Open Access Article
Open Access ArticleCobalt immobilized on hydroxyapatite as a low-cost and highly effective heterogeneous catalyst for alkenes epoxidation under mild conditions†
Pagasukon Mekrattanachaiab,
Changyan Cao*ab,
Zhaohua Liab,
Huining Liab and
Weiguo Song *ab
*ab
aBeijing National Laboratory for Molecular Sciences, Laboratory of Molecular Nanostructures and Nanotechnology, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: cycao@iccas.ac.cn; wsong@iccas.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 7th November 2018
Abstract
Transition metal Co immobilized on hydroxyapatite with a loading of 0.05 wt% (denoted 0.05 wt% Co/HAP) could catalyze partial oxidation of cyclic alkenes, aromatic alkenes and aliphatic alkenes to yield epoxide products with excellent selectivity at 30 °C with O2 and iso-butyraldehyde as co-oxidant. The TOF value was as high as 6261 h−1 for epoxidation of cyclohexene. In addition, the prepared 0.05 wt% Co/HAP catalyst can be re-used at least 6 times without significant loss of catalytic activity and selectivity.
Epoxides are very important intermediates for the production of fine chemicals and pharmaceuticals. Epoxidation of alkenes is one of the most effective routes to synthesize epoxides.1–3 In the past decades, many different kinds of catalysts and reaction systems have been developed for alkenes epoxidation with molecular oxygen, hydrogen peroxide or organic oxidants. Homogeneous catalysts usually exhibited high activity and selectivity but they cannot be recycled, resulting in inconvenience for separation. In comparison, heterogeneous catalysts have the obvious advantage of recyclability. Apart from the well-known heterogeneous catalyst based on titanium silicalite (TS-1),4 catalysts based on noble metals (such as Au, Pd, Ru, etc.) were usually used for highly selective epoxidation of alkenes.5–7 Scattered reports about using novel low-cost non-noble metal catalysts, for example, tungsten oxide, gallium oxide and graphitic oxide can also be found in the literature.8–10 However, their activity and selectivity remain relatively low. In order to obtain high yields, these heterogeneous reaction systems were usually operated under harsh conditions, e.g. high temperature, strong organic oxidizing agents. Regarding atom efficiency and cost, it is highly desirable to develop a highly effective heterogeneous catalyst for alkenes epoxidation under mild reaction conditions.
Hydroxyapatite (HAP) which possess Ca2+ sites surrounded by PO43− tetrahedral parallel to the hexagonal axis, have attracted more attention as solid support for recyclable catalysts due to their multiple functionalities. HAP is not only nontoxic and high stability, but it is can be also modified through substitutions of other anions, cations, or functional groups.11–13 This character makes HAP as an excellent support for metals and makes the preparation for heterogeneous catalysts very simple. Based on the ion-exchange mechanism, various HAP-supported transition metals, such as Pd, Ru, Cu and Au, have been prepared and used as heterogeneous catalysts for various organic transformations, including oxidation and Suzuki–Miyaura cross-coupling.14–22 In our previous work, we also found an excellent catalytic system for the epoxidation of alkenes based on 0.05 wt% of Ru species supported on HAP in the presence of molecular oxygen and iso-butyraldehyde as co-oxidant.23 However, Ru is noble metal which is expensive and the reaction was operated at 60 °C to complete the reaction. Therefore, we wondered other metals immobilized on HAP might be more efficiency for alkenes epoxidation, especially for Co because of the special oxidizing property reported in the literatures.24–33
Herein, we indeed find that Co immobilized on hydroxyapatite even with very low loading of 0.05 wt% (denoted as 0.05 wt% Co/HAP) is a highly effective heterogeneous catalyst for alkenes epoxidation under mild condition. It could catalyze partial oxidation of cyclic alkenes, aromatic alkenes and aliphatic alkenes to yield epoxide products with excellent selectivity at 30 °C with O2 and iso-butyraldehyde as co-oxidant. The TOF value could be reached as high as 6261 h−1 for epoxidation of cyclohexene. Such value is among the highest active heterogeneous catalyst for epoxidation of alkenes. In addition, the prepared 0.05 wt% Co/HAP catalyst can be re-used at least 6 times without significant loss of catalytic activity and selectivity.
Various metals immobilized on HAP were prepared through cation-exchange between metal ions in solution and calcium ions in HAP,34,35 and then tested for epoxidation of cyclohexene at 30 °C with O2 and iso-butyraldehyde as co-oxidant in CH3CN solvent. The metals loading in all of prepared catalysts were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Table S1†). No peaks of metals were observed in the XRD patterns (Fig. S1†). As shown in Table 1, 0.05 wt% Co/HAP showed the highest conversion and selectivity, even much higher than those noble metals of Ru and Pd. The TOF value was calculated to be 6261 h−1 at 85% conversion, which was nearly three times higher than that of 0.05 wt% Ru/HAP. In addition, oxygen and iso-butyraldehyde co-oxidant were both indispensable for this reaction system (Table 1, entries 7 and 8). The organic solvents with varying polarity also showed obvious effect on the catalytic activity for cyclohexene epoxidation. As shown in Table S2,† the moderate polarity solvent, CH2Cl2, showed the highest activity in this system. The effect of Co loading was also investigated and the results were presented with TOF value (Table 2). When the higher Co loading (1, 3 and 5 wt% of Co) were immobilized on HAP, more cyclohexene-1-one was observed and the % selectivity was slightly decreased. This might be due to the more Co active sites in the reaction system, the high possibility of over oxidized cyclohexene-1-one product could take place. It can be seen that 0.05 wt% Co/HAP showed the highest TOF value of 245 min−1 and 86% selectivity for 1,2-epoxycyclohexane. This indicated that 0.05 wt% Co/HAP exhibited superior catalytic activity which might be due to the low coordination of Co nanocluster on HAP.
| Entry | Catalyst | TOF (h−1) | Conv.% | Sel.% |
|---|---|---|---|---|
| a Reaction conditions: catalyst (20 mg), cyclohexene (1 mmol), CH3CN (5 mL) iso-butyraldehyde (5 mmol), 30 °C, O2 balloon, 2 h reaction time.b Without oxygen balloon.c Without iso-butyraldehyde. | ||||
| 1 | 0.05 wt% Co/HAP | 6261 | 85 | 93 |
| 2 | 0.05 wt% Ru/HAP | 2148 | 51 | 88 |
| 3 | 0.05 wt% Mn/HAP | 1614 | 47 | 90 |
| 4 | 0.05 wt% Cu/HAP | 1245 | 47 | 88 |
| 5 | 0.05 wt% Fe/HAP | 838 | 54 | 83 |
| 6 | 0.05 wt% Pd/HAP | 1786 | 47 | 64 |
| 7 | 0.05 wt% Co/HAPb | 3388 | 46 | 78 |
| 8 | 0.05 wt% Co/HAPc | 3094 | 42 | 11 |
| Entry | Catalyst | TOF (min−1) | Conv.% | Sel.% |
|---|---|---|---|---|
| a Reaction conditions: catalyst (20 mg), cyclohexene (1 mmol), CH3CN (5 mL) iso-butyraldehyde (5 mmol), 30 °C, O2 balloon, 30 min reaction time. | ||||
| 1 | 0.05 wt% Co/HAP | 245 | 50 | 86 |
| 2 | 1 wt% Co/HAP | 12 | 92 | 85 |
| 3 | 3 wt% Co/HAP | 7 | 95 | 84 |
| 4 | 5 wt% Co/HAP | 6 | 96 | 84 |
The 0.05 wt% Co/HAP catalyst also exhibited excellent catalytic property in epoxidation of other alkenes, including cyclic alkenes, aromatic alkenes and aliphatic alkenes (Table 3). All tested olefins could be converted to epoxides with excellent selectivities. Particularly, allylic alkenes and linear olefins could be converted to epoxide products with high yields under such mild reaction condition, confirming the highly active of 0.05 wt% Co/HAP catalyst in this catalytic system.
| Entry | Substrate | Time (h) | Conv.% | Sel.% |
|---|---|---|---|---|
| a Reaction conditions: catalyst (20 mg), alkene (1 mmol), CH2Cl2 (5 mL), iso-butyraldehyde (5 mmol), 30 °C, O2 balloon. | ||||
| 1 |  |
3.5 | 100 | 100 |
| 2 | 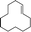 |
5 | 100 | 100 |
| 3 | 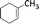 |
6 | 77 | 100 |
| 4 | 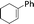 |
24 | 87 | 93 |
| 5 |  |
20 | 88 | 96 |
| 6 |  |
15 | 66 | 100 |
| 7 |  |
30 | 70 | 100 |
| 8 |  |
6 | 92 | 100 |
| 9 |  |
14 | 73 | 100 |
| 10 |  |
17 | 70 | 95 |
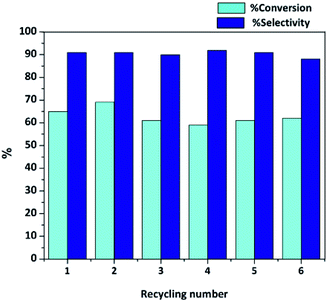
Besides the high activity and selectivity, 0.05 wt% Co/HAP showed the good recyclability. During recycles, the solid catalyst was recovered from reaction mixture by centrifugation, washed with dichloromethane and then dried at 60 °C under vacuum overnight. As shown in Fig. 1, the prepared 0.05 wt% Co/HAP catalyst can be re-used at least 6 times without significant loss of catalytic activity and selectivity. Additionally, the Co loading of the used 0.05 wt% Co/HAP after the 6th run reaction was determined by ICP-AES. The result showed that Co loading was 0.05 wt%, which was the same as the fresh catalyst. Therefore, this confirmed the high stability of the Co/HAP catalyst and the leaching of Co species was not observed. Furthermore, the BET surface area after the re-used catalyst was only a little decrease compared with the fresh catalyst (Fig. S2†). This might be due to the adsorption capacity of HAP support, which could adsorb some organic molecules on its surface.
Based on the above results, the facile synthesis procedures, extremely low percentage of Co, together with excellent catalytic performances and stability make 0.05 wt% Co/HAP a low-cost and highly effective heterogeneous catalyst for olefins epoxidation under mild reaction conditions.
Physical structure of 0.05 wt% Co/HAP was characterized by various techniques in order to preliminary clarifying the excellent catalytic property. In the XRD patterns, all the peaks of 0.05 wt% Co/HAP can be ascribed to the pure hexagonal structure HAP (JCPDS 74-0565).36,37 No peaks of Co species were observed (Fig. 2a), suggesting the extremely low loading and highly dispersion of Co species. In addition, no obvious dots or nanoparticles were observed in the dark-field TEM image (Fig. S3†), while highly dispersed Co species can be clearly seen in the EDS mapping image (Fig. 2b). These results indicated that Co species in 0.05 wt% Co/HAP might exist as single atoms or clusters. The 0.05 wt% Co/HAP catalyst in this study was prepared through cation-exchange between Co2+ in solution and Ca2+ on HAP.34,35 Co species should be existed as monomeric cations surrounded by oxygen, similar with that structure of Ru/HAP in the literature.38 Co–O linkage structure makes Co species electron deficient, leading to prefer affinity with double bond of alkenes. Thus, the highly dispersed Co species on HAP exhibited the superior catalytic activity.
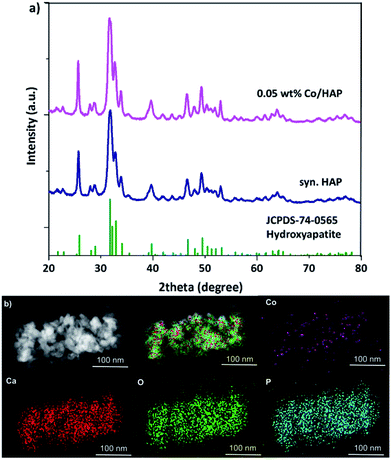 | ||
| Fig. 2 (a) XRD patterns of synthesized HAP and 0.05 wt% Co/HAP, (b) dark field TEM image of 0.05 wt% Co/HAP and EDS mapping images. | ||
Based on the above results and literatures about heterogeneous catalysts for alkenes epoxidation.19,39–41 We proposed a plausible reaction mechanism for cyclohexene epoxidation over our catalysis system (Scheme S1†). Firstly, acylperoxy radicals were formed through homo and heterolytic cleavage of oxygen molecules and iso-butyraldehyde; then, Co species react with oxygen atom of acylperoxy radical, which was subsequently coordinated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of alkenes; finally, the oxidative addition of acylperoxy radical to C
C bond of alkenes; finally, the oxidative addition of acylperoxy radical to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond take place, yielding epoxide product companying the formation of butyric acid.
C bond take place, yielding epoxide product companying the formation of butyric acid.
Conclusions
Co immobilized on hydroxyapatite with loading of 0.05 wt% was found to be a highly effective heterogeneous catalyst for alkenes epoxidation under mild conditions, e.g. reaction temperature at 30 °C, O2 and iso-butyraldehyde as co-oxidant. Various alkenes, including cyclic alkenes, aromatic alkenes and aliphatic alkenes can be converted to epoxide products with excellent selectivity. The TOF value for epoxidation of cyclohexene could be reached as high as 6261 h−1. In addition, the prepared 0.05 wt% Co/HAP catalyst also showed good recyclability, no significant loss of catalytic activity and selectivity were observed after the 6th runs.According to the excellent catalytic performances and high stability of 0.05 wt% Co/HAP catalyst, this might be indicated that Co species might exist as single atom or nanocluster with low coordination environment. Additionally, the initial success in achieving selective epoxidation over our prepared 0.05 wt% Co/HAP catalyst might provide a new avenue for developing new types of catalyst with small amount of metal loading.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the financial supports from the National Natural Science Foundation of China (NSFC 21333009, 21573244, 21573245) and the Youth Innovation Promotion Association of CAS (2017049).Notes and references
- Y. Nishiyama, Y. Nakagawa and N. Mizuno, Angew. Chem., Int. Ed., 2001, 40, 3639–3641 CrossRef CAS PubMed.
- A. Fingerhut, O. V. Serdyuk and S. B. Tsogoeva, Green Chem., 2015, 17, 2042–2058 RSC.
- R. Neumann and M. Dahan, Nature, 1997, 388, 353–355 CrossRef CAS.
- G. Grigoropoulou, J. H. Clark and J. A. Elings, Green Chem., 2003, 5, 1–7 RSC.
- M. Turner, V. B. Golovko, O. P. H. Vaughan, P. Abdulkin, A. B. Murcia, M. S. Tikhov, B. F. G. Johnson and R. M. Lambert, Nature, 2008, 454, 981–983 CrossRef CAS PubMed.
- M. Tada, S. Muratsugu, M. Kinoshita, T. Sasaki and Y. Iwasawa, J. Am. Chem. Soc., 2010, 132, 713–724 CrossRef CAS PubMed.
- X. He, L. Chen, X. Zhou and H. Ji, Catal. Commun., 2016, 83, 78–81 CrossRef CAS.
- K. Kamata, K. Yonehara, Y. Sumida, K. Hirata, S. Nojima and N. Mizuno, Angew. Chem., Int. Ed., 2011, 50, 12062–12066 CrossRef CAS PubMed.
- W. Lueangchaichaweng, N. R. Brooks, S. Fiorilli, E. Gobechiya, K. Lin, L. Li, S. Parres-Esclapez, E. Javon, S. Bals, G. V. Tendeloo, J. A. Martens, C. E. A. Kirschhock, P. A. Jacobs and P. P. Pescarmona, Angew. Chem., Int. Ed., 2014, 53, 1585–1589 CrossRef CAS PubMed.
- S. Pattisson, E. Nowicka, U. N. Gupta, G. Shaw, R. L. Jenkins, D. J. Morgan, D. W. Knight and G. J. Hutchings, Nat. Commun., 2016, 7(12855), 1–9 Search PubMed.
- K. Mori, T. Hara, M. Oshiba, T. Mizugaki, K. Ebitani and K. Kaneda, New J. Chem., 2005, 29, 1174–1181 RSC.
- Y. Masuyama, Y. Sugioka, S. Chonan, N. Suzuki, M. Fujita, K. Hara and A. Fukuoka, J. Mol. Catal. A: Chem., 2012, 352, 81–85 CrossRef CAS.
- C. Mondelli, D. Ferri and A. Baiker, J. Catal., 2008, 258, 170–176 CrossRef CAS.
- M. Gruselle, J. Organomet. Chem., 2015, 793, 93–101 CrossRef CAS.
- P. Zhang, T. Wu, T. Jiang, W. Wang, H. Liu, H. Fan, Z. Zhang and B. Han, Green Chem., 2013, 15, 152–159 RSC.
- R. Radhakrishnan, K. Kannan, S. Kumaravel and S. Thiripuranthagan, RSC Adv., 2016, 6, 45907–45922 RSC.
- B. Maaten, J. Moussa, C. Desmarets, P. Gredin, P. Beaunier, T. Kanger, K. Tonsuaadu, D. Villemin and M. Gruselle, J. Mol. Catal. A: Chem., 2014, 393, 112–116 CrossRef CAS.
- J. Guo, H. Yu, F. Dong, B. Zhu, W. Huang and S. Zhang, RSC Adv., 2017, 7, 45420–45431 RSC.
- K. E. Kabouss, M. Kacimi, M. Ziyad, S. Ammar, A. Ensuque, J. Y. Piquemal and F. B. Verduraz, J. Mater. Chem., 2006, 16, 2453–2463 RSC.
- A. Indra, C. S. Gopinath, S. Bhaduri and G. K. Lahiri, Catal. Sci. Technol., 2013, 3, 1625–1633 RSC.
- N. Jamwal, M. Gupta and S. Paul, Green Chem., 2008, 10, 999–1003 RSC.
- Y. Feng, W. Xue, H. Yin, M. Meng, A. Wang and S. Liu, RSC Adv., 2015, 5, 106918–106929 RSC.
- P. Mekrattanachai, J. Liu, Z. Li, C. Cao and W. Song, Chem. Commun., 2018, 54, 1433–1436 RSC.
- D. Xu, P. Lu, P. Dai, H. Wang and S. Ji, J. Phys. Chem. C, 2012, 116, 3405–3413 CrossRef CAS.
- Y. Li, S. Zhao, Q. Hu, Z. Gao, Y. Liu, J. Zhang and Y. Qin, Catal. Sci. Technol., 2017, 7, 2032–2038 RSC.
- J. J. Stracke and R. G. Finke, J. Am. Chem. Soc., 2011, 133, 14872–14875 CrossRef CAS PubMed.
- L. Ji, J. lin and H. C. Zeng, J. Phys. Chem. B, 2000, 104, 1783–1790 CrossRef CAS.
- I. I. Soykal, H. Sohn and U. S. Ozkan, ACS Catal., 2012, 2, 2335–2348 CrossRef CAS.
- C. Weerakkody, S. Biswas, W. Song, J. He, N. Wasalathanthri, S. Dissanayake, D. A. Kriz, B. Dutta and S. L. Suib, Appl. Catal., B, 2018, 221, 681–690 CrossRef CAS.
- Z. Wang, X. Hou, J. Shen and T. Li, RSC Adv., 2016, 6, 89503–89509 RSC.
- M. Zhao and C. D. Wu, Catal. Commun., 2017, 99, 146–149 CrossRef CAS.
- Z. Asgharpour, F. Farzaneh and A. Abbasib, RSC Adv., 2016, 6, 95729–95739 RSC.
- M. Jafarpour, H. Kargar and A. Rezaeifard, RSC Adv., 2016, 6, 79085–79089 RSC.
- M. E. Zilm, L. Chen, V. Sharma, A. Mcdannald, M. Jain, R. Ramprasad and M. Wei, Phys. Chem. Chem. Phys., 2016, 18, 16457–16465 RSC.
- Z. Opre, T. Mallat and A. Baiker, J. Catal., 2007, 245, 482–486 CrossRef CAS.
- C. M. Ho, W. Y. Yu and C. M. Che, Angew. Chem., Int. Ed., 2004, 43, 3303–3307 CrossRef CAS PubMed.
- A. Peeters, L. Claes, I. Geukens, I. Stassen and D. D. Vos, Appl. Catal., A, 2014, 469, 191–197 CrossRef CAS.
- K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2003, 125, 11460–11461 CrossRef CAS PubMed.
- J. Sebastian, K. M. Jinka and R. V. Jasra, J. Catal., 2006, 244, 208–218 CrossRef CAS.
- B. B. Wentzel, P. L. Alsters, M. C. Feiters and R. J. M. Nolte, J. Org. Chem., 2004, 69, 3453–3464 CrossRef CAS PubMed.
- T. C. Chou and S. V. Lee, Ind. Eng. Chem. Res., 1997, 36, 1485–1490 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, solvents effect and TEM image of catalyst. See DOI: 10.1039/c8ra07168c |
| This journal is © The Royal Society of Chemistry 2018 |