Open Access Article
Open Access Article1,2-Benzoxathiin-4(3H)-one 2,2-dioxide – new enol nucleophile in three-component interaction with benzaldehydes and active methylene nitriles†
Galyna V. Grygoriv a,
Dmitry A. Lega
a,
Dmitry A. Lega a,
Valentin P. Chernykh
a,
Valentin P. Chernykh a,
Lucjusz Zaprutko
a,
Lucjusz Zaprutko *b,
Andrzej K. Gzella
*b,
Andrzej K. Gzella b,
Anna Pawełczyk
b,
Anna Pawełczyk b and
Leonid A. Shemchuk
b and
Leonid A. Shemchuk *a
*a
aNational University of Pharmacy, Pushkinska Str. 53, Kharkiv, 61001, Ukraine. E-mail: orgchem@nuph.edu.ua
bPoznan University of Medical Sciences, Pharmaceutical Faculty, Organic Chemistry Department, 6 Grunwaldzka Str, 60-780 Poznan, Poland
First published on 6th November 2018
Abstract
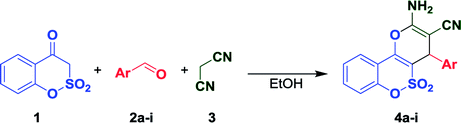
The reactivity of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide was studied in multicomponent type reactions for the first time, namely, in a three-component interaction with active methylene nitriles and aromatic aldehydes in order to construct condensed 2-amino-4H-pyran derivatives. The reaction outcome strongly depended on the nature of an active methylene nitrile and an arenecarbaldehyde. Application of malononitrile resulted in novel 2-amino-4-aryl-4H-pyrano[3,2-c][1,2]benzoxathiine-3-carbonitrile 5,5-dioxides in most cases, whereas the utilization of ethyl cyanoacetate resulted in a complex mixture of products. In the last case, three different products were isolated depending on the arenecarbaldehyde used, namely ethyl 2-amino-4-aryl-4H-pyrano[3,2-c][1,2]benzoxathiine-3-carboxylate 5,5-dioxides, ethyl 2-cyano-3-arylacrylates, and salts of 3,3′-(arylmethylene)bis(4-hydroxybenzo[e][1,2]oxathiine 2,2-dioxides). Attempts to obtain separately ethyl 2-amino-4-aryl-4H-pyrano[3,2-c][1,2]benzoxathiine-3-carboxylate 5,5-dioxides enabled us to propose reaction pathways leading to these products. The salts were obtained for the first time. The preparative method for the synthesis of triethylammonium salts of 3,3′-(arylmethylene)bis(4-hydroxybenzo[e][1,2]oxathiine 2,2-dioxides) was proposed by the direct interaction of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide with arenecarbaldehydes. The application of ammonium acetate as a catalyst allowed us to synthesize 7-aryl-7,14-dihydrobenzo[5,6][1,2]oxathiino[4,3-b]benzo[5,6][1,2]oxathiino[3,4-e]pyridine 6,6,8,8-tetraoxides containing a novel heterocyclic system. These facts, combined with our past investigations, allowed us to assert that the reactivity of enol nucleophiles that include the COCH2SO2X fragment has not been reported previously.
1. Introduction
To date, synthetic organic chemistry does a great deal of work and it is difficult to find a relatively simple organic compound which is poorly studied in respect of its chemical and/or biological properties. Nevertheless, 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) turned out to be one of these ‘dark places’ in medicinal chemistry (Fig. 1).Despite the presence of the synthetically attractive COCH2SO2 moiety in its structure, the chemistry of the 1,2-benzoxathiin-4(3H)-one 2,2-dioxide core is scarcely investigated. There are only a few reports dedicated to the study of its chemical transformations and are mainly disclosed in the works of Löwe et al.1 and also some other researchers.2 However, all of them are based on multi-stage processes, which reduce their attractiveness in terms of creating combinatorial libraries of new compounds.3 This could be related to the fact that the multicomponent processes are often associated with ambiguous reaction mechanisms, selectivity issues or unexpected outcomes.4 However, such reactions still remain the main provider of novel core-structures.5
At the beginning of the 20th century the concept of isosterism became relevant in medicinal chemistry and remains a powerful tool for the purposeful searching of new bioactive substances.6 In this regard, the 1,2-benzoxathiin-4(3H)-one 2,2-dioxide core can be considered as an isostere for two groups of heterocycles. The first of them comprises such a famous pharmacophore as the 4-hydroxycoumarin core A (Fig. 2). Its derivatives have become well-known drugs and among others are used as anticoagulants by means of vitamin K inhibiting.7 The second group includes 1H-2,1-benzothiazin-4(3H)-one 2,2-dioxide B derivatives, which have recently shown a high effectiveness in the treatment of pain and inflammation and are much stronger NSAIDs compared to the famous drugs Piroxicam® and Meloxicam®.8 In this way 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) conceals inside itself a great potential for drug creation.
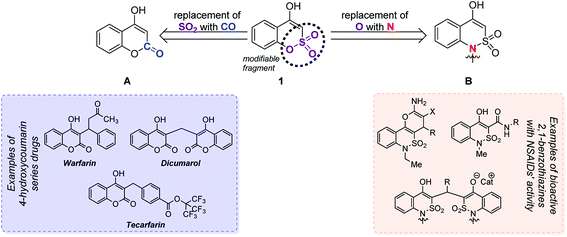 | ||
| Fig. 2 Isosteric relations of the 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) core with 4-hydroxycoumarin (A) and 1H-2,1-benzothiazin-4(3H)-one 2,2-dioxide (B) derivatives. | ||
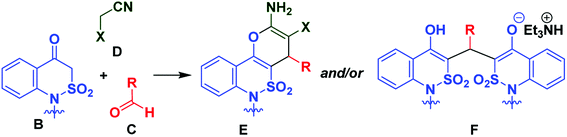
Previously, we explored 1H-2,1-benzothiazin-4(3H)-one 2,2-dioxide (B) as a synthetic analog of 1,3-dicarbonyls in the multicomponent synthesis of fused 2-amino-4H-pyrans E based on the three-component interaction of the former with wide range of aldehydes C and active methylene nitriles D (Scheme 1).9,10 This allowed us to isolate two product types – 2-amino-4H-pyrans E and/or salts F, depending on the starting reagents used and conditions applied in the reaction.
As for 2-amino-4H-pyrans, they have previously appeared to be a promising chemical class to search among for substances displaying valuable kinds of bioactivity such as anticancer,11 antibacterial,12,13 anti-rheumatic14 and others.
Thereby, as a logical continuation of our research, in this paper we, for the first time, have uncovered the chemical behavior of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) in multicomponent type reactions, particularly in the three-component interaction with active methylene nitriles and aromatic aldehydes in order to construct condensed 2-amino-4H-pyrans.
2. Results and discussion
1,2-Benzoxathiin-4(3H)-one 2,2-dioxide (1) was synthesized by a modified two-stage procedure described in the work15 using methyl salicylate as the starting compound (see Experimental section).Literature data reveal the application of various reaction conditions and catalysts in the three-component interaction of enol nucleophiles with active methylene nitriles and carbonyl compounds easily leading in most cases to 2-amino-4H-pyrans irrespective of the reactant nature.16 Previously found features gave us an opportunity to predict a non-conventional reactivity of SO2-containing enol nucleophiles in such types of interactions.9,10b Because of this, first of all we studied the model reaction of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) with malononitrile (3) as an active methylene nitrile and benzaldehyde (2a) as a representative of aromatic aldehydes to find acceptable reaction conditions resulting in the target condensed 2-amino-4-phenyl-4H-pyran-3-carbonitrile 4a (Table 1). Initially, the task was set to find the most suitable catalyst for the reaction using common reaction conditions according to the literature data,16,17 specifically the interaction of equimolar quantities of the reagents in ethanol. Results for this step are given in Table 1. As can be seen from the table, without any catalyst the reaction resulted in the formation of fused compound 4a with only 4% yield. Thereby, the range of basic and acidic catalysts was tested in order to increase the reaction yield. According to the obtained data, triethylamine turned out to be the most acceptable catalyst for the tested reaction (Table 1, entry 4). It should also be noted that the usage of the much stronger base 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU) led to a significant decrease of the 2-amino-4H-pyran 4a yield. Applying more than 0.1 equiv. of triethylamine as well as prolongation of the reaction time and carrying out of the reaction under room temperature did not result in improving the reaction efficiency. Despite the previously reported successful application of acidic catalysts in the synthesis of 2-amino-4H-pyrans,18,19 we failed in our efforts to obtain the target 2-amino-4H-pyran 4a when p-toluenesulfonic acid was applied.
| Entry | ArCHO | Ar | Catalysta | Time (h) | Yield of 4b, % |
|---|---|---|---|---|---|
| a A catalyst was used in the amount of 0.1 equiv. unless otherwise specified.b Isolated yields.c Reaction was carried out at room temperature. | |||||
| 1 | 2a | Ph | No cat. | 1 | 4 |
| 2 | 2a | Ph | L-Lysine | 1 | 60 |
| 3 | 2a | Ph | AcONH4 | 1 | 68 |
| 4 | 2a | Ph | Et3N | 1 | 89 |
| 5 | 2a | Ph | DBU | 1 | 23 |
| 6 | 2a | Ph | TsOH | 1 | Not isol. |
| 7 | 2a | Ph | Et3N | 1c | 75 |
| 8 | 2a | Ph | Et3N | 3 | 64 |
| 9 | 2a | Ph | Et3N (1 equiv.) | 1 | 83 |
| 10 | 2b | 2-MeO–C6H4 | Et3N | 1 | 77 |
| 11 | 2c | 4-MeO–C6H4 | Et3N | 1 | 85 |
| 12 | 2d | 4-HO-3-MeO–C6H4 | Et3N | 1 | 96 |
| 13 | 2e | 3,4,5-TriMeO–C6H2 | Et3N | 1 | 88 |
| 14 | 2f | 2-EtO2CCH2O–C6H4 | Et3N | 1 | 79 |
| 15 | 2g | 2-NCCH2O–C6H4 | Et3N | 1 | 66 |
| 16 | 2h | 4-Cl-C6H4 | Et3N | 1 | 87 |
| 17 | 2i | 4-O2N–C6H4 | Et3N | 1 | 81 |
According to the data given above, the reflux of equimolar quantities of the reactants in ethanol for 1 h in the presence of 0.1 equiv. of triethylamine was chosen as the general condition for further investigations. In this regard, a range of substituted aromatic aldehydes was employed at the next step and a series of 2-amino-4-aryl-4H-pyrano[3,2-c][1,2]benzoxathiine-3-carbonitrile 5,5-dioxides 4b–i was obtained in high yields (Table 1). All the condensed derivatives 4 were isolated as white or slightly colored crystalline precipitates that can be recrystallized from a mixture EtOH-DMF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
As it turned out, the application of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) as an enol nucleophile in the studied reaction had some limitations. Thus, the usage of benzaldehydes containing a strong electron donating substituent (4-dimethylaminobenzaldehyde), or a bulky aromatic residue (9-antraldehyde) resulted in the isolation of only the corresponding Knoevenagel products (β-aryl-α-cyanoacrylonitriles) with almost quantitative yields despite our efforts to vary the reaction conditions (reflux for 20 h, application of a range of basic catalysts). This result can be explained not only by means of a significant decrease of the double bond reactivity of the Knoevenagel products in the course of the Michael addition reaction to benzoxathiinone 1, but also by a reduced nucleophilicity of 3-C in O-containing benzoxathiinone 1 compared with its N-containing analog – benzothiazinone B.9
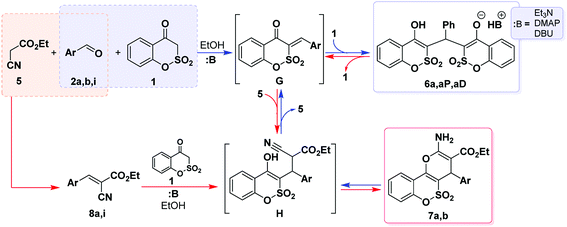
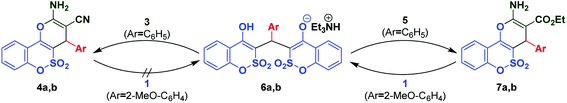
The next step of the current research was the utilization of ethyl cyanoacetate (5) instead of malononitrile (3) in order to investigate the scope of the studied reaction (Scheme 2). Applying unsubstituted benzaldehyde (2a) as a representative in the three-component interaction made it possible to get a new interesting result in the chemistry of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1). Thus, the use of equimolar amounts of the reagents under reflux for 4 h (Et3N, 1 equiv.) led to the isolation of triethylammonium salt 6a in 17% yield, which can be easily detected in its 1H NMR spectrum by the presence of an OH signal as the most downfield peak at about 17 ppm.
Similar salts were isolated for the first time under investigations of the three-component condensation involving N-ethyl-1H-2,1-benzothiazin-4(3H)-one 2,2-dioxide, ethyl cyanoacetate and benzaldehydes.9 This allowed us to assert that such chemical behavior is the general property of cyclic SO2-containing enol nucleophiles of the type XSO2CH2CO. Because of the low yield of 6a and still being surprised by its isolation, we additionally analyzed the residue obtained by removing the solvent under reduced pressure using a 1H NMR method. The obtained data evidenced the formation of the desired ethyl 2-amino-4-phenyl-4H-pyran-2-carboxylate 7a along with the salt 6a and ethyl α-cyano-β-phenylacrylate 8a in an approximately molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45. The application of other benzaldehydes gave us the following results: 2-methoxybenzaldehyde (2b), reacted under the above-mentioned conditions, gave the target 2-amino-4H-pyran 7b in 27% yield, while the application of 4-nitrobenzaldehyde (2i) resulted in the isolation of arylidene 8i.
0.45. The application of other benzaldehydes gave us the following results: 2-methoxybenzaldehyde (2b), reacted under the above-mentioned conditions, gave the target 2-amino-4H-pyran 7b in 27% yield, while the application of 4-nitrobenzaldehyde (2i) resulted in the isolation of arylidene 8i.
It could be assumed that the product mixture, containing 6, 7 and 8, was formed in the course of the three-component reaction involving ethyl cyanoacetate 5. In this regard, the isolation of various compositions of the products, depending on the benzaldehyde applied, is related to a different solubility of an individual product in ethanol.
To achieve the goal of the current research we tested several routes, namely the utilization of ethyl cyanoacetate 5 excess, applying different heating modes, variation of catalysts and also a two-component approach20 (8 + 1) towards 2-amino-4H-pyrans 7.
2.1. Excess of ethyl cyanoacetate 5
The formation of triethylammonium salts 6 and ethyl 2-amino-4H-pyran-3-carboxylates 7 was considered by us as two competitive processes (Scheme 2). Previously, the application of ethyl cyanoacetate excess allowed us to shift the equilibrium, established in the reaction mixture, towards the formation of the target ethyl 2-amino-4H-pyran-3-carboxylates.9,10b According to this, it was logical to use an excess of 5 in the studied reaction. Thus, the utilization of 2 equiv. of 5 (1 equiv. of Et3N, reflux for 4 h) also led to the isolation of salt 6a in 24% yield. Applying of 3 equiv. of 5 under the previous reaction conditions allowed us to isolate the mixture of the initial 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1), arylidene 8a and target 2-amino-4H-pyran-3-carboxylate 7a in an approximate molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to 1H NMR spectroscopy. It might have seemed like the reaction did not complete during the heating time. In this regard, the above-mentioned interaction was carried out for 10 h in similar conditions, but the mixture of equimolar amounts of 2-amino-4H-pyran-3-carboxylate 7a and triethylammonium salt 6a was isolated. The same mixture was also isolated when a 4-fold excess of 5 was used. This may serve as evidence of an equilibrium established between the products 6a and 7a in the course of the reaction performed. When a 5-fold or more excess of 5 was introduced to the reaction, no solid was isolated from the mixture.
1 according to 1H NMR spectroscopy. It might have seemed like the reaction did not complete during the heating time. In this regard, the above-mentioned interaction was carried out for 10 h in similar conditions, but the mixture of equimolar amounts of 2-amino-4H-pyran-3-carboxylate 7a and triethylammonium salt 6a was isolated. The same mixture was also isolated when a 4-fold excess of 5 was used. This may serve as evidence of an equilibrium established between the products 6a and 7a in the course of the reaction performed. When a 5-fold or more excess of 5 was introduced to the reaction, no solid was isolated from the mixture.
2.2. Variation of catalysts
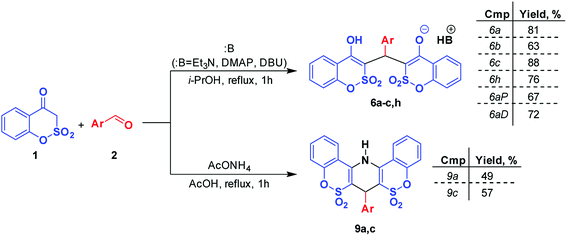
Literature data reveal that a wide range of basic catalysts can be applied in similar interactions that result in high yields of 2-amino-4H-pyrans irrespective of the nature of the 1,3-dicarbonyl and nitrile components.21 However, it was shown that, in certain instances, the catalyst is a crucial factor directing the reaction towards unexpected outcomes.16 We selected 4-dimethylaminopyridine (DMAP), DBU and ammonium acetate as the tested catalysts. The first was chosen since in the case of the N-containing analog of 1, DMAP appeared to be an effective catalyst in the synthesis of some 3-ethoxycarbonyl derivatives of 2-amino-4H-pyrans9 and DBU was selected because of its high basicity. However, reflux for 4 h of the alcohol solution of benzoxathiinone 1 with benzaldehyde (2a) and 3 equiv. of 5 (Scheme 2) under the catalysis of DMAP (1 equiv.) led to the isolation of 4-dimethylaminopyridinium salt 6aP in 37% yield. A similar result was also obtained when DBU was used as a catalyst in the reaction and salt 6aD was isolated with 43% yield. In the case of ammonium acetate utilization (1 equiv., AcOH, reflux for 4 h), analysis of the reaction mixture by a 1H NMR method showed the presence of the starting benzoxathiinone 1 together with ethyl 2-amino-4H-pyran-3-carboxylate 7a and arylidene 8a.2.3. Two-component approach
According to the above-mentioned, variation of the reaction conditions did not provide us with the desired outcome. Because of this, we focused our attention on the two-component format of ethyl 2-amino-4H-pyran-3-carboxylates 7 synthesis based on the reaction of the Knoevenagel products 8 with benzoxathiinone 1 (Scheme 2). One could assume that such an approach would allow us to avoid the formation of salts 6 (as the result of a possible direct interaction of 1 + 2) and additionally would help to clarify some aspects of the reaction mechanism. However, even such a stepwise format under the catalysis of Et3N (1 equiv., reflux for 4 h) led to the isolation of triethylammonium salt 6a in 78% yield. The application of DBU instead of Et3N in the two-component format led to the corresponding salt 6a (yield – 33%) even at room temperature. These facts suggest that the reaction proceeds through the formation of the highly reactive arylidene G formed by Michael cleavage of the initially generated adduct H (Scheme 2). Intermediate G subsequently adds benzoxathiinone 1 in the presence of a base giving salt 6. Apparently, the transformation of salt 6 into ethyl 2-amino-4H-pyran-3-carboxylate 7 is also possible by means of the reverse reactions.The use of ammonium acetate provided us with another new derivative of 1,2-benzoxathiine 2,2-dioxide and a novel product for such types of interactions – 7-phenyl-7,14-dihydrobenzo[5,6][1,2]oxathiino[4,3-b]benzo[5,6][1,2]oxathiino[3,4-e]pyridine 6,6,8,8-tetraoxide (9a) when a mixture of 1 and 8a was refluxed in AcOH for 1 h in the presence of 1 equiv. of ammonium acetate (Scheme 3). Apparently, the initially formed ammonium salt under the reaction conditions loses 2 molecules of water and cyclizes into 1,4-dihydropyridine 9a.
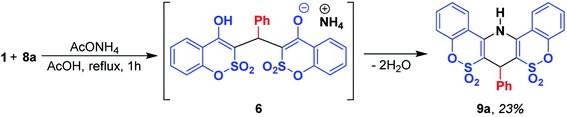 | ||
| Scheme 3 Formation of 7-phenyl-7,14-dihydrobenzo[5,6][1,2]oxathiino[4,3-b]benzo[5,6][1,2]oxathiino[3,4-e]pyridine 6,6,8,8-tetraoxide 9a. | ||
The interactions, presented in Scheme 2, resulted into salts 6 and/or 2-amino-4H-pyrans 7. Thus, there is a clear distinction between the reactivity of malononitrile and ethyl cyanoacetate in the three-component reactions. It is possible that these differences are associated with the different pKa values of the CH2 moiety of the two compounds; one other possible reason for this may be differences in the reactivity of the double bond in the resultant Knoevenagel condensation products. Taking into account the obtained results, we may conclude that the formation of ammonium salts 6 dominates over the formation of ethyl 2-amino-4H-pyran-3-carboxylates 7 and even the application of ethyl cyanoacetate (5) excess, as well as that the use of a two-component format of the synthesis did not allow us to manage the selectivity of the reaction. One could assume that the generation of stable ammonium salts continuously pushes the reaction equilibrium towards their formation. Because of this we decided to check the possibility that salts 6 could be converted into pyrans 7 (Scheme 4). This research we carried out together with the study of the transformation 6 → 4, since the utilization of malononitrile (3) unambiguously led to 2-amino-4H-pyran-3-carbonitriles 4. The reverse transformations 7 → 6 and 4 → 6 were also investigated. It was found that the interaction of salt 6a with malononitrile (3) or ethyl cyanoacetate (5) resulted in the isolation of a mixture of the starting salt and appropriate pyran 4a or 7a. At the same time, only the 3-ethoxycarbonyl derivative 7b was able to be converted into salt 6b when reacted with 1; in the case of the 3-cyano derivative 4b reaction with 1, only the starting materials were recovered. These results explain the facts that the 2-amino-4H-pyran-3-carbonitriles 4 are formed as single products in the three-component interaction when malononitrile (3) was used (Table 1), whereas the application of ethyl cyanoacetate (5) resulted in the isolation of the product mixture (Scheme 2).
 | ||
| Scheme 4 The study of the mutual transformation (EtOH, Et3N, reflux for 4h) of the compounds 4, 6 and 7. | ||
In respect of the interconversions established, the formation of relatively unstable salts in the course of the reaction might shift the equilibrium towards 2-amino-4H-pyrans 7. To check this hypothesis, we introduced into the two-component reaction such a base as sodium acetate. Its application in the reaction of 1 with 8a allowed us to isolate pure target ethyl 2-amino-4-phenyl-4H-pyran-3-carboxylate 7a in 24% yield. A very unexpected result was occasionally gathered by us during the measurement of the 1H NMR spectra of 7a. This essential fact referred to the stability of ethyl 2-amino-4H-pyran-3-carboxylates 7 (Fig. 3). 1H NMR spectra of 7a recorded at 24 h and 48 h showed the presence of a high percentage of the initial benzoxathiinone 1 and ethyl α-cyano-β-phenylacrylate (8a) increasing over time. Apparently, DMSO induces the retro-Michael cleavage of 7a, most probably as a result of its weak basic properties.22
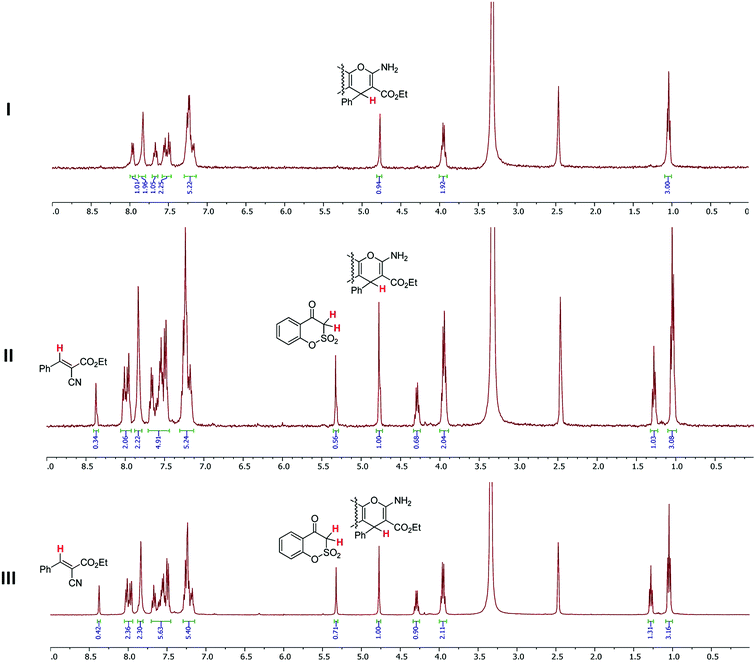 | ||
| Fig. 3 1H NMR spectra of 7a (DMSO-d6, r.t., analyzed by MestReNova v12.0.2) measured: I – immediately after dissolving; II – in 24 h after dissolving; III – in 48 h after dissolving. | ||
Additionally, preparative two-component approaches were worked out for the synthesis of salts 6 and 1,4-dihydropyridines 9. These routes are based on the interaction of benzoxathiinone 1 with benzaldehydes 2 in different reaction conditions (Scheme 5).
Structural features of the synthesized 2-amino-4-aryl-4H-pyrano[3,2-c][1,2]benzoxathiine-3-carbonitrile 5,5-dioxides and triethylammonium 3-((4-hydroxy-2,2-dioxidobenzo[e][1,2]oxathiin-3-yl)(aryl)methyl)benzo[e][1,2]oxathiin-4-olate 2,2-dioxides were confirmed by X-ray crystallographic analysis of example compounds 4b and 6a (Fig. 4).
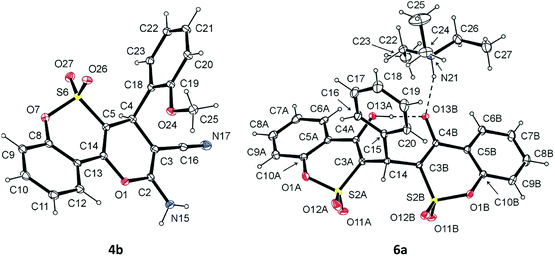 | ||
| Fig. 4 Molecular structure (ORTEP plot) of 4b (deposition no. CCDC 1585657) and 6a (deposition no. CCDC 1585656) showing the atomic labeling scheme. Non-H atoms are drawn as 30% probability displacement ellipsoids and H atoms are drawn as spheres of an arbitrary size. | ||
3. Conclusion
The three-component interaction of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) with benzaldehydes and active methylene nitriles is a complicated process. Its outcome was controlled by the nitrile nature. In the case of malononitrile (3), the products formed were solely 2-amino-4H-pyran-3-carbonitriles 4. When ethyl cyanoacetate (5) was used under basic catalysis, the reaction led to the product mixture as a result of a number of equilibrium processes existing between the starting compounds, ethyl 3-aryl-2-cyanoacrylates 8, ethyl 2-amino-4-aryl-4H-pyran-3-carboxylates 7 and triethylammonium salts 6. In this instance, the composition of the isolated mixture strongly depended on the reaction conditions applied and relative solubility of the components. The low stability established for ethyl 2-amino-4-aryl-4H-pyran-3-carboxylates 7 and the difficulties of obtaining them motivated us to search for a new effective route towards these condensed derivatives, for reasons including their pharmacological attractiveness. The study of the three-component interaction involving ethyl cyanoacetate (5) allowed us to isolate for the first time the ammonium salts 6 and 1,4-dihydropyridines 9.4. Experimental
4.1. Synthesis of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1)
To a cooled to 0 °C vigorously stirred mixture of methyl salicylate (64.50 mL, 0.5 mol), triethylamine (76.51 mL, 0.55 mol) and diethyl ether (700 mL), methanesulfonyl chloride (42.35 mL, 0.55 mol) was added dropwise. The temperature was slowly raised to 15–20 °C and the resulting mixture was stirred for 24 h. After the completion of this time, water (500 mL) was added to the suspension and the organic layer was separated. The ethereal solution was successively washed with a 1 M solution of sodium bicarbonate (100 mL) and water (200 mL), dried over Na2SO4 and concentrated in vacuo. The residue obtained was methyl 2-((methylsulfonyl)oxy)benzoate as a light-yellow solid (mp 35–37 °C) which was used in the next step without additional purification.A solution of methyl 2-((methylsulfonyl)oxy)benzoate (46.0 g, 0.2 mol) in dry DMF (100 mL) was added dropwise to a suspension of NaH (8.8 g of 60% suspension in mineral oil, 0.22 mol) in dry DMF (70 mL) at 0 °C. After completion of the hydrogen evolution, the mixture was additionally stirred for 1.5 h at 0 °C and to the resulted suspension diluted HCl was added to achieve pH 1. The precipitate of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) was collected by filtration, dried in air and recrystallized from ethanol. The total yield of 1 was 64%.
White crystalline powder; mp 87–89 °C (from EtOH) (lit. 2a 89–91 °C); anal. calcd for C18H12N2O4S: C, 48.48; H, 3.05; S, 16.18. Found, %: C 48.61; H 3.11; S, 16.04; 1H NMR (400 MHz, DMSO-d6)23: δ (ppm) 8.00 (d, J = 7.19 Hz, 1H, Ar), 7.85 (t, J = 7.05 Hz, 1H, Ar), 7.42–7.56 (m, 2H, Ar), 5.34 (br. s., 2H, CH2).
4.2. General procedure for the synthesis of 2-amino-4-aryl-4H-pyrano[3,2-c][1,2]benzoxathiine-3-carbonitrile 5,5-dioxides (4a–i)
To a solution of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) (0.198 g, 0.001 mol), the appropriate aromatic aldehyde 2a–i (0.001 mol) and malononitrile (3) (0.066 g, 0.001 mol) in ethanol (10 mL), triethylamine (0.014 mL, 0.0001 mol) was added. The mixture was refluxed for 1 h and cooled to room temperature. The resulting precipitates of 4a–i were filtered off, washed with ethanol, dried in air and recrystallized from the mixture EtOH–DMF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
4.3. Preparative procedure for the synthesis of triethylammonium 3-((4-hydroxy-2,2-dioxidobenzo[e][1,2]oxathiin-3-yl)(aryl)methyl)benzo[e][1,2]oxathiin-4-olate 2,2-dioxides (6a–c,h)24
To a solution of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) (0.198 g, 0.001 mol) and the appropriate aromatic aldehyde 2a–c,h (0.0005 mol) in propan-2-ol (10 mL), triethylamine (0.07 mL, 0.0005 mol) was added. The solution was stirred for 1 h under reflux. The obtained precipitates of 6a–c,h were filtered off, washed with propan-2-ol, dried in air and recrystallized from ethanol.4.4. Synthesis of ethyl 2-amino-4-phenyl-4H-pyrano[3,2-c][1,2]benzoxathiine-3-carboxylate 5,5-dioxides (7)
4.5. Preparative procedure for the synthesis of 7-aryl-7,14-dihydrobenzo[5,6][1,2]oxathiino[4,3-b]benzo[5,6][1,2]oxathiino[3,4-e]pyridine 6,6,8,8-tetraoxides (9)
A solution of 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) (0.396 g, 0.002 mol), the appropriate aromatic aldehyde 2a,c (0.001 mol) and ammonium acetate (0.77 g, 0.01 mol) in AcOH (10 mL) was refluxed for 1 h and cooled to room temperature. The resulting precipitates of 9a,c were filtered off, washed successively with AcOH and water and dried in air.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Mr Eugene S. Gladkov (SSI “ISC” NASU) for his support in obtaining the spectroscopic data for the compounds presented in this paper.Notes and references
- For some examples, see: (a) W. Löwe and C. Müller-Menke, Arch. Pharm., 1985, 318, 954–956 CrossRef; (b) W. Löwe and C. Müller-Menke, Arch. Pharm., 1988, 321, 755–756 CrossRef; (c) W. Löwe, P. Jeske and A. Kradepohl, J. Heterocycl. Chem., 1988, 25, 699–701 CrossRef.
- (a) V. R. Arava, U. B. R. Siripalli, V. Nadkarni and R. Chinnapillai, Beilstein J. Org. Chem., 2007, 3, 20 CrossRef PubMed; (b) C. Peixoto, P. Laurin, M. Klich, C. Dupuis-Hamelin, P. Mauvais, P. Lassaigne, A. Bonnefoy and B. Musicki, Tetrahedron Lett., 2000, 41, 1741–1745 CrossRef CAS.
- C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51–80 CrossRef CAS PubMed.
- (a) Y. V. Sedash, N. Y. Gorobets, V. A. Chebanov, I. S. Konovalova, O. V. Shishkin and S. M. Desenko, RSC Adv., 2012, 2, 6719–6728 RSC; (b) E. A. Muravyova, S. M. Desenko, R. V. Rudenko, S. V. Shishkina, O. V. Shishkin, Y. V. Sen'ko, E. V. Vashchenko and V. A. Chebanov, Tetrahedron, 2011, 67, 9389–9400 CrossRef CAS.
- V. A. Chebanov and S. M. Desenko, Chem. Heterocycl. Compd., 2012, 48, 566–583 CrossRef CAS.
- C. W. Thornber, Chem. Soc. Rev., 1979, 8, 563–580 RSC.
- N. Au and A. E. Rettie, Drug Metab. Rev., 2008, 40, 355–375 CrossRef CAS PubMed.
- (a) I. V. Ukrainets, L. A. Petrushova, S. P. Dzyubenko and G. Sim, Chem. Heterocycl. Compd., 2014, 50, 103–110 CrossRef CAS; (b) D. A. Lega, N. I. Filimonova, I. A. Zupanets, S. K. Shebeko, V. P. Chernykh and L. A. Shemchuk, J. Org. Pharm. Chem., 2016, 14, 3–11 CAS.
- D. A. Lega, N. Y. Gorobets, V. P. Chernykh, S. V. Shishkina and L. A. Shemchuk, RSC Adv., 2016, 6, 16087–16099 RSC.
- (a) D. A. Lega, V. P. Chernykh, L. Zaprutko, A. K. Gzella and L. A. Shemchuk, Chem. Heterocycl. Compd., 2017, 53, 219–229 CrossRef CAS; (b) D. A. Lega, V. P. Chernykh and L. A. Shemchuk, J. Org. Pharm. Chem., 2016, 14, 6–16 CAS.
- A.-G. E. Amr, A. M. Mohamed, S. F. Mohamed, N. A. Abdel-Hafez and A. E.-F. G. Hammam, Bioorg. Med. Chem., 2006, 14, 5481–5488 CrossRef CAS PubMed.
- P. Paliwal, S. Jetti and S. Jain, Med. Chem. Res., 2013, 22, 2984–2990 CrossRef CAS.
- D. Kumar, V. B. Reddy, S. Sharad, U. Dube and S. Kapur, Eur. J. Med. Chem., 2009, 44, 3805–3809 CrossRef CAS PubMed.
- C. W. Smith, J. M. Bailey, M. E. J. Billingham, S. Chandrasekhar, C. P. Dell, A. K. Harvey, C. A. Hicks, A. E. Kingston and G. N. Wishart, Bioorg. Med. Chem. Lett., 1995, 5, 2783–2788 CrossRef CAS.
- C. F. Schwender and B. R. Sunday, US pat. US4116961A, 1978.
- Y. M. Litvinov and A. M. Shestopalov, in Adv. Heterocycl. Chem., ed. A. R. Katritzky, Academic Press, 2011, vol. 103, pp. 175–260 Search PubMed.
- (a) K. Azizi and A. Heydari, RSC Adv., 2014, 4, 6508–6512 RSC; (b) M.-J. Yao, Z. Guan and Y.-H. He, Synth. Commun., 2013, 43, 2073–2078 CrossRef CAS; (c) J. Safaei-Ghomi, R. Teymuri, H. Shahbazi-Alavi and A. Ziarati, Chin. Chem. Lett., 2013, 24, 921–925 CrossRef CAS; (d) G. H. Mahdavinia, M. Mirzazadeh and B. Notash, Tetrahedron Lett., 2013, 54, 3487–3492 CrossRef CAS.
- L. Jalili-Baleh, N. Mohammadi, M. Khoobi, L. Ma'mani, A. Foroumadi and A. Shafiee, Helv. Chim. Acta, 2013, 96, 1601–1609 CrossRef CAS.
- B. Maheshwar Rao, G. N. Reddy, T. V. Reddy, B. L. A. P. Devi, R. B. N. Prasad, J. S. Yadav and B. V. S. Reddy, Tetrahedron Lett., 2013, 54, 2466–2471 CrossRef CAS.
- A. Thakur, M. Tripathi, U. C. Rajesh and D. S. Rawat, RSC Adv., 2013, 3, 18142–18148 RSC.
- G. Brahmachari, in Green Synthetic Approaches for Biologically Relevant Heterocycles, ed. G. Brahmachari, Elsevier, Boston, 2015, pp. 185–208 Search PubMed.
- W. S. MacGregor, Ann. N. Y. Acad. Sci., 1967, 141, 3–12 CrossRef CAS PubMed.
- According to 1H NMR spectrum, 1,2-benzoxathiin-4(3H)-one 2,2-dioxide (1) in DMSO-d6 solution exists in two tautomeric forms – 4-oxo form and 4-hydroxy (enol) form in aproximate molar ratio 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1. - The same synthetic procedure was applied for the preparation of 4-(dimethylamino)pyridin-1-ium 6aP and 3,4,5,6,7,8,9,10-octahydro-2H-pyrimido[1,2-a]azepin-5-ium 6aD salts using DMAP and DBU, respectively.
Footnote |
| † Electronic supplementary information (ESI) available: Full spectroscopic data (1H and 13C NMR, IR, MS) for compounds 4, 6, 7 and 9 as well as X-ray crystallographic data for compounds 4b and 6a. CCDC 1585657 and 1585656. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra06801a |
| This journal is © The Royal Society of Chemistry 2018 |