Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advancing and receding contact angle investigations for highly sticky and slippery aluminum surfaces fabricated from nanostructured anodic oxide†
Daiki Nakajima,
Tatsuya Kikuchi *,
Shungo Natsui and
Ryosuke O. Suzuki
*,
Shungo Natsui and
Ryosuke O. Suzuki
Faculty of Engineering, Hokkaido University, N13-W8, Kita-ku, Sapporo, Hokkaido 060-8628, Japan. E-mail: kiku@eng.hokudai.ac.jp
First published on 6th November 2018
Abstract
The fabrication of sticky and slippery superhydrophobic aluminum was achieved by anodizing in pyrophosphoric acid and modification with self-assembled monolayers (SAMs). In addition, the corresponding sliding behaviors of a water droplet were investigated by contact angle measurements and direct observations. For the formation of anodic alumina nanofibers, 4N aluminum plates were anodized in a concentrated pyrophosphoric acid solution at 25–75 V. The morphology of the anodic oxide successively changed to barrier oxide, porous oxide, nanofibers, bundle structures with many nanofibers, and then weak nanofibers during anodizing. The anodized specimens were immersed in a fluorinated phosphonic acid/ethanol solution to form SAMs on the surface of the anodic oxide. The contact angle hysteresis drastically changed with anodizing time: it increased with the formation of porous oxide, decreased for the nanofibers and bundle structures, and then increased once again for the weak nanofibers. Correspondingly, the adhesion interaction between the water droplet and the aluminum surface also drastically changed to show sticky, slippery, and sticky behaviors with anodizing time. More sticky and slippery aluminum surfaces can be obtained by anodizing at higher voltages. The slippery behavior was further improved through two distinct anodizing processes with the formation of ordered alumina nanofibers. A superhydrophobic aluminum surface with coexisting sticky and slippery properties was fabricated by the selective anodizing method.
Introduction
Anodizing (anodization) is one of the most important surface finishing processes for valve metals, such as aluminum, titanium, magnesium, and their alloys. In particular, aluminum is often anodized for mechanical property improvement,1–4 corrosion protection,5–9 electronic applications,10,11 and nanostructural engineering.12–14 The morphology of anodic aluminum oxide is determined by the electrolyte solution used during anodizing.15–20 Anodizing aluminum in a pyrophosphoric acid (H4P2O7) solution causes the formation of numerous nanoscale anodic alumina nanofibers.21,22 Although similar alumina nanofibers were formed by long-term anodizing in typical electrolyte solutions, such as oxalic and phosphoric acids, followed by chemical dissolution of the anodic oxide,23–25 pyrophosphoric acid anodizing (PyAA) has the following major advantages: (a) the rapid formation of alumina nanofibers within 2 min under the appropriate operating conditions;22 (b) the ability to precisely control the surface nanostructures, such as independent standing nanofiber arrays, bundle structures with different scales, and high-aspect-ratio bending nanofibers;26 and (c) the successful fabrication of highly ordered alumina nanofibers via two distinct anodizing processes.27 These fibrous oxides can also be fabricated on various commercially available aluminum alloys, such as 1N30, 3004, and 8021.28PyAA allows control of the water wettability over a wide range of values on the aluminum surface. A nanofiber-covered aluminum surface exhibits superhydrophilic properties with a water contact angle (WCA) of less than 10° observed within 0.1 seconds after placement of the water drop.29,30 Rapid drying and snow-sliding behaviors were observed on these aluminum surfaces covered with alumina nanofibers. Conversely, the WCA drastically changes to show superhydrophobic behavior and is over 150° when the surface of the alumina nanofibers is modified with a self-assembled monolayer (SAM).26 This superhydrophobic aluminum surface shows high light reflectance, measuring more than 99% over the whole visible spectrum (compared with an electropolished aluminum surface).
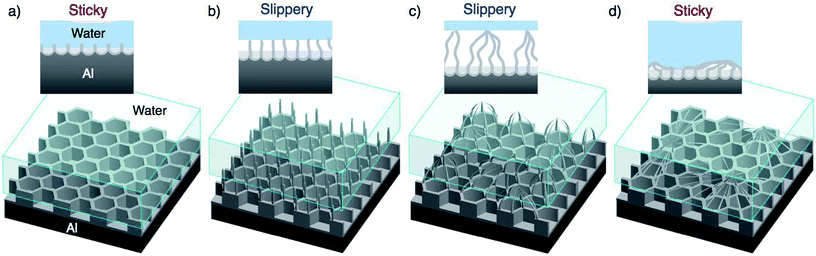
Although all superhydrophobic surfaces possess similar static WCAs that are greater than 150°, the adhesion interaction between the water droplet and the superhydrophobic surface may vary according to the surface morphology. For example, a lotus leaf exhibits superhydrophobic behavior, as observed by the WCA measurement, and the water droplet can easily slide from the surface (i.e., “slippery” surface).31,32 By contrast, while a rose petal also exhibits similar superhydrophobic behavior with respect to the contact angle, the water droplet strongly adsorbs on its surface (i.e., “sticky” surface).33 Because both slippery and sticky surfaces are of considerable interest for various industrial applications, such as self-cleaning surfaces,34,35 heat-exchange devices,36–38 single-molecule spectroscopy,39,40 and micromanipulators,41 the fabrication of slippery and sticky aluminum surfaces would expand the applicability of aluminum and its alloys.
Herein, we demonstrate the fabrication of slippery and sticky aluminum surfaces covered with anodic alumina nanofibers via PyAA under various electrochemical conditions and subsequent modification with fluorinated SAMs. The effects of the morphological properties of the alumina nanofibers, such as the length, density, and periodicity, on the adhesion interaction between the water droplet and the surface were investigated by WCA measurements. The adhesion interaction changed drastically with anodizing time and the consequent morphology. We successfully fabricated a highly slippery aluminum surface with a sliding angle of less than 2° and a highly sticky aluminum surface with a non-sliding angle of 180° by controlling the surface morphology via PyAA. Moreover, we also demonstrate a superhydrophobic aluminum surface with coexisting sticky and slippery properties that was fabricated via the selective PyAA method.
Experimental
Pretreatment of aluminum specimens
Commercially available aluminum plates (purity: 99.99 wt%, size: 20 mm × 40 mm, thickness: 400 μm, Nippon Light Metal, Japan) were used as the starting aluminum specimens. The aluminum plates were immersed in ethanol and then ultrasonically degreased for 10 min. The bottom half of the specimens (i.e., 20 mm × 20 mm area) was electrochemically polished in a 13.6 M acetic acid/2.56 M perchloric acid solution (T < 280 K) at 28 V for 1 min. A high-purity aluminum plate was used as the cathode, and the electropolishing solution was stirred by a magnetic stirrer bar.Anodizing of aluminum
A commercially available concentrated pyrophosphoric acid solution (concentration: 74.0 wt%, Kanto Chemical, Japan) was used as the anodizing electrolyte solution. An electropolished aluminum specimen was immersed in the pyrophosphoric acid solution (solution volume: 100 cm3, inner diameter of the glass cell: 55 mm, T = 293 K). A platinum plate (purity: 99.95 wt%, size: 16 mm × 28 mm, thickness: 100 μm, Furuya Metal, Japan) was used as the cathode. The aluminum anode and platinum cathode were set parallel at a distance of 20 mm in the electrochemical cell. Then, the aluminum specimen was anodized at 25–75 V for 60 min to form anodic alumina nanofibers (constant voltage PyAA). The anodizing solution was stirred by a magnetic stirrer bar at 1.0 s−1 during PyAA and maintained at a constant temperature using a water bath (UCT-1000, AS ONE, Japan). A direct current power supply (PWR-400H/400M, Kikusui, Japan) was used for PyAA. For comparison, typical porous alumina was also formed by anodizing in a 0.3 M oxalic acid solution at 293 K and 50 V for 60 min (oxalic acid anodizing: OAA).An ordered anodic alumina nanofiber array was fabricated by two distinct anodizing processes.27 First, the electropolished aluminum specimens were anodized in a 0.3 M oxalic acid solution at 293 K and 50 V for 2 h to form ordered porous alumina (OAA). The porous alumina was dissolved in a 0.2 M chromic acid/0.51 M phosphoric acid solution (T = 353 K) for 20 min, forming an ordered dimple array on the aluminum surface. After chemical dissolution, the specimens were anodized again in pyrophosphoric acid solution for 60 min to form an ordered alumina nanofiber array (PyAA).
Modification of SAMs
After anodizing, the surface of the anodic oxide was modified with 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctylphosphonic acid (FOPA). In this modification, (a) the anodized specimens were placed into a vial filled with 0.5 mM FOPA/ethanol solution (volume: 30 mL), (b) the vial was sealed with a screw cap, and (c) the vial was placed in an incubator (Heratherm IMC18, Thermo Fisher Scientific, USA) at 313 K for 24 h. After SAM modification, the specimens were stored in a desiccator after washing with ethanol.Characterization
The anodized specimens were observed by field-emission scanning electron microscopy (FE-SEM, JSM-6500F, JEOL, Japan). A platinum electroconductive layer was sputter-coated on the surface of the specimens before SEM observation (MSP-1S, Vacuum Device, Japan).The WCAs on the anodized specimens were examined by an optical contact angle meter at room temperature (DM-501, Kyowa Interface Science, Japan).42,43 Although high-precision WCA measurements such as high-precision drop shape analysis were already reported,44–46 standard advancing and receding contact angle measurements were used for the fabrication of sticky and slippery aluminum surfaces in the present investigation. Ultra pure water droplets with a resistance of 18.2 MΩ cm were formed on the surface using an auto dispenser and a microsyringe with a flow rate of 2 μL s−1, and the advancing contact angle (ACA) and receding contact angle (RCA) were recorded by a CCD camera. Here, the ACA was defined as the angle measured when the droplet had the maximum volume for the same interface area during water injection. Conversely, the RCA was defined as the angle measured when the droplet had the minimum volume for the same interface area during water suction. The RCA value was defined as zero when the triple point (three-phase contact point) was fixed during water suction. The ACA and RCA values of the water droplets were fitted using a circle fitting method. WCA measurements were performed at five different positions on each specimen, and then, the values were averaged without including the maximum and minimum values.
The slipping behavior of the water droplet formed on the anodized specimens was observed using a rotating stage with a stage controller (SKIDS-60YAW and Mark-102, Sigmakoki, Japan) and a digital microscope (Hidemicron Pro2, Tec, Japan). A water droplet (20 μL) was placed on the horizontal anodized specimen, and then, the specimen was rotated counterclockwise at a rate of 5° s−1 to cause the droplet to slide.
Results and discussion
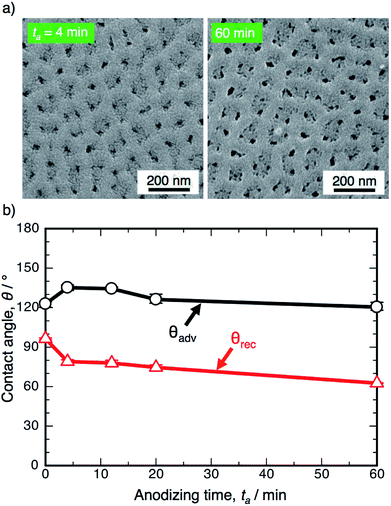
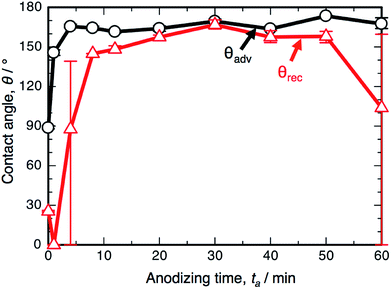
Fig. 1a shows the SEM images of the surface formed by OAA in a 0.3 M oxalic acid solution at 293 K and 50 V for ta = 4 min and 60 min. Many nanosize pores with diameters of approximately 15–35 nm were observed over the entire surface, and typical porous alumina was obtained in the initial stage of OAA. The morphology of the porous alumina was nearly unchanged by the additional anodizing process carried out for 60 min, even though the diameter of the pores increased slightly due to chemical dissolution in oxalic acid. The electropolished and anodized aluminum specimens were immersed in a 0.5 mM FOPA/ethanol solution to form SAMs on their surfaces, and the WCAs were measured using an optical contact angle meter.Fig. 1b shows the changes in the ACA, θadv, and RCA, θrec, on the SAM-modified aluminum specimens with the anodizing time, ta. The electropolished aluminum surface exhibits weak hydrophobicity with θadv = 122.8°, since FOPA molecules consisting of trifluoromethyl groups (–CF3) self-assembled on the aluminum oxide upon immersion in FOPA/ethanol solution.47–50 The θadv of the anodized specimens slightly increased to approximately 135° in the initial stage and then gradually decreased with increasing anodizing time. However, the θadv remained hydrophobic over the entire anodizing time. The θrec of the electropolished specimen was measured to be 96.4° and then decreased gradually with increasing anodizing time. Importantly, the difference between θadv and θrec corresponds to the spreading of the water droplet on the surface (i.e., contact angle hysteresis, θadv − θrec).51–53 The hysteresis of the electropolished surface was calculated to be 26.4°, and low spreading behavior was observed. The hysteresis increased to approximately 60° by OAA for each operating time, and the surfaces covered with porous alumina exhibited weak slippery behavior.
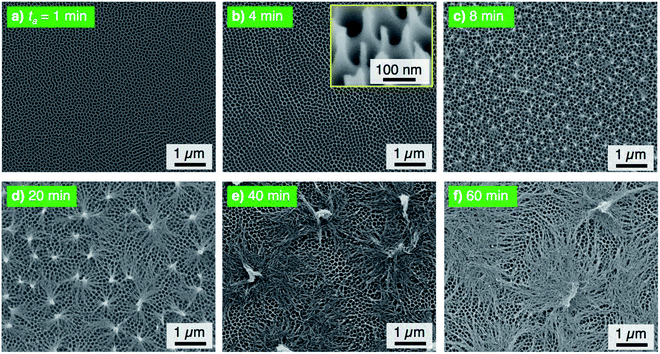
The electropolished aluminum specimens were anodized in a concentrated pyrophosphoric acid solution under the same operating conditions at 293 K and 50 V. Fig. 2 shows the SEM images of the surface formed by PyAA for (a) ta = 1 min through (f) 60 min. A thin, flat barrier anodic oxide was formed on the surface by PyAA for 1 min (Fig. 2a). As the anodizing time increased to 4 min, porous oxide with numerous nanopores was formed on the surface (Fig. 2b). The diameter of the pores formed by PyAA was larger than that of the pores formed by OAA (Fig. 1a) due to rapid chemical dissolution into the concentrated pyrophosphoric acid solution, and several continu ous grooves were also observed. The alumina cell was located around the pores dissolved by further PyAA for 8 min, and porous oxide with narrow polygonal walls was obtained on the surface (Fig. 2c). In addition, the tiled image shows that nanoscale alumina fibers were formed at the apices of each network structure (inset of Fig. 2c). These alumina nanofibers grow via further PyAA and bend due to their flexibility. As a result, pyramidal bundle structures consisting of several tens of alumina nanofibers were fabricated on the surface (Fig. 2d). These nanofibers were composed of amorphous aluminum oxide without an electrolyte anion. The average size of the alumina bundles increased with anodizing time due to the formation of longer alumina nanofibers, and large bundle structures consisting of several hundreds of alumina nanofibers were fabricated (Fig. 2e). However, excess PyAA led to chemical dissolution of the pure alumina nanofibers into the pyrophosphoric acid solution, and a weak nanofiber structure was observed after PyAA for 60 min (Fig. 2f). In sum, the anodic nanostructures formed by PyAA changed into barrier oxide, porous oxide, alumina nanofibers, bundle structures with many alumina nanofibers, and weak nanofibers. These anodized specimens were also modified with FOPA-SAMs, and then, their WCAs were measured.
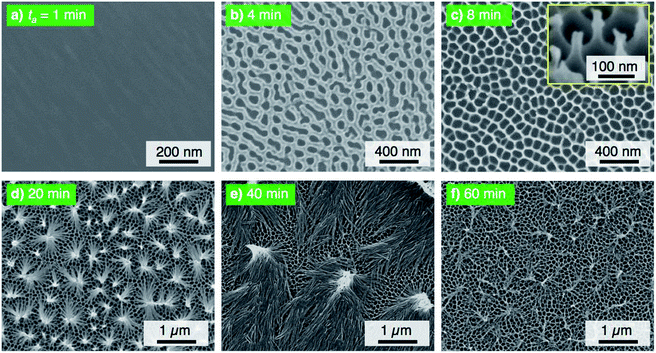 | ||
| Fig. 2 SEM images of the surface of the specimen anodized in concentrated pyrophosphoric acid solution at 293 K and 50 V for (a) 1 min through (f) 60 min. | ||
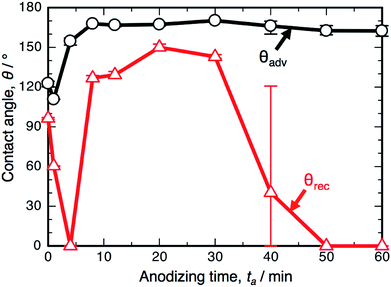
Fig. 3 shows the changes in θadv and θrec on the SAM-modified aluminum specimens with ta. The shape of the θadv curve was relatively simple: θadv rapidly increased to more than 150° after an initial slight decrease, and high θadv values of 162–170° were maintained upon further PyAA for up to 60 min. Thus, superhydrophobic aluminum surfaces with contact angles over 150° were successfully fabricated by PyAA after 4 min. On the other hand, θrec drastically changed with anodizing time. First, θrec decreased rapidly during the initial anodizing stage, and the lowest θrec value was obtained after anodizing for 4 min. Then, θrec rapidly increased to over 120° with further PyAA, and the highest θrec of 150° was obtained after PyAA for 20 min. Finally, θrec gradually decreased with anodizing time, and the lowest θrec values were measured once again after PyAA for more than 50 min. As a result, the contact angle hysteresis (θadv − θrec) varied strongly with anodizing time and is divided into the following two regions: high hysteresis values of 154–162° were obtained with PyAA times of 4 min and over 50 min, and low hysteresis values of 17–40° were obtained with PyAA times of 8–30 min. Compared to the WCA measurements obtained by OAA (Fig. 1b), more slippery (low hysteresis) or sticky (high hysteresis) superhydrophobic aluminum surfaces can be obtained by PyAA for an appropriate operating time. Such slippery and sticky surfaces strongly depend on the morphology of the anodic oxide formed by PyAA. Therefore, further WCA measurements were performed on the aluminum specimens formed by various anodizing voltages.
The electropolished aluminum specimens were anodized at a relatively low voltage of 25 V in pyrophosphoric acid solution (293 K) for (a) ta = 1 min through (f) 60 min, and the corresponding SEM images of the 20° tiled surface of the specimen are shown in Fig. 4. High-density alumina nanofibers gradually grew on the surface with increasing anodizing time up to 20 min (Fig. 4a–d) because the number of growth nuclei of the alumina nanofibers increases with decreasing anodizing voltage due to the formation of a smaller honeycomb structure at the bottom of the nanofibers, showing behavior that is similar to that observed in the formation of porous alumina.54,55 Then, linear bundle structures consisting of numerous alumina nanofibers were fabricated by further PyAA for 40 min (Fig. 4e). Finally, a weak nanofiber structure was observed upon excess PyAA for 60 min due to chemical dissolution (Fig. 4f). Compared to the growth behavior of the alumina nanofibers at 50 V (Fig. 2), the following major differences can be observed by PyAA at low voltage: (a) slow growth rate, (b) high density, and (c) formation of linear bundles.
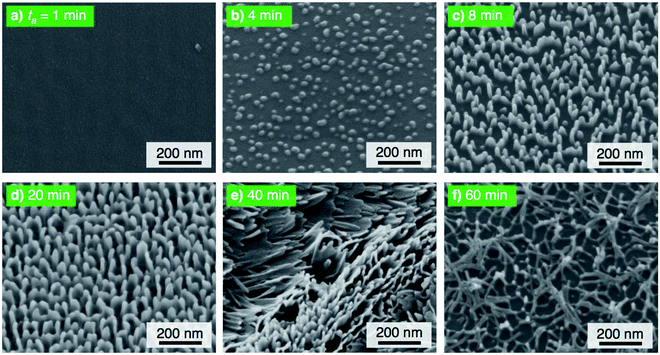 | ||
| Fig. 4 SEM images of the surface of the specimen anodized in concentrated pyrophosphoric acid solution at 293 K and 25 V for (a) 1 min through (f) 60 min. | ||
The electropolished specimens were also anodized at a relatively high voltage of 75 V, and the surface morphologies of the anodized specimens are shown in Fig. 5. Because the anodic current density increased with the applied voltage, porous oxide, nanofibers, and pyramidal bundle structures grew rapidly on the surface with PyAA for 1–8 min (Fig. 5a–c). In addition, the density of the alumina nanofibers was lower than that obtained at the lower anodizing voltages. Because longer alumina nanofibers were formed by long-term PyAA, the pyramidal bundles became larger due to the absorption of surrounding alumina nanofibers (Fig. 5d and e). Finally, the aluminum surface was covered with longer alumina nanofibers after PyAA for 60 min (Fig. 5f). Compared to the growth behavior at 50 V, the following major differences can be observed by PyAA at high voltage: (a) high growth rate, (b) low density, and (c) formation of large bundle structures.
 | ||
| Fig. 5 SEM images of the surface of the specimen anodized in concentrated pyrophosphoric acid solution at 293 K and 75 V for (a) 1 min through (f) 60 min. | ||
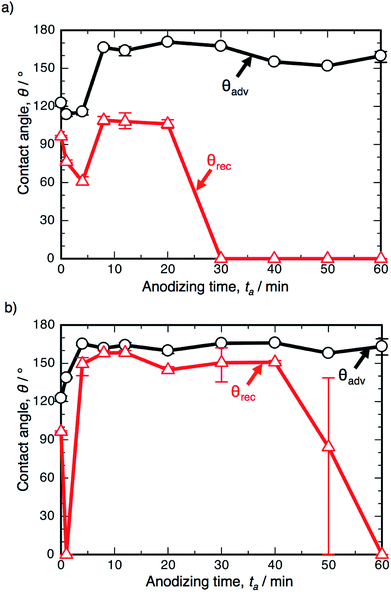
Fig. 6 shows the results of the WCA measurements on the SAM-modified aluminum specimens formed by PyAA at (a) 25 V and (b) 75 V. At the low voltage of 25 V (Fig. 6a), θadv increased rapidly to over 150° in the initial stage of PyAA, and this high value was maintained on the anodized surface for up to 60 min. The shape of the θadv curve is similar to that obtained at 50 V (Fig. 3), even though the surface slowly changed to show superhydrophobic behavior. The shape of the θrec curve is also similar to the results obtained at 50 V, but the variation is smaller; θrec decreased slightly to 60° after 4 min, slightly increased to 109° after 8 min, and then rapidly decreased to 0° after more than 30 min.
When the anodizing voltage was increased to 75 V (Fig. 6b), θadv rapidly increased to over 150° within 4 min, and this high value was maintained upon further PyAA. Therefore, the surface formed by high-voltage anodizing rapidly changed to show superhydrophobic behavior. In addition, θrec changed drastically with anodizing time; θrec rapidly decreased to 0° upon anodizing for 1 min, rapidly increased to over 150° after 4 min, maintained its value for up to 40 min, and then gradually decreased to 0° upon further PyAA. Such changes are more drastic than those obtained at 50 V. Notably, the highest hysteresis measured at 163.2° and the lowest hysteresis measured at 3.8° were obtained by PyAA at 75 V.
The WCA measurements and the corresponding nanostructures formed by PyAA can be summarized as follows. As the alumina nanofibers were formed by PyAA, the surface exhibits superhydrophobic behavior with θadv = 150°. This superhydrophobicity is maintained by the subsequent bundle and weak structures formed by further PyAA. In contrast, the change in θrec is much more complex; θrec decreases as the porous oxide is formed on the surface, increases as the alumina nanofibers and the bundle structures are formed, and decreases once again as the weak nanofiber structures are formed. As a result, the contact angle hysteresis increases, decreases, and then increases once again with increasing anodizing time.
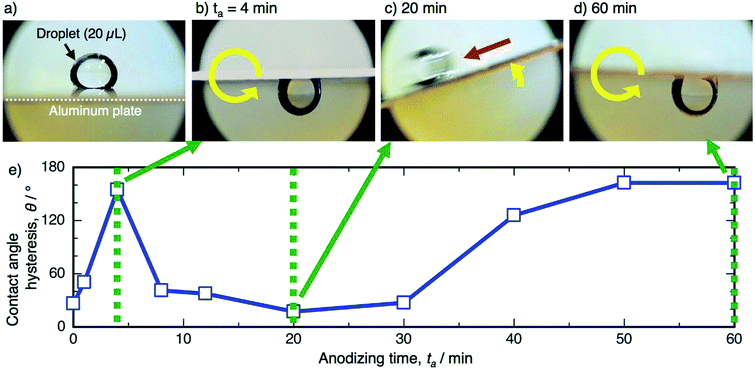
The contact angle hysteresis corresponds to the sliding behavior of the water droplet on the surface. A direct observation of the sliding behavior was recorded by a digital microscope, and still images and the corresponding contact angle hysteresis curve are shown in Fig. 7. Here, a water droplet (20 μL) was placed on the horizontal specimen anodized at 50 V (Fig. 7a), and then, the specimens were rotated counterclockwise at a rate of 5° s−1. The water droplet strongly adhered on the specimen anodized for 4 min after the 180° rotation, and a highly sticky aluminum surface was obtained, as shown by the high hysteresis of 154.7° (Fig. 7b). As the contact angle hysteresis decreased to 17.3° after PyAA for 20 min, the water droplet easily slid to the left side upon tilting the specimen (Fig. 7c). As the hysteresis increased once again by further anodizing for 60 min, the droplet strongly adhered on the surface after the 180° rotation (Fig. 7d). Consequently, sticky aluminum surfaces could be obtained via short- and long-term anodizing processes, and slippery surfaces could also be obtained via the mid-term anodizing process.
Fig. 8 shows schematic illustrations of the morphological variations and the expected results for a water droplet placed on the surface. The magnitude of the adhesive force of the water droplet increases in the order of:56–58
| Area-contact > point-contact |
The porous oxide film formed by short-term PyAA has a continuous alumina area, and this area-contact surface may generate stronger surface adhesion than the point contact. Therefore, the water droplet is supported by the continuous area, and the surface exhibits sticky behavior (Fig. 8a). As the anodizing time increased, numerous independent alumina nanofibers and pyramidal bundle structures were formed on the surface (Fig. 8b and c). The water droplet is supported by the points of the alumina nanostructures, and this point-contact with the lowest contact area causes the highly slippery behavior (Cassie–Baxter state).33,40 Finally, as weak nanofiber structures were formed by excess PyAA, the surface once again exhibits sticky behavior due to its area-contact surface (Fig. 8d). Here, the water droplet is supported by the area-contact surface of the weak nanofiber structure (Cassie-impregnating wetting state).33,40 However, further investigations are required in order to develop a deeper understanding of these unique behaviors.
Typical PyAA leads to the formation of disordered alumina nanofibers on the aluminum surface, and the nanostructure has many defects, such as doubled-nanofibers and non-uniform shapes.21,22 The sticky and slippery behaviors will be improved by the formation of ordered alumina nanofibers due to the complete point-contact. We previously reported that ordered alumina nanofibers were fabricated via two distinct anodizing processes.27 In this technique, an ordered aluminum dimple array was fabricated by typical OAA and porous alumina dissolution, and then, anodic alumina nanofibers were grown at the six apexes of the ordered dimples through PyAA at the same applied voltage. As a result, highly ordered alumina nanofibers without any defects were fabricated on the surface. Fig. 9 shows the WCA measurements on the SAM-modified aluminum specimens formed by the two distinct anodizing processes at 50 V. Compared to the surface obtained by one-step PyAA at 50 V (Fig. 3), (a) the θadv values were slightly higher and (b) the contact angle hysteresis was smaller after PyAA for 8–50 min. Therefore, the water droplet easily slid to the left side after slightly tilting the specimen at an angle of less than 2°, and a highly slippery aluminum surface was successfully obtained by the two distinct anodizing processes.
Finally, we demonstrate the fabrication of a superhydrophobic aluminum surface showing both sticky and slippery properties through the selective anodizing method. In this technique, the electropolished aluminum specimens were selectively covered with a non-electroconductive nitrocellulose layer, and the exposed aluminum surface without the nitrocellulose layer was anodized in a pyrophosphoric acid solution at 293 K and 75 V for 11 min. The anodized specimens were immersed in acetone to dissolve the nitrocellulose layer and then anodized once again under the same operating conditions for 1 min. Consequently, two different anodized regions of the sticky surface (anodizing time: 1 min) and the slippery surface (total anodizing time: 12 min) were fabricated on the aluminum substrate. The water-sliding behavior of the anodized specimen is shown in Fig. 10 (a movie is also available in the ESI†). Here, two water droplets (20 μL) were placed on the surfaces anodized for 1 min and 12 min, and then, the specimen was slowly tilted to the front. The reflection of the digital camera was observed on the aluminum due to the highly mirror-finished surface formed by anodizing. The water droplet easily slid from the slippery surface anodized for 12 min, even though the droplet strongly adhered to the sticky surface anodized for 1 min. Such a superhydrophobic aluminum surface with the coexisting sticky and slippery properties could be easily fabricated via the selective anodizing method.
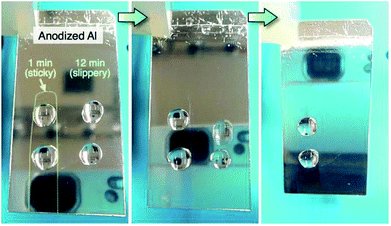 | ||
| Fig. 10 Water-sliding behavior of the aluminum surface fabricated via the selective anodizing method at 293 K and 75 V for 1 min (sticky surface) and 12 min (slippery surface). | ||
Conclusions
We investigated the WCAs of nanofiber-covered aluminum surfaces fabricated by anodizing in pyrophosphoric acid and the modification of fluorinated SAMs. We successfully fabricated highly slippery and sticky superhydrophobic aluminum surfaces. The following conclusions were reached in our investigations.(1) Anodizing aluminum in pyrophosphoric acid solution results in the growth of anodic alumina nanofibers through barrier and porous oxide formation. The nanofibers are bundled as the anodizing time increases, and the bundle structures grow during long-term anodizing. Finally, weak nanofiber structures are obtained on the surface by excess anodizing due to chemical dissolution of anodic alumina. The growth rate, density, and morphology of the alumina nanofibers depend strongly on the anodizing voltage.
(2) The contact angle hysteresis changes drastically with anodizing time and the corresponding morphology of the alumina nanofibers. The hysteresis increases with the formation of porous oxide, decreases for the nanofibers and bundle structures, and then increases once again for the weak nanostructures. Therefore, the adhesion interaction between the water droplet and the surface also drastically changes with anodizing time to sticky, slippery, and sticky. Higher sticky and slippery aluminum surfaces can be obtained by anodizing at higher voltages.
(3) The slippery behavior exhibited on the aluminum surfaces can be further improved by the use of two distinct anodizing processes through the formation of an aluminum dimple array and subsequent alumina nanofibers.
(4) A superhydrophobic aluminum surface with the coexisting sticky and slippery properties can be fabricated by the selective anodizing method.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Japan Society for the Promotion of Science (JSPS); the Tokyo Ohka Foundation for The Promotion of Science and Technology, Japan; and the Light Metal Educational Foundation, Japan for financial support. The authors also thank the “Nanotechnology Platform” Program of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, Mr Nobuyuki Miyazaki, and Mr Takashi Endo for the SEM observations.References
- D. R. Gabe, Met. Finish., 2002, 100, 52–58 CrossRef CAS.
- K. Kuroda and M. Okido, J. Biomater. Nanobiotechnol., 2018, 9, 26–40 CrossRef.
- S. Moon and Y. Jeong, Corros. Sci., 2009, 51, 1506–1512 CrossRef CAS.
- T. Kikuchi, A. Takenaga, S. Natsui and R. O. Suzuki, Surf. Coat. Technol., 2017, 326, 72–78 CrossRef CAS.
- X. Li, X. Nie, L. Wang and D. O. Northwood, Surf. Coat. Technol., 2005, 200, 1994–2000 CrossRef CAS.
- S. Moon and S. Pyun, J. Solid State Electrochem., 1998, 2, 156–161 CrossRef CAS.
- Y.-I. Choi, S. Salman, K. Kuroda and M. Okido, Electrochim. Acta, 2013, 97, 313–319 CrossRef CAS.
- V. R. Capelossi, M. Poelman, I. Recloux, R. P. B. Hernandez, H. G. de Melo and M. G. Olivier, Electrochim. Acta, 2014, 124, 69–79 CrossRef CAS.
- M. Zheng, M. Sakairi and H. Jha, Corros. Sci., 2012, 55, 332–338 CrossRef CAS.
- J. Li, C. Papadopoulos, J. M. Xu and M. Moskovits, Appl. Phys. Lett., 1999, 75, 367–369 CrossRef CAS.
- W. Lee and P. S. Joon, Chem. Rev., 2014, 114, 7487–7556 CrossRef CAS PubMed.
- T. Yanagishita, T. Kondo and H. Masuda, J. Vac. Sci. Technol., B: Nanotechnol. Microelectron.: Mater., Process., Meas., Phenom., 2018, 36, 031802 Search PubMed.
- H. Jha, T. Kikuchi, M. Sakairi and H. Takahashi, Nanotechnology, 2008, 19, 395603 CrossRef PubMed.
- W. Lee, K. Schwirn, M. Steinhart, E. Pippel, R. Scholz and U. Gösele, Nat. Nanotechnol., 2008, 3, 234–239 CrossRef CAS PubMed.
- L. Zaraska, G. D. Sulka and M. Jaskuła, Surf. Coat. Technol., 2010, 204, 1729–1737 CrossRef CAS.
- A. P. Li, F. Müller, A. Birner, K. Nielsch and U. Gösele, J. Vac. Sci. Technol., A, 1999, 17, 1428–1431 CrossRef CAS.
- T. Kikuchi, D. Nakajima, O. Nishinaga, S. Natsui and R. O. Suzuki, Curr. Nanosci., 2015, 11, 560–571 CrossRef CAS.
- L. Zaraska, M. Jaskuła and G. D. Sulka, Mater. Lett., 2016, 171, 315–318 CrossRef CAS.
- D. Nakajima, T. Kikuchi, S. Natsui and R. O. Suzuki, Appl. Surf. Sci., 2014, 321, 364–370 CrossRef CAS.
- S. Akiya, T. Kikuchi, S. Natsui and R. O. Suzuki, Appl. Surf. Sci., 2017, 403, 652–661 CrossRef CAS.
- T. Kikuchi, O. Nishinaga, D. Nakajima, J. Kawashima, S. Natsui, N. Sakaguchi and R. O. Suzuki, Sci. Rep., 2014, 4, 7411 CrossRef CAS PubMed.
- D. Nakajima, T. Kikuchi, S. Natsui, N. Sakaguchi and R. O. Suzuki, Appl. Surf. Sci., 2015, 356, 54–62 CrossRef CAS.
- L. A. Meier, A. E. Alvarez, D. R. Salinas and M. C. del Barrio, Mater. Lett., 2012, 85, 146–148 CrossRef CAS.
- N.-Q. Zhao, X.-X. Jiang, C.-S. Shi, J.-J. Li, Z.-G. Zhao and X.-W. Du, J. Mater. Sci., 2007, 42, 3878–3882 CrossRef CAS.
- M. Altomare, O. Pfoch, A. Tighineanu, R. Kirchgeorg, K. Lee, E. Selli and P. Schmuki, J. Am. Chem. Soc., 2015, 137, 5646–5649 CrossRef CAS PubMed.
- D. Nakajima, T. Kikuchi, S. Natsui and R. O. Suzuki, Appl. Surf. Sci., 2018, 440, 506–513 CrossRef CAS.
- D. Nakajima, T. Kikuchi, S. Natsui and R. O. Suzuki, ECS Electrochem. Lett., 2015, 4, H14–H17 CrossRef CAS.
- R. Kondo, D. Nakajima, T. Kikuchi, S. Natsui and R. O. Suzuki, J. Alloys Compd., 2017, 725, 379–387 CrossRef CAS.
- P. Rong, W. Yang, L. Fu, J. Zhu, D. Li and L. Zhou, Mater. Res. Express, 2017, 4, 065007 CrossRef.
- D. Nakajima, T. Kikuchi, S. Natsui and R. O. Suzuki, Appl. Surf. Sci., 2016, 389, 173–180 CrossRef CAS.
- A. Otten and S. Herminghaus, Langmuir, 2004, 20, 2405–2408 CrossRef CAS PubMed.
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1–8 CrossRef CAS.
- L. Feng, Y. Zhang, J. Xi, Y. Zhu, N. Wang, F. Xia and L. Jiang, Langmuir, 2008, 24, 4114–4119 CrossRef CAS PubMed.
- J. Lomga, P. Varshney, D. Nanda, M. Satapathy, S. S. Mohapatra and A. Kumar, J. Alloys Compd., 2017, 702, 161–170 CrossRef CAS.
- W.-T. Cao, Y.-J. Liu, M.-G. Ma and J.-F. Zhu, Colloids Surf., A, 2017, 529, 18–25 CrossRef CAS.
- X. Yao, Y. Song and L. Jiang, Adv. Mater., 2011, 23, 719–734 CrossRef CAS PubMed.
- F. Wang, C. Liang, M. Yang, C. Fan and X. Zhang, Appl. Therm. Eng., 2015, 75, 1126–1132 CrossRef.
- N. Rungraeng, Y.-C. Cho, S. H. Yoon and S. Jun, J. Food Eng., 2012, 111, 218–224 CrossRef CAS.
- A. Winkleman, G. Gotesman, A. Yoffe and R. Naaman, Nano Lett., 2008, 8, 1241–1245 CrossRef CAS PubMed.
- D. Ebert and B. Bhushan, J. Colloid Interface Sci., 2012, 384, 182–188 CrossRef CAS PubMed.
- X. Hong, X. Gao and L. Jiang, J. Am. Chem. Soc., 2007, 129, 1478–1479 CrossRef CAS PubMed.
- M. Schmitt and F. Heib, in Advances in Contact Angle, Wettability and Adhesion 3, ed. K. L. Mittal, Wiley-Scrivener, Beverly, MA, 2018, section 1, pp. 1–57 Search PubMed.
- A. Marmur, in Contact Angle Wettability and Adhesion 6, ed. K. L. Mittal, CRC Press, Boca Raton, FL, 2009, pp. 3–18 Search PubMed.
- M. Schmitt and F. Heib, J. Chem. Phys., 2013, 139, 134201 CrossRef PubMed.
- M. Schmitt, R. Hempelmann, S. Ingebrandt, W. Munief, D. Durneata, K. Groβ and F. Heib, Int. J. Adhes. Adhes., 2014, 55, 123–131 CrossRef CAS.
- M. Schmitt, J. Grub and F. Heib, J. Colloid Interface Sci., 2015, 447, 248–253 CrossRef CAS PubMed.
- W. Ma, H. Wu, Y. Higaki, H. Otsuka and A. Takahara, Chem. Commun., 2012, 48, 6824–6826 RSC.
- P. Vengatesh and M. A. Kulandainathan, ACS Appl. Mater. Interfaces, 2015, 7, 1516–1526 CrossRef CAS PubMed.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed.
- K. Nakayama, E. Tsuji, Y. Aoki and H. Habazaki, RSC Adv., 2014, 4, 30927–30933 RSC.
- A. Marmur, C. D. Volpe, S. Siboni, A. Amirfazli and J. W. Drelich, Surf. Innovations, 2017, 5, 3–8 CrossRef.
- L. Gao and T. J. McCarthy, Langmuir, 2006, 22, 6234–6237 CrossRef CAS PubMed.
- E. Chibowski, Adv. Colloid Interface Sci., 2003, 103, 149–172 CrossRef CAS PubMed.
- G. E. Thompson, Thin Solid Films, 1997, 297, 192–201 CrossRef CAS.
- G. D. Sulka and W. J. Stępniowski, Electrochim. Acta, 2009, 54, 3683–3691 CrossRef CAS.
- Y. Lai, X. Gao, H. Zhuang, J. Huang, C. Lin and L. Jiang, Adv. Mater., 2009, 21, 3799–3803 CrossRef CAS.
- D. Öner and T. J. McCarthy, Langmuir, 2000, 16, 7777–7782 CrossRef.
- W. Chen, A. Y. Fadeev, M. C. Hsieh, D. Öner, J. Youngblood and T. J. McCarthy, Langmuir, 1999, 15, 3395–3399 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: A movie of the water-sliding behaviour of the anodized specimen is provided. See DOI: 10.1039/c8ra07712f |
| This journal is © The Royal Society of Chemistry 2018 |