Open Access Article
Open Access ArticleOptimized cesium and potassium ion-exchanged zeolites A and X granules for biogas upgrading†
Kritika Naranga,
Kristina Fodora,
Andreas Kaiserb and
Farid Akhtar *a
*a
aDivision of Materials Science, Luleå University of Technology, Luleå 97187, Sweden. E-mail: farid.akhtar@ltu.se
bDepartment of Energy Conversion, Technical University of Denmark, Roskilde 4000, Denmark
First published on 6th November 2018
Abstract
Partially ion-exchanged zeolites A and X binderless granules were evaluated for CO2 separation from CH4. The CO2 adsorption capacity and CO2-over-CH4 selectivity of binderless zeolites A and X granules were optimized by partial exchange of cations with K+ and Cs+, while retaining the mechanical strength of 1.3 MPa and 2 MPa, respectively. Single gas CO2 and CH4 adsorption isotherms were recorded on zeolites A and X granules and used to estimate the co-adsorption of CO2–CH4 using ideal adsorbed solution theory (IAST). The IAST co-adsorption analysis showed that the partially ion-exchanged binderless zeolites A and X granules had a high CO2-over-CH4 selectivity of 1775 and 525 respectively, at 100 kPa and 298 K. Optimally ion-exchanged zeolite X granules retained 97% of CO2 uptake capacity, 3.8 mmol g−1, after 5 breakthrough adsorption–desorption cycles while for zeolite A ion-exchanged granules the reduction in CO2 uptake capacity was found to be 18%; CO2 uptake capacity of 3.4 mmol g−1. The mass transfer analysis of breakthrough experimental data showed that the ion-exchanged zeolite X had offered a higher mass transfer coefficient, (κ) through the adsorption column compared to zeolite A; 0.41 and 0.13 m s−1 for NaK4.5Cs0.3X and CaK2.5Cs0.2A, respectively.
Introduction
The combustion of biomass produces approximately the same amount of CO2 with respect to its decomposition cycle, which leads to biomass carbon neutrality. In this respect, biomass conversion to biogas is a viable choice of a renewable fuel.1 Biogas is produced from a large diversity of biomass feedstocks, such as sewage, industrial waste, agricultural waste, dairy manure, landfill waste, food processing by products, etc., via anaerobic digestion.2 Methane is the primary component of raw biogas (45–75%) in addition to carbon dioxide (typically 25–55%) and other components like hydrogen sulfide, ammonia, water and trace gas compounds like oxygen and hydrocarbons.3 In order to use biogas as vehicle fuel or its induction to existing natural gas grids, upgrading of biogas to biomethane with a CH4 mole fraction over 96% is required to meet the standards laid out by respective industries.4 Several technologies have been developed to upgrade raw biogas via adsorption5 absorption,6 permeation7 and cryogenic technologies8 resulting in biogas with a content of CH4 > 90%.9 Moreover, adsorption-based technologies offer advantages like low upgrading costs and ease of scalability with biogas production unit over other technologies.Various microporous adsorbents have been explored during the last two decades in the field of biogas upgrading such as activated carbon, zeolites and metal–organic frameworks (MOFs).10 Zeolites A and X have many advantageous properties like flexibility in tailoring the pore opening, high CO2 uptake capacity, chemical and thermal stability, hence these materials have been investigated for biogas and natural gas upgrading.11–13 Analogues of zeolite A with 8-ring pore openings have pore apertures with the diameters similar to the kinetic diameters of CO2 (0.33 nm), N2 (0.364 nm) and CH4 (0.38 nm).14 The aperture size for zeolite A depends on the extra framework cation present in the structure, thus it varies between 0.3–0.5 nm.15 Due to these characteristics, researchers have tailored the pore size using partial ion exchange process on zeolite A for CO2 separation from gas streams.16 Liu et al., ion exchanged Na+ cations with K+ and reported ideal CO2-over-N2 selectivity of 172 with 17 atomic percentage (at%) K+ content in zeolite A.15 Bacsik et al., investigated NaKA zeolites for CO2-over-CH4 separation, and suggested that |Na12−xKx|-LTA, with x ranging between 1.8 ≤ x ≤ 3.2 (x being the number of ions in pseudocell) offers high CO2-over-CH4 selectivity.17 Cheung et al., reported a high CO2-over-CH4 selectivity, of around 1500 for |Na10.2KCs0.8|-LTA, which was achieved by partial ion exchanging Na+ to K+, and then replacing some of the K+ with Cs+.18 Zeolite NaX has been studied extensively for CO2 separation from flue-gas streams due to its superior CO2 adsorption capacity and selectivity over N2.19–21 Harlick and Tezel showed that NaX and NaY had the highest CO2 adsorption capacities and proposed these zeolites as best candidates for CO2 separation,22 while Walton et al., showed that in all cases X zeolites possess more CO2 molecules per supercage than the Y by examining their Li, K, Na, Rb, and Cs ion exchanged derivatives.23 Next to this, Cavenati et al., conducted a study of high pressure adsorption of CO2, CH4 and N2 on zeolite NaX, and concluded that the adsorbent was highly selective to CO2 thus rendering it suitable for purification purposes.20
The microporous zeolites are used in structured forms in adsorption-based separation technologies using pressure and/or temperature cycling. The mechanically stable structured microporous materials are required to minimize the pressure drop, promote high gas flow and rapid mass transfer kinetics throughout the adsorption column in a swing adsorption process.24 The earlier reports on the partially ion-exchanged are focused on powdered zeolite. The processing of these partially ion-exchange powders to structured forms using clay binders (typically 25–45%)25 can influence the adsorption properties significantly.26 Moreover, the addition of clay binders to zeolites to achieve desired mechanical stability reduces the durability of the structured product.27 The reports on structuring of partially ion-exchange zeolites are sparse despite the fact that structuring is an important aspect of practical separation of CO2 from methane for biogas upgrading using adsorption-based technologies.28 In this regard, current work reports a robust method to process partially ion-exchange binderless structured granules of zeolite CaA and NaX with reasonably high mechanical stability and adsorption performance for biogas upgrading. The binderless granules of zeolite CaA and NaX were subjected to partial ion-exchange with K+ and Cs+ cations to optimize the adsorptive properties for CO2 separation from CH4 in a pressure swing adsorption process. The evaluation of adsorption properties such as CO2 uptake capacity, CO2-over-CH4 selectivity and cyclic performance of mechanically strong partially ion-exchanged zeolites A and X granules are reported. Cyclic breakthrough measurements and calculated mass transfer coefficients of zeolite NaX and CaA granules as well as their optimal ion exchanged derivatives are reported in this study to evaluate the performance.
Experimental
Materials and methods
Commercially available zeolite NaX and CaA powder and binderless granules were purchased from Luoyang Jianlong Chemical Industrial Co. Ltd., Yanshi, Henan, China, with zeolite crystal size 2–4.5 μm. Potassium chloride (optical grade 99%) and cesium chloride (optical grade ≥99.5%) were purchased from Sigma Aldrich Chemie GmBH, Germany. The partial ion exchange is a simple and a robust method. The K+ and Cs+ ion exchange process for the granules was performed in two stages. First, the CaA and NaX zeolite granules were partially ion-exchanged by K+ cations and then the partially K+ exchanged zeolite granules (CaKA and NaKX) were collected and further ion exchanged with Cs+ cations. The detailed experimental description of the ion exchange process on the powders of zeolite NaX and CaA and their CO2/CH4 selectivities are provided in the ESI (Tables 1S and 2S,† respectively). The optimum combination of CO2 uptake capacity and CO2/CH4 selectivity obtained from the partially K+ and Cs+ ion exchanged powders of zeolite CaA and NaX was used as a guideline to prepare partially K+ and Cs+ ion exchanged granules of CaA and NaX to obtain optimum combination of CO2 uptake capacity and CO2-over-CH4 selectivity as shown in Table 1.| Starting zeolite | Amount of zeolite (g) | Amount of KCl (g) | Amount of CsCl (g) | Amount of water (ml) | Stirring time (min) | Final zeolite compositiona |
|---|---|---|---|---|---|---|
| a The final composition of the zeolite after ion-exchange was determined by EDS analysis. | ||||||
| NaX powder | ||||||
| NaX | 5 | 1 | — | 500 | 30 | NaK3.7X |
| NaK3.7X | 1.2 | — | 0.02 | 120 | 30 | NaK3.27Cs0.19X |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| NaX granule | ||||||
| NaX | 10 | 8 | — | 1000 | 60 | NaK3.9X |
| NaK3.9X | 5 | — | 0.20 | 500 | 60 | NaK4.5Cs0.3X |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| CaA powder | ||||||
| CaA | 1 | 0.8 | — | 100 | 30 | CaK3.4A |
| CaK3.4A | 1.8 | — | 0.06 | 180 | 30 | CaK2.5Cs0.45A |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| CaA granule | ||||||
| CaA | 5.1 | 4 | — | 1000 | 60 | CaK2.6A |
| CaK2.6A | 5.9 | — | 0.20 | 500 | 60 | CaK2.5Cs0.2A |
Characterization
The elemental composition of the partial ion exchanged NaX and CaA powders as well as of granules were determined by energy dispersive spectroscopy (EDS) using JEOL JCM-6000Plus scanning electron microscope (SEM). The zeolites granules were crushed into powder and were spread onto a double-sided copper tape and examined at an accelerating voltage of 15 kV. The same procedure was followed for the zeolite powders. Scanning electron microscope (SEM) micrographs were obtained on JEOL JSM-IT300 microscope. The X-ray powder diffraction pattern (PXRD) of NaX, CaA zeolites including its ion-exchange derivatives were analyzed using PANalytical's X-ray diffractometer (Empyrean, Nederland) over the 2θ range from 5 to 60°.The CO2, CH4 equilibrium adsorption isotherms were collected on a Gemini VII 2390 Surface Area Analyzer (Micromeritics, Norcross, USA). Apart from the adsorption isotherms, the surface area of the samples was measured by N2 adsorption at liquid nitrogen temperature (77 K) using Brunauer–Emmett–Teller analysis technique. Prior to the measurements, the specimens were degassed using VacPrep 061 for 24 h at 573 K under a dynamic vacuum. All the adsorption measurements were done at 298 K within a pressure range of 1 to 90 kPa. The CO2-over-CH4 selectivities of all zeolites were calculated using Henry's Law constants (KH) and ideal adsorbed solution theory. The co-adsorption of CO2–CH4 was estimated using ideal adsorbed solution theory (IAST). Single gas adsorption data and Langmuir model were utilized to obtain the parameters needed to estimate the binary selectivity coefficients using IAST model for a mixed gas containing 50–50 mol% of CO2–CH4 at various total pressures. The equilibrium cyclic performance of the ion-exchanged zeolites was recorded on a Gemini VII 2390 Surface Area Analyzer (Micromeritics, Norcross, USA).
The mechanical strength of the granules was determined by performing a compression test between the steel plates in a 1 kN load cell on Zwick Z050 universal test machine. A pre-load of 5 N was used. The test was carried out on five identical granules of each type to obtain a statistically significant data distribution. Mercury intrusion porosimetry of the zeolites granules was performed on a Micromeritics AutoPore III-9400 (Norcross, GA, USA) over a wide range of pressures (0.03–420 MPa). The pore size distributions were obtained from the Washburn equation using 0.485 N m−1 surface tension and 130° contact angle of mercury. To investigate the biogas upgrading, cyclic breakthrough measurements on NaX, CaA granules as well as their potassium and cesium ion exchange analogues were obtained using a Pressure Swing Adsorption PSA-300LC (L&C Science and Technology, Florida, USA) system. Prior to the measurement, the zeolite granules were regenerated at 200 °C in helium gas passing through the column at a flow rate of 20 ml min−1 for 12 h. Moreover, the dew point of −15 °C was used as a benchmark to ensure the drying of the zeolite column. A binary mixture of CO2/CH4 (45 mol%/55 mol%) gas was passed in the fixed bed of length 100 mm and 15 mm in diameter to study the adsorption performance of zeolites and its optimum ion exchange derivatives at 2 bar and 293 K up to 5 cycles. The flow rate was set to 19 ml min−1 and 15 ml min−1 for CO2 and CH4 gases respectively. The amount of the adsorbent in the PSA column were 4.96 g, 5.30 g, 4.28 g, 4.70 g for NaX, CaA, optimal ion exchanged NaX, optimal ion exchanged CaA zeolites, respectively. For the cyclic measurements, the zeolite column was regenerated by evacuation. A pressure of 0.01 bar was chosen to be reached in the columns for regeneration in the cyclic measurements.
Results and discussion
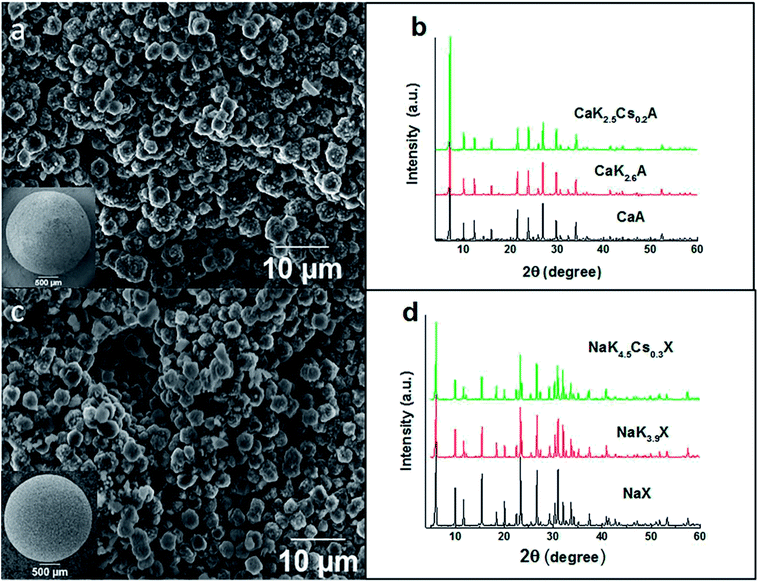
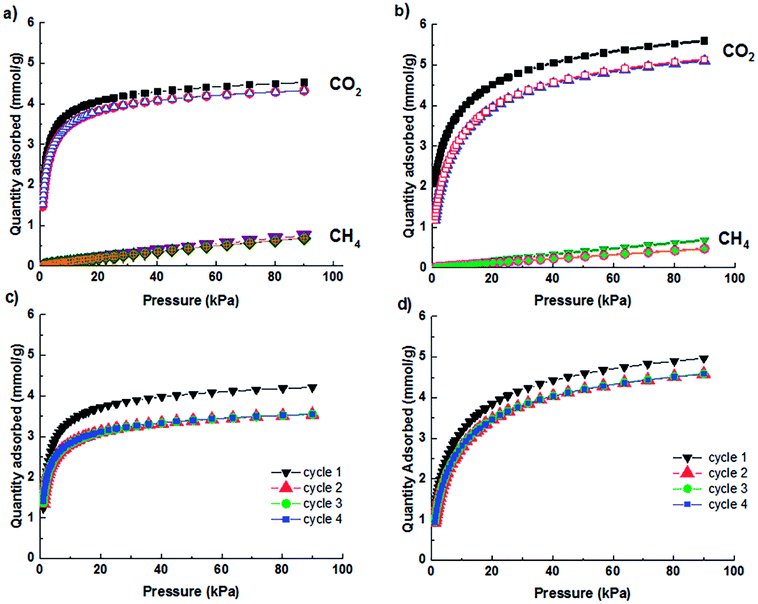
The zeolite granules, 1.6 mm in diameter, in Fig. 1 consist of primary zeolite particles with a size distribution of 2.5 to 4.0 microns for CaA granules and 2.5 to 4.5 microns for NaX granules. The partial ion-exchanged derivatives of zeolites CaA and NaX granules with K+ and Cs+ were prepared following the adsorptive properties and energy dispersive spectroscopy analysis of elemental composition of partial ion exchange of CaA and NaX powders with varying fraction of K+ and Cs+ cations (ESI, Table 3S†). The compositions of the optimal ion exchange zeolite A and X granules determined from energy dispersive spectroscopy (EDS) analysis were CaK2.6A, CaK2.5Cs0.2A, NaK3.9X and NaK4.5Cs0.3X, respectively. The microstructure and X-ray diffraction data in Fig. 1 and ESI (Fig. 1S and 2S†) confirms that the optimal ion exchanged zeolite A and X crystals preserve the morphology and crystallinity after the ion exchange process.In (Fig. 2a), the uptake capacities of CO2 of zeolite CaA and ion-exchanged CaK2.5Cs0.2A granules are 4.5 mmol g−1 and 4.3 mmol g−1, respectively. In (Fig. 2b), the uptake capacity of CO2 for zeolite NaX and NaK4.5Cs0.3X granules are 5.6 mmol g−1 and 5.1 mmol g−1, respectively. The CO2 uptake capacity of binderless granules of CaA and NaX is comparable to the structured zeolite A and X monoliths prepared without addition of binders.25,29,30 Moreover, it has been established that the binderless structured adsorbents offer advantages of high CO2 adsorption capacity and durability over traditional adsorbents structured using inorganic clay binders up to 45% to achieve the mechanical strength.27,31 After partial ion-exchange, the binderless zeolite granules show small decrease in the uptake capacities, which is attributable to the presence of extra framework cations. The charge density decreases in order of Na > K > Cs, resulting in a decrease of electrostatic interaction between the extra frameworks cations and the permanent quadruple moment of CO2 and hence the reduction in CO2 capacities is observed for the optimal ion-exchanged granules. Similar decrease in the CO2 uptake capacity was shown by the partially ion exchange CaA and NaX powders (ESI, Fig. 3S†). This result agrees with studies of Walton et al., who showed that the CO2 capacity increased with the small alkali cations in X and Y zeolites.23 Similar results have been reported in the literature on alkali exchanged faujasite zeolites.32 The CO2 uptake capacities for the ion exchanged zeolite granules are higher than the previously reported ion exchanged zeolites,18 3D-printed NaX and CaA zeolite monoliths29 while maintaining the high CO2/CH4 selectivity of the granules.
In (Fig. 2a), the CH4 adsorption capacities of zeolite CaA and optimal ion-exchanged CaK2.5Cs0.2A granules are 0.74 mmol g−1 and 0.69 mmol g−1, respectively and in (Fig. 2b), the uptake capacity of CH4 for zeolite NaX and NaK4.5Cs0.3X granules are 0.67 mmol g−1 and 0.46 mmol g−1, respectively. The reduction in CH4 uptake is connected to the hindrance to the percolation of CH4 due to enhanced diffusion barrier in ion-exchanged cages.33 The partial ion exchange of the CaA and NaX powders shows the similar reduction in CH4 adsorption capacities (ESI, Fig. 4S†). Moreover, linear isotherms were obtained in case of CH4 adsorption due to the absence of a quadruple moment in CH4 giving rise to weak interaction primarily via dispersion–repulsion forces exist between CH4 molecules and the adsorbent.34 CO2 cyclic adsorption isotherms for the optimal ion exchange derivatives of CaA and NaX granules are reported in (Fig. 2c and d) respectively. The cyclic stability was investigated over up to 4 cycles. For the first cycle, the zeolite granules were regenerated at 300 °C under vacuum and for the further cycles, the zeolites were regenerated under vacuum at room temperature.
In (Fig. 2c and d), the reduction in the CO2 adsorption capacity is observed after cycle 1. The reason for this reduction is attributed to chemisorption of CO2.35,36 No further reduction is observed in the following cycles. For the CaK2.5Cs0.2A granules, 15% of the reduction in the CO2 uptake is noticed from 1st cycle to 4th cycle, while the reduction of 7.9% is observed in the case of NaK4.5Cs0.3X granules. However, after the first cycle, the CaK2.5Cs0.2A and NaK4.5Cs0.3X granules keep the CO2 adsorption capacity.
The textural properties and the CO2-over-CH4 selectivity of zeolites granules and the optimal ion exchange derivatives are shown in Table 2. The specific surface areas of granulated CaK2.5Cs0.2A and NaK4.5Cs0.3X are 351 m2 g−1 and 474 m2 g−1, respectively. The decrease in the surface area is observed with the increase of cation size (Na < K < Cs), as reported earlier.37 The external surface area and the micropore volume of the NaK4.5Cs0.3X granules were 33 m2 g−1 and 0.25 cm3 g−1 and for CaK2.5Cs0.2A granules were 64.2 m2 g−1 and 0.16 cm3 g−1 respectively. There is no significant decrease in micropore volume and external surface area for the zeolites with its corresponding optimal ion exchange derivatives. These results suggest that the ion-exchange process of zeolite granules did not result in surface contamination of the zeolite particles and the granules. The reduction in the total surface area is mainly determined by the reduction in micropore area due to size difference of the cations. Similar observations have been reported for ion exchanged zeolite powders.38,39 The calculated CO2-over-CH4 selectivities in Table 2, using Langmuir adsorption model and ideal adsorbed solution theory (IAST), show higher values for optimal ion-exchanged zeolites CaA and NaX with K+ and Cs+ cations (ESI, Table 4S† for Langmuir parameter and the Henry's constant for the powders and granules). The CO2-over-CH4 selectivity increases with partial exchange of K+ cations. It is also noticed, that the CO2-over-CH4 selectivity increased with the addition of Cs+ in the structure. The role of the Cs+ in the structure is determined to be two-fold. First, due to its size it is expected to reduce the pore opening of the super cage to achieve molecular sieving effect, however the significant size difference of Cs+ compared to Na+ ion will generate a stronger interaction for the bigger cation, because the molecular shielding from the surrounding oxygen atoms is less, thus the interaction with CH4 will not be limited, as it is the case with the Na+.40
| Granules | BET surface area (m2 g−1) | Micropore area (m2 g−1) | External surface area (m2 g−1) | Micropore volume (cm3 g−1) | IAST selectivity (CO2/CH4) |
|---|---|---|---|---|---|
| CaA | 436 | 370 | 66.6 | 0.21 | 542 |
| CaK2.6A | 354 | 304 | 50.1 | 0.17 | 906 |
| CaK2.5Cs0.2A | 351 | 287 | 64.2 | 0.16 | 1773 |
| NaX | 496 | 461 | 35 | 0.26 | 329 |
| NaK3.9X | 458 | 425.4 | 32.8 | 0.24 | 384 |
| NaK4.5Cs0.3X | 474 | 441.4 | 33 | 0.25 | 525 |
Furthermore, it is deduced that two factors will affect the selectivity of the potassium and cesium ion-exchanged zeolite granules: (a) steric selectivity, due to the reduced pore opening and pore volume and (b) the difference in the interactions of CO2 and CH4 gases with the individual cations. Even though zeolite NaX contains cations at all sites, position I and I′ are within the sodalite cages and these are usually not reachable for most adsorbate molecule (ESI, Fig. 5S†).23 The non-accessibility of certain sites due to the size difference of the cations leads to a significant decrease in CH4 uptake of the cesium and potassium ion exchanged zeolites, generating only a slight reduction in the CO2 uptake. This difference in the amount of reduction in the CH4 and CO2 uptake capacities give rise to high CO2-over-CH4 selectivity. The interaction between the cations and the CO2 is higher as compared CH4 due to the stronger polarizability and quadrupole moment of CO2.41
The use of zeolites in industrial applications requires a strong macroscopic structure with good mechanical stability and hierarchical porosity which can provide a low pressure drop and rapid mass transfer kinetics.42 High mechanical strength is crucial for the adsorbents as they undergo large and rapid pressure and thermal variations.27,43 Table 3 shows that the mechanical strength of the optimal ion-exchanged granules is comparable to pure CaA and NaX granules. Nevertheless, a drop in the mechanical strength has been noticed for the optimal cesium ion exchanged granules. Optimal cesium exchanged granules underwent two times the ion exchange process which may result in erosion and dissolution of contact points/bridges between the zeolite crystals in the granules and reduce the mechanical strength.31 The mechanical strength of ion-exchanged binderless CaA and NaX granules (Table 3) is higher than the mechanical strength reported for conventional structured CaA and NaX zeolite monoliths.25 The retention of mechanical strength after the ion-exchange process is related to the binderless nature of the zeolite granules. It has been reported that the addition of clay binders to the zeolites results in significant reduction in the mechanical durability of the structured body on exposure to acidic and basic aqueous media.27
| Granules | Macroporosity (%) | Compressive strength (MPa) |
|---|---|---|
| CaA | 24.7 | 3 ± 0.7 |
| CaK2.6A | 25 | 3.6 ± 1.1 |
| CaK2.5Cs0.2A | 29.2 | 1.3 ± 0.4 |
| NaX | 21 | 4.4 ± 2.0 |
| NaK3.9X | 23 | 3.4 ± 0.9 |
| NaK4.5Cs0.3X | 19.5 | 2 ± 0.4 |
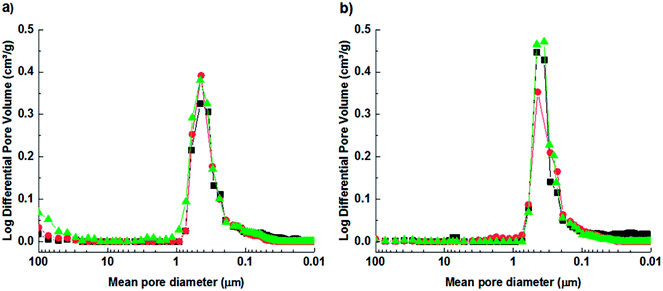
Fig. 3 shows the differential pore size distribution of the CaA, NaX and its optimal ion exchange derivative, measured by mercury porosimetry. The macroporosity of the zeolites is listed in Table 3. The CaA and the NaX zeolite granules show a homogeneous pore size distribution with a maximum centered at 0.4 μm. The peak corresponds to interparticle voids between the zeolites particles in the binderless granules. Such large pores are required for efficient mass transfer of gas molecules to the adsorption sites in the zeolite crystals.
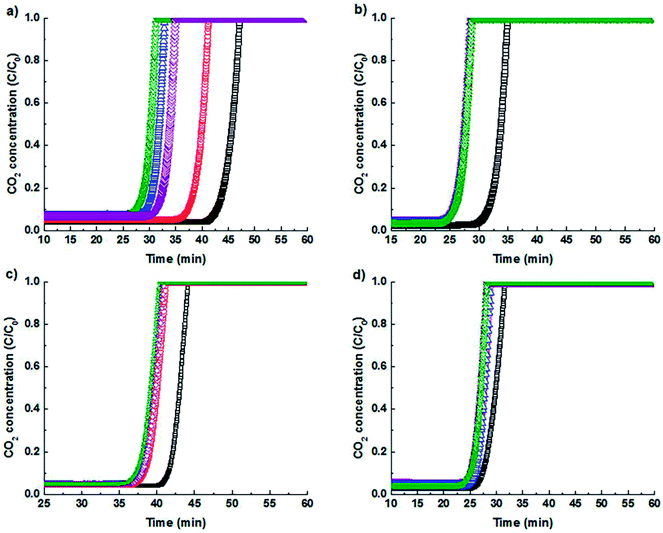
Fig. 4 shows the cyclic breakthrough experiments on adsorption column of binderless CaA and NaX granules and their optimum ion exchanged derivatives to evaluate and compare the performance. In breakthrough curve, C/C0 implies relative concentration of CO2, where C and C0 represent the concentration at the outlet and the inlet of the fixed bed respectively. The first part of the breakthrough curve in which the relative concentration (C/C0) is zero implies that all the CO2 gas molecules are being adsorbed. The second part of the curves with a steep increase in relative concentration implies that the adsorbent is saturated, and an increasing concentration of CO2 gas molecules is slipping (through the column). The zone between zero to one is described as mass transfer zone (MTZ). The fixed bed is completely saturated when C/C0 is equal to one.
After the first cycle, the reduction in the breakthrough point for further cycles is due to the chemisorbed CO2 molecules, as observed in cyclic experiment in (Fig. 2c and d) also, that could not be released through regeneration by evacuation. Both of the optimal ion exchange zeolite granules show stability in the adsorption capacity after three cycles. The amount of CO2 adsorbed by the zeolites until the breakthrough point for pure NaX and CaA zeolites were determined to be 4.6 mmol and 3.3 mmol of CO2 per g of the zeolite, respectively, for the final cycle. NaK4.5Cs0.3X shows the adsorption capacity of 3.8 mmol g−1 in the final cycle and is higher than the CaK2.5Cs0.2A zeolite, which adsorbs 3.4 mmol of CO2 per g of zeolite.
The CO2 uptake rate was 2.8 mg of CO2 per g per s for NaK4.5Cs0.3X and 2.5 mg of CO2 per g per s for CaK2.5Cs0.2A granules. The amount of CO2 adsorbed in breakthrough experiment is less than the amount adsorbed in the equilibrium gas adsorption experiment shown in Fig. 2. The sharp front in the breakthrough curve as shown in Fig. 4 implies that the zeolite granules have low mass transfer resistance and the fluid flow distribution is even within the granules. The mass transfer coefficient which corresponds to the speed of the molecules to diffuse and amount adsorb within the adsorbent can be derived from eqn (1) which was adapted from Klinkenberg44 and was fitted to the experimental breakthrough.
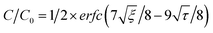 | (1) |
| ξ = κKz((1 − ε)/υε) | (2) |
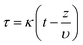 | (3) |
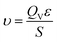 | (4) |
In above equations, κ is mass transfer coefficient, K is Henry constant of the adsorbate, ε is the total void fraction, z is the bed length, t is the time in minutes, υ is interstitial velocity which is given by eqn (4), where QV is the volumetric flow rate of the gas and S is the cross-sectional surface area of the bed.
The simulated mass transfer coefficient κ is 1.01 and 0.6 m s−1 for pure NaX and CaA granules, respectively. High mass transfer indicates rapid diffusion of CO2 molecules into the adsorbent. The mass transfer is fast in NaX due to relatively large pore size (0.74 nm) in the zeolite framework. The mass transfer coefficient was reduced to 0.41 and 0.13 m s−1 for NaK4.5Cs0.3X and CaK2.5Cs0.2A granules respectively. Due to the presence of extra framework cations in the zeolite, the diffusion of CO2 molecules is slower in the adsorbents and hence resulted in the relatively low mass transfer coefficient. The mass transfer coefficients for pure and ion-exchanged derivates are comparable to laminated NaA zeolites prepared by freeze casting.28
Conclusions
The optimal ion exchange of CaA and NaX zeolites and their adsorption properties were studied. The optimal concentration was determined according to the performance of the ion exchange zeolites by evaluating the CO2-over-CH4 selectivity using IAST theory. The CaKCsA with the 2.5 (at%) K+ and 0.2 (at%) Cs+ content and NaKCsX with the 4.5 (at%) K+ and 0.3 (at%) Cs+ content gives the CO2 uptake value of 4.3 mmol g−1 and 5.1 mmol g−1 respectively with high CO2-over-CH4 selectivity as compared to pure CaA and NaX. The optimal ion exchanged CaA and NaX zeolites shows high mechanical strength of 1.3 MPa and 2 MPa respectively. The breakthrough curves suggest low mass transfer resistance for both NaX and CaA granules including its optimal ion exchanged derivatives. NaX ion exchange granules have shown better performance as compared to CaA ion exchange zeolite, by having a CO2 uptake rate of 2.8 mg of CO2 per g per s while achieving the stability after few cycles with a mass transfer coefficient of 0.41 m s−1.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Innovation Fund Denmark (grant no. 5157-00008A), Formas (grant no. 2016–01099), Kempestiftelserna (grant no. JCK 1437) for their financial support. The authors acknowledge Hanzhu Zhang for the help in the experimental work.References
- A. Whiting and A. Azapagic, Energy, 2014, 70, 181–193 CrossRef.
- Ş. Y. Balaman and H. Selim, Appl. Energy, 2014, 130, 289–304 CrossRef.
- Q. Zhao, E. Leonhardt, C. MacConnell, C. Frear and S. Chen, CSANR Research Report, 2010, 9, 1–24 Search PubMed.
- M. Scholz, T. Melin and M. Wessling, Renewable Sustainable Energy Rev., 2013, 17, 199–212 CrossRef CAS.
- M. P. S. Santos, C. A. Grande and A. E. Rodrigues, Ind. Eng. Chem. Res., 2011, 50, 974–985 CrossRef CAS.
- O. I. Maile, E. Muzenda and H. Tesfagiorgis, Procedia Manufacturing, 2017, 7, 639–646 CrossRef.
- X. Y. Chen, H. Vinh-Thang, A. A. Ramirez, D. Rodrigue and S. Kaliaguine, RSC Adv., 2015, 5, 24399–24448 RSC.
- A. M. I. Yousef, Y. A. Eldrainy, W. M. El-Maghlany and A. Attia, Alexandria Eng. J., 2016, 55, 1143–1150 CrossRef.
- L. Deng and M. Hägg, Int. J. Greenhouse Gas Control, 2010, 4, 638–646 CrossRef CAS.
- T. A. Makal, J. Li, W. Lu and H. Zhou, Chem. Soc. Rev., 2012, 41, 7761–7779 RSC.
- T. Montanari, E. Finocchio, E. Salvatore, G. Garuti, A. Giordano, C. Pistarino and G. Busca, Energy, 2011, 36, 314–319 CrossRef CAS.
- S. J. Chen, Z. C. Tao, Y. Fu, M. Zhu, W. L. Li and X. D. Li, Appl. Energy, 2017, 205, 1435–1446 CrossRef CAS.
- Y. Gao, J. Zhou, H. Sheng, X. Liu, X. Yong, S. Wang, Z. Yan and T. Zheng, Xiandai Huagong, 2014, 34, 50–54 CAS.
- R. T. Yang, Adsorbents: Fundamentals and Applications, WileyInterscience, New York, 2003 Search PubMed.
- Q. Liu, A. Mace, Z. Bacsik, J. Sun, A. Laaksonen and N. Hedin, Chem. Commun., 2010, 46, 4502–4504 RSC.
- B. Yang, Y. Liu and M. Li, Chin. Chem. Lett., 2016, 27, 933–937 CrossRef CAS.
- Z. Bacsik, O. Cheung, P. Vasiliev and N. Hedin, Appl. Energy, 2016, 162, 613–621 CrossRef CAS.
- O. Cheung, D. Wardecki, Z. Bacsik, P. Vasiliev, L. B. McCusker and N. Hedin, Phys. Chem. Chem. Phys., 2016, 18, 16080–16083 RSC.
- D. Ko, R. Siriwardane and L. Biegler, Ind. Eng. Chem. Res., 2003, 42, 339–348 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, J. Chem. Eng. Data, 2004, 49(4), 1095–1101 CrossRef CAS.
- A. Eskandari, M. Jahangiri and M. Anbia, Int. J. Eng., Trans. A, 2016, 29, 1–7 CAS.
- P. J. E. Harlick and F. H. Tezel, Microporous Mesoporous Mater., 2004, 76, 71–79 CrossRef CAS.
- K. S. Walton, M. B. Abney and M. D. LeVan, Microporous Mesoporous Mater., 2006, 91, 78–84 CrossRef CAS.
- F. Rezaei and P. Webley, Chem. Eng. Sci., 2009, 64, 5182–5191 CrossRef CAS.
- F. Akhtar and L. Bergström, J. Am. Ceram. Soc., 2011, 94, 92–98 CrossRef CAS.
- R. V. Jasra, B. Tyagi, Y. M. Badheka, V. N. Choudary and T. S. G. Bhat, Ind. Eng. Chem. Res., 2003, 42, 3263–3272 CrossRef CAS.
- F. Akhtar, L. Andersson, S. Ogunwumi, N. Hedin and L. Bergström, J. Eur. Ceram. Soc., 2014, 34, 1643–1666 CrossRef CAS.
- A. Ojuva, M. Järveläinen, M. Bauer, L. Keskinen, M. Valkonen, F. Akhtar, E. Levänen and L. Bergström, J. Eur. Ceram. Soc., 2015, 35, 2607–2618 CrossRef CAS.
- H. Thakkar, S. Eastman, A. Hajari, A. A. Rownaghi, J. C. Knox and F. Rezaei, ACS Appl. Mater. Interfaces, 2016, 8, 27753–27761 CrossRef CAS PubMed.
- F. Akhtar, Q. Liu, N. Hedin and L. Bergström, Energy Environ. Sci., 2012, 5, 7664–7673 RSC.
- N. Keshavarzi, F. Akhtar and L. Bergström, J. Mater. Res., 2013, 28, 2253–2259 CrossRef CAS.
- S. Salehi and M. Anbia, J. Inorg. Organomet. Polym. Mater., 2017, 27, 1281–1291 CrossRef CAS.
- T. Remy, E. Gobechiya, D. Danaci, S. A. Peter, P. Xiao, L. Van Tendeloo, S. Couck, J. Shang, C. E. A. Kirschhock, R. K. Singh, J. A. Martens, G. V. Baron, P. A. Webley and J. F. M. Denayer, RSC Adv., 2014, 4, 62511–62524 RSC.
- Q. Jiang, J. Rentschler, G. Sethia, S. Weinman, R. Perrone and K. Liu, Chem. Eng. J., 2013, 230, 380–388 CrossRef CAS.
- B. P. Spigarelli and S. K. Kawatra, J. CO2 Util., 2013, 1, 69–87 CrossRef CAS.
- L. Bertsch and H. W. Habgood, J. Phys. Chem., 1963, 67, 1621–1628 CrossRef CAS.
- G. P. Dangi, K. Munusamy, R. S. Somani and H. C. Bajaj, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2012, 51, 1238–1251 Search PubMed.
- N. Sue-aok, T. Srithanratana, K. Rangsriwatananon and S. Hengrasmee, Appl. Surf. Sci., 2010, 256, 3997–4002 CrossRef CAS.
- S. Yang, J. Kim and W. Ahn, Microporous Mesoporous Mater., 2010, 135, 90–94 CrossRef CAS.
- G. Sethia, R. S. Somani and H. C. Bajaj, Ind. Eng. Chem. Res., 2014, 53, 6807–6814 CrossRef CAS.
- F. Akhtar, N. Keshavarzi, D. Shakarova, O. Cheung, N. Hedin and L. Bergström, RSC Adv., 2014, 4, 55877–55883 RSC.
- P. Vasiliev, F. Akhtar, J. Grins, J. Mouzon, C. Andersson, J. Hedlund and L. Bergström, ACS Appl. Mater. Interfaces, 2010, 2, 732–737 CrossRef CAS PubMed.
- F. Akhtar, S. Ogunwumi and L. Bergström, Sci. Rep., 2017, 7, 10988 CrossRef PubMed.
- A. Klinkenberg, Ind. Eng. Chem., 1954, 46, 2285–2289 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra08004f |
| This journal is © The Royal Society of Chemistry 2018 |