Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of 5-amino-N′-(9H-fluoren-9-ylidene)-8-nitro-7-aryl-1,2,3,7-tetrahydroimidazo[1,2-a]pyridine-6-carbohydrazide derivatives based on heterocyclic ketene aminals†
Hajar Hosseini and
Mohammad Bayat
and
Mohammad Bayat *
*
Department of Chemistry, Faculty of Science, Imam Khomeini International University, Qazvin, Iran. E-mail: bayat_mo@yahoo.com; m.bayat@sci.ikiu.ac.ir; Tel: +98 (28) 33780040
First published on 11th December 2018
Abstract
A new class of tetrahydroimidazo[1,2-a]pyridine derivatives has been successfully prepared via a five-component domino reaction using cyanoacetohydrazide, 9-fluorenone, aromatic aldehydes, 1,1-bis(methylthio)-2-nitroethene and ethylenediamine in ethanol at reflux. The new efficient cascade approach involves a sequence of N,N-acetal formation, Knoevenagel condensation, Michael addition, imine–enamine tautomerization and N-cyclization as key steps. The merit of this protocol is highlighted by its available and economical starting compounds, operational simplicity, clean reaction profile and tolerance of a wide diversity of functional groups.
Introduction
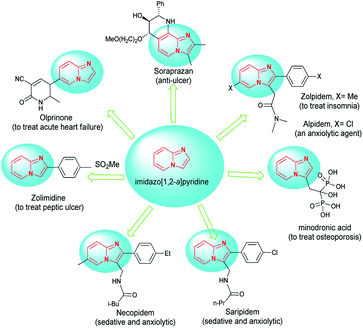
Imidazopyridines have shown a broad spectrum of pharmacological and biological activities.1 Among the various derivatives, the imidazo[1,2-a]pyridine framework is likely the most important construction due to its vital role as a key structure in drugs and biologically active compounds with properties such as anti-inflammatory,2,3 antiviral,4–6 antiulcer agents,7,8 antifungal,9 anticancer,10 anxiolytic,11 anti-ulcer,12 and antiprotozoal.13 They are included in marketed drugs such as the clinical anti-ulcer compound zolpidem and alpidem,14 olprinone,15 zolimidine,16 necopidem and saripidem,17 soraprazan and minodronic acid18 (Fig. 1).The design of reactions that minimize the number of synthetic steps for the rapid formation of functionalized molecules is one of the goals of modern synthesis. One way to achieve this purpose involves the development of multicomponent processes. Multicomponent reactions (MCRs) present a wide range of possibilities for the construction of complex molecules in a single step. The benefits of this approach include minimum time, labor and cost, high atom economy, and straight experimental procedures.19 These advantages are highlights for multicomponent cascade reactions, which contain in situ production of an intermediate with a reactive site for subsequent variations.20
By now, various synthetic methods have been developed to prepare imidazo[1,2-a]pyridines. The common strategies were the cyclocondensations of 2-aminopyridines with α-halocarbonyl compounds,21 1,3-dicarbonyl compounds,22 nitroolefins or alkynes.23 Besides, the condensation of 2-aminopyridines, aldehydes and isonitriles or alkynes in a one-pot process was also an efficient method for the synthesis of imidazo[1,2-a]pyridines.24
There are still many efforts to the development of new methods for the synthesis of imidazo[1,2-a]pyridine derivatives with a variety of substituents at two rings. Some other novel synthetic approaches have been established in recent years for the synthesis of tetrahydroimidazo[1,2-a]pyridines by heterocyclic ketene aminals (HKAs).25–29 Heterocyclic ketene aminals (HKAs) have been proven to be efficient synthons in the synthesis of heterocyclic systems. During the past few years, reactions of cyclic ketene aminals with a variety of bis-electrophilic compounds have been applied to make five- and six-membered fused heterocycles.30
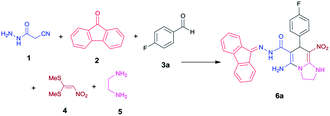
As a part of our current studies on synthesis of novel heterocyclic compounds using cyanoacetohydrazide, we describe herein an efficient one-pot five-component synthesis of novel imidazo[1,2-a]pyridine-6-carbohydrazides via in situ preparation of nitroketene aminal. These structures are completely new and there is no report on their synthesis.
Results and discussion
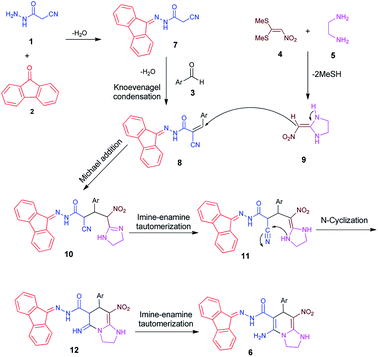
We have developed an efficient synthesis of tetrahydroimidazo[1,2-a]pyridine-6-carbohydrazides via a one-pot five-component reaction. We used cyanoacetohydrazide 1, 9-fluorenone 2, aromatic aldehyde 3, 1,1-bis(methylthio)-2-nitroethene 4 and ethylenediamine 5 for the synthesis of title compounds.Optimization of the conditions
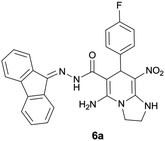
Initially, to identify the optimum reaction condition, 4-fluorobenzaldehyde was used as model substrate (since 4-fluorobenzaldehyde has clear reaction with obvious TLC at appropriate Rf value). At first, ethanol was used as solvent without any catalyst at reflux conditions and it was observed the desired product was not formed (Table 1, entry 1). The use of piperidine catalyst resulted in a yield of 40% in the product (entry 2). In order to improve yield, two other types of catalysts were used. With p-TSA, the five-component product did not form, and with acetic acid in a mixture of water and ethanol the efficiency did not change significantly (entry 3 and 5). The use of water and ethanol or water or acetonitrile without any catalyst resulted in no product formation (entry 4, 7 and 8). It was found that the reaction proceeded with high yield to formation of 5-amino-N′-(9H-fluoren-9-ylidene)-7-(4-fluorophenyl)-8-nitro-1,2,3,7-tetrahydroimidazo[1,2-a]pyridine-6-carbohydrazide 6a when ethanol was used as solvent and acetic acid was applied as catalyst at reflux conditions (entry 6).| Entry | Solvent | Catalyst (mol%) | Time (h) | Temp. (°C) | Yield (%) |
|---|---|---|---|---|---|
| a Reagents and conditions: cyanoacetohydrazide (1 mmol), 9-fluorenone (1 mmol), 4-fluorobenzaldehyde (1 mmol), 1,1-bis(methylthio)-2-nitroethene (1 mmol), ethylenediamine (1 mmol), solvent (20 mL), catalyst (0.2 mmol). | |||||
| 1 | EtOH | — | 24 | 78 | No reaction |
| 2 | EtOH | Piperidine | 24 | 78 | 40 |
| 3 | EtOH | p-TSA | 24 | 78 | No reaction |
| 4 | H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
— | 24 | 78 | No reaction |
| 5 | H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
AcOH | 24 | 78 | 35 |
| 6 | EtOH | AcOH | 9 | 78 | 87 |
| 7 | H2O | — | 24 | 100 | No reaction |
| 8 | CH3CN | — | 24 | 82 | No reaction |
It should be noted that initially a two-component reaction of cyanoacetohydrazide and 9-fluorenone is performed in the presence of acetic acid and then, without separating the product, aldehyde and ketene aminal are added.
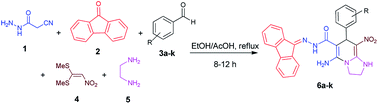
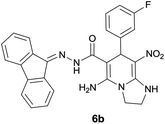
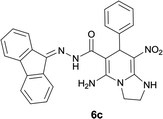
With information obtained from optimization conditions table, we could synthesize target compounds 6a–k using cyanoacetohydrazide 1, 9-fluorenone 2, various aromatic aldehydes 3a–k, 1,1-bis(methylthio)-2-nitroethene 4 and ethylenediamine 5 as starting materials (Scheme 1).
The reactions were completed after 8–12 h overall to afford corresponding heterocyclic systems 6a–k in good to high yields (65–87%). The results are summarized in Table 2.
| Entry | Aromatic aldehyde | Product | Time (h) | Yield (%) | Mp (°C) |
|---|---|---|---|---|---|
| a The reaction was performed using cyanoacetohydrazide (1 mmol), 9-fluorenone (1 mmol), aromatic aldehyde (1 mmol), 1,1-bis(methylthio)-2-nitroethene (1 mmol), ethylenediamine (1 mmol), EtOH (20 mL), AcOH (1 mL), reflux. | |||||
| 1 |  |
 |
9 | 87 | 246–248 |
| 2 |  |
 |
10 | 80 | 240–242 |
| 3 |  |
 |
11 | 75 | 249–251 |
| 4 |  |
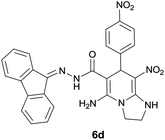 |
8 | 87 | 225–228 |
| 5 | 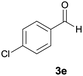 |
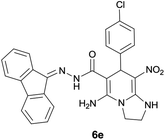 |
9 | 85 | 210–212 |
| 6 | 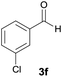 |
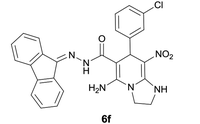 |
10 | 78 | 209–211 |
| 7 | 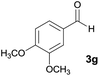 |
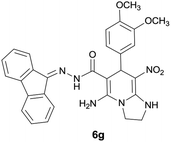 |
12 | 65 | 218–220 |
| 8 | 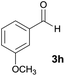 |
 |
12 | 70 | 198–200 |
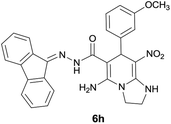
| 9 | 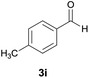 |
 |
11 | 83 | 218–220 |
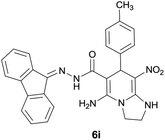
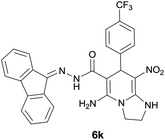
| 10 | 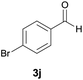 |
 |
9 | 80 | 212–214 |
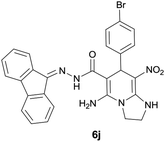
| 11 | 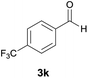 |
 |
9 | 75 | 226–229 |
Effect of substituents
This reaction was performed with other derivatives of diamines (1,3-diaminopropane, 1,4-diaminobutane and 1,2-diaminocyclohexane) under the same conditions, which did not result in the desired product. Also the reaction with ortho derivatives of benzaldehyde (2-chloro and 2-nitro) did not produce the product, probably due to steric effects. For aldehydes with an electron-withdrawing group on para position of ring (nitro and halogens), the reaction rate is the highest and with methoxy group, the rate is the lowest.It was found that the most important side product in these reactions was a four-component structure that was previously synthesized using two equivalents of aldehyde which will be further explained in the Mechanism section.
Structure determination
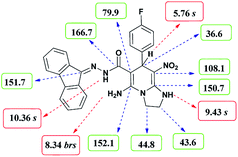
The structures of compounds 6a–k were deduced from their IR, 1H NMR, 13C NMR spectroscopic and mass spectrometric data (see the ESI†).The formation of proposed products 6a–k is clearly confirmed by the 1H and 13C NMR spectra of the crude products. As a representative case, the key signals of 1H and 13C NMR chemical shifts for 5-amino-N′-(9H-fluoren-9-ylidene)-7-(4-fluorophenyl)-8-nitro-1,2,3,7-tetrahydroimidazo[1,2-a]pyridine-6-carbohydrazide 6a are shown in Fig. 2.
The 1H NMR spectrum of 6a showed two NH groups at δ 9.43 and 10.36 ppm. The NH2 group appeared at δ 8.34 ppm. The protons of three aromatic rings were seen at δ 6.99–7.84 ppm. The proton of CH at pyridine ring was observed at δ 5.76 ppm. Two protons of two methylene groups appeared at δ 3.75–3.86 and 4.04–4.07 ppm.
The 1H-decoupled 13C NMR spectrum of 6a displayed 25 distinct signals in accordance to desired structure. The characteristic signals of three aliphatic carbons (CH and two CH2 groups) were observed at δ 36.6, 43.6 and 44.8 ppm respectively. Two signals at δ 79.9 and 108.1 ppm were related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO and C–NO2 respectively. The carbonyl group appeared at δ 166.7 ppm (Fig. 2).
C–CO and C–NO2 respectively. The carbonyl group appeared at δ 166.7 ppm (Fig. 2).
The mass spectrum of 6a displayed a molecular-ion peak at m/z 496 in agreement with the proposed product. The IR spectrum of this compound showed absorption bands at 3431, 3344, 3272 due to NH and NH2 groups, stretching vibration of aliphatic C–H bands at 2920, strong absorption of carbonyl group at 1654, stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of aromatic ring at 1445 and C–N stretching band at 1259 cm−1. Two absorption bands due to nitro group appeared at 1363 and 1528 cm−1.
C of aromatic ring at 1445 and C–N stretching band at 1259 cm−1. Two absorption bands due to nitro group appeared at 1363 and 1528 cm−1.
Mechanism
A typical plausible mechanism for the formation of 5-amino-N′-(9H-fluoren-9-ylidene)-7-aryl-8-nitro-1,2,3,7-tetrahydroimidazo[1,2-a]pyridine-6-carbohydrazide 6 is depicted in Scheme 2. On the basis of well-established chemistry of 1,1-bis(methylthio)-2-nitroethene, initially, addition of ethylenediamine 5 to 1,1-bis(methylthio)-2-nitroethene 4 leads to the formation of ketene aminal 9. On the other hand condensation of cyanoacetohydrazide 1 with 9-fluorenone 2 leads to hydrazone 7. Further, with increasing aldehyde 3, the Knoevenagel condensation affords intermediate 8. Then, Michael addition of ketene aminal 9 to adduct 8 leads to the intermediate 10, which undergoes successive imine–enamine tautomerization followed by an intramolecular cyclization via nucleophilic addition of –NH to nitrile group. Finally, another imine–enamine tautomerization gives the corresponding products 6 (Scheme 2). The most important side product in these reactions was a four-component structure without participation of 9-fluorenone.25 To prevent the formation of this product, firstly, the reaction of cyanoacetohydrazide with fluorenone was completed in the acidic medium within 5 hours, and then the aldehyde and the corresponding enamine are added simultaneously.Experimental
Materials
All commercially available reagents and other solvents were purchased from Aldrich and Merck Chemical Co. and used without further purification. The NMR spectra were recorded with a Bruker DRX-300 AVANCE instrument (300 MHz for 1H and 75.4 MHz for 13C) with DMSO-d6 as solvent. Chemical shifts are given in ppm (δ) relative to internal TMS, and coupling constant (J) are reported in Hertz (Hz). Melting points were measured with an electrothermal 9100 apparatus. Mass spectra were recorded with an Agilent 5975C VL MSD with Triple-Axis detector operating at an ionization potential of 70 eV. IR spectra were measured with Bruker Tensor 27 spectrometer. Elemental analyses for C, H and N were performed using a PerkinElmer 2004 series [II] CHN elemental analyzer.General procedure of the synthesis of tetrahydroimidazo[1,2-a]pyridine-6-carbohydrazide derivatives
A mixture of ethylenediamine (66 mL, 1 mmol), 1,1-bis(methylthio)-2-nitroethylene (0.165 g, 1 mmol) and 10 mL EtOH in a 50 mL flask was refluxed for 6 hours. In another 50 mL flask the stoichiometric mixture of cyanoacetohydrazide (1 mmol, 0.99 g) and 9-fluorenone (1 mmol, 0.180 g) in EtOH (10 mL) and AcOH (1 mL) was stirred at reflux conditions for 5 hours. After this time, it is observed that precipitate is formed and TLC shows the consumption of the starting components. Then, aromatic aldehyde (1 mmol) and the first solution (HKA) was added to this mixture simultaneously. The progress of the reaction was monitored by TLC using ethyl acetate/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After completion of the reaction, the precipitated product was collected by filtration and washed with warm ethanol to give the pure products 6a–k.
1). After completion of the reaction, the precipitated product was collected by filtration and washed with warm ethanol to give the pure products 6a–k.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 108.1 (C
C–CO), 108.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 115.0, 120.6, 120.9, 121.8, 126.9, 128.0, 128.5, 130.0, 130.1, 130.3, 131.1, 137.4, 139.5, 141.2, 141.8, 160.0 (Ar), 150.7 (C
C–NO2), 115.0, 120.6, 120.9, 121.8, 126.9, 128.0, 128.5, 130.0, 130.1, 130.3, 131.1, 137.4, 139.5, 141.2, 141.8, 160.0 (Ar), 150.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.7 (C
C–NH), 151.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.1 (C
N), 152.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.7 (C
C–NH2), 166.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (EI, 70 eV): m/z (%) = 496 (0.07) [M]+, 453 (0.17), 407 (0.10), 356 (100), 327 (35), 276 (4), 254 (4), 230 (4), 194 (3), 178 (12), 164 (15), 150 (2), 133 (1), 97 (1), 69 (1); anal. calcd for C27H21FN6O3: C, 65.32; H, 4.26; N, 16.93. Found: C, 65.1; H, 4.2; N, 16.7.
O); MS (EI, 70 eV): m/z (%) = 496 (0.07) [M]+, 453 (0.17), 407 (0.10), 356 (100), 327 (35), 276 (4), 254 (4), 230 (4), 194 (3), 178 (12), 164 (15), 150 (2), 133 (1), 97 (1), 69 (1); anal. calcd for C27H21FN6O3: C, 65.32; H, 4.26; N, 16.93. Found: C, 65.1; H, 4.2; N, 16.7.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 107.6 (C
C–CO), 107.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 114.9, 120.6, 120.9, 121.8, 124.2, 127.0, 128.5, 130.1, 130.3, 131.1, 137.4, 139.5, 141.2, 148.5 (Ar), 150.8 (C
C–NO2), 114.9, 120.6, 120.9, 121.8, 124.2, 127.0, 128.5, 130.1, 130.3, 131.1, 137.4, 139.5, 141.2, 148.5 (Ar), 150.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.8 (C
C–NH), 151.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.3 (C
N), 152.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.8 (C
C–NH2), 166.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (EI, 70 eV): m/z (%) = 496 (0.03) [M]+, 453 (1), 407 (0.4), 356 (100), 327 (40), 276 (40), 230 (56), 194 (79), 178 (24), 164 (90), 150 (9), 133 (9), 95 (7), 69 (8); anal. calcd for C27H21FN6O3: C, 65.32; H, 4.26; N, 16.93. Found: C, 65.7; H, 4.1; N, 16.8.
O); MS (EI, 70 eV): m/z (%) = 496 (0.03) [M]+, 453 (1), 407 (0.4), 356 (100), 327 (40), 276 (40), 230 (56), 194 (79), 178 (24), 164 (90), 150 (9), 133 (9), 95 (7), 69 (8); anal. calcd for C27H21FN6O3: C, 65.32; H, 4.26; N, 16.93. Found: C, 65.7; H, 4.1; N, 16.8.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 108.2 (C
C–CO), 108.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 120.6, 120.9, 121.7, 126.9, 127.1, 128.2, 128.5, 128.7, 129.1, 130.0, 130.2, 131.0, 137.4, 139.4, 141.1, 145.5 (Ar), 149.9 (C
C–NO2), 120.6, 120.9, 121.7, 126.9, 127.1, 128.2, 128.5, 128.7, 129.1, 130.0, 130.2, 131.0, 137.4, 139.4, 141.1, 145.5 (Ar), 149.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.8 (C
C–NH), 151.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.2 (C
N), 152.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.7 (C
C–NH2), 166.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (EI, 70 eV): m/z (%) = 478 (0.02) [M]+, 435 (2), 419 (0.9), 356 (78), 327 (35), 258 (33), 212 (54), 194 (67), 178 (22), 164 (100), 150 (8), 115 (8), 96 (0.9), 77 (3); anal. calcd for C27H22N6O3: C, 67.77; H, 4.63; N, 17.56. Found: C, 67.4; H, 4.9; N, 17.3.
O); MS (EI, 70 eV): m/z (%) = 478 (0.02) [M]+, 435 (2), 419 (0.9), 356 (78), 327 (35), 258 (33), 212 (54), 194 (67), 178 (22), 164 (100), 150 (8), 115 (8), 96 (0.9), 77 (3); anal. calcd for C27H22N6O3: C, 67.77; H, 4.63; N, 17.56. Found: C, 67.4; H, 4.9; N, 17.3.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 107.0 (C
C–CO), 107.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 120.6, 120.9, 121.8, 123.7, 127.0, 128.6, 129.6, 130.2, 130.4, 131.1, 137.4, 139.6, 141.3, 146.5 (Ar), 151.7 (C
C–NO2), 120.6, 120.9, 121.8, 123.7, 127.0, 128.6, 129.6, 130.2, 130.4, 131.1, 137.4, 139.6, 141.3, 146.5 (Ar), 151.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.8 (C
C–NH), 151.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.1 (C
N), 152.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 153.4 (CAr–NO2), 166.8 (C
C–NH2), 153.4 (CAr–NO2), 166.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C27H21N7O5: C, 61.95; H, 4.04; N, 18.73. Found: C, 61.6; H, 4.3; N, 18.5.
O); anal. calcd for C27H21N7O5: C, 61.95; H, 4.04; N, 18.73. Found: C, 61.6; H, 4.3; N, 18.5.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 107.7 (C
C–CO), 107.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 120.6, 120.9, 121.8, 126.9, 127.9, 128.4, 128.5, 129.2, 130.1, 130.3, 131.1, 131.5, 137.4, 139.5, 141.2, 144.6 (Ar), 150.9 (C
C–NO2), 120.6, 120.9, 121.8, 126.9, 127.9, 128.4, 128.5, 129.2, 130.1, 130.3, 131.1, 131.5, 137.4, 139.5, 141.2, 144.6 (Ar), 150.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.7 (C
C–NH), 151.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.1 (C
N), 152.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.7 (C
C–NH2), 166.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (EI, 70 eV): m/z (%) = 356 (9), 327 (5), 292 (21), 275 (19), 246 (41), 220 (35), 194 (15), 164 (100), 135 (6), 95 (5), 69 (9), 44 (21); anal. calcd for C27H21ClN6O3: C, 63.22; H, 4.13; N, 16.38. Found: C, 63.6; H, 4; 5 N, 16.1.
O); MS (EI, 70 eV): m/z (%) = 356 (9), 327 (5), 292 (21), 275 (19), 246 (41), 220 (35), 194 (15), 164 (100), 135 (6), 95 (5), 69 (9), 44 (21); anal. calcd for C27H21ClN6O3: C, 63.22; H, 4.13; N, 16.38. Found: C, 63.6; H, 4; 5 N, 16.1.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 107.5 (C
C–CO), 107.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 120.6, 120.9, 121.8, 126.8, 127.0, 128.1, 128.2, 128.5, 130.1, 130.3, 130.5, 131.0, 133.0, 137.4, 139.5, 141.2, 148.1 (Ar), 150.9 (C
C–NO2), 120.6, 120.9, 121.8, 126.8, 127.0, 128.1, 128.2, 128.5, 130.1, 130.3, 130.5, 131.0, 133.0, 137.4, 139.5, 141.2, 148.1 (Ar), 150.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.7 (C
C–NH), 151.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.3 (C
N), 152.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.8 (C
C–NH2), 166.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C27H21ClN6O3: C, 63.22; H, 4.13; N, 16.38. Found: C, 63.4; H, 4.4; N, 16.3.
O); anal. calcd for C27H21ClN6O3: C, 63.22; H, 4.13; N, 16.38. Found: C, 63.4; H, 4.4; N, 16.3.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 108.3 (C
C–CO), 108.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 112.3, 112.7, 119.7, 120.6, 121.0, 121.7, 126.7, 127.9, 128.5, 129.3, 130.1, 131.1, 137.4, 138.0, 139.4, 141.2, 148.1, 148.7 (Ar), 149.3 (C
C–NO2), 112.3, 112.7, 119.7, 120.6, 121.0, 121.7, 126.7, 127.9, 128.5, 129.3, 130.1, 131.1, 137.4, 138.0, 139.4, 141.2, 148.1, 148.7 (Ar), 149.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.8 (C
C–NH), 151.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.3 (C
N), 152.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.6 (C
C–NH2), 166.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C29H26N6O5: C, 64.68; H, 4.87; N, 15.60. Found: C, 64.3; H, 4.6; N, 15.7.
O); anal. calcd for C29H26N6O5: C, 64.68; H, 4.87; N, 15.60. Found: C, 64.3; H, 4.6; N, 15.7.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 108.0 (C
C–CO), 108.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 111.6, 114.8, 120.2, 120.6, 120.9, 121.7, 126.9, 128.1, 128.5, 129.7, 130.0, 130.2, 131.0, 137.4, 139.4, 141.1, 147.1 (Ar), 150.8 (C
C–NO2), 111.6, 114.8, 120.2, 120.6, 120.9, 121.7, 126.9, 128.1, 128.5, 129.7, 130.0, 130.2, 131.0, 137.4, 139.4, 141.1, 147.1 (Ar), 150.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.8 (C
C–NH), 151.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.3 (C
N), 152.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 159.5 (CAr–OMe), 166.7 (C
C–NH2), 159.5 (CAr–OMe), 166.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (EI, 70 eV): m/z (%) = 508 (0.06) [M]+, 435 (0.1), 356 (4), 327 (1), 292 (5), 276 (4), 220 (7), 194 (6), 163 (100), 134 (55), 105 (14), 85 (7), 57 (12); anal. calcd for C28H24N6O4: C, 66.13; H, 4.76; N, 16.53. Found: C, 66.3; H, 4.5; N, 16.3.
O); MS (EI, 70 eV): m/z (%) = 508 (0.06) [M]+, 435 (0.1), 356 (4), 327 (1), 292 (5), 276 (4), 220 (7), 194 (6), 163 (100), 134 (55), 105 (14), 85 (7), 57 (12); anal. calcd for C28H24N6O4: C, 66.13; H, 4.76; N, 16.53. Found: C, 66.3; H, 4.5; N, 16.3.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 108.3 (C
C–CO), 108.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 120.6, 120.9, 121.7, 126.9, 128.1, 128.5, 129.0, 130.0, 130.1, 131.0, 136.1, 137.4, 139.4, 141.1, 142.5 (Ar), 149.6 (C
C–NO2), 120.6, 120.9, 121.7, 126.9, 128.1, 128.5, 129.0, 130.0, 130.1, 131.0, 136.1, 137.4, 139.4, 141.1, 142.5 (Ar), 149.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.7 (C
C–NH), 151.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.2 (C
N), 152.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.6 (C
C–NH2), 166.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); MS (EI, 70 eV): m/z (%) = 492 (0.04) [M]+, 435 (0.08), 356 (100), 327 (39), 272 (20), 226 (30), 194 (50), 165 (59), 115 (7), 69 (8); anal. calcd for C28H24N6O3: C, 68.28; H, 4.91; N, 17.06. Found: C, 68.7; H, 4.6; N, 17.2.
O); MS (EI, 70 eV): m/z (%) = 492 (0.04) [M]+, 435 (0.08), 356 (100), 327 (39), 272 (20), 226 (30), 194 (50), 165 (59), 115 (7), 69 (8); anal. calcd for C28H24N6O3: C, 68.28; H, 4.91; N, 17.06. Found: C, 68.7; H, 4.6; N, 17.2.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 107.7 (C
C–CO), 107.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 120.0, 120.6, 120.9, 121.8, 126.9, 127.9, 128.5, 130.1, 130.3, 130.5, 131.1, 131.3, 137.4, 139.6, 141.2, 145.0 (Ar), 151.0 (C
C–NO2), 120.0, 120.6, 120.9, 121.8, 126.9, 127.9, 128.5, 130.1, 130.3, 130.5, 131.1, 131.3, 137.4, 139.6, 141.2, 145.0 (Ar), 151.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.7 (C
C–NH), 151.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.1 (C
N), 152.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.7 (C
C–NH2), 166.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C27H21BrN6O3: C, 58.18; H, 3.80; N, 15.08. Found: C, 58.4; H, 3.5; N, 15.3.
O); anal. calcd for C27H21BrN6O3: C, 58.18; H, 3.80; N, 15.08. Found: C, 58.4; H, 3.5; N, 15.3.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CO), 108.0 (C
C–CO), 108.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NO2), 115.0, 115.2, 120.6, 120.9, 120.9, 121.8, 126.9, 128.5, 130.0, 130.1, 130.3, 131.0, 137.4, 139.5, 141.2, 141.8 (Ar), 150.7 (C
C–NO2), 115.0, 115.2, 120.6, 120.9, 120.9, 121.8, 126.9, 128.5, 130.0, 130.1, 130.3, 131.0, 137.4, 139.5, 141.2, 141.8 (Ar), 150.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH), 151.7 (C
C–NH), 151.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.1 (C
N), 152.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH2), 166.7 (C
C–NH2), 166.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C28H21FN6O3: C, 61.54; H, 3.87; N, 15.38. Found: C, 61.8; H, 3.7; N, 15.2.
O); anal. calcd for C28H21FN6O3: C, 61.54; H, 3.87; N, 15.38. Found: C, 61.8; H, 3.7; N, 15.2.Conclusion
A green and highly efficient method for the synthesis of imidazo[1,2-a]pyridine core has been created by annulation of heterocyclic ketene aminals (HKAs) and a three-component product of cyanoacetohydrazide, 9-fluorenone and aromatic aldehydes that is produced under reaction conditions and can not be separated. The reactions are completed within 8–12 h in EtOH in the presence of acetic acid at reflux conditions. This methodology provides a novel approach for easy construction of highly substituted imidazopyridines in good yields. The present synthesis shows significant properties such as high regioselectivity, cascade one-pot reaction, relatively short reaction times, simple purification of products and high atom economy.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran is gratefully acknowledged.Notes and references
- C. E. Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888 CrossRef.
- L. Almirante, L. Polo, A. Mugnaini, E. Provinciali, P. Rugarli, A. Biancotti, A. Gamba and W. Murmann, J. Med. Chem., 1965, 8, 305 CrossRef CAS.
- A. Chiacchio, M. Rimoli and L. Avllone, Arch. Pharm. Pharm. Med. Chem., 1998, 331, 273 CrossRef.
- S. Kotovskaya and Z. Baskakova, Pharm. Chem. J., 2005, 39, 574 CrossRef CAS.
- M. Lhassani and O. Chavignon, Eur. J. Med. Chem., 1999, 34, 271 CrossRef CAS.
- G. Puerstinger and J. Paeshuyse, Bioorg. Med. Chem. Lett., 2007, 17, 390 CrossRef CAS PubMed.
- J. Kaminsky, C. Puchalski and D. Solomon, J. Med. Chem., 1989, 32, 1686 CrossRef.
- J. Kaminsky and A. Doweyko, J. Med. Chem., 1997, 40, 427 CrossRef.
- Y. Rival, G. Grassy and A. Taudou, Eur. J. Med. Chem., 1991, 26, 13 CrossRef CAS.
- S. Kataev and B. Syropyatov, Pharm. Chem. J., 2004, 38, 181 CrossRef CAS.
- H. Huang and X. Ji, Org. Lett., 2013, 15, 24 Search PubMed.
- J. J. Kaminski and A. M. Doweyko, J. Med. Chem., 1997, 40, 427 CrossRef CAS.
- M. Bollini, J. J. Casal, D. E. Alvarez, L. Boiani, M. Gonzalez, H. Cerecetto and A. M. Bruno, Bioorg. Med. Chem., 2009, 17, 1437 CrossRef CAS.
- S. Z. Langer, S. Arbilla, J. Benavides and B. Scatton, Adv. Biochem. Psychopharmacol., 1990, 46, 61 CAS.
- K. Mizushige, T. Ueda, K. Yukiiri and H. Suzuki, Cardiovasc. Drug Rev., 2002, 20, 163 CrossRef CAS PubMed.
- L. Almirante, L. Polo, A. Mugnaini, E. Provinciali, P. Rugarli, A. Biancotti, A. Gamba and W. Murmann, J. Med. Chem., 1965, 8, 305 CrossRef CAS PubMed.
- R. J. Boerner and H. Moller, J. Psychopharmakother., 1997, 4, 145 Search PubMed.
- A. Bagdi and S. Santra, Chem. Commun., 2015, 51, 1555 RSC.
- J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300 CrossRef CAS PubMed.
- K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS.
- S. Ulloora, A. V. Adhikari and R. Shabaraya, Chin. Chem. Lett., 2013, 24, 853–856 CrossRef CAS.
- X. Wang, L. Ma and W. Yu, Synthesis, 2011, 2445–2453 Search PubMed.
- R. L. Yan, H. Han and C. Ma, J. Org. Chem., 2012, 77, 2024–2028 CrossRef CAS PubMed.
- S. K. Guchhait and A. L. Chandgude, J. Org. Chem., 2012, 77, 4438–4444 CrossRef CAS.
- M. Bayat and F. S. Hosseini, Tetrahedron Lett., 2017, 58, 1616–1621 CrossRef CAS.
- A. Alizadeh and A. C. R. Rezvanian, C. R. Chim., 2014, 17, 103–107 CrossRef CAS.
- C. Y. Yu, P. H. Yang, M. X. Zhao and Z. T. Huang, Synlett, 2006, 1835–1840 CrossRef CAS.
- W. Y. Xu, Y. M. Jia, J. K. Yang and Z. T. Huang, Synlett, 2010, 1682–1684 CAS.
- H. Wang, Y. Wang, D. Liang, L. Liu, J. Zhang and Q. Zhu, Angew. Chem., Int. Ed., 2011, 50, 5678 CrossRef CAS.
- (a) K. M. Wang, S. J. Yan and J. Lin, Eur. J. Org. Chem., 2014, 1129–1145 CrossRef CAS; (b) P. H. Yang, Res. Chem. Intermed., 2016, 42, 5617–5637 CrossRef CAS; (c) L. Zhang, J. Dong, X. Xu and Q. Liu, Chem. Rev., 2016, 116, 287–322 CrossRef CAS PubMed; (d) F. Sun, F. Zhu, Z. Shao and Z. Li, Synlett, 2015, 26, 2306–2312 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra09308c |
| This journal is © The Royal Society of Chemistry 2018 |