Open Access Article
Open Access ArticleDesign, synthesis and fungicidal activity of isothiazole–thiazole derivatives†
Qi-Fan Wu‡
 a,
Bin Zhao‡
a,
Bin Zhao‡ a,
Zhi-Jin Fan*a,
Jia-Bao Zhaoa,
Xiao-Feng Guoa,
Dong-Yan Yang*a,
Nai-Lou Zhanga,
Bin Yua,
Tatiana Kalinina*b and
Tatiana Glukharevab
a,
Zhi-Jin Fan*a,
Jia-Bao Zhaoa,
Xiao-Feng Guoa,
Dong-Yan Yang*a,
Nai-Lou Zhanga,
Bin Yua,
Tatiana Kalinina*b and
Tatiana Glukharevab
aState Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, P. R. China. E-mail: fanzj@nankai.edu.cn; yangdy@nankai.edu.cn; Fax: +86-22-23503620; Tel: +86-23499464
bThe Ural Federal University named after the First President of Russia B. N. Yeltsin, 19 Mira Str., Ekaterinburg 620002, Russia. E-mail: t.a.kalinina@urfu.ru
First published on 26th November 2018
Abstract
3,4-Dichloroisothiazoles can induce systemic acquired resistance (SAR) to enhance plant resistance against a subsequent pathogen attack, and oxathiapiprolin exhibits excellent anti-fungal activity against oomycetes targeting at the oxysterol-binding protein. To discover novel chemicals with systemic acquired resistance and fungicidal activity, 21 novel isothiazole–thiazole derivatives were designed, synthesized and characterized according to the active compound derivatization method. Compound 6u, with EC50 values of 0.046 mg L−1 and 0.20 mg L−1 against Pseudoperonospora cubensis (Berk. et Curt.) Rostov and Phytophthora infestans in vivo, might act at the same target as oxysterol binding protein (PcORP1) of oxathiapiprolin; this result was validated by cross-resistance and molecular docking studies. The expression of the systemic acquired resistance gene pr1 was significantly up-regulated after treating with compound 6u for 24 h (43-fold) and 48 h (122-fold). These results can help the development of isothiazole–thiazole-based novel fungicides.
1 Introduction
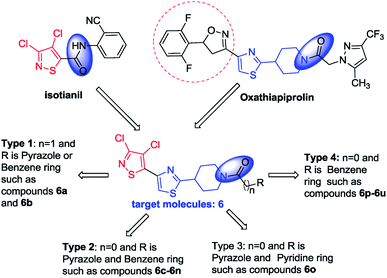
Crops are often infected by various pathogens, which results in yield reduction. Oomycetes are filamentous eukaryotic microorganisms1 that attack a large number of plants2 and animals3 and therefore, they can sometimes threaten agricultural ecosystems. Some pathogens cause humanitarian disasters; for example, the Great Famine was caused by oomycetes attacking potato fields.4 The oomycetes classified as Phytophthora are one of the most serious pathogenic threats all over the world.5 Only Phytophthora infestans (Mont.) de Bary caused the failure of the potato crop protection in 1840s and decreased the population of Ireland nearly by 25%.6Heterocyclic compounds are widely used in novel agrochemical researches and developments.7–10 Thiazoles have attracted the interest of pharmaceutical and agrochemical anti-fungal research since the development of thiabendazole as a fungicide in 1962 by Merck.11 Oxathiapiprolin was developed by DuPont in 2007 as a piperidinyl thiazole isoxazoline fungicide targeting at oxysterol binding protein (PcORP1) against Peronospora belbahrii, Phytophthora parasitica var. Nicotianae (Breda de Hean) Tucker, Phytophthora capsici Leonian and downy mildew.12–16 However, fungicides acting at a single site of action have a high level of resistance risk.12
As a derivative of 1,3-thiazole, isotianil has a wide range of biological activities17–19 including fungicidal, insecticidal, herbicidal and antiviral activities via activating the plant induced systemic resistance and affecting multiple links in the life cycle of pathogens. Particularly, 3,4-dichloroisothiazoles not only have antifungal activity, but also show good systemic acquired resistance.17 Novel fungicide development is one of the important directions and measures for fungicide resistance management.20
On the basis of our former findings,4,5,21–23 to continue our aim of finding novel highly anti-fungal active compounds with novel modes of action and without resistance risks, 4 types of novel target molecules were designed and synthesized for fungicidal activity and systemic acquired resistance evaluation based on PcORP1 as the target and N and S containing five-membered heterocycles as bioactive substructures by the combination of bioactive substructures of plant elicitor isotianil and fungicide oxathiapiprolin according to the active compound derivatization method24 (Fig. 1 and Table 1). The mode of action of the active compound was validated by cross resistance, molecular biology and molecular docking studies.
2 Results and discussion
2.1 Chemistry
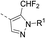
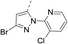
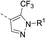
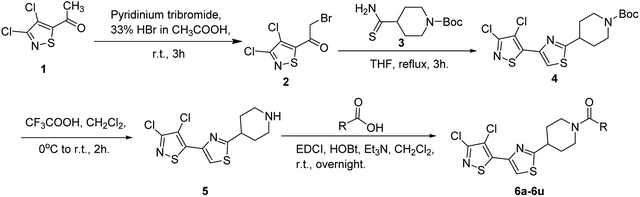
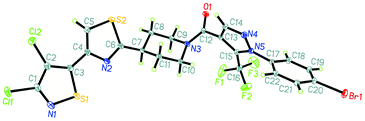
The synthesis route of isothiazole–thiazole derivatives is designed and shown in Scheme 1. Compound 1 was constructed according to reported procedures.17 3,4-Dichloro-N-methoxy-N-methylisothiazole-5-carboxamide was obtained by the reaction between 3,4-dichloroisothiazole-5-carboxylic acid and N,O-dimethylhydroxylamine hydrochloride to produce the Weinreb amide, which was then reacted with methyl magnesium bromide to obtain 1-(3,4-dichloroisothiazol-5-yl)ethan-1-one 1 with a good yield. Compound 2 could be prepared in one step from compound 1 by a substitution reaction with pyridinium tribromide. Compound 4 was synthesized by condensation between intermediate 2 and tert-butyl 4-carbamothioylpiperidine-1-carboxylate 3. After removal of protection group at N-Boc of compound 4under trifluoroacetic acid, intermediate 5 was obtained. By the activation of the corresponding acid using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride and 1-hydroxybenzotriazole and the succeeding condensation with compound 5, isothiazole–thiazole derivatives 6 were effectively afforded. A crystal of the representative compound 6j was obtained from the mixture of dichloromethane and ethyl acetate for X-ray diffraction (Fig. 2).2.2 Compound 6u has the same potent target as oxathiapiprolin
| Fungi | Compd | Regression equation | R2 | EC50/mg L−1 |
|---|---|---|---|---|
| a Azoxystrobin, the data cited from the ref. 21.b Azoxystrobin, the data cited from the ref. 22. | ||||
| A. solani | 6o | y = 4.2714 + 0.7903x | 0.9642 | 8.92 |
| 6s | y = 4.3901 + 0.6928x | 0.9839 | 7.84 | |
| Oxathiapiprolin | y = 2.3920 + 1.0678x | 0.9857 | 296.60 | |
| Azoxystrobina | y = 3.7648 + 0.5446x | 0.9251 | 185.42 | |
| G. zeae | 6a | y = 3.5238 + 1.2423x | 0.9917 | 15.03 |
| 6f | y = 4.2432 + 0.8867x | 0.9828 | 7.67 | |
| 6o | y = 3.8997 + 0.9485x | 0.9858 | 15.60 | |
| Oxathiapiprolin | y = 4.8606 + 0.8936x | 0.9944 | 1.47 | |
| Azoxystrobinb | y = 4.3881 + 0.7278x | 0.9777 | 6.92 | |
| S. sclerotiorum | 6b | y = 6.8128 + 2.7830x | 0.9298 | 0.22 |
| 6c | y = 5.1267 + 0.4377x | 0.9284 | 0.53 | |
| 6l | y = 4.5982 + 0.7369x | 0.9611 | 3.83 | |
| 6r | y = 3.1831 + 1.4723x | 0.9582 | 17.36 | |
| Oxathiapiprolin | y = 3.9644 + 1.3662x | 0.9855 | 5.98 | |
| Azoxystrobina | y = 4.6795 + 0.5283x | 0.8375 | 4.04 | |
Both compounds 6o and 6s showed higher activities, with EC50 values of 8.92 mg L−1 and 7.84 mg L−1, respectively, than oxathiapiprolin (EC50 = 296.60 mg L−1) and azoxystrobin (EC50 = 185.42 mg L−1) against Alternaria solani. The EC50 values of compounds 6b (EC50 = 0.22 mg L−1) and 6c (EC50 = 0.53 mg L−1) were about 1/20 and 1/10, respectively, as compared to that of commercial fungicides oxathiapiprolin (EC50 = 5.98 mg L−1) and azoxystrobin (EC50 = 4.04 mg L−1) against Sclerotinia sclerotiorum.
| Strains | Inhibition ratio (mean ± SD) | ||
|---|---|---|---|
| 6u (10 mg L−1) | Oxathiapiprolin (10 mg L−1) | Oxathiapiprolin (1 mg L−1) | |
| LP3 (wild-type) | 32 ± 3% | 100% | 100% |
| LP3-m (oxathiapiprolin-resistant) | 12 ± 2% | 78 ± 2% | 26 ± 3% |
| LP3-h (oxathiapiprolin-resistant) | 7 ± 2% | 71 ± 5% | 17 ± 5% |
2.3 Results of compounds 6a–6u against oomycetes in vivo
The fungicidal activities of 6a–6u against oomycetes of P. cubensis and P. infestans at 100 mg L−1 in vivo are listed in Table 4. Most of the designed isothiazole–thiazole derivatives displayed excellent in vivo anti-oomycete activity (100%) against P. cubensis and P. infestans at 100 mg L−1, and the EC50 values of the compounds with inhibition over 90% at 100 mg L−1 against P. cubensis (Table 5) and P. infestans (Table 6) in vivo were determined.| Compd | Inhibition rate (%) | |
|---|---|---|
| P. cubensis | P. infestans | |
| 6a | 0 | 0 |
| 6b | 100 | 100 |
| 6c | 100 | 100 |
| 6d | 100 | 100 |
| 6e | 100 | 100 |
| 6f | 30 ± 2 | 0 |
| 6g | 0 | 0 |
| 6h | 100 | 0 |
| 6i | 40 ± 1 | 100 |
| 6j | 0 | 0 |
| 6k | 100 | 100 |
| 6l | 100 | 100 |
| 6m | 100 | 100 |
| 6n | 100 | 100 |
| 6o | 100 | 60 ± 4 |
| 6p | 100 | 100 |
| 6q | 100 | 0 |
| 6r | 100 | 100 |
| 6s | 100 | 100 |
| 6t | 80 ± 4 | 100 |
| 6u | 100 | 100 |
| Isotianil | 100 | 100 |
| Oxathiapiprolin | 100 | 100 |
| Compd | Regression equation | R2 | EC50 (mg L−1) | 95% CIa of EC50 |
|---|---|---|---|---|
| a 95% confidence interval.b Not determined. | ||||
| 6a | —b | — | >100 | — |
| 6b | y = 5.7565 + 0.7424x | 0.9808 | 0.10 | 0.01–0.70 |
| 6c | y = 2.9999 + 3.1494x | 0.9330 | 4.61 | 0.97–22.46 |
| 6d | y = 2.9744 + 3.1505x | 0.9273 | 4.71 | 0.91–23.79 |
| 6e | y = 0.4131 + 4.9556x | 0.9901 | 12.49 | 7.59–22.36 |
| 6f | — | — | >100 | — |
| 6g | — | — | >100 | — |
| 6h | y = 0.4131 + 4.9556x | 0.9901 | 12.49 | 7.59–22.36 |
| 6i | — | — | >100 | — |
| 6j | — | — | >100 | — |
| 6k | y = 4.7655 + 1.5431x | 0.9665 | 1.42 | 0.35–6.39 |
| 6l | y = 4.7479 + 0.8326x | 1.0000 | 2.02 | 1.99–2.18 |
| 6m | y = 5.1520 + 2.2378x | 0.9609 | 0.91 | 0.16–4.94 |
| 6n | y = 4.9881 + 1.0722x | 0.9998 | 1.05 | 0.93–1.24 |
| 6o | y = 0.4131 + 4.9556x | 0.9901 | 12.49 | 7.59–22.36 |
| 6p | y = 7.2490 + 2.3175x | 0.9788 | 0.11 | 0.05–0.24 |
| 6q | y = 0.4710 + 4.9817x | 0.9858 | 13.58 | 7.23–24.05 |
| 6r | y = 3.4658 + 2.9562x | 0.9253 | 3.49 | 0.62–19.46 |
| 6s | y = 4.1499 + 2.9208x | 1.0000 | 2.13 | 2.06–2.11 |
| 6t | — | — | — | — |
| 6u | y = 9.1621 + 3.0757x | 0.9621 | 0.046 | 0.016–0.13 |
| Oxathiapiprolin | y = 8.7940 + 1.6011x | 0.9997 | 0.0046 | 0.0042–0.0051 |
| Isotianil | y = 5.1578 + 5.0000x | 0.9985 | 1.01 | 0.78–1.22 |
| Compd | Regression equation | R2 | EC50 (mg L−1) | 95% CIa of EC50 |
|---|---|---|---|---|
| a 95% confidence interval.b Not determined. | ||||
| 6a | —b | — | >100 | — |
| 6b | y = 5.3634 + 1.9964x | 0.9634 | 0.68 | 0.21–2.10 |
| 6c | y = 3.0649 + 3.1028x | 0.9704 | 4.44 | 1.73–11.03 |
| 6d | y = 0.2101 + 4.8366x | 0.9889 | 13.00 | 7.16–22.02 |
| 6e | y = 0.2101 + 4.8366x | 0.9889 | 13.00 | 7.16–22.02 |
| 6f | — | — | >100 | — |
| 6g | — | — | >100 | — |
| 6h | — | — | >100 | — |
| 6i | y = 3.0513 + 3.1078x | 0.9662 | 4.39 | 1.67–11.19 |
| 6j | — | — | >100 | — |
| 6k | y = 4.7053 + 1.0010x | 0.9969 | 2.14 | 1.61–2.86 |
| 6l | y = 3.2938 + 3.0991x | 0.9770 | 3.66 | 1.76–8.40 |
| 6m | y = 5.1537 + 1.0510x | 0.9921 | 0.74 | 0.48–1.25 |
| 6n | y = 5.2728 + 1.0510x | 0.9352 | 0.75 | 0.17–3.47 |
| 6o | — | — | >100 | — |
| 6p | y = 6.3442 + 2.9208x | 0.9167 | 0.38 | 0.06–2.31 |
| 6q | — | — | >100 | — |
| 6r | y = 3.0513 + 3.1078x | 0.9662 | 4.39 | 1.67–11.19 |
| 6s | y = 5.1960 + 1.7904x | 0.9555 | 0.81 | 0.26–2.67 |
| 6t | y = 4.7813 + 2.0405x | 0.9050 | 1.32 | 0.22–7.80 |
| 6u | y = 6.8798 + 2.6878x | 0.9651 | 0.20 | 0.06–0.75 |
| Oxathiapiprolin | — | — | <0.10 | — |
| Isotianil | y = 5.4639 + 2.0486x | 0.9749 | 0.61 | 0.23–1.71 |
All compounds except 6a, 6f, 6g, 6i, 6j, 6o and 6q exhibited good to excellent inhibitory activity against P. cubensis and P. infestans with EC50 values of 0.046–12.49 mg L−1 and 0.20–13.00 mg L−1, respectively. For P. cubensis, compounds 6k, 6l, 6n and 6s displayed the same level of fungicidal activity as that of isotianil (EC50 = 1.01 mg L−1) with EC50 from 1.05 mg L−1 to 2.13 mg L−1, whereas compounds 6b, 6m, 6p and 6u with EC50 of less than 1.00 mg L−1 exhibited higher activity than the positive control of isotianil against P. cubensis. The anti-oomycete activity of compound 6u (EC50 = 0.046 mg L−1) was 10 times less effective than that of the positive control oxathiapiprolin (EC50 = 0.0046 mg L−1) against P. cubensis; however, it was 20 times better than that of the positive control isotianil (EC50 = 1.01 mg L−1). It is worth noting that compounds 6b, 6m, 6p and 6u also displayed excellent inhibitory activities against P. infestans with EC50 values less than 1.00 mg L−1. Moreover, EC50 of compound 6n was 0.75 mg L−1. Ultimately, compound 6u exhibited the best fungicidal activity against P. infestans with EC50 of 0.20 mg L−1 among all the isothiazole–thiazoles designed and synthesized in this study.
2.4 Compound 6u can activate SAR in plants
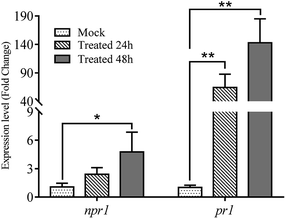
“*” and “**” indicate significant difference between treated and mock at P < 0.05 and P < 0.01, respectively.
The expressions of npr1 and pr1 were analyzed in A. thaliana. The pr1 expression was significantly up-regulated by 64-fold and 143-fold when treated with 6u for 24 hours and 48 hours, respectively (Fig. 4). The npr1 expression was also significantly up-regulated by 4.7-fold after 48 hours of treatment, but the change was not very significant when treated for 24 hours. Isotianil, discovered by Bayer CropScience AG in 1997 and developed jointly with Sumitomo Chemical Co., Ltd, is used as a crop protectant against rice blast and rice leaf blight. The difference in gene expressions identified after isotianil treatment for 24 hours and 48 hours in Oryza sativa by microarray analysis showed that 17 PR genes including pr1a, pbz, npr1 and PR involved in antifungal activities such as chitinase and β-glucanase were upregulated.28 Based on these results, it is possible that the activation of pr1 can induce SAR and increase the fungicidal activity of compound 6u in vivo.
3 Experimental
3.1 General information
All solvents were of analytical grade unless otherwise noted. 1-Substituted phenyl-5-trifluoromethyl-4-pyrazole carboxylic acid and substituted phenyl-5-difluoromethyl-4-pyrazole carboxylic acid were provided by Jia-xing Huang of China Agricultural University (Beijing, China). Melting points (temperature uncorrected) were recorded on an X-4 binocular microscope. 1H and 13C Nuclear Magnetic Resonance (NMR) spectra in CDCl3 or dimethyl sulfoxide-d6 were recorded on a Bruker AV400 spectrometer with tetramethylsilane as the internal standard. Mass spectrometry was conducted using Agilent 6520 Q-TOF LC/MS. The single crystal diffraction was carried out on a Rigaku 007HF XtaLAB P200 diffractometer. Fungicidal activities in vitro and in vivo were tested at Nankai University (Tianjin, China) and Shenyang Research Institute of Chemical Industry (Shenyang, China), respectively.3.2 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 to obtain 2 as a white solid (15.3 mmol, 100%). Mp 52–53 °C; 1H NMR (400 MHz, CDCl3) δ 4.47 (d, J = 2.9 Hz, 2H, CH2). HRMS (ESI) [M − H]− calcd For C5H2BrCl2NOS (M − H)−: 271.8418, found: 271.8344
50 to obtain 2 as a white solid (15.3 mmol, 100%). Mp 52–53 °C; 1H NMR (400 MHz, CDCl3) δ 4.47 (d, J = 2.9 Hz, 2H, CH2). HRMS (ESI) [M − H]− calcd For C5H2BrCl2NOS (M − H)−: 271.8418, found: 271.8344![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 to obtain 4 as a white solid (3.48 mmol, 42%). Mp 153–155 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H, thiazole-CH), 4.21 (t, J = 10.7 Hz, 2H, piperidine-CH2), 3.19 (m, 1H, piperidine-CH), 2.93 (t, J = 12.1 Hz, 2H, piperidine-CH2), 2.13 (m, 2H, piperidine-CH2), 1.77 (m, 2H, piperidine-CH2), 1.46 (s, 9H, CH3). HRMS (ESI) (M + H)+ calcd for C16H19Cl2N3O2S2: 420.0296, found: 420.0345; HRMS (ESI) (M + Na)+ calcd for C16H19Cl2N3O2S2: 442.0296, found: 442.0183.
16 to obtain 4 as a white solid (3.48 mmol, 42%). Mp 153–155 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H, thiazole-CH), 4.21 (t, J = 10.7 Hz, 2H, piperidine-CH2), 3.19 (m, 1H, piperidine-CH), 2.93 (t, J = 12.1 Hz, 2H, piperidine-CH2), 2.13 (m, 2H, piperidine-CH2), 1.77 (m, 2H, piperidine-CH2), 1.46 (s, 9H, CH3). HRMS (ESI) (M + H)+ calcd for C16H19Cl2N3O2S2: 420.0296, found: 420.0345; HRMS (ESI) (M + Na)+ calcd for C16H19Cl2N3O2S2: 442.0296, found: 442.0183.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.001 to obtain 5 as a white solid (3.19 mmol, 92%). Mp 97–99 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H, thiazole-CH), 5.31 (s, 1H, NH), 3.22 (t, J = 11.6 Hz, 2H, piperidine-CH2), 3.17 (s, 1H, piperidine-CH), 2.78 (t, J = 11.7 Hz, 2H, piperidine-CH2), 2.14 (m, 2H, piperidine-CH2), 1.76 (m, 2H, piperidine-CH2). HRMS (ESI) [M + H]+ calcd for C11H11Cl2N3S2: 319.9771, found: 319.9850.
0.001 to obtain 5 as a white solid (3.19 mmol, 92%). Mp 97–99 °C; 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H, thiazole-CH), 5.31 (s, 1H, NH), 3.22 (t, J = 11.6 Hz, 2H, piperidine-CH2), 3.17 (s, 1H, piperidine-CH), 2.78 (t, J = 11.7 Hz, 2H, piperidine-CH2), 2.14 (m, 2H, piperidine-CH2), 1.76 (m, 2H, piperidine-CH2). HRMS (ESI) [M + H]+ calcd for C11H11Cl2N3S2: 319.9771, found: 319.9850.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–1
9–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to obtain target compound 6a.
3 to obtain target compound 6a.Compounds 6b to 6u were synthesized according to a similar procedure as that used for 6a by changing the raw materials, as shown in Scheme 1.
Data for 6a. White solid, mp > 200 °C, yield 60%, 1H NMR (400 MHz, DMSO) δ 8.08 (s, 1H, thiazole-CH), 6.35 (s, 1H, pyrazole-CH), 5.01 (s, 2H, CH2), 4.58 (d, J = 13.3 Hz, 1H, piperidine-CH2), 4.08 (d, J = 12.5 Hz, 1H, piperidine-CH2), 3.33 (s, 2H, piperidine-CH2), 2.93 (m, 1H, piperidine-CH), 2.34 (s, 3H, CH3), 2.22 (m, 2H, piperidine-CH2), 1.91–1.68 (m, 2H, piperidine-CH2). HRMS (ESI) [M + H]+ calcd for C18H16Cl2F3N5OS2: 510.0125, found: 510.0203. Anal. calcd for C18H16Cl2F3N5OS2: C, 42.36; H, 3.16; N, 13.72. Found: C, 42.15; H, 3.69; N, 13.74.
Data for 6c. White solid, mp 125–127 °C, yield 95%, 1H NMR (400 MHz, CDCl3) δ 8.10 (s, 1H, thiazole-CH), 7.78 (s, 1H, pyrazole-CH), 7.54 (s, 5H, Ph-H), 6.93 (t, J = 53.0 Hz, 1H, CHF2), 4.76 (s, 1H, piperidine-CH2), 4.24 (d, J = 64.7 Hz, 1H, piperidine-CH2), 3.38 (s, 2H, piperidine-CH2), 3.14 (s, 1H, piperidine-CH), 2.27 (m, 2H, piperidine-CH2), 1.91 (m, 2H, piperidine-CH2). HRMS (ESI) [M + H]+ calcd for C22H17Cl2F2N5OS2: 540.0220, found: 540.0299. Anal. calcd for C22H17Cl2F2N5OS2: C, 48.89; H, 3.17; N, 12.96. Found: C, 48.23; H, 3.30; N, 13.08.
Data for 6g. White solid, mp 181–183 °C, yield 92%, 1H NMR (400 MHz, CDCl3) δ 8.09 (s, 1H, thiazole-CH), 7.76 (s, 1H, pyrazole-CH), 7.50 (d, J = 8.2 Hz, 5H, Ph-H), 4.79 (d, J = 12.8 Hz, 1H, piperidine-CH2), 3.91 (d, J = 12.7 Hz, 1H, piperidine-CH2), 3.34 (t, J = 14.6 Hz, 2H, piperidine-CH2), 3.10 (m, 1H, piperidine-CH), 2.26 (m, 2H, piperidine-CH2), 1.87 (m, 2H, piperidine-CH2). HRMS (ESI) [M + H]+ calcd for C22H16Cl2F3N5OS2: 558.0125, found: 558.0190. Anal. calcd for C22H16Cl2F3N5OS2: C, 47.32; H, 2.89; N, 12.54. Found: C, 47.14; H, 3.41; N, 12.88.
Data for 6k. White solid, mp 167–169 °C, yield 90%, 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 74.7 Hz, 1H, thiazole-CH, pyrazole-CH), 7.50–7.04 (m, 5H, Ph-H), 4.60 (s, 1H, piperidine-CH2), 3.59 (d, J = 64.5 Hz, 1H, piperidine-CH2), 3.12 (t, J = 64.9 Hz, 2H, piperidine-CH2), 2.99–2.75 (m, 1H, piperidine-CH), 2.12 (m, 2H, piperidine-CH2), 1.71 (m, 2H, piperidine-CH2). HRMS (ESI) [M + H]+ calcd for C22H16Cl2F3N5OS2: 591.9736, found: 591.9812. Anal. calcd for C22H16Cl2F3N5OS2: C, 44.57; H, 2.55; N, 11.81. Found: C, 44.42; H, 2.68; N, 12.28.
Data for 6p. White crystals, mp 163–164 °C, yield 100%, 1H NMR (400 MHz, CDCl3) δ 7.95 (s, 1H, thiazole-CH), 7.33 (s, 5H, Ph-H), 4.68 (s, 1H, piperidine-CH2), 3.81 (s, 1H, piperidine-CH2), 3.23 (m, 1H, piperidine-CH), 3.04 (t, J = 55.1 Hz, 2H, piperidine-CH2), 2.10 (m, 2H, piperidine-CH2), 1.74 (m, 2H, piperidine-CH2). HRMS (ESI) [M + H]+ calcd for C18H15Cl2N3OS2: 424.0034, found: 424.0109. Anal. calcd for C18H15Cl2N3OS2: C, 50.95; H, 3.56; N, 9.90. Found: C, 50.33; H, 3.36; N, 9.87.
3.3 In vitro antifungal activity test
The fungicidal activities of title compounds against Cercospora arachidicola (C. A), Alternaria solani (A. S), Botrytis cinereal (B. C), Gibberella zeae (G. Z), Physalospora piricola (P. P), Sclerotinia sclerotiorum (S. S), Rhizoctonia cerealis (R. C) and Pellicularia sasakii (P. S) were tested at 50 mg L−1 in vitro by the mycelium growth rate method according to our previous publications.30,31 Commercial fungicides oxathiapiprolin, isotianil and azoxystrobin were chosen as positive controls. The EC50 values of the compounds with fungi growth inhibition greater than 70% at 50 mg L−1 were measured.3.4 Oomycete sensitivity in wild-type and oxathiapiprolin-resistant strains of P. capsici
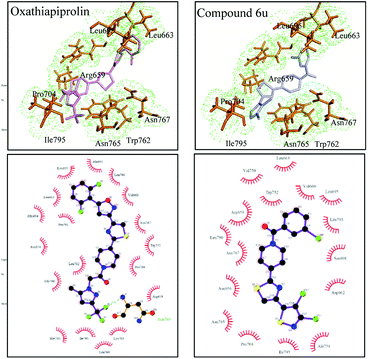
The P. capsici strains of the oxathiapiprolin-resistant mutants LP3-m, LP3-h and wild-type LP3 (ref. 32) were used for the effectiveness tests of compound 6u and oxathiapiprolin by measuring their colony diameters after dark-incubation at 25 °C for 5 days. Each strain and concentration were measured in three plates and the experiments were performed for three times.3.5 Molecular modelling studies and docking analysis of PsORP1
For molecular modelling, the structure of PsORP1 (ref. 32) (Protein ID: 558498) in Phytophthora capsici was generated using the homology model application within the YASARA program33 with default parameters. The binding center of PsORP1 was identified by aligning to the template structure in Autodock Tools.34,35 The coordinate of the binding center in PsORP1 was identified by the model structures of 4B2Z and 5H2D. The structures of the ligands were optimized with the ligand minimization protocol. The molecular docking analysis was performed by Autodock Tools and ten random conformations were generated for each ligand. The rest of the parameters were set to default values. The optimal structure of the complex was selected based on visual inspection and the docking score. The structures of the complexes were shown by Pymol36 and LigPlot.373.6 In vivo antifungal activity test
The protective activities in potted plants of the title compounds were determined by the procedures described below:31 compounds 6a–6u (10.0 mg) and positive controls oxathiapiprolin and isotianil were dissolved in 0.5 mL DMF solutions. Then, they were diluted using 95.5 mL of distilled water (containing 0.1% Tween 80) to obtain the working solutions of 100 mg L−1. The working solutions were sprayed on to the host plant using a sprayer when the plants were grown to 1 to 3 leaf stages. After 24 h, the leaves treated by working solution were inoculated by the fungi of P. cubensis and P. infestans; 7 days later, the inhibitory activities in vivo of all the compounds were assessed. Two concentrations of 10 mg L−1 and 1 mg L−1 were further tested for the compounds with activity over 90% against P. cubensis at 100 mg L−1. Then, the compounds with more than 90% inhibition against P. cubensis at 1 mg L−1 were investigated at 0.1 mg L−1, 0.01 mg L−1 and 0.001 mg L−1. The compounds with 100% inhibition rate against P. infestans were tested for 3 concentrations of 10 mg L−1, 1 mg L−1 and 0.1 mg L−1. An inhibition rate of 100% represents complete control of the fungi growth and an inhibition rate of 0% represents no control of the fungi growth.3.7 RNA extraction and Q-PCR analysis of the expression of defence genes in the salicylic acid pathway
Based on the difference between anti-oomycete activities in vivo and in vitro, we proposed that compound 6u might be able to induce systemic acquired resistance of plants. The RNAs of Arabidopsis thaliana, which was treated with 6u (10 mg L−1) and DMF, were isolated by using E.Z.N.A.® Plant RNA Kit (Omega, USA) and the mRNA was transcribed into cDNA reversely. Q-PCR was performed using the 2−ΔΔCT method38 with TransStart Top Green Q-PCR Super Mix (K31102, TransStart, China). The expressions of defense genes pr1 and npr1 in the salicylic acid pathway were analyzed and the primers used for Q-PCR are shown in Table 7.| Gene ID | Gene name | Primer-F | Primer-R |
|---|---|---|---|
| AT3G41768 | 18s | tgtttgatggtaactactactc | gaatgatgcctcgccagcacaga |
| AT1G64280 | npr1 | acgaagagaacatcaccggg | cgggaagaatcgtttcccga |
| AT2G14610 | pr1 | attttactggctattctcgatt | agttgcctcttagttgttctgcg |
4 Conclusions
A series of novel isothiazole–thiazole derivatives were designed, synthesized and rationally characterized. The novel compounds were screened for antifungal activity in vitro and anti-oomycete activity in vivo. Compound 6u exhibited excellent anti-oomycete activity in vivo with EC50 values of 0.046 mg L−1 and 0.27 mg L−1 against P. cubensis and P. infestans, respectively. Although 6u was not as effective as oxathiapiprolin, it was much better than isotianil, a compound which can induce plant systemic acquired resistance. Therefore, isothiazole–thiazole derivatives are worthy of further research. Since the docking experiments indicated that oxathiapiprolin can form a hydrogen bond with Asn 765 of PsORP1, analogues with appropriately placed N–H, O–H and F–H units in a target molecule will be designed for further improvement of anti-fungal activity of compound 6u.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31571991 and No. 31872007), the Tianjin Natural Science Foundation (No. 18JCZDJC33500), the China Postdoctoral Science Foundation (No. 2017M611156) and the International Science & Technology Cooperation Program of China (No. 2014DFR41030). The authors also thank Dr Jiaxing Huang for providing pyrazole compounds (synthesized in the Key Technologies R&D program of China, 2015BAK45B01, CAU) for model reaction. The authors are grateful to the research team of Professor Xi-Li Liu at CAU for their help of determination of the active compounds against oxathiapiprolin-resistant fungi. Kalinina T. A. thanks the Russian Foundation for Basic Research (Grant No. 18-316-20018).Notes and references
- M. Larousse and E. Galiana, PLoS Pathog., 2017, 13, e1006028 CrossRef PubMed.
- S. Kamoun, O. Furzer, J. D. G. Jones, H. S. Judelson, G. S. Ali, R. J. D. Dalio, S. G. Roy, L. Schena, A. Zambounis and F. Panabières, Mol. Plant Pathol., 2015, 16, 413–434 CrossRef PubMed.
- P. V. West and G. W. Beakes, Fungal Biol., 2014, 118, 525–526 CrossRef PubMed.
- Y. J. Zhu, J. Q. Huo, Z. J. Fan, Q. F. Wu, X. Li, S. Zhou, L. X. Xiong, T. Kalinina and T. Glukhareva, Org. Biomol. Chem., 2018, 16, 1163–1166 RSC.
- Z. J. Fan, Z. K. Yang, H. K. Zhang, M. Na, H. Wang, C. Fei, X. Zuo, Q. X. Zheng and H. B. Song, J. Agric. Food Chem., 2010, 58, 2630–2636 CrossRef CAS PubMed.
- W. Fry, Mol. Plant Pathol., 2008, 9, 385–402 CrossRef PubMed.
- M. A. Castro, A. M. Gamito, V. Tangarife-Castano, V. Roa-Linares, M. D. C. J. Ma, A. C. Mesa-Arango, L. Betancur-Galvis, A. M. Francesch and A. S. Feliciano, RSC Adv., 2015, 46, 1244–1261 RSC.
- B. L. Wang, H. W. Zhu, Z. M. Li, L. Z. Wang, X. Zhang, L. X. Xiong and H. B. Song, Pest Manage. Sci., 2018, 74, 726–736 CrossRef CAS PubMed.
- S. K. Li, K. U. Huang, B. A. Cao, J. W. Zhang, W. J. Wu and X. M. Zhang, Angew. Chem., Int. Ed., 2012, 51, 8573–8576 CrossRef CAS PubMed.
- L. Zhang, W. Li, T. F. Xiao, Z. H. Song, R. Csuk and S. K. Li, J. Agric. Food Chem., 2018, 66, 8957–8965 CrossRef CAS PubMed.
- H. D. Brown, A. R. Matzuk, I. R. Ilves, L. H. Peterson, S. A. Harris, L. H. Sarett, J. R. Egerton, J. J. Yakstis, W. C. Campbell and A. C. Cuckler, J. Am. Chem. Soc., 1961, 83, 174–175 Search PubMed.
- R. J. Pasteris and M. A. Hanagan, US Pat., WO/2013/0261154, 2014.
- J. Q. Miao, X. Dong, D. Lin, Q. S. Wang, P. F. Liu, F. R. Chen, Y. X. Du and X. L. Liu, Pest Manage. Sci., 2016, 72, 1572–1577 CrossRef CAS PubMed.
- P. Ji, A. S. Csinos, L. L. Hickman and U. Hargett, Plant Dis., 2014, 98, 1551–1554 CrossRef CAS.
- P. Ji and A. S. Csinos, Ann. Appl. Biol., 2015, 166, 229–235 CrossRef CAS.
- Y. Cohen, PLoS One, 2015, 10, e0140015 CrossRef PubMed.
- L. Chen, X. F. Guo, Z. J. Fan, N. L. Zhang, Y. J. Zhu, Z. M. Zhang, I. Khazhieva, M. Y. Yurievich, N. P. Belskaya and V. A. Bakulev, RSC Adv., 2017, 7, 3145–3151 RSC.
- J. G. Samaritoni, J. M. Babcock, M. L. Schlenz and G. W. Johnson, J. Agric. Food Chem., 1999, 47, 3381–3388 CrossRef CAS PubMed.
- X. Y. Chen, L. Y. Dai, Y. D. Li, W. T. Mao, Z. Fang, J. J. Li, D. Wang, K. Tatiana and Z. J. Fan, Chin. J. Pestic. Sci., 2013, 15, 140–144 CAS.
- D. Worrall, G. H. Holroyd, J. P. Moore, M. Glowacz, P. Croft, J. E. Taylor, N. D. Paul and M. R. Roberts, New Phytol., 2012, 193, 770–778 CrossRef CAS PubMed.
- L. Chen, B. Zhao, Z. J. Fan, X. M. Liu, Q. F. Wu, H. P. Li and H. X. Wang, J. Agric. Food Chem., 2018, 66, 7319–7327 CrossRef CAS PubMed.
- L. Chen, Y. J. Zhu, Z. J. Fan, X. F. Guo, Z. M. Zhang, J. H. Xu, Y. Q. Song, Y. Y. Morzherin, N. P. Belskaya and V. A. Bakulev, J. Agric. Food Chem., 2017, 65, 745–751 CrossRef CAS PubMed.
- B. Zhao, S. J. Fan, Z. J. Fan, H. X. Wang, N. L. Zhang, X. F. Guo, D. Y. Yang, Q. F. Wu, B. Yu and S. Zhou, J. Agric. Food Chem., 2018, 66, 12439–12452 CrossRef CAS PubMed.
- A. Y. Guan, C. L. Liu, X. P. Yang and M. Dekeyser, Chem. Rev., 2014, 114, 7079–7107 CrossRef CAS PubMed.
- F. Sun, P. Y. Zhang, M. R. Guo, W. Q. Yu and K. S. Chen, Food Chem., 2013, 138, 539–546 CrossRef CAS PubMed.
- L. C. Van Loon, M. Rep and C. M. Pieterse, Annu. Rev. Phytopathol., 2006, 44, 135–162 CrossRef CAS PubMed.
- Y. Wu, D. Zhang, J. Y. Chu, P. Boyle, Y. Wang, I. D. Brindle, L. V. De and C. Després, Cell Rep., 2012, 1, 639–647 CrossRef CAS PubMed.
- V. Toquin, C. Sirven, L. Assmann and H. Sawada, in Modern Crop Protection Compounds, 2012, ed. W. Krämer, U. Schirmer, P. Jeschke and M. Witschel, Wiley-VCH, Weinheim, Germany, pp. 739–748 Search PubMed.
- H. Kim, S. J. Cho, M. Yoo, S. K. Kang, K. R. Kim, H. H. Lee, J. S. Song, S. D. Rhee, W. H. Jung and J. H. Ahn, Bioorg. Med. Chem. Lett., 2017, 27, 5213–5220 CrossRef CAS PubMed.
- Z. J. Fan, Z. G. Shi, H. K. Zhang, X. F. Liu, L. L. Bao, L. Ma, X. Zuo, Q. X. Zheng and N. Mi, J. Agric. Food Chem., 2009, 57, 4279–4286 CrossRef CAS PubMed.
- F. Y. Li, X. F. Guo, Z. J. Fan, Y. Q. Zhang, G. N. Zong, X. L. Qian, L. Y. Ma, L. Chen, Y. J. Zhu and K. Tatiana, Chin. Chem. Lett., 2015, 26, 1315–1318 CrossRef CAS.
- J. Q. Miao, C. Meng, D. Xue, L. Li, L. Dong, C. Zhang, Z. L. Pang and X. L. Liu, Front. Microbiol., 2016, 7, 615 Search PubMed.
- H. Land and M. S. Humble, Methods Mol. Biol., 2018, 1685, 43–67 CrossRef PubMed.
- E. D. Muzio, D. Toti and F. Polticelli, J. Comput.-Aided Mol. Des., 2017, 31, 1–6 CrossRef PubMed.
- G. M. Morris, D. S. Goodsell, R. Huey and A. J. Olson, J. Mol. Recognit., 1996, 10, 293–304 CAS.
- D. Seeliger and B. L. D. Groot, J. Comput.-Aided Mol. Des., 2010, 24, 417–422 CrossRef CAS PubMed.
- A. C. Wallace, R. A. Laskowski and J. M. Thornton, Protein Eng., 1995, 8, 127–134 CrossRef CAS PubMed.
- K. J. Livak and T. D. Schmittgen, Methods, 2001, 25, 402–408 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystal data of 6j (CCDC 1867013), antifungal activities of target compounds in vivo and vitro and the physico-chemical data of the title compound. CCDC 1867013. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra07619g |
| ‡ Qifan Wu and Bin Zhao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |