Open Access Article
Open Access ArticleSynthesis and application of a cationic waterborne polyurethane fixative using quaternary ammonium diol as a chain extender
Chenghao Dong ,
Wei Xin and
Yunjun Luo
,
Wei Xin and
Yunjun Luo *
*
School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: yjluo@bit.edu.cn
First published on 18th December 2018
Abstract
In order to improve the stability of waterborne polyurethane fixative during the color-fixing treatment, a novel environment-friendly cationic waterborne polyurethane (CWPU) containing quaternary ammonium groups was synthesized from isophorone diisocyanate, polypropylene glycol, butanone oxime, and N-methyl dihydroxyethyl allyl ammonium chloride (MDAAC) and dispersed in water by using diethylenetriamine as the post-chain extender. The structures of the MDAAC and CWPU were characterized by 1H-NMR and FTIR. Effects of R-value, MDAAC content, post-chain extender and blocking agent on the properties of the waterborne polyurethane emulsion and the rubbing fastness of the treated dyed cotton fabrics were investigated. The investigation results showed that the R-value and the cationic group content of the cationic waterborne polyurethane had no significant effect on the color-fixing performance, but the content of DETA had a great influence on the wet-rubbing fastness. The wet rubbing fastness of the treated cotton fabrics was promoted from grade 1–2 to grade 3. Furthermore, the quaternary ammonium groups were able to provide the surface charges for the stabilization of the resulting polymer in alkaline electrolyte solution which met the requirements of practical applications.
Introduction
As one of the most popular fabrics, cotton fabrics have many advantages such as softness, breathability, moisture absorption, warmth and so on.1 Reactive dyes which have reactive groups such as monochloro-triazine, vinyl sulfone, etc. that can form covalent bonds with cotton fabrics2 are the most common dyes in cotton dyeing because of their relatively high color fastness. However, due to the presence of water-soluble groups such as sulphonic groups3 in the reactive dye molecules and the hydrolysis of covalent bonds among the dye molecules and fabrics,4 the binding force among the dye molecules and cotton fabrics is insufficient, so the dye molecules are easily detached from fabrics, resulting in a decrease in color fastness, especially for deep dark cotton fabrics dyed with reactive dyes, the wet-rubbing fastness often fails to meet the requirements of use.To solve this problem, numerous dye-fixing agents were prepared to improve the color fastness of dyed cotton fabrics.5,6 Among them, waterborne polyurethane (WPU) fixative might be a promising choice. It is well known that WPU is environmentally friendly and has excellent mechanical properties, abrasion resistance, and good film-forming properties due to its unique structure.7 The cationic group of WPU can be combined with the anion group of dye molecule to form a water-insoluble lake,8 and polyurethane molecules can enclose the dye molecules among the fibers because of their excellent film-forming ability. Furthermore, if reactive group (such as blocked –NCO group9–11 or epoxy group9,12) was incorporated into WPU, the polyurethane molecules can form covalent bonds with fabrics or dyes during the high-temperature baking process.13,14 By applying these methods, the color fastness of dyed fabrics was significantly improved.
Cellulosic fibers build up negative surface charges in the dyebath and these negative charges repel reactive dyes to reduce dye exhaustion.15 Thus a high concentration of electrolytes such as sodium carbonate was used to overcome the repulsion in dyeing progress.16 Given a consideration of the presence of residual electrolyte between the cotton fibers, the WPU fixatives need to have good alkali resistance, that is, it should be stably dispersed in an alkaline electrolyte solution for a certain period of time.
However, cationic groups on the WPU using acid as neutralizer would be transformed into uncharged tertiary amine groups in the alkali solution.17 The preparations of cationic WPU with a good alkali resistance are rarely reported. Nonionic hydrophilic groups can improve the stability of WPU in alkaline electrolyte solution,18,19 but the water resistance of WPU decreases significantly after the incorporation of nonionic groups,20 which results in a poor wet-rubbing fastness. Zenglu et al.13,21 used alkylating agent to synthesized cationic WPU. Since the cationic groups of these WPUs are still charged in an alkaline solution, these WPU should have good alkali resistance. However, they are not suitable for fabric finishing because of the toxicity of alkylating agents.
Herein, the quaternary ammonium diol, N-methyl dihydroxyethyl allyl ammonium chloride (MDAAC), was synthesized and used as the hydrophilic chain extender. Moreover, the non-toxic CWPU synthesized in this paper containing quaternary ammonium structure showed positive charge in a wide pH range,22 especially under the alkaline conditions so that it could be stable dispersed in the finishing solution during application. Furthermore, the test results showed that this CWPU fixative could improve the wet-rubbing fastness of deep black cotton fabrics from grade 1–2 to grade 3. To the best of our knowledge, the synthesis of no-toxic cationic WPU fixative with good alkali resistance and excellent application performance had not been previously reported.
Experimental
Materials
The deep black twill cotton fabric was kindly provided by Transfar Group Co., Ltd. 3-Chloropropene (98% purity) was purchased from Shanghai Macklin Biochemical Co., Ltd; N-methyl diethanolamine (AR), isophorone diisocyanate (IPDI, 98% purity), butanone oxime (MEKO, AR), 1-methyl-2-pyrrlione (NMP, AR), dibutyltin dilaurate (DBTDL, AR), diethylenetriamine (DETA, AR) were purchased from Tianjin Guangfu Fine Chemical Research Institute and used without further purification. Butanone (MEK, AR) purchased from Beijing Chemical Plant was dehydrated by immersed in 4 Å molecular sieve for 7 days. Polypropylene glycol (Mn = 1000, PPG1000, industrial grade, Tianjin petrochemical plant) was vacuum dried at 110 °C and 1–2 mmHg for 2 h before used.The synthesis of MDAAC
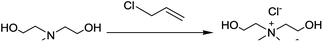
22.96 g 3-chloropropene (0.3 mol) and 29.79 g MDEA (0.25 mol) were charged into a 250 ml round-bottom four-necked flask,23,24 which was equipped with a mechanical stirrer, thermometer, nitrogen apparatus, and condenser. The reactants were stirred for 1 h at the constant temperature of 50 °C. Then adding crystal nucleus to crystallize the product, unreacted 3-chloropropene was removed by vacuum, the resulting white crystal was ground and dried in a vacuum oven at 50 °C until a constant weight was reached. The synthesis scheme of MDAAC is illustrated in Scheme 1.The synthesis of waterborne polyurethane color-fixing agent
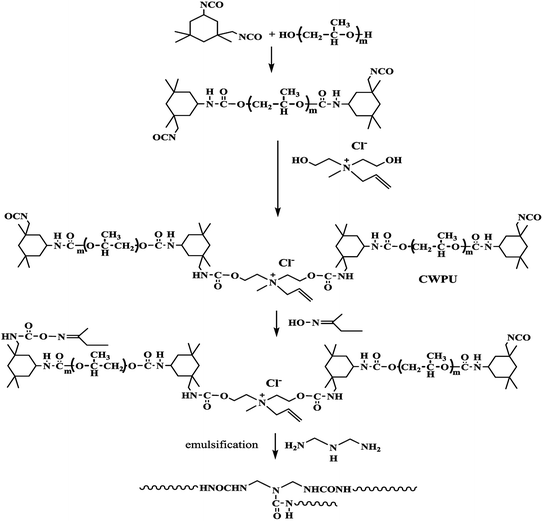
Quantitative mass of PPG1000 and IPDI were placed into a 500 ml four-necked flask equipped with a stirrer, thermometer, nitrogen inlet, and outlet apparatus. The reaction proceeded at 85 °C until isocyanate value reached the theoretical value, under the catalysis of DBTDL. After cooling the reaction mixture to 70 °C, MDAAC dissolved in NMP was added to the flask and reacted for 3 h, then a calculated amount of MEKO was fed into the reactor and reacted for another 3 h. MEK was added to reduce viscosity during the above processes. After that, the prepolymer was cooled to the room temperature and emulsified with deionized water under a high speed for 5 min. Finally, DETA was added dropwise to the above emulsion and the emulsion was emulsified for another 15 minutes. The MEK in emulsion was removed by rotary vacuum evaporation and the resulting product was a transparent or translucent emulsion with 20% solid content. The synthesis route of the cationic waterborne polyurethane (CWPU) is shown in Scheme 2 and the compositions of CWPU prepared in this paper with different R-value and MDAAC content are shown in Table 1. Moreover, the compositions of CWPU blocked by MEKO and extended by DETA with fixed R-value and MDAAC content are shown in Table 2.| Samples | R | w(MDAAC) (%) | IPDI (g) | PPG1000 (g) | MDAAC (g) | Water (g) |
|---|---|---|---|---|---|---|
| CWPU1 | 1.1 | 12 | 7.34 | 14.66 | 3 | 100 |
| CWPU2 | 1.2 | 12 | 7.86 | 14.14 | 3 | 100 |
| CWPU3 | 1.3 | 12 | 8.37 | 13.63 | 3 | 100 |
| CWPU4 | 1.4 | 12 | 8.86 | 13.14 | 3 | 100 |
| CWPU5 | 1.5 | 12 | 9.34 | 12.66 | 3 | 100 |
| CWPU6 | 1.4 | 10 | 8.37 | 14.13 | 2.5 | 100 |
| CWPU7 | 1.4 | 8 | 7.88 | 15.12 | 2 | 100 |
| CWPU8 | 1.4 | 6 | 7.41 | 16.09 | 1.5 | 100 |
| Samplesa | DETA (g) | MEKO (g) | Water (g) |
|---|---|---|---|
| a The amount of PPG1000, IPDI and MDAAC for each sample in this table is the same as the amount of CWPU6 sample. | |||
| CWPU6-D25% | 0.19 | 0 | 100.76 |
| CWPU6-D50% | 0.37 | 0 | 101.48 |
| CWPU6-D75% | 0.56 | 0 | 102.24 |
| CWPU6-D100% | 0.74 | 0 | 102.96 |
| CWPU6-M100% | 0 | 1.88 | 107.52 |
| CWPU6-D25%M75% | 0.19 | 1.40 | 106.36 |
| CWPU6-D50%M50% | 0.37 | 0.94 | 105.24 |
| CWPU6-D75%M25% | 0.56 | 0.47 | 104.12 |
Treatment of cotton fabric with CWPU emulsion
The CWPU emulsion was diluted in deionized water to provide a 1.6 wt% color-fixing agent solution. The dyed cotton samples were dipped and padded twice with the solutions of CWPU to give 80% wet pick-up at a liquor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, then curing at 160 °C for 90 s.
30, then curing at 160 °C for 90 s.
Preparation of the CWPU films
The CWPU emulsions were cast on horizontal Teflon plates to allow them to dry at the room temperature for 7 days, and then at 60 °C in vacuo for 24 h. After demolding, the films were kept in a desiccator to avoid moisture.Characterization
| Water absorption = (m − m0)/m0 × 100%, |
Results and discussion
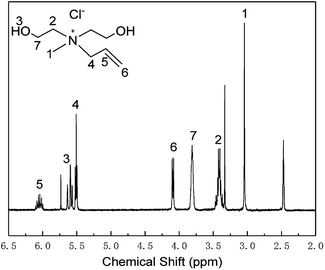
The 1H-NMR result of MDAAC
The 1H-NMR spectrum of MDAAC is shown in Fig. 1. The peaks at 2.48 ppm and 3.33 ppm were attributed to the solvent DMSO-d6 and water individually. Other peaks are listed as follow: 3.04 (s, 3H, N–CH3), 3.37–3.46 (m, 4H, N–CH2–C), 3.80–3.82 (m, 4H, N–C–CH2–), 4.08–4.10 (d, 2H, N–C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.57–5.73 (m, 2H,–OH), 5.50–5.52 (t, 2H, N–CH2–C
CH2), 5.57–5.73 (m, 2H,–OH), 5.50–5.52 (t, 2H, N–CH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 6.00–6.10 (m, 1H, N–C–CH
C), 6.00–6.10 (m, 1H, N–C–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The 1H-NMR result demonstrated the successful synthesis of MDAAC.
C). The 1H-NMR result demonstrated the successful synthesis of MDAAC.
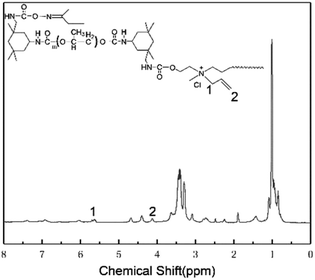
1H-NMR and FTIR analysis of CWPU
The 1H-NMR spectrum of all CWPUs were similar. As an example, the 1H-NMR test result of CWPU6-D50%M50% is shown in Fig. 2. The chemical shift of the methylene group in allyl was 5.66 ppm and the chemical shift of H at the end of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was 4.12 ppm, and the integral area of the two were equal, indicating the allyl in MDAAC did not participate in polymerization resulting from the self-inhibition effect of allyl27 and the effect of steric hindrance.
C was 4.12 ppm, and the integral area of the two were equal, indicating the allyl in MDAAC did not participate in polymerization resulting from the self-inhibition effect of allyl27 and the effect of steric hindrance.
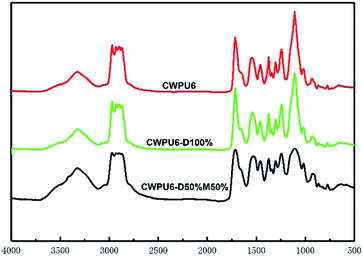
All CWPU emulsions had the similar infrared spectra and Fig. 3 shows FTIR spectroscopic of the CWPU6, CWPU6-D100% and CWPU-D50%M50% respectively. The absorption band near 3321 cm−1 was ascribed to the presence of –NH– stretching. The absorption peak corresponding to the carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) appeared at 1717 cm−1. Meanwhile, characteristic peak at 1660 cm−1 was assigned to the stretching vibration of C
O) appeared at 1717 cm−1. Meanwhile, characteristic peak at 1660 cm−1 was assigned to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. A narrow peak around 1108 cm−1 belonged to stretching vibration of ether linkage in PPG. The FTIR and 1H-NMR spectra demonstrated the successful synthesis of CWPU.
C. A narrow peak around 1108 cm−1 belonged to stretching vibration of ether linkage in PPG. The FTIR and 1H-NMR spectra demonstrated the successful synthesis of CWPU.
The properties of CWPU emulsions
| Samples | Sizea (PDI) (nm) | Zeta potential (mV) | Storage stability at room temperatureb | The stability of emulsion in Na2CO3 solution |
|---|---|---|---|---|
| a PDI refers to particle distribution index.b Storage stability refers to the time when deposition or delamination occurs in the latex. | ||||
| CWPU1 | 20.33(0.264) | 48.1 | Above 6 months | Precipitated after 12 h |
| CWPU2 | 21.12(0.235) | 35.5 | Above 6 months | Stable |
| CWPU3 | 25.37(0.173) | 32.6 | Above 6 months | Stable |
| CWPU4 | 30.28(0.147) | 33.9 | Above 6 months | Stable |
| CWPU5 | 33.13(0.099) | 33.0 | Above 6 months | Stable |
| CWPU6 | 46.16(0.147) | 33.7 | Above 6 months | Stable |
| CWPU7 | 52.73(0.140) | 27.0 | Above 6 months | Precipitated after 2 h |
| CWPU8 | 68.94(0.247) | 15.7 | Above 6 months | Gelled |
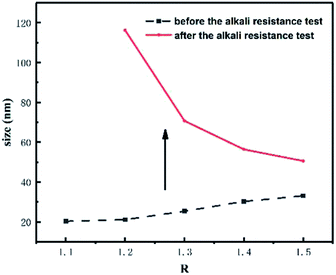
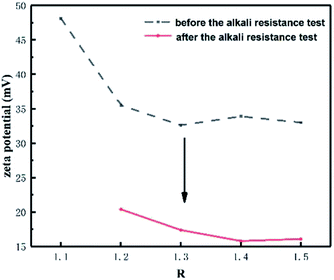
As can be seen from Table 3, the particle size increased and the zeta potential decreased with the increase of R-value for CWPU1–CWPU5. This could be explained by that the content of hard segment increased with the ratio of n(–NCO)/n(–OH) increasing, which made the flexibility of polyurethane molecules decreased and difficult to dispersed. In addition, the –NCO at the end of the prepolymer reacted with water to form polar urea groups during emulsification, resulting in adhesion of the emulsion particles during collision, which also increased the particle size of the emulsion. It can be concluded from Fig. 4 and 5 that after the addition of Na2CO3, the zeta potential of the CWPU emulsion was significantly reduced, and the particle size increased. This illustrated that the CO32− destroyed the electric double layer structure of the emulsion, and the electrostatic repulsion among the emulsion particles decreased, thus the emulsion particles aggregated with each other. In addition, as the R-value increased, the stability of the emulsion in Na2CO3 solution increased, which was attributed to the formation of more urea groups during emulsification. The polarity of the urea group is greater than that of the carbamate28 and more urea can form a thicker hydration layer with water molecules by the hydrogen bond. This makes it difficult for the CO32− to enter the diffusion layer formed by Cl− and destroy the electric double layer structure of emulsion.29 For CWPU4, CWPU6–CWPU8 samples, the particle size of the emulsion became smaller and the zeta potential increases with the increasing MDAAC content. The hydration layer of the emulsion was thicker,30 so it takes longer time for the CO32− to destroyed the electric double layer, thus the stability of the emulsion in the Na2CO3 solution became better.
| Samples | Sizea (PDI) (nm) | Zeta potential (mV) | Storage stability at room temperatureb | The stability of emulsion in Na2CO3 solution |
|---|---|---|---|---|
| a PDI refers to particle distribution index.b Storage stability refers to the time when deposition or delamination occurs in the latex. | ||||
| CWPU6 | 46.16(0.147) | 33.7 | Above 6 months | Stable |
| CWPU6-D25% | 64.02(0.579) | 38.3 | Above 6 months | Stable |
| CWPU6-D50% | 67.84(0.473) | 37.6 | Above 6 months | Stable |
| CWPU6-D75% | 101.1(0.684) | 36.5 | Above 6 months | Stable |
| CWPU6-D100% | 105.4(0.706) | 57.8 | Above 6 months | Stable |
| CWPU6-D25%M75% | 115.6(0.342) | 64.8 | Above 6 months | Precipitated after 4 h |
| CWPU6-D50%M50% | 366.3(0.293) | 37.6 | Above 6 months | Precipitated after 6 h |
| CWPU6-D75%M25% | 66.35(0.293) | 32.0 | Above 6 months | Stable |
| CWPU6-D25%M75% | 115.6(0.342) | 64.8 | Above 6 months | Precipitated after 4 h |
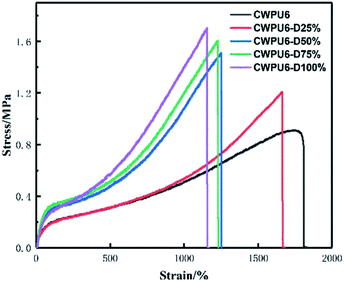
| Samples | CWPU6 | CWPU6-D25% | CWPU6-D50% | CWPU6-D75% | CWPU6-D100% |
|---|---|---|---|---|---|
| Tensile strength (MPa) | 0.9145 | 1.2078 | 1.5121 | 1.6069 | 1.7027 |
| Elongation at break (%) | 1751 | 1661 | 1250 | 1228 | 1154 |
| Water absorption (%) | Dissolved | 350.8 | 301.1 | 268.6 | 201.0 |
The color fixation properties of CWPU emulsions
| Samples | Rub fastness | Zeta potential of treated cotton fiber (mV) | |
|---|---|---|---|
| Dry | Wet | ||
| Untreated | 4 | 1–2 | −24.8 |
| CWPU1 | 4 | 2 | −1.74 |
| CWPU2 | 4 | 2 | −2.77 |
| CWPU3 | 4 | 2 | −0.92 |
| CWPU4 | 4 | 2 | −2.63 |
| CWPU5 | 4 | 2 | −2.49 |
| CWPU6 | 4 | 2 | −3.17 |
| CWPU7 | 4 | 2 | −3.53 |
| CWPU8 | 4–5 | 2–3 | −10.4 |
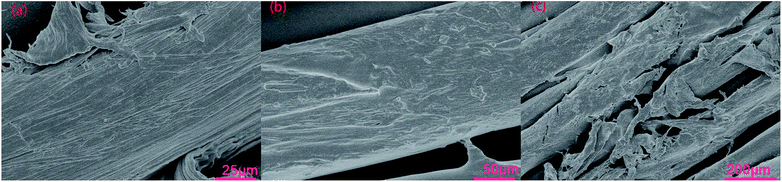 | ||
| Fig. 7 The SEM figures of treated or untreated dye cotton fabric surface: (a) untreated; (b) treated by CWPU6; (c) treated by CWPU6 after the wet-rubbing fastness test. | ||
Compared with the rubbing fastness of the cotton fabrics treated with CWPU4, CWPU6 and CWPU7, it is clear that the change of MDAAC content had no significant effect on the rubbing fastness. For the cotton fabric treated by CWPU8, since the CWPU8 was unstable in Na2CO3 solution, polyurethane molecules precipitated on the surface of the fabrics during fixing treatment, so that more polyurethane molecules were attached to the surface of the fabric, resulting in a half grade improvement of dry and wet-rubbing fastness compared with other samples.
The zeta potential of treated fabrics was significantly reduced even close to zero, suggesting that the quaternary ammonium salt groups in the cationic waterborne polyurethane combined with the anions in the reactive dye and formed water-insoluble lakes. Moreover, the polyurethane film enclosed the dye particles in the film, making it difficult for the dye molecules to fell off the fabric into the water.
| Samples | Rub fastness | |
|---|---|---|
| Dry | Wet | |
| Untreated | 4 | 1–2 |
| CWPU6 | 4 | 2 |
| CWPU6-D25% | 4 | 2–3 |
| CWPU6-D50% | 4 | 2–3 |
| CWPU6-D75% | 4 | 2–3 |
| CWPU6-D100% | 4–5 | 3 |
| CWPU6-M100% | 4 | 3 |
| CWPU6-D25%M75% | 4 | 2–3 |
| CWPU6-D50%M50% | 4 | 2 |
| CWPU6-D75%M25% | 4 | 2–3 |
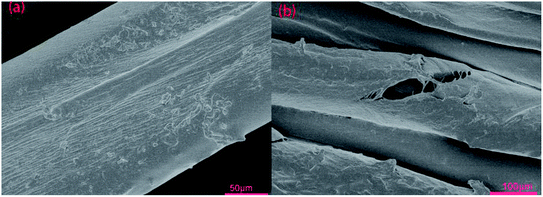 | ||
| Fig. 8 The SEM figures of treated dye cotton fabric surface: (a) treated by CWPU6-D100%; (b) treated by CWPU6-D100% after the wet-rubbing fastness test. | ||
Compared with the wet-rubbing fastness of cotton fabric treated by CWPU6 which was unblocked, the wet-rubbing fastness of the cotton fabric treated by CWPU6-M100% increased from grade 2 to grade 3. This illustrated that the chain extended by DETA and the use of MEKO to block –NCO groups both could improve the wet-rubbing fastness of the treated cotton. Nevertheless, for the CWPU which blocked by MEKO then use DETA as the post-chain extender, the rubbing fastness remained unchanged or slightly decreased compared with that of CWPU6-D25% to CWPU6-D100% which were unblocked. This was caused by that the effect of blocked –NCO groups was twofold, (i) to decrease the color fastness of dye cotton fabrics by decreasing the film-forming properties of fixative films, and (ii) to increase the color fastness by the covalent bonds among dye molecules and cotton fabrics.
Conclusions
In this work, a novel cationic WPU using quaternary ammonium diol as the chain extender was successfully prepared and its structure was demonstrated by the FTIR and 1H-NMR. It was found that the R-value and the cationic group content of the cationic waterborne polyurethane had no significant effect on the color-fixing performance, but the content of DETA which significantly affected the water resistance and tensile strength of polyurethane films, having a great influence on the wet-rubbing fastness. Furthermore, the blocked NCO groups also improved the rubbing fastness. The wet-rubbing fastness of the deep black cotton fabric treated by the cationic waterborne polyurethane prepared in this paper was significantly increased from grade 1–2 to grade 3, and the emulsions could be stably dispersed in the Na2CO3 solution, which met requirements of the practical application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Transfar Group Co., Ltd. for the financial support and their assistance in application testing.References
- M. A. R. Bhuiyan, M. A. Hossain, M. Zakaria, M. N. Islam and M. Z. Uddin, J. Polym. Environ., 2017, 25, 334–342 CrossRef.
- U. H. Siddiqua, S. Ali, M. Iqbal and T. Hussain, J. Mol. Liq., 2017, 241, 839–844 CrossRef CAS.
- D. Juan, Z. Li and C. Shuilin, Color. Technol., 2010, 121, 29–36 Search PubMed.
- U. Sahm, D. Knittel and E. Schollmeyer, Fresenius' J. Anal. Chem., 1990, 338, 824–830 CrossRef CAS.
- A. Marsal, S. Cuadros, L. Ollé, A. Bacardit, A. M. a. Manich and J. Font, J. Cleaner Prod., 2018, 186, 45–56 CrossRef CAS.
- Y. Yu and Y. Zhang, J. Vinyl Addit. Technol., 2010, 16, 277–283 CrossRef CAS.
- S. Xinrong, W. Nanfang, S. Kunyang, D. Sha and C. Zhen, J. Ind. Eng. Chem., 2014, 20, 3228–3233 CrossRef.
- Y. Yu and Y. Zhang, J. Vinyl Addit. Technol., 2013, 19, 219–224 CrossRef CAS.
- M. S. Yen, C. N. Huang and P. D. Hong, J. Appl. Polym. Sci., 2007, 106, 2907–2916 CrossRef CAS.
- X. Lai, Y. Song and M. Liu, J. Polym. Res., 2013, 20, 1–6 CAS.
- H. Mao, S. Qiang, F. Yang, C. Zhao, C. Wang and Y. Yin, J. Appl. Polym. Sci., 2015, 132, 42780 Search PubMed.
- Z. Liu, Y. Tian, S. Kang and X. Zhang, J. Appl. Polym. Sci., 2012, 125, 3490–3499 CrossRef CAS.
- Z. Fan, Q. Li, X. Cai and Z. Li, J. Text. Inst., 2017, 108, 1227–1233 Search PubMed.
- H. H. Wang and M. S. Lin, J. Polym. Res., 2000, 7, 81–90 CrossRef CAS.
- L. Liu and J. Yao, Fibers Polym., 2011, 12, 42–49 CrossRef CAS.
- K. Srikulkit and P. Santifuengkul, Color. Technol., 2000, 116, 398–402 CAS.
- A. Dong, G. Hou, Y. Wang and D. Sun, J. Polym. Sci., Part B: Polym. Phys., 2002, 40, 972–979 CrossRef CAS.
- M. S. Yen, P. Y. Chen and H. C. Tsai, J. Appl. Polym. Sci., 2003, 90, 2824–2833 CrossRef CAS.
- C. K. Kim and B. K. Kim, J. Appl. Polym. Sci., 1991, 43, 2295–2301 CrossRef CAS.
- H. Lijie, D. Yongtao, Z. Zhiliang, S. Zhongsheng and S. Zhihua, Colloids Surf., A, 2015, 467, 46–56 CrossRef.
- Y. Nakayama, T. Inaba, Y. Toda, R. Tanaka, Z. Cai, T. Shiono, H. Shirahama and C. Tsutsumi, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4423–4428 CrossRef CAS.
- A. F. Martins, S. P. Facchi, H. D. Follmann, A. G. Pereira, A. F. Rubira and E. C. Muniz, Int. J. Mol. Sci., 2014, 15, 20800–20832 CrossRef CAS PubMed.
- T. Mao, C. Zheng, J. Lin, X. Chen, X. Huang, X. Xing and L. Zhang, CIESC J., 2013, 5, 1884–1889 Search PubMed.
- R. F. Alamdari, F. G. Zamani and M. Shekarriz, J. Mol. Liq., 2015, 211, 831–838 CrossRef.
- F. Zhang, Y. Chen, H. Lin and Y. Lu, Color. Technol., 2007, 123, 351–357 CAS.
- Z. Ge and Y. Luo, Prog. Org. Coat., 2013, 76, 1522–1526 CrossRef CAS.
- R. Laible, Chem. Rev., 1958, 58, 807–843 CrossRef CAS.
- K. Zhu, X. Li, H. Wang, G. Fei and J. Li, J. Appl. Polym. Sci., 2016, 133, 43211 Search PubMed.
- H. Wu, Z. Li, L. Zhu and J. Gu, J. Appl. Polym. Sci., 2015, 132, 43211 CrossRef.
- L. Bao, H. Fan, Y. Chen, J. Yan, J. Zhang and Y. Guo, Adv. Polym. Technol., 2016, 37, 21736 Search PubMed.
- H. Wang, J. Fan, G. Fei, J. Lan and Z. Zhao, J. Appl. Polym. Sci., 2015, 132, 42354 Search PubMed.
- C. Hou, G. Ma and G. Wang, Juanzhi Gongye, 2013, 28, 6–9 Search PubMed.
- Y. H. Guo, J. J. Guo, H. Miao, L. J. Teng and Z. Huang, Prog. Org. Coat., 2014, 77, 988–996 CrossRef CAS.
- A. Morel, F. Salaün, G. Bedek, D. Dupont and S. Giraud, J. Mater. Sci., 2017, 52, 1014–1027 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |