Open Access Article
Open Access ArticleNanocharacterization of liposomes for the encapsulation of water soluble compounds from Cordyceps sinensis CS1197 by a supercritical gas anti-solvent technique†
G. M. Shashidhar*a and
B. Manohar b
b
aSarda Gums & Chemicals, Pali–Marwar – 306401, India. E-mail: shashigalaxy@gmail.com; Fax: +91 2932 281324
bDepartment of Food Engineering, CSIR-CFTRI, Mysore, India
First published on 9th October 2018
Abstract
Nano-liposomes were designed for the sustained release of water soluble compounds from C. sinensis CS1197 using a supercritical gas anti-solvent (SC-GAS) method at various pressures, temperatures and Tween 80 concentrations. The SC-GAS method was compared to the Bangham method of liposome production in terms of mean diameter, coefficient of uniformity (Cu), encapsulation efficiency, morphology, viscosity and actual energy required for liposome formation. Liposome production via the SC-GAS method under optimized formulation conditions, i.e., 180 bar; 50 °C; 0.75% Tween 80; and a depressurization rate of 25 bar min−1, yielded nano-liposomes exhibiting the lowest Cu value (1.10 ± 0.012) with a mean diameter of 0.072 ± 0.002 μm and better encapsulation efficiencies of 75.48 ± 2.5, 74.9 ± 2.1 and 70.23 ± 2.9% for adenosine, cordycepin and polysaccharides, respectively. Nano-liposomes were characterized using FTIR, XRD, DSC and TGA techniques. The stability indices and viscosities of the prepared liposome suspensions indicated good stability of up to 2 months and near-Newtonian behavior. The in vitro release of CS1197 water soluble compounds exhibited biphasic and sustained release patterns.
Introduction
Cordyceps sinensis (CS) is a highly valued traditional Chinese medicine with various health benefits. C. sinensis is an excellent nourishment option for the kidneys, lungs and liver and is an effective aphrodisiac. Water soluble compounds, such as nucleosides and polysaccharides, from C. sinensis possess unbelievable pharmacological properties.1,2 C. sinensis water extract has been effectively used to treat various pathological conditions and has also been used in various functional food preparations. C. sinensis based therapies may face difficulties, such as a low therapeutic effect, due to their polar nature, which may cause increased intracellular absorption. Nucleosides in C. sinensis water extract are small molecules (<500 D), which are affected by rapid clearance and suboptimal biodistribution. To address current challenging tasks, like the development of sustained and targeted drugs or active principles, the development of nano-carriers with the best information from nano-science is a sound potential approach and is a major focus of current research.Among the available nano-carriers, nano-liposomes have attracted much attention as potential carriers of drugs.3 The idea of nano-processing C. sinensis based active ingredients for the development of smart drug delivery systems, like those involving liposomes, has become an emerging trend to achieve targeted and sustained release. This is because liposomes are potent drug delivery systems because of their ability to meet several requirements, such as the suitable stability of active molecules, yield and efficiency of drug encapsulation, reproducibility of the micro/nano-particle quality and the drug release profile, and residual levels of organic solvent in the particles. Basically, liposomes are composed of one or more bilayers of amphipathic molecules (e.g., phospholipids) surrounded by an aqueous continuous phase. The major advantage of liposomal encapsulation is that both hydrophilic and hydrophobic molecules can be intercalated.4 Moreover the surfaces of liposomes offer flexibility for any modifications to achieve the site specific unloading of active principles and/or drugs.
Many methods have been developed for liposome production.5 The supercritical method is a novel approach through which liposomes can be made in a single step by making use of supercritical carbon dioxide (SCCO2).6 Zarena et al. (2011) reviewed variants of the method to produce submicron- and nano-particles using SCCO2, such as: the rapid expansion of supercritical solutions (RESS); the gas anti-solvent (GAS) method; the precipitation with compressed anti-solvent process (PCA); solution enhanced dispersion via supercritical fluids (SEDS); and the particles from gas-saturated solutions (PGSS) method.7 Further, GAS is a more universal process and offers greater flexibility than the other variants because the compounds to be encapsulated do not need to be dissolved in SCCO2. Both hydrophilic and hydrophobic compounds can be entrapped in lipid vesicles. Kadimi et al. (2007) claimed an intercalation efficiency (IE) of 20% for amphotericin B and, in a similar study, an IE of 28% for seabuckthorn leaf extract with a narrow range of size distribution (0.48–1.07 μm), using a SCCO2 method.8,9 Liposome preparation in batch mode via a modified supercritical fluid method improved particle sizes in the range of 265 and 214 nm.10,11 Further efforts have been made to decrease the particle size to 146 nm, with the superior characteristics of liposomes over traditional methods, and a similar study proposed the production of liposomes in a continuous mode using a supercritical method.12,13
Preliminary attempts to design liposomes using the GAS method8,9 have been successful in the submicron range (<1 μm) in the author's lab. To the best of our knowledge, this is the first study reporting the fed-batch mode development of nano-sized liposomes for the entrapment of water soluble compounds from C. sinensis CS1197. The major objective of the current study is to optimize the formulation variables, i.e., pressure, temperature, and Tween 80 concentration, to design nano-liposomes via a SC GAS method. The nano-liposomes produced via the SC method were well characterized using FTIR, DSC, TGA and XRD techniques. Liposomes prepared with the SC method were compared to those obtained with the Bangham method.
Experimental
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) in the extract
1 w/w) in the extract![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liposomal content ratio of 1
liposomal content ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w). The liposomal contents were dissolved using a solvent mixture of chloroform and ethanol (3
10 (w/w). The liposomal contents were dissolved using a solvent mixture of chloroform and ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at a ratio of 1
1 v/v) at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/v) and loaded into a 500 ml high pressure reactor vessel (Berghof Autoclave, HPR, Germany). Initially, experiments were carried out in batch mode to optimize a favorable operating range for pressure, temperature and surfactant content. The experimental process was optimized via tuning the process parameters, such as pressure, temperature and Tween 80 concentration, between 50 and 200 bar, 40 and 65 °C, and 0 and 3%, respectively.
3 (w/v) and loaded into a 500 ml high pressure reactor vessel (Berghof Autoclave, HPR, Germany). Initially, experiments were carried out in batch mode to optimize a favorable operating range for pressure, temperature and surfactant content. The experimental process was optimized via tuning the process parameters, such as pressure, temperature and Tween 80 concentration, between 50 and 200 bar, 40 and 65 °C, and 0 and 3%, respectively.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at a ratio of 1
1, v/v) at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v). The organic solvents were removed under reduced pressure in a rotary evaporator at 65 °C (above the lipid-transition temperature). After 1 h of equilibration, a phosphate buffer solution (PBS) (pH 7.2)/Tween 80 (0.75%, w/v) solution was introduced under vacuum and hydrated for 2 h for better encapsulation.9
20 (w/v). The organic solvents were removed under reduced pressure in a rotary evaporator at 65 °C (above the lipid-transition temperature). After 1 h of equilibration, a phosphate buffer solution (PBS) (pH 7.2)/Tween 80 (0.75%, w/v) solution was introduced under vacuum and hydrated for 2 h for better encapsulation.9Characterization of CS liposomes
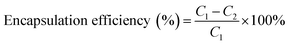 | (1) |
Morphology of CS liposomes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (7
methanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v), followed by the addition of 50 μl of both 10 mM xylenol orange solution and ferric chloride solution. After incubation for 5 min in the dark, the absorbance of the mixture was taken at 560 nm using a UV/vis spectrophotometer (UV-1800, Shimadzu, Japan). The standard curve is plotted from a series of Fe(III) standard solutions. PVs were expressed as milli-equivalents (meq) O2/kg liposome:19
3 v/v), followed by the addition of 50 μl of both 10 mM xylenol orange solution and ferric chloride solution. After incubation for 5 min in the dark, the absorbance of the mixture was taken at 560 nm using a UV/vis spectrophotometer (UV-1800, Shimadzu, Japan). The standard curve is plotted from a series of Fe(III) standard solutions. PVs were expressed as milli-equivalents (meq) O2/kg liposome:19
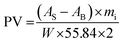 | (2) |
(1) ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C = ln C = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0 − K1t (first order model) C0 − K1t (first order model)
| (3) |
| (2) Q = K2t1/2 (Higuchi model) | (4) |
Results and discussion
Preparation of nano-sized CS liposomes via the SC-GAS method
The phenomena involved in liposome formation using the SCCO2-gas anti-solvent technique have been illustrated earlier.5 The basic principle of the GAS method involves the saturation of lipid components with SCCO2, reducing solubility in organic solvents and resulting in precipitation. The lipid drying step was optimized at 15 min, during which lipid saturation with SCCO2 facilitates the rapid diffusion of CO2, with the attainment of an expanded lipid bilayer with the complete removal of remnant solvent. Hydration under pressure and equilibration for 1 h yields LUVs with a bimodal particle size distribution. The depressurization of the SC liquid to reduce the large unilamellar vesicles (LUVs) to small unilamellar vesicles (SUVs) on the nano-scale is an essential step in the GAS technique. A slow depressurization rate (25 bar min−1) offers better control over particle size and distribution, as observed in a previous study.5 Similar observations were made by other authors.20,21 The importance of the depressurization of the SC liposome suspension near the lipid transition temperature is discussed later.Effects of pressure
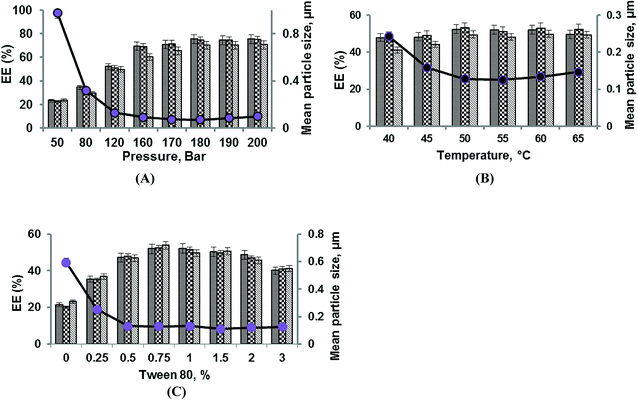
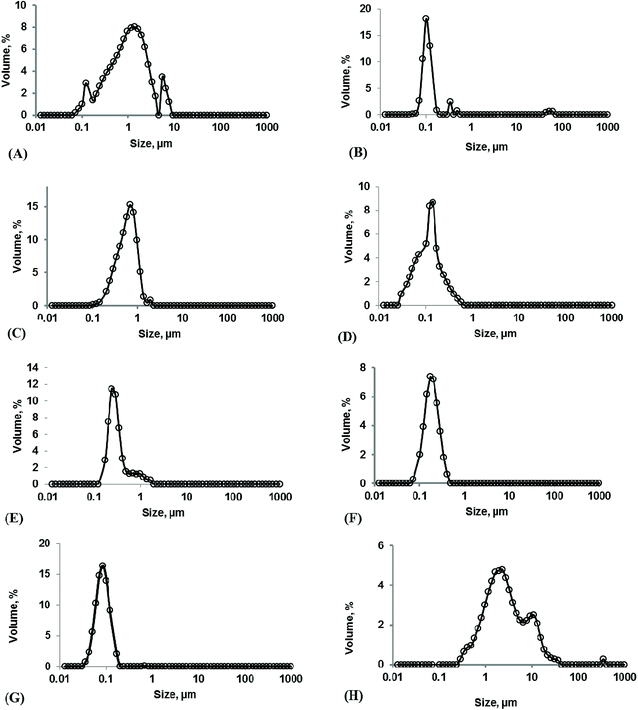
The effects of pressure at a constant temperature (50 °C) and Tween 80 concentration (0.75%) on the mean diameter, EE, and Cu of liposomes prepared via the SC-GAS technique are shown in Fig. 1A. The pressure was varied between 50 and 200 bar and it was found that increasing the pressure has a significant (p < 0.05) positive effect on particle size, EE and Cu. The extent of free and diffused CO2 in the phospholipid bi-layer is the determining parameter for the particle size of liposomes. Fig. 1A indicates that a lower pressure of <80 bar is not enough to reduce the particle size to the nano-level (<100 nm) due to the ineffective diffusion of CO2 in the lipid bi-layer. Liposome formation at pressures of 50 and 80 bar cannot ensure the sphericity of particles due to the unequal diffusion of CO2 throughout the lipid layer and its ineffectiveness in the complete unpacking of the bi-layer structure to discrete lipid molecules. Low pressure treatment yields liposomes with a broad range particle size distribution (Fig. 2A). As the pressure was increased from 120 to 200 bar, there was a significant reduction in particle size and this pressure range is effective for producing spherical particles possessing a narrow particle size distribution range (Fig. 2B). Lisha Zhao and co-workers reported operating pressure range to obtain spherically shaped submicron liposomes.10,11 A higher pressure range facilitated the CO2/aqueous/Tween80/phospholipids quaternary system to become more favorable for uniform dispersion of phospholipid molecules, allowing them to unite together after depressurization to form a larger number of nano-sized liposomes per unit volume; thus a better EE can be achieved. Increasing the pressure from 50 to 200 bar drastically reduces the liposome size from 0.978 ± 0.003 to 0.099 ± 0.004 μm, with a greater number of liposomes per unit volume achieving a maximum EE of 75.48 ± 2.45, 74.78 ± 3.2 and 70.31 ± 2.9 for adenosine, cordycepin and polysaccharides, respectively.The current configuration of the nano-liposome production setup was proven to engineer unimodal nano-liposomes over the tested pressure range (50–200 bar), provided the pressure inside the pressure vessel was necessarily kept constant during depressurization. Throughout depressurization, CO2 was released uniformly from every portion of the liquid phase, leading to each particle being influenced by dense CO2 and leading to a low Cu. The increasing pressure reasonably improved the uniformity of the liposomes from 8.32 ± 0.013 to 1.19 ± 0.012 (Table S1†) over the tested pressure range. Further experiments were carried out at pressures between 160 and 200 bar to select the best effective pressure for nano-liposome production. The liposomes produced at a pressure of 180 bar were the smallest liposomes, with a mean particle size of 0.072 ± 0.003 μm (Fig. 2G) and Cu of 1.10 ± 0.012 (Table S1†), with better EE.
Effects of Tween 80 concentration
The presence of a surfactant (Tween 80) plays an important role in the CO2/aqueous/Tween 80/phospholipids quaternary system, wherein the concentration of Tween 80 majorly governs the extent of lipid dispersion into the aqueous phase, which helps liposome recovery during the depressurization step. The concentration of Tween 80 at a constant pressure (120 bar) and temperature (50 °C) was varied between 0 and 3% to evaluate its effect on the mean particle size, EE and Cu of liposomes (Fig. 1C). Even though one can obtain liposomes in the absence of a surfactant, the particle mean diameter is larger (0.593 ± 0.005 μm) with a broad particle size distribution (Fig. 2C) and poor EEs of 21.34 ± 3.1, 20.12 ± 1.9 and 23.2 ± 2.7 are achieved for adenosine, cordycepin and polysaccharides, respectively. This is due to the fact that no repulsive force exists around the liposomes after depressurization to stop coalescence, and moreover there is incomplete dispersion of lipids in the aqueous phase affecting the CO2/aqueous/surfactant/lipids quaternary homogenous phase, and about 50% (by wt) of the liposome material still remained in the pressure vessel, thus affecting the EE. Experiments were carried with small amounts of Tween 80 (0.25%) to produce liposomes, where the particle size was reduced to 0.252 ± 0.003 μm and Cu was 4.65 ± 0.012 (Table S1†). Increasing the concentration of Tween 80 from 0.25 to 0.75% shows a positive effect, from which a minimum particle size of 0.129 ± 0.002 μm (Fig. 1B), maximum EEs of 51.96 ± 1.98, 52.36 ± 2.6 and 53.78 ± 2.1 for adenosine, cordycepin and polysaccharides, respectively, and improved Cu of 3.14 ± 0.011 (Table S1†) were obtained for 0.75%. Liposome production using a Tween 80 concentration in the range of 0.25 to 2% resulted in an 80 to 90% (data not shown) liposome yield, and the rest remained in the vessel due to foam formation, which was unavoidable. A further increase in surfactant concentration from 1 to 3% resulted in a slight increase in the mean diameter (Fig. 2D). This may be due to continued foam formation causing negative effects.22Effects of temperature
Variations in temperature at a constant pressure (120 bar) and Tween 80 concentration (1%) around the phase transition temperature (40–65 °C) involve melting transitions of lecithin, which is due to van der Waals interactions between adjacent lipid molecules. In the absence of CO2, lecithin exists in a lamellar gel phase at lower temperatures, whereas at higher temperatures it changes to a fluid liquid-crystalline phase. In the presence of dense CO2 and cholesterol, the gel-fluid phase transition temperature is reduced and the fluid liquid-crystalline phase region is broadened.23 In a high pressure system, whatever the temperature, the fluid liquid-crystalline phase is favoured, which is required for SC mediated liposome production as it enables liposomal encapsulation even at mild temperatures.24 In the present study, the effects of temperature at a constant pressure and surfactant content were evaluated with regards to the particle size, EE and Cu of liposomes (Fig. 1B). Temperature has a significant effect (p < 0.05) on the CO2/aqueous/Tween 80/lipids quaternary system, as an increase in temperature from 40 to 50 °C results in the reduction of the particle size from 0.243 ± 0.005 to 0.128 ± 0.002 μm (Fig. 2E). This is due to an increased density of CO2 under the interactive effects of pressure (120 bar) and temperature (45 and 50 °C), which creates the most favorable conditions for increased CO2 solubility in the aqueous phase and the formation of small particles. But at higher temperatures (55–65 °C) the particle size was increased to 0.147 ± 0.004 μm (Fig. 2F) and there was a slight decrease in the EE; this is due to the fact that elevated temperatures decrease the cohesive energy density of CO2, lowering its solubility in the aqueous phase and also causing the partitioning of the surfactant towards the aqueous phase, creating a slightly higher CO2-lecithin phase and a lower surfactant-aqueous phase.25 The unequal availability of surfactant results in the aggregation of liposomes during depressurization. An increase in temperature also results in the formation of a greater number of fine particles, thus increasing the EEs to 52.40 ± 1.68, 53.25 ± 2.9 and 49.57 ± 2.6 for adenosine, cordycepin and polysaccharides, respectively. Cu decreased from 3.18 ± 0.012 to 2.80 ± 0.015 as the temperature increased from 40 to 50 °C (Table S1†) and later increased with temperature to 2.98 ± 0.018, due to phase separation causing a non-uniform distribution of surfactant molecules around the liposomes. Hence, an optimal temperature of 50 °C is recommended for the production of nano-sized liposomes, where better size uniformity and EE can be obtained.Importance of depressurization and depressurization at the phase inversion temperature to the particle size and EE
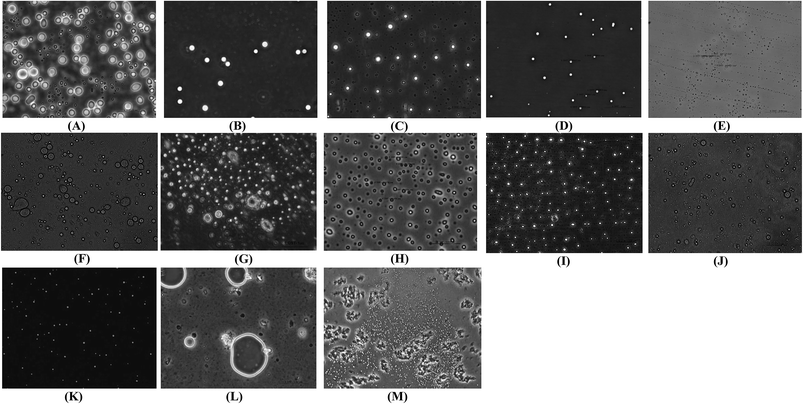
The importance of the depressurization step lies in the conversion of LUVs to SUVs via creating a pressure drop through micrometric high pressure tubing. Experiments were carried out under optimized conditions, i.e., 180 bar, 50 °C and 0.75% Tween 80, to understand the importance of depressurization on the particle size and EE. Liposome samples were collected from the pressure vessel just after the hydration step, and liposomes exhibited a large multimodal size distribution with a Cu value of 3.01 ± 0.021 with EEs of 59.23 ± 2.3, 57.13 ± 2.1 and 53.12 ± 1.9 for adenosine, cordycepin and polysaccharides, respectively. A unique feature of the current configuration of the SC-GAS process is that particle formation and encapsulation happen twice, i.e. during hydration under pressure and after depressurization, which is one possible way to obtain liposomes with superior characteristics exhibiting better EE.It is well understood that the presence of dense CO2 in the CO2/water/surfactant/lipids quaternary system facilitates the phase transition of lecithin at a mild temperature. During the depressurization step, the release of CO2 causes cooling of the liquid phase, creating a sudden shock, which is un-favorable for the re-assembling of lipid molecules to form liposomes. Due to an increase in the phase transition temperature, phospholipids undergo a phase transition from a fluid liquid-crystalline phase to a lamellar gel phase. Fig. 3M clearly proves that depressurization of the SC liquid phase at ambient temperature causes the formation of fewer liposomes, and most of the lipid molecules remain discrete, exhibiting poor EEs of 47.34 ± 2.5, 45.98 ± 3.1 and 41.56 ± 2.1 for adenosine, cordycepin and polysaccharides, respectively. So it is recommended that depressurization to atmospheric pressure occurs at a certain temperature above the lipid phase transition to obtain the highest number of small uniform liposomes.
Characterization of CS nano-liposomes
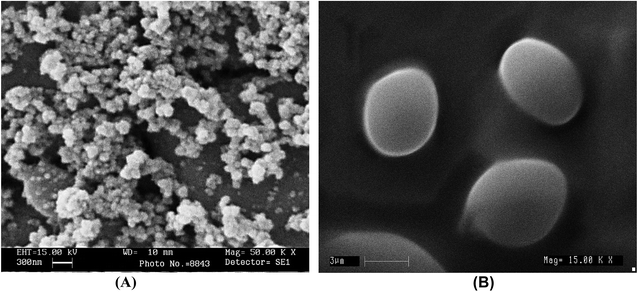
The occurrence of very much smaller sized liposomes (<100 nm) from the SC-GAS method than from the conventional method is evident in SEM pictures of the liposomes, where their occurrence was abundant. Smaller sized liposomes are delivered more efficiently to an infected site compared with larger sized liposomes. The SEM images in Fig. 4A and B confirm the occurrence of particles, supporting the micrographs from phase contrast microscopy. By observing the results regarding liposome size (Fig. 2G), and phase contrast and SEM images (Fig. 3K and 4A, respectively), the proposed configuration for SC-GAS liposome production, at 180 bar and 50 °C with 0.75% Tween 80 and a depressurization rate of 25 bar min−1, can undoubtedly be employed to produce mono-dispersed nano-liposomes with reproducible features.
The SC-GAS method in fed-batch mode is strongly supported as a superior method of nano-liposome production compared to other variants of SC techniques and high pressure based competitive methods that were discussed in an earlier report.5 The present configuration of the SC-GAS set-up facilitates nano-liposomes with a mean size of 72 nm and an EE of about 75%, which is 40% less than other SC methods and 70–90% less than high pressure homogenization and micro-fluidizer based techniques. This is the first study to report the production of stabilized superior liposomes that are less than 100 nm with a narrow range size distribution for the encapsulation of water soluble compounds from C. sinensis CS1197 via a SC-GAS method.
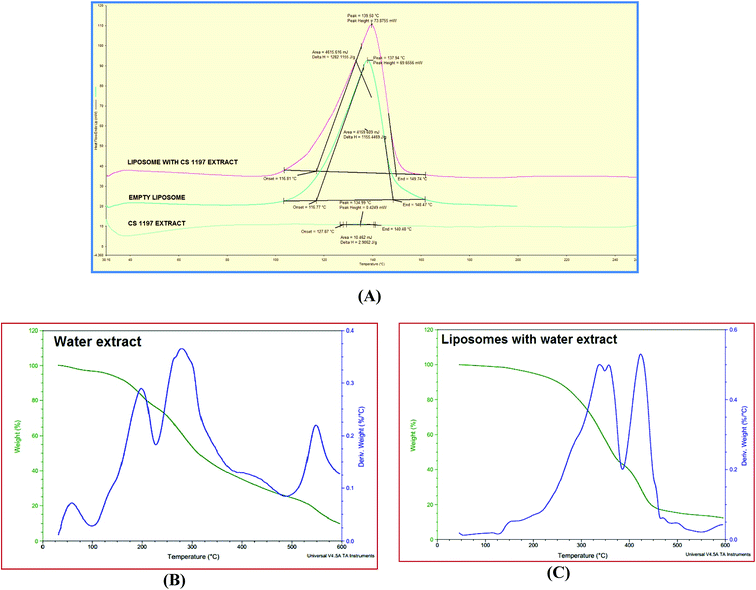
TGA was used to measure physical and chemical changes in compounds as a function of temperature. TGA curves for C. sinensis CS1197 WE and CS nano-liposomes are shown in Fig. 6B and C, respectively. During heating from 30 to 600 °C, about 90% wt loss was observed at a temperature of 198 °C for free C. sinensis CS1197 WE and 290 °C for the CS nano-liposomes. This wt loss may be due to a loss of moisture and/or a change in the molecular structure causing phase transitions (as shown by the secondary Y-axis). Liposomal encapsulation has improved the thermal stability of the extract.
| Time (weeks) | Mean particle size (μm) | Coefficient of uniformity (Cu) | Viscosity (mPa s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature effect | |||||||||
| 4 °C | 25 °C | 37 °C | 4 °C | 25 °C | 37 °C | 4 °C | 25 °C | 37 °C | |
| 0 | 0.072 ± 0.002 | 1.101 ± 0.010 | 1.781 ± 0.01 | ||||||
| 1 | 0.072 ± 0.001 | 0.073 ± 0.001 | 0.072 ± 0.003 | 1.102 ± 0.081 | 1.111 ± 0.013 | 1.110 ± 0.013 | 1.779 ± 0.02 | 1.782 ± 0.02 | 1.778 ± 0.01 |
| 2 | 0.073 ± 0.001 | 0.073 ± 0.003 | 0.074 ± 0.002 | 1.120 ± 0.013 | 1.119 ± 0.019 | 1.121 ± 0.018 | 1.778 ± 0.01 | 1.779 ± 0.01 | 1.777 ± 0.02 |
| 3 | 0.073 ± 0.001 | 0.074 ± 0.001 | 0.075 ± 0.001 | 1.126 ± 0.012 | 1.125 ± 0.018 | 1.126 ± 0.018 | 1.779 ± 0.02 | 1.777 ± 0.02 | 1.777 ± 0.02 |
| 4 | 0.075 ± 0.003 | 0.076 ± 0.001 | 0.079 ± 0.002 | 1.125 ± 0.112 | 1.126 ± 0.021 | 1.130 ± 0.013 | 1.776 ± 0.01 | 1.775 ± 0.03 | 1.759 ± 0.03 |
| 6 | 0.076 ± 0.001 | 0.077 ± 0.003 | 0.082 ± 0.004 | 1.130 ± 0.011 | 1.131 ± 0.011 | 1.139 ± 0.019 | 1.759 ± 0.01 | 1.750 ± 0.04 | 1.750 ± 0.01 |
| 8 | 0.076 ± 0.001 | 0.077 ± 0.005 | 0.089 ± 0.003 | 1.202 ± 0.018 | 1.139 ± 0.026 | 1.149 ± 0.010 | 1.758 ± 0.02 | 1.749 ± 0.02 | 1.733 ± 0.01 |
| Time (weeks) | pH effect | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 7 | 10 | 2 | 4 | 7 | 10 | 2 | 4 | 7 | 10 | |
| 0 | 0.072 ± 0.002 | |||||||||||
| 1 | 0.072 ± 0.002 | 0.072 ± 0.002 | 0.072 ± 0.004 | 0.073 ± 0.003 | 1.110 ± 0.013 | 1.109 ± 0.013 | 1.111 ± 0.013 | 1.120 ± 0.013 | 1.779 ± 0.02 | 1.781 ± 0.02 | 1.782 ± 0.02 | 1.780 ± 0.02 |
| 2 | 0.078 ± 0.003 | 0.076 ± 0.001 | 0.073 ± 0.003 | 0.077 ± 0.002 | 1.131 ± 0.019 | 1.121 ± 0.019 | 1.119 ± 0.019 | 1.124 ± 0.019 | 1.763 ± 0.01 | 1.773 ± 0.01 | 1.779 ± 0.01 | 1.779 ± 0.01 |
| 3 | 0.081 ± 0.002 | 0.078 ± 0.004 | 0.074 ± 0.001 | 0.082 ± 0.001 | 1.170 ± 0.018 | 1.130 ± 0.018 | 1.125 ± 0.018 | 1.155 ± 0.018 | 1.729 ± 0.02 | 1.769 ± 0.02 | 1.777 ± 0.02 | 1.759 ± 0.02 |
| 4 | 0.080 ± 0.001 | 0.077 ± 0.003 | 0.076 ± 0.002 | 0.087 ± 0.004 | 1.174 ± 0.021 | 1.154 ± 0.021 | 1.126 ± 0.021 | 1.163 ± 0.021 | 1.665 ± 0.03 | 1.765 ± 0.03 | 1.775 ± 0.03 | 1.565 ± 0.03 |
| 6 | 0.090 ± 0.004 | 0.080 ± 0.002 | 0.077 ± 0.003 | 0.108 ± 0.004 | 1.271 ± 0.011 | 1.201 ± 0.011 | 1.131 ± 0.011 | 1.298 ± 0.011 | 1.557 ± 0.04 | 1.757 ± 0.04 | 1.750 ± 0.04 | 1.367 ± 0.04 |
| 8 | 0.108 ± 0.002 | 0.087 ± 0.003 | 0.077 ± 0.005 | 0.127 ± 0.005 | 1.340 ± 0.026 | 1.289 ± 0.026 | 1.139 ± 0.026 | 1.450 ± 0.026 | 1.510 ± 0.02 | 1.740 ± 0.02 | 1.749 ± 0.02 | 1.240 ± 0.02 |
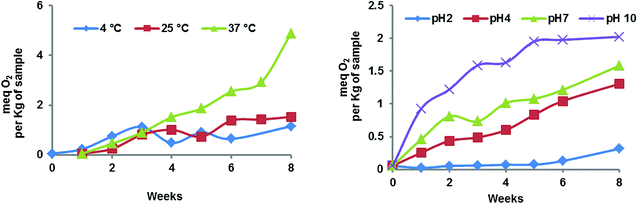
The nano-liposomes prepared via the SC-GAS method stored at 4, 25 and 37 °C were highly stable for up to 2 months (Table 1) with no significant changes (p > 0.05) in mean size, Cu and viscosity. This is due to the fact that uniformly distributed nano-liposomes possess a uniform charge density around the surface, attaining a thermodynamically stabilized state. Liposomes prepared via the SC-GAS and Bangham methods and stored at different temperatures were observed under a phase contrast microscope (Fig. 9). Liposomes prepared via the SC-GAS method showed no aggregation, but liposomes prepared via the Bangham method showed fast aggregation after 2 months. Large variations in the mean size caused an unequal distribution of the charge density i.e., large particles possessed a larger charge density compared to smaller particle, and as a result larger particles had the tendency to show dominance over smaller particles because the large potential difference caused aggregation. Fig. 10A–C shows the effects of storage temperature on the EE over two months. The EEs of liposomes for adenosine, cordycepin and polysaccharides decreased by less than 2.3% at 4 °C, 4.4% at 25 °C and 9.8% at 37 °C after one month, and less than 3.2% at 4 °C, 7.3% at 25 °C and 20.3% at 37 °C after two months. The study suggests that liposome storage at 4 and 25 °C favors minimal compound leakage, assuring 92–97% drug retention in the nano-liposomes, whereas 79–81% drug retention can be assured for liposomes stored at 37 °C (Table 2).
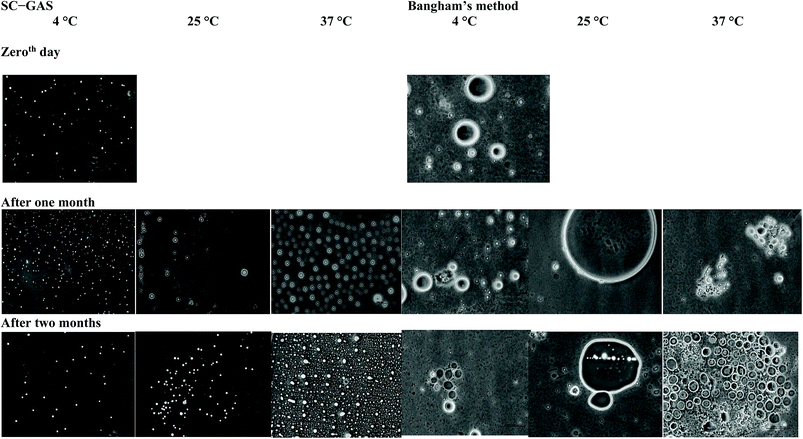 | ||
| Fig. 9 Phase contrast micrographs of liposomes prepared via the SC-GAS and Bangham methods, stored at different temperatures. | ||
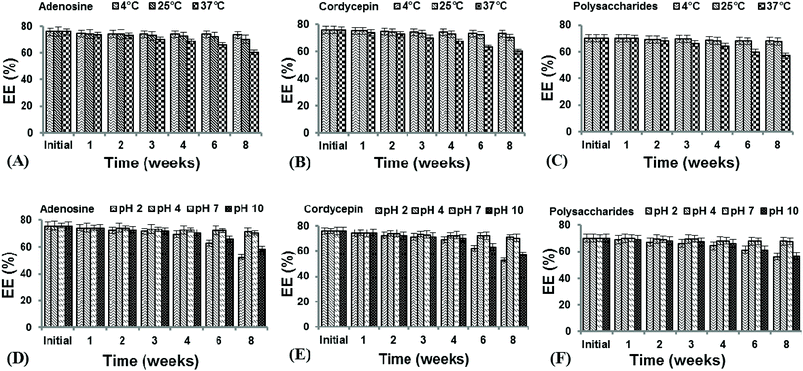 | ||
| Fig. 10 The effects of storage time, temperature and pH on the encapsulation efficiencies of CS nano-liposomes. | ||
| Parameter | Retention after 8 weeks (%) | |||
|---|---|---|---|---|
| Adenosine | Cordycepin | Polysaccharides | ||
| Temperature (°C) | 4 | 97.07 | 96.26 | 96.79 |
| 25 | 92.65 | 92.83 | 96.51 | |
| 37 | 79.6 | 78.82 | 81.17 | |
| pH | 2 | 68.79 | 69.42 | 79.9 |
| 4 | 93.64 | 93.44 | 96.66 | |
| 7 | 92.65 | 92.83 | 96.51 | |
| 10 | 76.96 | 75.07 | 80.69 | |
The CS nano-liposomes stored at different pH values of 2, 4, 7 and 10 were assessed for their EEs (Fig. 10D–F), mean diameters, Cu values and viscosities over two months (Table 1). The nano-liposomes stored at pH 4 and 7 were stable for up to 2 months with no significant changes (p > 0.05) in mean size, Cu and viscosity. This is due to the fact that a pH value of 4 to 7 favored the maximum retention of surfactant molecules on the surfaces of the liposomes, thus arresting aggregation. However, nano-liposomes stored under extreme pH conditions, i.e., 2 and 10, were stable until the 3rd week and were later destabilized due to fast aggregation. Under extreme pH conditions, higher ionic strength caused a lowering of the surfactant charge density over the liposome surfaces, resulting in fast aggregation. This study suggests that liposome storage at pH 4 and 7 favors minimal compound leakage, assuring 92–96% drug retention in nano-liposomes, whereas 68–80% drug retention can be assured for liposomes stored at pH 2 and 10 (Table 2).
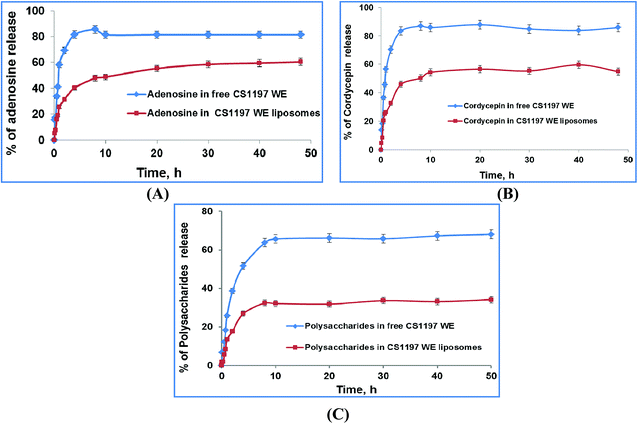 | ||
| Fig. 12 The in vitro kinetic release profiles of CS nano-liposome dispersions prepared via the SC-GAS method in pH 6.8 hydration medium at 37 °C. | ||
The mechanism of the release phenomenon through the nano-liposomes was studied via applying two kinetic models. The kinetic data for the two models is shown in Table 3 and the regression of the kinetic models to the in vitro kinetic release data from the CS nano-liposome formulations is shown in Fig. S3.† A first order model fits the in vitro experimental release data of free C. sinensis CS1197 WE well (R2 = 0.927–0.943), where a fast release pattern is adopted. But the release of C. sinensis CS1197 WE through the nano-liposomes followed the Higuchi model (R2 = 0.931–0.957), proving that release through the liposomes involves a diffusion-controlled mechanism.
| First order | Higuchi model | ||||
|---|---|---|---|---|---|
| K1 | R2 | K2 | R2 | ||
| Free CS1197 WE | Adenosine | 0.1794 | 0.927 | 26.622 | 0.833 |
| Cordycepin | 0.191 | 0.943 | 27.594 | 0.847 | |
| Polysaccharides | 0.017 | 0.929 | 22.512 | 0.969 | |
| CS liposomes | Adenosine | 0.027 | 0.849 | 17.877 | 0.953 |
| Cordycepin | 0.031 | 0.844 | 17.274 | 0.931 | |
| Polysaccharides | 0.047 | 0.869 | 11.593 | 0.957 | |
Conclusions
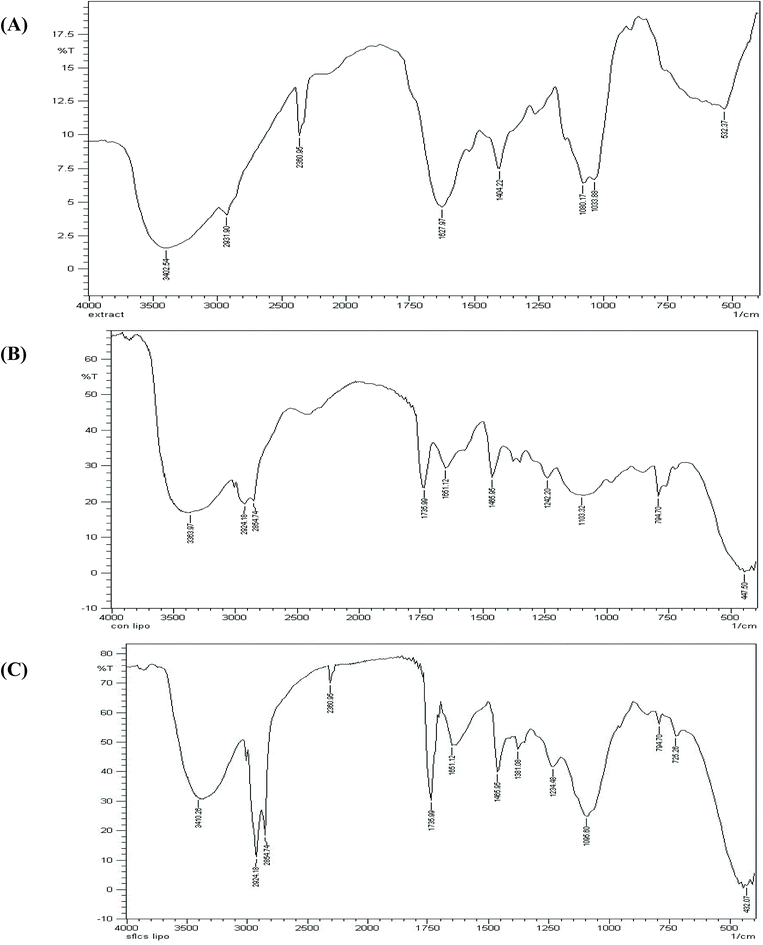
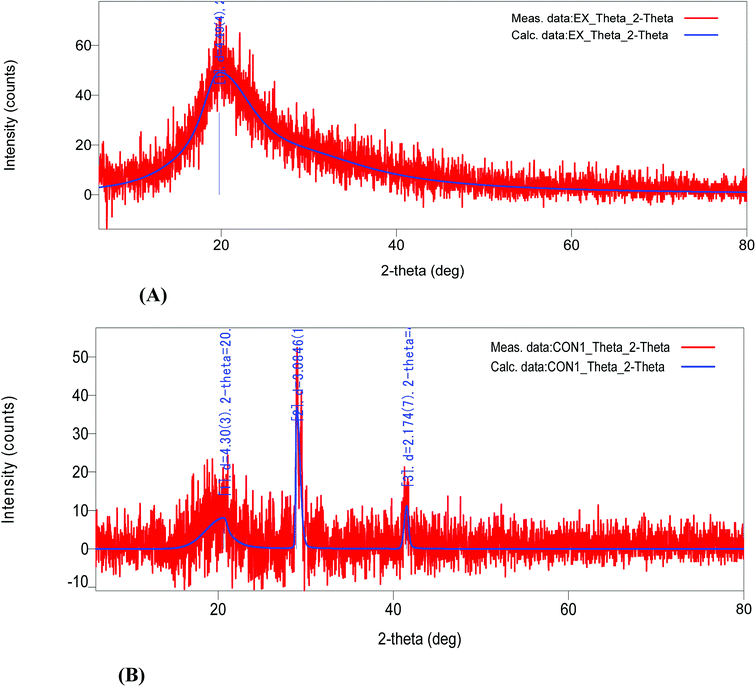
The SC-GAS method provides a simpler way to prepare liposomes with a characteristic nano-size and better morphology (i.e., a uniform size and shape). The current configuration of the SC-GAS mediated liposome production unit can be operated in fed-batch mode, with the additional feature of ensuring reproducibility for the encapsulation of both hydrophilic and hydrophobic active compounds. CS nano-liposome production under optimized conditions, i.e., 180 bar, 50 °C and 0.75% Tween 80, offers stabilized liposomes exhibiting good LSI and rheological properties and achieving about 75% EE. DSC and TGA studies reveal that encapsulation within a lipid bilayer improves the thermal stability of C. sinensis water soluble compounds. FTIR analysis proved that the liposomal encapsulation of C. sinensis WE ensures the efficient unloading of active ingredients at target sites. The ordered structure of the lipid bi-layer was attributed to crystallinity, which was confirmed via XRD analysis. Storage and oxidative stability studies suggest that maintaining the CS nano-liposomes at 4 and 25 °C ensures maximum drug retention and minimum lipid oxidation. In vitro kinetic release studies show that the active compounds are delivered through liposomes via a diffusion controlled mechanism. The proposed model can be successfully implemented on a pilot scale, indicating that supercritical processes can provide promising technical and economic conditions for large scale production. Supercritical technology shows advantages over conventional preparation methods and offers great potential for the encapsulation of valuable compounds relevant to nutraceutical, functional food and pharmaceutical applications.Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.Acknowledgements
The author (SMG) acknowledges encouragement from the Director of the CSIR – Central Food Technological Research Institute, Mysore, India.References
- P. LI, H. JI, T. Tina and W. Karl, Chin. J. Pharm. Anal., 2001, 21, 77–78 Search PubMed.
- M. Shashidhar, P. Giridhar, K. U. Sankar and B. Manohar, J. Funct. Foods, 2013, 5, 1013–1030 CrossRef CAS.
- G. Gregoriadis, N. Engl. J. Med., 1976, 295, 704–710 CrossRef CAS PubMed.
- S.-L. Huang, Adv. Drug Delivery Rev., 2008, 60, 1167–1176 CrossRef CAS PubMed.
- G. Shashidhar and P. Gadkari, RSC Adv., 2016, 6, 57739–57750 RSC.
- S. Varona, Á. Martín and M. a. J. Cocero, Ind. Eng. Chem. Res., 2011, 50, 2088–2097 CrossRef CAS.
- A. S. Zarena and K. U. Sankar, Ther. Delivery, 2011, 2, 259–277 CrossRef CAS.
- U. S. Kadimi, D. R. Balasubramanian, U. R. Ganni, M. Balaraman and V. Govindarajulu, Nanomedicine, 2007, 3, 273–280 CrossRef CAS PubMed.
- S. M. Ghatnur, R. S. Sonale, M. Balaraman and U. S. Kadimi, J. Liposome Res., 2012, 22, 215–223 CrossRef CAS PubMed.
- L. Zhao and F. Temelli, J. Supercrit. Fluids, 2015, 100, 110–120 CrossRef CAS.
- L. Zhao and F. Temelli, J. Food Eng., 2015, 158, 104–112 CrossRef CAS.
- L. Zhao, F. Temelli, J. M. Curtis and L. Chen, Food Res. Int., 2015, 77, 63–72 CrossRef CAS.
- I. E. Santo, R. Campardelli, E. C. Albuquerque, S. V. de Melo, G. Della Porta and E. Reverchon, Chem. Eng. J., 2014, 249, 153–159 CrossRef CAS.
- S. M. Ghatnur, G. Parvatam and M. Balaraman, Pharmacogn. Mag., 2015, 11, 448 CrossRef CAS PubMed.
- A. Bangham, M. M. Standish and J. Watkins, J. Mol. Biol., 1965, 13, 238–IN227 CrossRef CAS PubMed.
- R. F. Craig, Craig's soil mechanics, CRC Press, 2004 Search PubMed.
- K. N. Pearce and J. E. Kinsella, J. Agric. Food Chem., 1978, 26, 716–723 CrossRef CAS.
- P. V. Gadkari and M. Balaraman, J. Food Eng., 2015, 147, 14–23 CrossRef CAS.
- R. Wrolstad, T. Acree, E. Decker, M. Penner, D. Reid, S. Schwartz, C. Shoemaker, D. Smith and P. Sporns, Lipids, and Carbohydrates. Wiley, New Jersey, 2005 Search PubMed.
- T. P. Castor and L. Chu, US Pat., US5776486A, 1998.
- T. Castor, WO9427581, 1994.
- R. Campardelli and E. Reverchon, J. Food Eng., 2015, 149, 131–136 CrossRef CAS.
- G. D. Bothun, B. L. Knutson, H. J. Strobel and S. E. Nokes, Langmuir, 2005, 21, 530–536 CrossRef CAS PubMed.
- L. Lesoin, O. Boutin, C. Crampon and E. Badens, Colloids Surf., A, 2011, 377, 1–14 CrossRef CAS.
- C. T. Lee, P. A. Psathas, K. P. Johnston, J. deGrazia and T. W. Randolph, Langmuir, 1999, 15, 6781–6791 CrossRef CAS.
- A. Zarena, S. Bhattacharya and U. S. Kadimi, Food Bioprocess Technol., 2012, 5, 3007–3013 CrossRef.
- J. Rao and D. J. McClements, J. Agric. Food Chem., 2011, 59, 5026–5035 CrossRef CAS PubMed.
- P. P. Gaikwad and T. Desai Maya, Int. J. Pharma Res. Rev., 2013, 2, 40–52 Search PubMed.
- B. Akbari, M. P. Tavandashti and M. Zandrahimi, Iran. J. Mater. Sci. Eng., 2011, 8, 48–56 CAS.
- W. Zhou, W. Liu, L. Zou, W. Liu, C. Liu, R. Liang and J. Chen, Colloids Surf., B, 2014, 117, 330–337 CrossRef CAS PubMed.
- V. Venkateswarlu and K. Manjunath, J. Controlled Release, 2004, 95, 627–638 CrossRef CAS PubMed.
- P. Panwar, B. Pandey, P. Lakhera and K. Singh, Int. J. Nanomed., 2010, 5, 101–108 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07601d |
| This journal is © The Royal Society of Chemistry 2018 |