus D., Shwartz Y., Hug B. and Krajcik J., (2009), Developing a Learning Progression for Scientific Modeling: Making scientific Modeling Accessible and Meaningful for Learners, J. Res. Sci. Teach., 46(6), 632–654.
- Sendur G., Polat, M. and Kazanci C., (2017), Does a course on the history and philosophy of chemistry have any effect on prospective chemistry teachers’ perceptions? The case of chemistry and the chemist, Chem. Educ. Res. Pract., 18, 601–629.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15, 10–23.
- Siegel M. A. and Ranney M. A., (2003), Developing the Changes in Attitude about the Relevance of Science (CARS) Questionnaire and Assessing Two High School Science Classes, J. Res. Sci. Teach., 40(8), 757–775.
- Sjöström J. and Talanquer V., (2014), Humanizing chemistry education: from simple contextualization to multifaceted problematization, J. Chem. Educ., 91(8), 1125–1131.
- Stuckey M., Hofstein A., Mamlok-Naaman R. and Eilks I., (2013), The meaning of ‘relevance’ in science education and its implications for the science curriculum, Stud. Sci. Educ., 49(1), 1–34.
- Talanquer V., (2013), Chemistry education: Ten Facets to Shape Us, J. Chem. Educ., 90(7), 832–838.
- Tosun C., (2000), The Beliefs of Preservice Elementary Teachers Towards Science and Science Teaching, Sch. Sci. Math., 100(7), 374–379.
- Ucar S., (2012), How do pre-service science teachers’ views on science, scientists, and science teaching change over time in a science teacher training program? J. Sci. Educ. Technol., 21(2), 255–266.
- Varelas M., Pappas C. C. and Rife A., (2006), Exploring the Role of Intertextuality in Concept Construction: Urban Second Graders Make Sense of Evaporation, Boiling, and Condensation, J. Res. Sci. Teach., 43(7), 637–666.
- Wang C. and Burris M. A., (1997), Photovoice: Concept, Methodology, and Use for Participatory Needs Assessment, Health Educ. Behav., 24(3), 369–387.
| This journal is © The Royal Society of Chemistry 2018 |