Open Access Article
Open Access ArticleFabrication of eco-friendly nanofibrous membranes functionalized with carboxymethyl-β-cyclodextrin for efficient removal of methylene blue with good recyclability†
Yinli Liuab,
Dequn Wu *ab,
Xueli Wangc,
Jianyong Yuc and
Faxue Li
*ab,
Xueli Wangc,
Jianyong Yuc and
Faxue Li *abc
*abc
aKey Laboratory of Textile Science & Technology, Ministry of Education, Donghua University, Shanghai 201620, China. E-mail: fxlee@dhu.edu.cn; dqwu@dhu.edu.cn
bCollege of Textiles, Donghua University, Shanghai 201620, China
cInnovation Center for Textile Science and Technology, Donghua University, Shanghai 201620, China
First published on 9th November 2018
Abstract
Considering the excellent thermo-mechanical properties, chemical stability and low cost of biodegradable aliphatic–aromatic copolyesters, they are an ideal matrix when functionalized for capturing pollutants in wastewater. In this work, biodegradable poly((butylene succinate-co-terephthalate)-co-serinol terephthalate) (PBSST) copolyesters with amino side group (–NH2) were first synthesized through copolymerization, followed by grafting carboxymethyl-β-cyclodextrin (CM-β-CD) into PBSST molecular chains via amidation reaction to prepare PBSST-g-β-CD. The corresponding nanofibrous membranes were then fabricated by electrospinning as adsorbents for efficiently removing cationic dye methyl blue (MB) from aqueous solutions. The adsorption performance of the nanofibrous membranes was fitted well with pseudo-second-order model and Langmuir isotherm model. The maximum adsorption capacity was 543.48 mg g−1 for MB along with a removal efficiency of 98% after five regeneration cycles, indicating the high adsorption capacity and good recyclability of nanofibrous membranes. The adsorbents possess features of high adsorption capacity, eco-friendliness and easy operation, and exhibit great potential for disposing of printing-dying wastewater.
Introduction
Nowadays, various dyes are widely used in food, medicine, printing, dyeing, and cosmetics industries, so more and more organic and inorganic pollutants have been produced posing great threat to human health and the stability of the ecological environment. Various techniques including chemical precipitation,1 photodegradation,2 electro-chemical treatment,3 evaporative recovery,4 solvent extraction,5 ultrafiltration,6 ion exchange,7 filtration,8 adsorption,9 and electrodialysis10 have been utilized to remove pollutants from wastewater. Among these methods, adsorption is always confirmed to be an efficient route to capture organic and inorganic pollutants in the ecological water environment due to its easy operation, wide adaptability and excellent remediation. However, the application of some adsorbents is restricted due to their high cost,11 low recyclability,12 and low adsorption capacity.13 Membrane technology is one of the most important and effective strategies for wastewater purification. Water separation through membrane technology is highly efficient, cost-effective, energy-saving, and eco-friendly. Nanofibrous membranes obtained via electrospinning as adsorbents for removing pollutants have become of interest because of their relatively simple fabrication and cost effectiveness,14,15 and they can improve the adsorption capacity of substrate material.Cyclodextrin (CD) is a cyclic oligosaccharide produced by starch under the action of amylase, and possesses a rigid, truncated-cone structure with a hydrophobic internal cavity and hydrophilic external surface, which can be utilized as a sustainable adsorbent for selectively encapsulating pollutants (various small molecules, polymers, ions, or even radicals, etc.) to form well-defined host-guest complexes.16 Therefore, CD and its derivatives have been widely utilized to remove organic and inorganic pollutants, especially β-CD for application due to its wide availability, low cost and eco-friendliness. However, the solubility of β-CD in water limits its application in sewage purification,17 so β-CD functionalized polymers have been studied intensively as adsorbents for removing pollutants.18–22
As a biodegradable aliphatic-aromatic copolyester, poly(butylene succinate-co-terephthalate) (PBST) has excellent thermo-mechanical properties, chemical stability and resistance to deformation,23–25 and is an ideal matrix functionalized for capturing pollutants in wastewater. Recently, we fabricated PBST nanofibrous membranes physically compounded with β-CD, which is used to adsorb the cationic dye methyl blue (MB) in aqueous solution and the maximum adsorption capacity is 90.9 mg g−1.26 After the service life is terminated, the membranes can be biodegraded in soil without secondary pollution. Nevertheless, the prepared membranes have poor adsorption capacity and low recyclability since the β-CD derivatives cannot be chemically linked with PBST nanofibrous membranes at the absence of reactive functional groups in PBST molecular chains, which results into easily peeling-off of β-CD derivatives from the matrix. In addition, the porous structure of PBST nanofibrous membranes is destroyed by β-CD physically coated on the surface of nanofibrous membranes, resulting in the poor adsorption capacity of PBST nanofibrous membranes physically compounded with β-CD. Therefore, it is necessary to immobilize β-CD or its derivatives on the surface of PBST nanofibrous membranes to improve the adsorption capacity and extend the recyclability of adsorbents in wastewater purification.
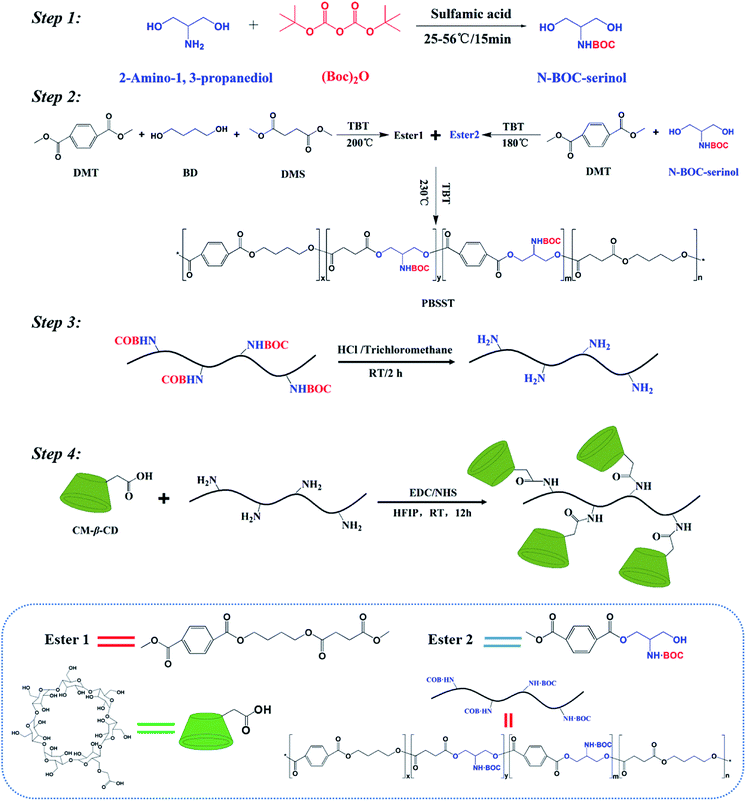
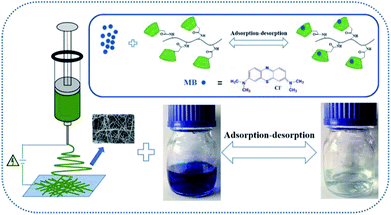
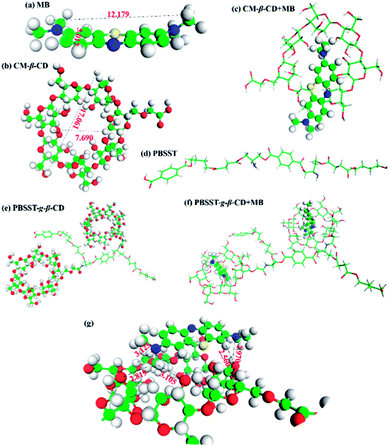
In this work, novel PBSST copolyesters with amino side group (–NH2) were first synthesized on the basis of PBST synthesis, followed by grafting carboxymethyl-β-cyclodextrin (CM-β-CD) into the copolyesters via amidation reaction between amino groups of PBSST and carboxyl groups of CM-β-CD to prepare the eco-friendly materials (the schematic diagram of synthesis route of PBSST-g-β-CD in Fig. 1). The corresponding nanofibrous membranes were then fabricated via electrospinning to adsorb MB in aqueous solutions, and they showed large adsorption capacity to MB with good recyclability. The experiment results indicate that the PBSST-g-β-CD nanofibrous membranes present a great potential in disposing the printing-dying wastewater.
Experimental section
Materials
Dimethyl terephthalate (DMT), dimethyl succinate (DMS), 1,4-butanediol (BD), chloroform and dichloromethane (DCM), sodium hydroxide (NaOH) and hydrochloric acid (HCl) were commercially supplied by Shanghai Lingfeng Chemical Reagent Co. Ltd. Serinol was supplied by Shanghai Yijing industrial Co. Ltd. Sodium chloride (NaCl) was supplied by Sinopharm Chemical Reagent Co. Ltd. Tetrabutyl titanate (TBT), ditertbutyl dicarbonate ((Boc)2O), sulfamic acid, methylene blue (MB), 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC), hexafluoroisopropanol (HFIP) and N-hydroxysuccinimide (NHS) were supplied by Aladdin Chemical Regents Co. Ltd. Carboxymethyl-β-cyclodextrin (CM-β-CD) was supplied by Zhiyuan Bio-Technology Co. Ltd (Shandong, China). All chemicals were of analytical grade and used as received without further purification.Preparation of N-BOC-serinol
N-BOC protection of serinol was catalyzed by sulfamic acid under the followed conditions. (Boc)2O (1.1 mol) and sulfamic acid (0.05 mol) were put in 500 mL round-bottom flask at 25 °C. Serinol (1 mol) was added into the flask with stirring at 55 °C for 15 min. Saturated NaCl aqueous solution (100 mL) and ethyl acetate (150 mL) were put in the reaction mixture, and then aqueous and organic layers appeared. The aqueous layer was extracted with ethyl acetate (150 mL) for three times, while the organic layer were collected, dried with MgSO4, and then concentrated.Preparation of N-BOC-PBSST copolyesters
In order to increase the conversion rate of monomer, N-BOC-PBSST copolyesters were synthesized with the stepwise method with DMT, DMS, BD and N-BOC-serinol. DMT (0.2 mol), DMS (0.2 mol), BD (0.42 mol) and TBT with a certain fraction were mixed together in a round-bottom flask and stirred at about 200 °C for 2–3 h to gain Ester 1. DMT (0.2 mol), N-BOC-serinol (0.21 mol) and TBT with a certain fraction were mixed together in a round-bottom flask and stirred at about 180 °C for 2–3 h to gain Ester 2. Subsequently, Ester 1 and Ester 2 were mixed together with different molar ratios (5%, 7%, 10%, 20% of Ester 2 to Ester 1), and heated to 210 °C at a pressure of 70 kPa with stirring for 2 h. Then the polycondensation occurred under the gradually reduced pressure and increasing temperature to 230 °C. The reaction was terminated as the stirrer torque approached the maximum. The as-products were dissolved in chloroform, and then precipitated from cold methanol for purification. Then white solid products were collected after drying under high vacuum overnight.Deprotection of N-BOC-PBSST copolyesters
N-BOC-PBSST copolyesters (10 g) were dissolved in anhydrous chloroform (200 mL) at room temperature and bubbled with HCl for 2 h. The resulting solution was added dropwise into cold methanol and then precipitated. White solid products (PBSST) were obtained after dried to constant weight for the next grafting reaction.Synthesis of PBSST-g-β-CD copolymers
CM-β-CD (8.000 g), EDC (0.863 g) and NHS (1.436 g) were dissolved in 100 mL of HFIP aqueous solution (90 v/v%). The reaction was stirred in ice bath for 6 h before PBSST was added with stirring for another 6 h at room temperature. The resulting solution was added dropwise into cold methanol and reprecipitated to obtain white solid products. The products were thoroughly washed with hot deionized water for several times. Then white solid products were received after dried to constant weight.Fabrication of nanofibrous membranes
PBST, PBSST and PBSST-g-β-CD nanofibrous membranes were fabricated through electrospinning. The as-products were dissolved in DCM to prepare 20 wt% spinning solutions with stirring for 12 h continuously. The spinning solution was loaded into syringe with a metallic needle (inner diameter 0.5 mm), and high voltage of 16 kV, feed rate of 1.5 mL h−1, the distance between needle tip and collector of 15 cm were applied to perform electrospinning. The temperature and relevant humidity were kept at 25 °C and 35%, respectively.Characterization
Microstructures of as-prepared products were characterized by different techniques. Nuclear magnetic resonance (1H NMR, AVANCE400, Bruker) spectra were recorded with the resonance frequency of 400 MHz. Fourier transform infrared spectroscopy (FTIR) analyzer (Nicolet 6700, Thermo Fisher) equipped with the Smart iTR operated on the attenuated total reflectance (ATR) mode in the wave-number range of 4000–400 cm−1. X-ray diffraction (XRD, max-2550VB, Rigaku) patterns were collected using a diffractometer with a Cu-Kα source (λ = 1.5418 Å). Differential scanning calorimetry (DSC4000, PerkinElmer) and thermogravimetric analysis (TGA4000, PerkinElmer) were used to investigate the thermal properties of PBST and PBSST. The morphologies of nanofibrous membranes were examined by scanning electron microscopy (SEM, JSM-5600LV, JEOL) at an accelerating voltage of 10 kV. The water contact angles of the nanofibrous membranes were measured by using a contact angle goniometer (OCA15EC, Dataphysics Instruments).Adsorption–desorption studies
Adsorption experiments were performed on a model BETS-M1 shaker (Kylin-Bell Lab Instruments Co. Ltd, China) with a shaking speed of 120 rpm at room temperature. The pH values of MB solution were adjusted by a pH meter with adding small amount of 0.1 mol L−1 HCl or 0.1 mol L−1 NaOH solution. PBST, PBSST and PBSST-g-β-CD nanofibrous membranes were individually immersed in MB solution with initial concentration of 10 mg L−1 and desired pH value of 2–12 for a certain time at room temperature; 50 mg of PBSST-g-β-CD nanofibrous membranes were immersed in MB solution (10 mg L−1, pH = 9) at different times (0–120 min) to explore the adsorption kinetics; 50 mg of PBSST-g-β-CD nanofibrous membranes were immersed in MB solution with different initial concentrations (5–200 mg L−1, pH = 9) to reveal the adsorption isotherms.For desorption experiment, PBSST-g-β-CD nanofibrous membranes adsorbed MB were washed with deionized water, then were put into methanol solution containing 5% (v/v) HCl (0.1 mol L−1) solution. After desorption equilibrium, the membranes were washed for several times with deionized water and reused for MB adsorption (adsorbent, 50 mg; MB solution, 50 mL, 50 mg L−1, initial pH = 9). The adsorption–desorption process was repeated for 5 times. The concentration of MB solution was measured by a UV-vis spectroscopy (UV-2550, Shimadzu) at the wavelength of 665 nm. MB removal efficiency (Rt) and adsorption capacity (qt) at time t were determined via the following equations respectively:
| Rt = 100 × (C0 − Ct)/C0 |
| qt = V × (C0 − Ct)/W |
Results and discussion
Characterization of raw materials and synthetic products
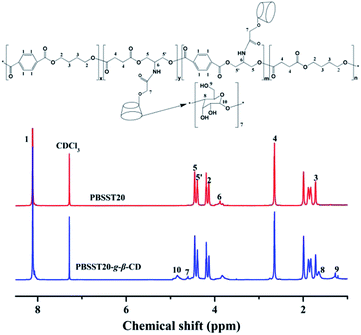
Fig. 3 plots the 1H NMR spectra of PBSST20 and PBSST20-g-β-CD, where “20” means the feed ratio of Ester 2 to Ester 1 is 20 mol% (the same meaning in other PBSST copolyesters). Peak at 3.8 ppm ((6) –CH–NH–) for PBSST20 corresponding to the hydrogen proton of methyne (–CH) of serinol appears (Fig. S1(a and b)†), indicating serinol unit is introduced into the molecular chains successfully. According to 1H NMR results in Fig. S1,† conversion ratio of N-BOC-serinol is calculated to be 75%, 73%, 73% and 72% for PBSST5, PBSST7, PBSST10 and PBSST20 respectively as listed in Table 1. Furthermore, new peaks appear at 4.82 ppm ((10) –O–CH–O–), 4.59 ppm ((7) –O–CH2–CO–), 1.68 ppm ((8) –CH–CH–) and 1.25 ppm ((9) –CH) for PBSST20-g-β-CD in CDCl3, indicative of the formation of covalent bonds between CM-β-CD and PBSST20.| Sample | Conversion ratio of N-BOC-serinol (%)a | Tm (°C) | Td (°C) | Crystallinity (%)b |
|---|---|---|---|---|
| a Conversion ratio is determined by calculating the area ratio of peak at 3.8 ppm to peak at 2.64 ppm in Fig. S1.b Crystallinity is determined by fitting the XRD curves through Origin software. | ||||
| PBST | — | 140 | 370 | 14.23 |
| PBSST5 | 75 | 136 | 364 | 11.43 |
| PBSST7 | 73 | 133 | 362 | 11.16 |
| PBSST10 | 73 | 130 | 359 | 10.84 |
| PBSST20 | 72 | 126 | 356 | 4.70 |
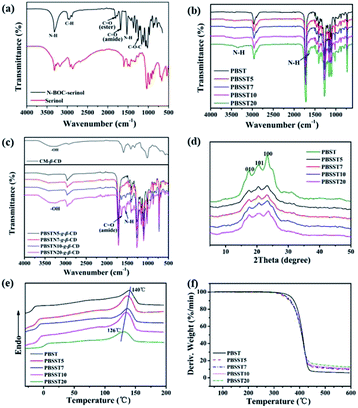
FTIR spectra of serinol, N-BOC-serinol, PBST, PBSST, CM-β-CD and PBSST-g-β-CD are shown in Fig. 4(a–c), and the absorption bands of serinol at about 3410 cm−1 correspond to the stretching vibrations of –OH, N–H, and those at about 2960 cm−1 correspond to the stretching vibration of –CH. Compared to serinol, new absorption bands appear at about 1686, 1531 and 1250 cm−1 in the spectrum of N-BOC-serinol, which are attributed to the stretching vibrational modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, N–H and C–O–C respectively.27 Different from PBST spectrum, the stretching vibration of N–H at around 3360 and 1656 cm−1 can be observed in the spectra of PBSST copolyesters, confirming the N-BOC-serinol involved in the copolymerization. This is in accordance with the results obtained from 1H NMR. New absorption bands at around 1643, 1539 cm−1 for PBSST-g-β-CD are the indication of C
O, N–H and C–O–C respectively.27 Different from PBST spectrum, the stretching vibration of N–H at around 3360 and 1656 cm−1 can be observed in the spectra of PBSST copolyesters, confirming the N-BOC-serinol involved in the copolymerization. This is in accordance with the results obtained from 1H NMR. New absorption bands at around 1643, 1539 cm−1 for PBSST-g-β-CD are the indication of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H groups formation, confirming that CM-β-CD is chemically grafted into PBSST through amide bonds. In addition, the as-prepared PBSST-g-β-CD shows the absorption bands of oxygen-containing functional groups stretching vibrations of CM-β-CD such as –OH (3410 cm−1),28 which further verifies that the grafting reaction between CM-β-CD and PBSST occurs.
O and N–H groups formation, confirming that CM-β-CD is chemically grafted into PBSST through amide bonds. In addition, the as-prepared PBSST-g-β-CD shows the absorption bands of oxygen-containing functional groups stretching vibrations of CM-β-CD such as –OH (3410 cm−1),28 which further verifies that the grafting reaction between CM-β-CD and PBSST occurs.
 | ||
| Fig. 4 (a–c) FTIR spectra of serinol and N-BOC-serinol, PBST and PBSST, CM-β-CD and PBSST-g-β-CD; (d) XRD curves of PBST and PBSST; (e) DSC curves of PBST and PBSST; (f) TGA curves of PBST and PBSST. | ||
Fig. 4(d) displays the XRD curves of PBST and PBSST. It is obvious that the XRD patterns of PBSST have similar diffraction peaks at 17.2° (010), 20.2° (101), 23.2° (100) to PBST, indicating that the crystal structures of PBSST do not alter significantly with the introduction of N-BOC-serinol unit. However, the intensities of diffraction peaks of PBSST are reduced with the increasing feed ratio of N-BOC-serinol, indicating the crystallinity decreases due to the increasing atacticity as shown in Table 1.
DSC and TGA measurements were conducted to investigate the thermal properties of PBST and PBSST as shown in Fig. 4(e and f). It can be seen that melting temperature (Tm) decreases from 140 °C to 126 °C and decomposition temperature (Td) decreases from 370 °C to 356 °C with the increasing feed ratio of N-BOC-serinol since the structure tacticity of copolymers is deteriorated with the introduction of new comonomer.
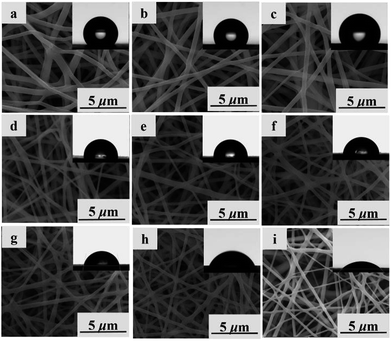
SEM technique was applied to investigate the morphological structures of PBST, PBSST and PBSST-g-β-CD nanofibrous membranes as shown in Fig. 5. The nanofibrous membranes are randomly oriented and the average diameter of fibers decreases to 469 nm from 653 nm (listed in Table S1†) with the increasing feed ratio of N-BOC-serinol. Actually, the effect of conductance of solution on the diameter of single nanofiber is a critical factor. In the research, the content of amino groups (–NH2) increases with the increasing feed ratio of N-BOC-serinol, which causes the increase of conductance of solution. In addition, the electrostatic repulsion among nanofibers increase with the increasing content of amino groups (–NH2), which causes the decrease of diameter of fibers.29,30
The contact angles of nanofibrous membranes influence their adsorption capacity to MB. As seen in Table S1,† the hydrophobic PBST nanofibrous membrane has the water contact angle of 135°, while the contact angles of PBSST nanofibrous membranes are reduced to 102° from 129° with the increasing feed ratio of N-BOC-serinol. After grafted with CM-β-CD, the prepared nanofibrous membranes have the sharply decreasing contact angle from 98° of PBSST5-g-β-CD nanofibrous membrane to 46° of PBSST20-g-β-CD nanofibrous membrane, indicating that the as-prepared PBSST20-g-β-CD membrane is hydrophilic, which in turn is beneficial to the MB adsorption in aqueous solution.
Overall, combining the results from characterization, one can concluded that CM-β-CD is chemically grafted into PBSST with covalent bonding, while the fabricated PBSST20-g-β-CD nanofibrous membrane has good hydrophilic and abundant active sites for the removal of pollutants in wastewater. Hence, PBSST20-g-β-CD nanofibrous membrane is selected for the MB adsorption in the following research.
Adsorption experiments
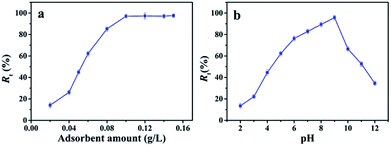
However, as the pH value of MB solution is higher than 9, the electrostatic repulsion between deprotonated adsorption sites of nanofibrous membrane and deprotonated dimethylamine group of MB restricts the interaction between MB molecules and nanofibrous membrane.31 Therefore, the Rt decreases with the continued increasing pH value of MB solution. These results suggest that the pH value of MB solution is significant to determine the removal efficiency of nanofibrous membrane to MB. Therefore, the adsorption process is dominated not only by the host-guest inclusion with the specific cavum from cyclodextrin derivative, but also the electrostatic interaction between hydroxy groups from nanofibrous membrane and groups containing nitrogen of MB molecules, which can be attributed to hydrogen bonds and van der Waals forces.
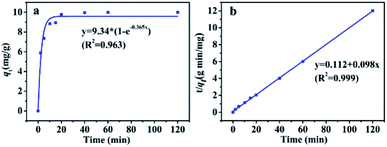 | ||
| Fig. 7 Adsorption kinetics plots of PBSST20-g-β-CD nanofibrous membrane to MB: (a) pseudo-first order fitting; (b) pseudo-second order fitting. | ||
To further study the adsorption process (e.g., mass transfer or chemical reaction), the kinetic data were fitted by pseudo-first-order and pseudo-second-order kinetic models. The pseudo-first-order kinetic model eqn (1) and pseudo-second-order kinetic model eqn (2) are usually given as followed:32
Pseudo-first order:
| qt = qe(1 − e−k1t) | (1) |
Pseudo-second order:
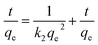 | (2) |
Langmuir model:
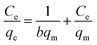 | (3) |
Freundlich model:
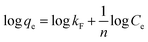 | (4) |
MB adsorption isotherm is displayed in Fig. 8(a and b) and the corresponding parameters calculated from the two models are listed in Table 2. Obviously, MB adsorption isotherm is well fitted by the Langmuir model (R2 > 0.99), showing that the MB adsorption on PBSST20-g-β-CD nanofibrous membrane is mainly a monolayer adsorption and relatively homogeneous. The maximum adsorption capacity (qm) of MB onto PBSST20-g-β-CD nanofibrous membrane calculated from the Langmuir model is 543.48 mg g−1, significantly higher than PBST and PBSST nanofibrous membranes shown in Table 2, indicating the introduction of the cavum from cyclodextrin is very beneficial to the MB adsorption. It is also interesting to found that the maximum adsorption capacity of PBSST nanofibrous membranes to MB is increased with the increasing feed ratio of serinol, suggesting that the increase of amino group (–NH2) in PBSST is propitious to MB adsorption due to the formation of hydrogen bonds between MB and PBSST nanofibrous membrane. Meanwhile, PBSST20-g-β-CD nanofibrous membrane has significantly higher qm value than the available adsorbents reported in literatures as listed in Table 3. The high adsorption capacity of PBSST20-g-β-CD nanofibrous membrane is contributed to the peculiar hydrophobic cavum of cyclodextrin, which can capture MB molecules by host-guest complexes. In addition, the porous structure in the nanofibrous membrane can adsorb the MB molecules to some extent. The synergy of the two factors is contributed to the high adsorption capacity.
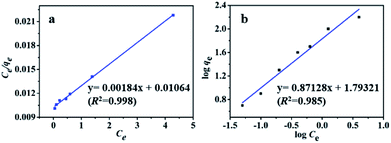 | ||
| Fig. 8 Adsorption isotherm and the corresponding Langmuir plot (a) and Freundlich plot (b) for MB adsorbed by PBSST20-g-β-CD nanofibrous membrane. | ||
| Adsorbent (nanofibrous membranes) | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | b (L mg−1) | R2 | KF | n | R2 | |
| PBST | 49.90 | 0.048 | 0.996 | 3.18 | 1.73 | 0.933 |
| PBSST5 | 55.80 | 0.045 | 0.996 | 3.24 | 1.67 | 0.937 |
| PBSST7 | 62.20 | 0.040 | 0.997 | 3.26 | 1.63 | 0.945 |
| PBSST10 | 67.60 | 0.038 | 0.998 | 3.40 | 1.61 | 0.955 |
| PBSST20 | 74.20 | 0.036 | 0.998 | 3.44 | 1.56 | 0.957 |
| PBSST20-g-β-CD | 543.48 | 0.173 | 0.998 | 62.12 | 1.15 | 0.985 |
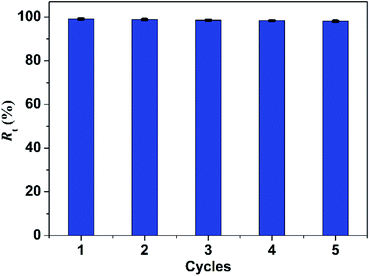
Reusability of adsorbent is essential for protecting ecological environment and reducing the overall cost in practical applications. Thus multiple experiments were conducted to demonstrate the good recyclability of PBSST20-g-β-CD nanofibrous membrane. As shown in the above study, PBSST20-g-β-CD nanofibrous membrane tends to adsorb few MB molecules at a low pH value, so methanol solution containing 5% (v/v) HCl (0.1 mol L−1) was used to regenerate the MB-adsorbed PBSST20-g-β-CD nanofibrous membrane. In this research, the adsorption–desorption cycle was repeated five times and the results are presented in Fig. 9. It can be seen that the Rt value remains at 98% after five regeneration cycles, which suggests that PBSST20-g-β-CD nanofibrous membrane has good recyclability in MB adsorption–desorption. Hence the prepared β-cyclodextrin-included nanofibrous membrane is a good candidate for efficiently removing MB in the printing-dying wastewater.
Conclusions
Novel eco-friendly PBSST-g-β-CD nanofibrous membranes were fabricated as adsorbents for removing MB from aqueous solutions. The results of adsorption experiment indicated that adsorption process of MB was well fitted with the pseudo-second order kinetic model and the Langmuir isotherm model. The maximum adsorption capacity was 543.48 mg g−1 for MB, much higher than many other adsorbents. After five regeneration cycles, MB removal efficiency still remained at 98%, suggesting that the nanofibrous membranes had good recyclability. The excellent adsorption of PBSST-g-β-CD nanofibrous membranes shows that they are promising materials for possible application in disposing printing-dying wastewater.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by Natural Science Fund of Shanghai City (18ZR1400500) and Application Fundamental Projects of China National Textile and Apparel Council.Notes and references
- N. Meunier, P. Drogui, C. Montane, R. Hausler, G. Mercier and J. F. Blais, J. Hazard. Mater., 2006, 137, 581–590 CrossRef CAS PubMed.
- Y. Feng, N. Feng, Y. Wei and G. Zhang, RSC Adv., 2014, 4, 7933 RSC.
- R.-F. Yu, C.-H. Lin, H.-W. Chen, W.-P. Cheng and M.-C. Kao, Chem. Eng. J., 2013, 218, 341–349 CrossRef CAS.
- L. Chen, D. Huang, S. Ren, T. Dong, Y. Chi and G. Chen, Nanoscale, 2013, 5, 225–230 RSC.
- A. L. Ahmad, S. Sumathi and B. H. Hameed, Chem. Eng. J., 2006, 118, 99–105 CrossRef CAS.
- F. Peng, T. Luo and Y. Yuan, New J. Chem., 2014, 38, 4427 RSC.
- C. Zhang, J. Hu, X. Wang, H. Toyoda, M. Nagatsu, X. Zhang and Y. Meng, J. Power Sources, 2012, 198, 112–116 CrossRef CAS.
- H. Wang, L. Ma, K. Cao, J. Geng, J. Liu, Q. Song, X. Yang and S. Li, J. Hazard. Mater., 2012, 229–230, 321–330 CrossRef CAS PubMed.
- Y. Zou, X. Wang, Y. Ai, Y. Liu, J. Li, Y. Ji and X. Wang, Environ. Sci. Technol., 2016, 50, 3658–3667 CrossRef CAS PubMed.
- R. Hu, X. Wang, S. Dai, D. Shao, T. Hayat and A. Alsaedi, Chem. Eng. J., 2015, 260, 469–477 CrossRef CAS.
- D. Li, Q. Li, D. Mao, N. Bai and H. Dong, Bioresour. Technol., 2017, 245, 649–655 CrossRef CAS PubMed.
- X. Gong, D. Huang, Y. Liu, G. Zeng, R. Wang, J. Wei, C. Huang, P. Xu, J. Wan and C. Zhang, Bioresour. Technol., 2018, 253, 64–71 CrossRef CAS PubMed.
- Y. Jiang, B. Liu, J. Xu, K. Pan, H. Hou, J. Hu and J. Yang, Carbohydr. Polym., 2018, 182, 106–114 CrossRef CAS PubMed.
- J. Xiao, W. Lv, Y. Song and Q. Zheng, Chem. Eng. J., 2018, 338, 202–210 CrossRef CAS.
- M.-Y. Lim, Y.-S. Choi, J. Kim, K. Kim, H. Shin, J.-J. Kim, D. M. Shin and J.-C. Lee, J. Membr. Sci., 2017, 521, 1–9 CrossRef CAS.
- L. Szente and J. Szeman, Anal. Chem., 2013, 85, 8024–8030 CrossRef CAS PubMed.
- E. M. M. Del Valle, Process Biochem., 2004, 39, 1033–1046 CrossRef CAS.
- A. I. Schäfer, K. Stelzl, M. Faghih, S. Sen Gupta, K. R. Krishnadas, S. Heißler and T. Pradeep, ACS Sustainable Chem. Eng., 2017, 6, 2942–2953 CrossRef.
- N. Morin-Crini and G. Crini, Prog. Polym. Sci., 2013, 38, 344–368 CrossRef CAS.
- D. M. Alzate-Sánchez, B. J. Smith, A. Alsbaiee, J. P. Hinestroza and W. R. Dichtel, Chem. Mater., 2016, 28, 8340–8346 CrossRef.
- M. Forouharshad, M. Putti, A. Basso, M. Prato and O. Monticelli, ACS Sustainable Chem. Eng., 2015, 3, 2917–2924 CrossRef CAS.
- A. Leudjo Taka, K. Pillay and X. Yangkou Mbianda, Carbohydr. Polym., 2017, 159, 94–107 CrossRef CAS PubMed.
- F. Li, S. Luo, J. Zhang and J. Yu, J. Therm. Anal. Calorim., 2012, 113, 915–921 CrossRef.
- S. Luo, F. Li, J. Yu and A. Cao, J. Appl. Polym. Sci., 2012, 125, 2426–2432 CrossRef CAS.
- J. Zhang, X. Wang, F. Li and J. Yu, Fibers Polym., 2012, 13, 1233–1238 CrossRef CAS.
- Z. Wei, Y. Liu, H. Hu, J. Yu and F. Li, RSC Adv., 2016, 6, 108240–108246 RSC.
- E. Busto, V. Gotor-Fernández, J. Montejo-Bernardo, S. García-Granda and V. Gotor, Tetrahedron, 2009, 65, 8393–8401 CrossRef CAS.
- A. Z. M. Badruddoza, G. S. S. Hazel, K. Hidajat and M. S. Uddin, Colloids Surf., A, 2010, 367, 85–95 CrossRef CAS.
- J. Ju, Z. Shi, L. Fan, Y. Liang, W. Kang and B. Cheng, Mater. Lett., 2017, 205, 190–193 CrossRef CAS.
- S. Haider, F. F. Binagag, A. Haider and W. A. Al-Masry, J. Polym. Res., 2014, 21, 371 CrossRef.
- R. Zhao, Y. Wang, X. Li, B. Sun and C. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 26649–26657 CrossRef CAS PubMed.
- Q. Liu, L. B. Zhong, Q. B. Zhao, C. Frear and Y. M. Zheng, ACS Appl. Mater. Interfaces, 2015, 7, 14573–14583 CrossRef CAS PubMed.
- Y. Zou, X. Wang, Y. Ai, Y. Liu, Y. Ji, H. Wang, T. Hayat, A. Alsaedi, W. Hu and X. Wang, J. Mater. Chem. A, 2016, 4, 14170–14179 RSC.
- J. Liu, G. Liu and W. Liu, Chem. Eng. J., 2014, 257, 299–308 CrossRef CAS.
- R. Zhao, Y. Wang, X. Li, B. Sun, Z. Jiang and C. Wang, Colloids Surf., B, 2015, 136, 375–382 CrossRef CAS PubMed.
- M. Massaro, C. G. Colletti, G. Lazzara, S. Guernelli, R. Noto and S. Riela, ACS Sustainable Chem. Eng., 2017, 5, 3346–3352 CrossRef CAS.
- J. N. Eildal, G. Hultqvist, T. Balle, N. Stuhr-Hansen, S. Padrah, S. Gianni, K. Stromgaard and P. Jemth, J. Am. Chem. Soc., 2013, 135, 12998–13007 CrossRef CAS PubMed.
- C. H. Wu, K. Ito, A. M. Buytendyk, K. H. Bowen and J. I. Wu, Biochemistry, 2017, 56(33), 4318–4322 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectra of serinol and N-BOC-serinol, PBST, PBSST5, PBSST7, PBSST10, PBSST20 and CM-β-CD. UV-vis spectra of MB solution adsorbed by PBSST20-g-β-CD nanofibrous membrane. Average diameters, contact angles and adsorption capacity of PBST, PBSST and PBSST-g-β-CD nanofibrous membranes. See DOI: 10.1039/c8ra07523a |
| This journal is © The Royal Society of Chemistry 2018 |