Open Access Article
Open Access ArticleHair growth promotion effect of cedrol cream and its dermatopharmacokinetics
Yan Zhanga,
Jie-wen Wanga,
Fan-zhi Qua,
Yu-meng Zhanga,
Guang-yue Su*ab and
Yu-qing Zhao *ab
*ab
aShenyang Pharmaceutical University, No. 103, Wenhua Road, Shenyang 110016, People's Republic of China. E-mail: zyq4885@126.com; suggyy@163.com; Fax: +86 24 43520300; Tel: +86 24 43520309
bKey Laboratory of Structure-based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang 110016, China
First published on 18th December 2018
Abstract
Topical use of cedrol ethanol has been reported to have a beneficial effect on hair loss. However, the use of cedrol has been limited by application-related issues, such as poor water solubility and volatile features. Therefore, the present study developed a cream formulation of cedrol and evaluated various physicochemical parameters of the prepared cream. The optimized cedrol cream was selected after orthogonal tests and determined further. The dermatopharmacokinetics were studied to investigate the absorption difference between cedrol cream and cedrol ethanol after dermal application, and the concentrations of cedrol in skin were analysed by the gas chromatography-mass spectrometry (GC-MS) method. By comparison, the area under the curve (AUC0–24 h) of cedrol cream was almost three times higher than that of cedrol ethanol. Moreover, this study was undertaken to evaluate the hair growth promoting efficacy of cedrol cream in C57BL/6 mice and Wistar rats. Macroscopic assessment and alopecia score showed that C57BL/6 mice treated with cedrol cream showed a faster production of pigmentation and a higher score at different growth stages than other groups. The hair length of the cedrol cream-treated group was much longer than those of the cedrol ethanol and minoxidil groups. Histological analyses indicated that in the cedrol ethanol group, most follicles of the C57BL/6 mice were in the catagen phase, whereas nearly 83% of hair follicles in the cedrol cream group remained in the anagen phase. Taken together, our data strongly suggest that the cream formulation of cedrol has a stronger hair growth promotion effect, gave no irritation and was safe for topical administration.
1. Introduction
Hair makes an important contribution to the appearance of people, and it plays an interesting role in human interaction.1 Hair loss or alopecia is a common skin disorder, which can have a devastating effect on the patient's psychology and life quality.2,3 Even though numerous products or surgical procedures have been promoted, many patients still suffer from hair loss or hair thinning. Presently, finasteride and minoxidil are the only two drugs approved by the FDA to treat hair loss. However, their side effects and unpredictable efficacy have limited their clinical use.4–7 Therefore, there is an urgent need to develop novel anti-hair loss agents with high efficiency and low toxicity. Natural products are reported to be rich sources of anti-hair loss agents and have a long history of treating hair loss.8,9Cedrol is the major ingredient in the volatile oil of Platycladus orientalis (L.) Franco, which is widely used to resist alopecia and promote hair growth.10–12 It is a sesquiterpene compound with a high boiling point (molecular weight, 222.37), which has been reported to have antispasmodic, sedative, anti-cancer, antifungal, analgesic and anti-inflammatory effects.13–18 Its safety for topical administration has been confirmed.19,20 Our previous studies demonstrated that topically applied cedrol (dissolved in 85% ethanol) could effectively promote hair growth and prevent chemotherapy-induced alopecia by stimulating hair regeneration and prolonging the anagen phase of hair follicles.21,22 However, long-term use of high concentrations of ethanol may lead to many skin problems, including dandruff, dry skin and allergies. Cedrol also exhibits a series of application-related issues, such as poor water solubility (21.88 mg l−1, 25 °C), volatile features and lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow 4.67).23 As mentioned above, it is necessary to develop a new alternative topical formulation of cedrol to overcome these disadvantages.
Kow 4.67).23 As mentioned above, it is necessary to develop a new alternative topical formulation of cedrol to overcome these disadvantages.
In the present study, with an aim to increase the skin retention and dermal bioavailability of cedrol, the cream formulations were designed according to an orthogonal experiment. The skin retention of cedrol cream and cedrol ethanol in vivo was also evaluated by GC-MS method. A skin irritation test was also carried out. Furthermore, the hair growth-promoting activities of cedrol cream were investigated in mice and rats.
2. Experiment
2.1. Materials
Minoxidil was purchased from a local market and used as a positive control. Liquid paraffin, stearic acid and monostearate glyceride were purchased from Xinxing (Tieling) Pharmaceutical Co., Ltd, China. Tween 80 and glycerin were purchased from China Pharmaceutical Group Chemical Reagent Co., Ltd, China. White Vaseline was purchased from Tianjin Hengxing Chemical Reagent Co., Ltd, China.2.2. Animals
Mice (Animal License no. SCXK (Liao) 2015-0001) were obtained from The Laboratory Animal Center of Shenyang Pharmaceutical University (Shenyang, PR China). Female KM mice aged 5–6 weeks old and weighing 20 ± 2 g were employed for dermatopharmacokinetic studies. Female C57BL/6 mice and male Wistar rats, weighing 20 ± 2 g and 140 ± 10 g, respectively, were employed for evaluating the hair-growth promoting effects. Mice were housed in cages and allowed to acclimatize for a minimum of 7 days before initiating the experiment. All the animals were kept at ambient temperature with a 12 h night/day cycle, and supplied with a standard pellet diet and water. This study was performed in strict accordance with the Guidelines for the Care and Use of Laboratory Animals of Shenyang Pharmaceutical University, and approved by the Institutional Animal Ethics Committee (approval protocol number: SYPU-IACUC-C2018-3-23-104; Liaoning, China).2.3. Development of cedrol cream
2.4. Dermatopharmacokinetics
Cedrol was analysed by GC-MS using nitrogen as the carrier gas with a flow of 1.0 ml min−1. The initial column temperature was held at 110 for 2 min and then increased at a rate of 15 °C min−1 to 180 °C for 2 min and then increased at a rate of 10 °C min−1 to 240 °C for a further 2 min. The mass spectrometer was operated in positive electron impact ionization mode (70 eV). The MS interface and ion source temperatures were set at 280 °C and 230 °C, respectively. The quantification was performed using selected ion detection (SIM): m/z 150 and 207 for cedrol, m/z 119 for IS.
Each sample was homogenized with 1.0 ml of saline with IS, then 1.0 ml of n-hexane was added and vortex mixed for 3 min. The samples were ultrasonicated for 5 min and centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. 1.0 μl of supernatant was injected for GC-MS analysis.
000 rpm for 5 min. 1.0 μl of supernatant was injected for GC-MS analysis.
Pharmacokinetic parameters were calculated by noncompartmental method using the pharmacokinetic program DAS 2.0. The maximum concentration (Cmax), maximum drug concentration time (Tmax), elimination half-life (T1/2), mean residence time (MRT) and area under the curve (AUC0–24 h) were calculated.
2.5. Hair-growth studies
C57BL/6 mice (n = 10) and Wistar mice (n = 6) were randomly divided into four groups. The test drugs for C57BL/6 mice were cedrol ethanol (20 mg ml−1, 100 μl), cedrol cream (5% w/w, 0.04 g), 2% minoxidil and vehicle control. 2% minoxidil was used as the positive control. A double dosage of drugs was given to Wistar rats. The dorsal skin of C57BL/6 mice (2 cm × 3 cm) and Wistar mice (3 cm × 3 cm) in the telogen phase was shaved 24 h before the experiment. The drug/vehicle was topically administered once a day to the mice and rats on the test area, for 21 d and 30 d, respectively. The hair regrowth activities of different groups were evaluated by hair growth condition, new hair length and quantitative histomorphometry.8,92.6. Statistical analysis
Values are expressed as mean ± standard deviation (S.D.). Statistical analyses were performed using Student's t-test (SPSS for Windows, version 16) to determine significant differences between treatments; a P value < 0.05 was considered statistically significant.3. Results and discussion
3.1. Development of cedrol cream
Nine different batches of oil/water (O/W) cream were prepared according to the L9 (34) matrix. The batches were scored and judged with the above-mentioned parameters for optimizing the best batch. The cream formulations were prepared by the melt emulsification method. In order to obtain a stable and high-quality cream, Tween 80 and glyceryl monostearate were chosen as the emulsifier and auxiliary emulsifier, respectively.The results of the orthogonal experiments exhibited that the factors' levels of significance were as follows: the dosage of Tween 80 > glyceryl monostearate > liquid paraffin > white Vaseline. The intuitionistic analysis and variance analysis of the experimental results showed that the optimized cedrol formulation was 5% (w/w) cedrol, 4% (w/w) Tween 80, 5% (w/w) white Vaseline, 6% (w/w) stearic acid, 10% (w/w) glyceryl monostearate, 10% (w/w) liquid paraffin, 11% (w/w) glycerol and 0.2% (w/w) sorbic acid. The optimized formulation of cedrol was white in appearance with high-quality organoleptic properties and could still maintain stability under the centrifugal (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 1 h), freezing (−20 °C, 24 h) and high temperature (50 °C, 6 h) conditions. The particle size of the cream was less than 50 μm (Fig. 1a).
000 rpm, 1 h), freezing (−20 °C, 24 h) and high temperature (50 °C, 6 h) conditions. The particle size of the cream was less than 50 μm (Fig. 1a).
3.2. Dermatopharmacokinetics
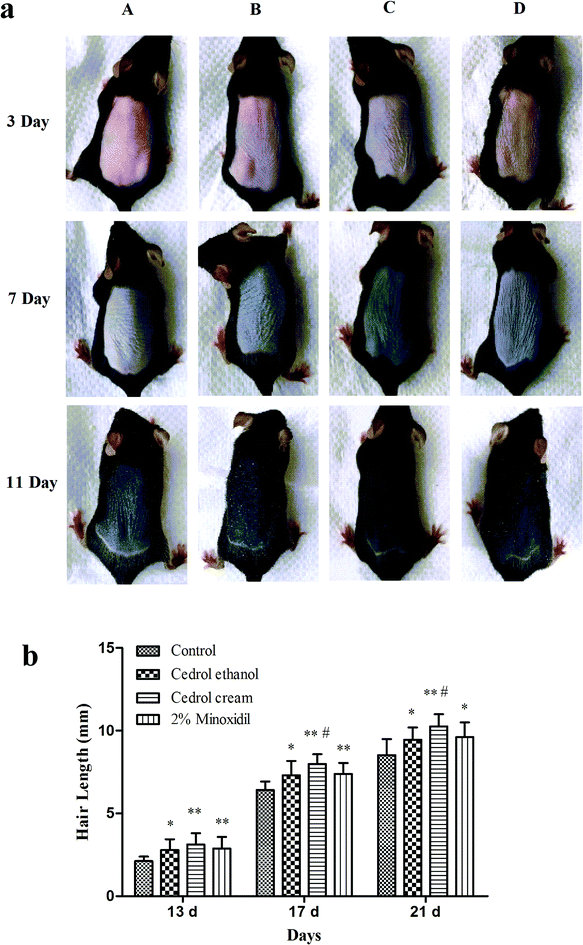
A gas chromatographic system combined with a triple quadrupole mass spectrometer was used to determine the content of cedrol in the skin samples. The specificity of the method was demonstrated by chromatograms for cedrol and the internal standard (IS) for a drug-free skin sample, a skin sample spiked with cedrol, a skin simple spiked with IS and a skin sample from a mouse 2 h after topical administration (Fig. 1b). The retention times for cedrol and the IS were approximately 8.63 and 10.68 min, respectively. No interference with other peaks was observed in the homogenate.To assess linearity, the calibration curve was established by the plotting peak area ratio of cedrol to the IS versus the corresponding concentrations using linear regression analysis with 1/x2 weighting. The standard equation was y = 0.283x + 0.202 and the concentration range for cedrol in the homogenate was 0.15–40 μg ml−1. The correlation coefficient for the standard curves was 0.9960. The average extraction recoveries from skin samples were 79.11–83.04% for cedrol and 81.02% for the IS. The accuracy and precision were less than 15% and were acceptable.
The presented method was applied for the determination of the cedrol cream and cedrol ethanol concentrations in mice skin for 24 h after topic administration of a single 100 mg kg−1 dose (n = 5). All main pharmacokinetic parameters were estimated by non-compartmental analysis after topical administration of cedrol cream and cedrol ethanol.
As the results show in Fig. 1b and Table 1, the mean peak concentrations of cedrol cream and cedrol ethanol were 16.83 μg ml−1 and 7.76 μg ml−1 at 1 h after administration, respectively. Compared with the cedrol ethanol (10.03 h), the T1/2 of the cedrol cream (18.56 h) was prolonged, with an almost twofold increase. Furthermore, there were significant differences in the AUC0–24 h for cedrol cream and cedrol ethanol (82.60 μg ml−1 × h vs. 28.19 μg ml−1 × h, p < 0.01). Obviously, the cedrol had better skin retention properties in the form of a cream. This may be credited to the presence of surfactants in the cream base that could contribute to the solubilization of cedrol. However, in the cedrol ethanol group, the volatilization of ethanol leads to the precipitation of cedrol.
| Parameters | Cedrol cream | Cedrol ethanol | p |
|---|---|---|---|
| a The results are shown as the mean ± standard deviation (S.D.). | |||
| AUC0–24 h (μg × h ml−1) | 82.60 ± 22.44 | 28.19 ± 6.98 | 0.001 |
| AUC0–∞ (μg × h ml−1) | 119.05 ± 36.95 | 33.48 ± 10.58 | 0.001 |
| MRT (h) | 7.12 ± 0.29 | 6.94 ± 0.20 | 0.29 |
| T1/2 (h) | 18.56 ± 7.17 | 10.03 ± 5.14 | 0.06 |
| Cmax (μg ml−1) | 16.83 ± 3.15 | 7.76 ± 1.83 | 0.001 |
The results also revealed that cedrol could penetrate into the skin instantaneously and the skin retention reached a peak in a short time (Tmax, 1 h). The results in Table 1 demonstrate that cedrol cream has a longer half-life and residence time, indicating that cedrol cream can maintain a high concentration for a long time in skin, which is beneficial for the effect of the drug in the skin epidermis and dermis. Therefore, the cream was more appropriate for the topical use of cedrol.
3.3. Effects of cedrol on hair regeneration
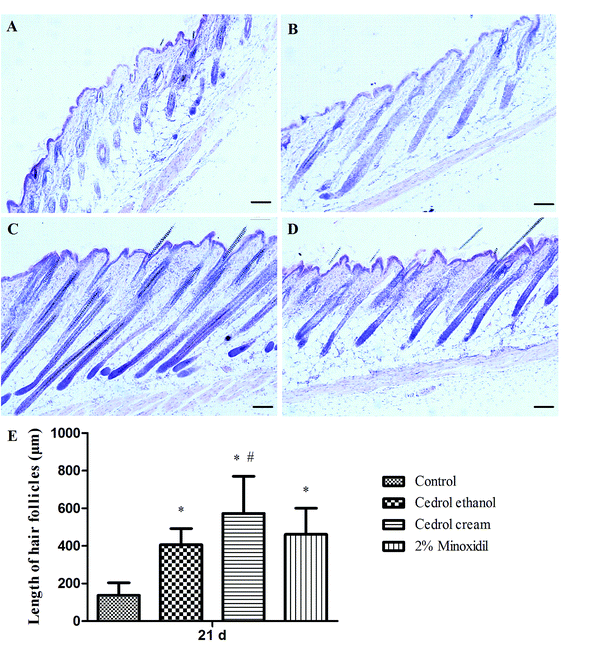
Hair growth is controlled by a unique dynamic cycle comprising growth (anagen), regression (catagen) and rest (telogen) phases.25,26 Hair follicles remodel themselves repeatedly throughout adult life.27 It is generally known that the human hair cycle lasts several years; in contrast, the mouse hair cycle is only three weeks. The hair cycle in mice is similar to that of humans. Therefore, to evaluate the hair-growth conditions, C57BL/6 mice were selected and the production of pigmentation was taken as evidence during only the anagen phase.28,29Hair growth was observed from the denuded area of C57BL/6 mice. The skin color of C57BL/6 mice in the telogen phase was pink, which darkens along with anagen initiation and then becomes grey. In comparison with the application of the control, blackened skin areas, especially in the cedrol cream group, were observed 7 d after shaving (Fig. 2a). At 11 d, the cedrol cream-treated group was covered with hair in the shaved skin, whereas other groups showed relatively less hair growth. Macroscopic assessment and alopecia score (Table 2) showed that cedrol cream significantly stimulated hair growth. The scores at 6 d and 8 d were estimated to be 1.9 and 2.8, respectively. The results for cedrol ethanol were almost the same as those for minoxidil.
| Compounds | The hair growth score of the mice | |||
|---|---|---|---|---|
| 4 day | 6 day | 8 day | 10 day | |
| a The hair-regrowth efficacies were scored according to different hair growth stages. The scoring criteria were as follows: 0 points: the skin of the depilatory area was pink; 1 point: the skin of the depilatory area was grey; 2 points: the skin of the depilatory area was black; 3 points: the hair in the depilatory area had started to grow; 4 point: the short-hair growth was complete. The results are shown as the mean ± standard deviation (S.D.). *P < 0.05, **P < 0.01, when compared to respective control values by Student's t-test (n = 10). | ||||
| Control | 0.3 ± 0.5 | 1.0 ± 0.7 | 1.7 ± 0.7 | 2.1 ± 0.7 |
| Cedrol ethanol | 0.5 ± 0.5 | 1.6 ± 0.7 | 2.2 ± 0.6 | 3.1 ± 0.7** |
| Cedrol cream | 0.7 ± 0.5 | 1.9 ± 0.7** | 2.8 ± 0.6** | 3.8 ± 0.4** |
| 2% minoxidil | 0.5 ± 0.5 | 1.7 ± 0.5* | 2.1 ± 0.7 | 3.3 ± 0.7** |
Regrown hairs were plucked randomly from the shaved dorsal center parts of all mice on 13 d, 17 d, and 21 d. The longest 10 hairs were measured and the average hair length was calculated. According to the data (Fig. 2b), mice treated with drugs exhibited significant activity in terms of the hair length when compared to the control group. The cedrol cream group showed the best hair growth promotion effects. Compared with the cedrol ethanol (7.31 mm) and minoxidil groups (7.39 mm), the cedrol cream group (7.99 mm) showed a remarkable effect on length of hair at 17 d. At 21 d, the hair length for the cedrol cream-treated group (10.26 mm) was significantly longer than that of the vehicle treated group (8.52 mm). The mice treated with cedrol cream exhibited significant results when compared with the cedrol ethanol group at 17 d and 21 d.
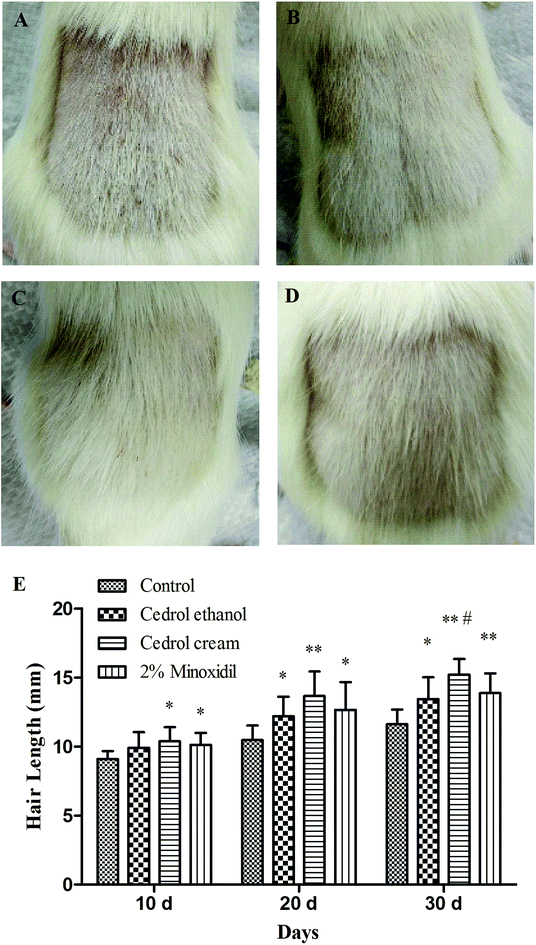
To investigate the effect of the drugs on the hair follicle cyclic, hematoxylin–eosin staining was performed. In the representative longitudinal sections, the majority of hair follicles in the vehicle group were in the telogen phase, whereas nearly 83% of the hair follicles in the cedrol cream group remained in the anagen phase. In the cedrol ethanol group, most follicles were in the catagen phase with only a few in the anagen phase. However, in the mice in the minoxidil-treated group, the majority of hair follicles were in the catagen and anagen phases (Fig. 3). Histological analyses indicated that cedrol cream slowed down hair follicles into the catagen phase.
Histomorphometric analysis was performed to quantify the hair growth promoting activities. Fig. 3E showed that cedrol cream had a great influence on hair follicle length; the hair follicles in the cedrol cream group mice were significantly longer than for other groups. The average hair follicle length in the cedrol cream and cedrol ethanol groups were 573.28 and 406.26 μm respectively. Minoxidil (461.23 μm) showed approximately the same efficacy as the cedrol ethanol treated group.
To further confirm this result, we subjected Wistar rats to the same regimen for 30 d. As shown in Fig. 4, the coverage of hair in the cedrol and minoxidil groups was significantly higher than that in the control group, and rats treated with cedrol cream were the best. In the cedrol cream group, new hair in the depilatory area was similar to that in the untreated part, while in the other three groups of rats a distinct boundary between the hair removal and the untreated areas was still observed. The effects of the cedrol ethanol were similar to those of the minoxidil. According to the data (Fig. 4E), the hair length in the cedrol and minoxidil groups was obviously superior to that of the control group. At 30 d, the cedrol cream group (15.2 mm) showed a remarkable effect on hair length when compared to the cedrol ethanol group (13.4 mm).
3.4. Skin irritation study
Wistar rats were selected to detect the irritation of cedrol cream. There was no erythema or edema of skin when cedrol cream was applied on the shaved back of rats within 72 h. This indicated that the cream formulation of cedrol was a safe agent for topical administration.Conflicts of interest
The authors report no conflicts of interest.Acknowledgements
This work was supported by the Liaoning (FGW) Engineering Technology Research Center for industrial chromatographic preparation of natural innovative drugs materials (grant number 2017-1007).References
- D. Stough, K. Stenn, R. Haber, W. M. Parsley, J. E. Vogel, D. A. Whiting and K. Washenik, Psychological effect pathophysiology and management of androgenetic alopecia in men, Mayo Clin. Proc., 2005, 80, 1316–1322 CrossRef PubMed.
- V. H. Price, Treatment of hair loss, N. Engl. J. Med., 1999, 341, 964–1773 CrossRef CAS PubMed.
- N. Hunt and S. McHale, The psychological impact of alopecia, Br. Med. J., 2005, 331, 951–953 CrossRef PubMed.
- P. Myung and M. Ito, Dissecting the bulge in hair regeneration, J. Clin. Invest., 2012, 122, 448–454 CrossRef CAS PubMed.
- N. Amberg, M. Holcmann, G. Stulnig and M. Sibilia, Effects of Imiquimod on Hair Follicle Stem Cells and Hair Cycle Progression, J. Invest. Dermatol., 2016, 136, 2140–2149 CrossRef CAS PubMed.
- B. A. Morgan, The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle, Cold Spring Harbor Perspect. Med., 2014, 4, a015180 CrossRef PubMed.
- J. S. Crabtree, E. J. Kilbourne, B. J. Peano, S. Chippari, T. Kenney and C. McNally, et al., A mouse model of androgenetic alopecia, Endocrinology, 2010, 151, 2373–2380 CrossRef CAS PubMed.
- J. I. Yoon, S. M. Al-Reza and S. C. Kang, Hair growth promoting effect of Ziziphus jujuba essential oil, Food Chem. Toxicol., 2010, 48, 1350–1354 CrossRef CAS PubMed.
- R. K. Roy, M. Thakur and V. K. Dixit, Hair growth promoting activity of Eclipta alba in male albino rats, Arch. Dermatol. Res., 2008, 300, 357–364 CrossRef CAS PubMed.
- B. Zhang, R. W. Zhang, X. Q. Yin, Z. Z. Lao, Z. Zhang, Q. G. Wu, L. W. Yu, X. P. Lai, Y. H. Wan and G. Li, Inhibitory activities of some traditional Chinese herbs against testosterone 5α-reductase and effects of Cacumen platycladi on hair re-growth in testosterone-treated mice, J. Ethnopharmacol., 2016, 177, 1–9 CrossRef PubMed.
- N. N. Zhang, D. K. Park and H. J. Park, Hair growth-promoting activity of hot water extract of Thuja orientalis, BMC Complementary Altern. Med., 2013, 13, 9–20 CrossRef CAS PubMed.
- S. R. Seo, G. Kang, J. W. Ha and J. C. Kim, In vivo hair growth-promoting efficacies of herbal extracts and their cubosomal suspensions, J. Ind. Eng. Chem., 2013, 19, 1331–1339 CrossRef CAS.
- B. A. Moharam, I. Jantan, J. Jalil and K. Shaari, Inhibitory effects of phylligenin and quebrachitol isolated from Mitrephora vulpina on platelet activating factor receptor binding and platelet aggregation, Molecules, 2010, 15, 7840–7848 CrossRef CAS PubMed.
- K. Kar, V. N. Puri, G. K. Patnaik, R. N. Sur, B. N. Dhawan, D. K. Kulshrestha and R. P. Rastogi, Spasmolytic constituents of Cedrus deodara (Roxb.) Loud: pharmacological evaluation of himachalol, J. Pharm. Sci., 1975, 64, 258–262 CrossRef CAS PubMed.
- Y. Yada, H. Sadachi, Y. Nagashima and T. Suzuki, Overseas survey of the effect of cedrol on the autonomic nervous system in three countries, J. Physiol. Anthropol., 2007, 26, 349–354 CrossRef PubMed.
- D. Kagawa, H. Jokura, R. Ochiai, I. Tokimitsu and H. Tsubone, The sedative effects and mechanism of action of cedrol inhalation with behavioral pharmacological evaluation, Planta Med., 2003, 69, 637–641 CrossRef CAS PubMed.
- Y. C. Su, K. P. Hsu, E. I. Wang and C. L. Ho, Composition anticancer and antimicrobial activities in vitro of the heartwood essential oil of Cunninghamia lanceolata var. konishii from Taiwan, Nat. Prod. Commun., 2012, 7, 1245–1247 CAS.
- I. Oh, W. Y. Yang, J. Park, S. Lee, W. Mar, K. B. Oh and J. Shin, In Vitro Na+/K+ – ATPase inhibitory activity and antimicrobial activity of sesquiterpenes isolated from Thujopsis dolabrata, Arch. Pharmacal Res., 2011, 34, 2141–2147 CrossRef CAS PubMed.
- RIFM (Research Institute for Fragrance Materials Inc.), Report on human maximization studies, RIFM, Woodcliff Lake, NJ, USA, 1973b Search PubMed.
- A. M. Kligman and W. Epstein, Updating the maximization test for identifying contact allergens, Contact Dermatitis, 1975, 1, 231–239 CrossRef CAS PubMed.
- Y. Zhang, L. Han, S. S. Chen, L. Guan, F. Z. Qu and Y. Q. Zhao, Hair growth promoting activity of cedrol isolated from the leaves of Platycladus orientalis, Biomed. Pharmacother., 2016, 83, 641–647 CrossRef CAS PubMed.
- S. S. Chen, Y. Zhang, Q. L. Lu, Z. Lin and Y. Zhao, Preventive effects of cedrol against alopecia in cyclophosphamide-treated mice, Environ. Toxicol. Pharmacol., 2016, 46, 270–276 CrossRef CAS PubMed.
- S. P. Bhatia, D. McGinty, C. S. Letizia and A. M. Api, Fragrance material review on cedrol, Food Chem. Toxicol., 2008, 46, S100–S102 Search PubMed.
- N. Aggarwal, S. Goindi and R. Khurana, Formulation, characterization and evaluation of an optimized microemulsion formulation of griseofulvin for topical application, Colloids Surf., B, 2013, 105, 158–166 CrossRef CAS PubMed.
- J. M. Adams and S. Cory, The Bcl-2 protein family: arbiters of cell survival, Science, 1998, 281, 1322–1326 CrossRef CAS PubMed.
- Y. AI-Nuaimi, G. Baier, R. E. Watson, C. M. Chuong and R. Paus, The cycling hair follicle as an ideal systems biology research model, Exp. Dermatol., 2010, 19, 707–713 CrossRef PubMed.
- S. Müller-Rover, B. Handjiski, C. van der Veen, S. Eichmüller, K. Foitzik, I. A. McKay, K. S. Stenn and R. Paus, A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages, J. Invest. Dermatol., 2001, 117, 3–15 CrossRef PubMed.
- H. B. Chase, Growth of the hair, Physiol. Rev., 1954, 34, 113–126 CrossRef CAS PubMed.
- P. M. Plonka, D. Michalczyk, M. Popik, B. Handjiski, A. Slominski and R. Paus, Splenic eumelanin differs from hair eumelanin in C57BL/6 mice, Acta Biochim. Pol., 2005, 52, 433–441 CAS.
| This journal is © The Royal Society of Chemistry 2018 |