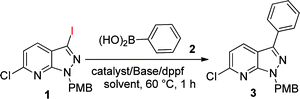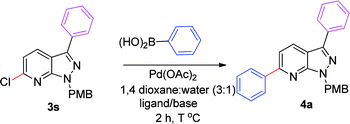Open Access Article
Open Access ArticleSynthesis of 3,6-diaryl-1H-pyrazolo[3,4-b]pyridines via one-pot sequential Suzuki–Miyaura coupling†
Urvashia,
Vibha Tandon *b,
Parthasarathi Das
*b,
Parthasarathi Das *c and
S. Kukreti
*c and
S. Kukreti a
a
aDepartment of Chemistry, University of Delhi, Delhi-110007, India
bSpecial Centre for Molecular Medicine, Jawaharlal Nehru University, New Delhi-110067, India. E-mail: vtandon@mail.jnu.ac.in
cDepartment of Applied Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad-826004, Jharkhand, India. E-mail: partha@iitism.ac.in
First published on 11th October 2018
Abstract
A practical synthesis of diarylpyrazolo[3,4-b]pyridine derivatives by a combination of chemoselective Suzuki–Miyaura cross-coupling reactions was developed. The sequential arylation strategy can be performed in a one-pot manner without much loss of efficiency when compared to the corresponding stepwise synthesis. These conditions are applicable to the coupling of a wide variety of aryl and heteroaryl-boronic acids with pyrazolo[3,4-b]pyridines with high selectivity of the C3 over the C6 position, thus enabling the rapid construction of a diverse array of medicinally important diarylpyrazolo[3,4-b]pyridines.
1. Introduction
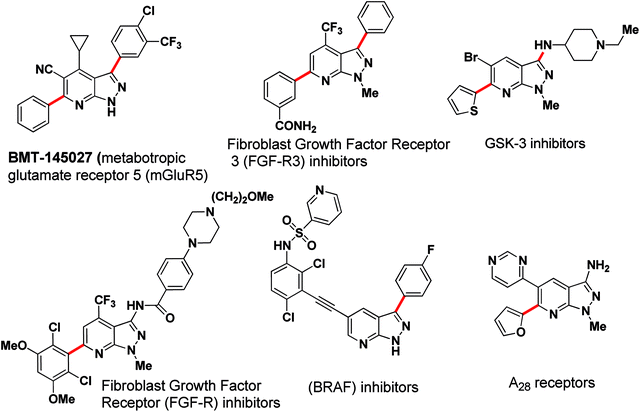
The transition-metal-catalysed cross-coupling reaction has been well-established as a powerful synthetic tool in C–C bond formation. In particular, the Suzuki–Miyaura cross-coupling reaction has shown widespread applications in natural product synthesis, the synthesis of medicinally important pharmacophores and organic materials.1 To explore further synthetic applications of transition-metal-catalyzed cross-coupling reactions, organocatalytic reactions and arylation of heteroaromatics have attracted attention in recent years.2 In the last decade a selective arylation strategy in a sequential manner has emerged as a valuable tool for cross-coupling techniques.3a–d In pursuit of this, selective and/or sequential arylation were studied on a few pharmaceutically important heterocycles e.g. indazoles,3e,f azaindoles,3g,h and imidazopyrazines.3i This strategy, which explores the different reactivities of electrophilic and nucleophilic cross-coupling partners, allows for multiple C-aryl bond formations in a “controlled” manner to rapidly construct functional molecules with complex structures applicable to pharmaceutical and material science.4,5Pyrazolo[3,4-b]pyridine, a privileged heterocyclic core, has been actively pursued in medicinal research due to its wide spectrum of biological activities; viz: glycogen synthase kinase-3 (GSK-3) inhibitors6 and A1 adenosine receptors7 (Fig. 1). Recently, arylated pyrazolo[3,4-b]pyridine has been outlined as a fibroblast growth factor inhibitor (FGF-R and FGFR3) specific for the treatment of bladder cancer,8 as a Raf inhibitor to inhibit B-RafV600E,9 as metabotropic glutamate receptor 5 (mGluR5) positive allosteric modulators (PAM's)10 and as a neuroprotector in MPP+-induced neurodegeneration (Fig. 1).11
A number of methods have been developed to functionalize this fused heterocyclic system.12 Recently, Guillaumet reported the direct heteroarylation of 2H-pyrazolo[3,4-b]pyridines using “on water” conditions13a whereas, Popowycz has reported Pd-catalyzed late-stage C-3 functionalization of pyrazolo[3,4-b]pyridines.13b Despite many advances in the synthesis of C-arylated pyrazolopyridines, a selective and high-yielding method from accessible starting materials remains a goal within the synthetic community. Herein, we report an efficient synthesis of 3,6-diarylpyrazolo[3,4-b]pyridine via site selective sequential Suzuki–Miyaura coupling (SMC).14 The salient features of this protocol are its high chemoselectivity, easy removal of the protecting group and tolerance towards functional groups. The designed 3,6-diarylpyrazolo[3,4-b]pyridines can be synthesized sequentially in a one-pot reaction.
2. Results and discussion
The synthesis of the 6-chloro-3-iodo-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine (1) core was accomplished through cyclisation of 2-chloro-3-cyanopyridine and hydrazine followed by in situ diazotization and iodination at the C3 position of the ring.12a C6-chlorination was undertaken on its N-oxide product and, lastly, removal of the N-protection.15 We commenced the optimization of arylation by coupling 6-chloro-3-iodo-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine (1) with phenylboronic acid (2) using Pd(OAc)2 as a catalyst (5 mol%) and dppf as a ligand (5 mol%) in the presence of Cs2CO3 at 60 °C in THF, giving 68% yield (Table 1, entry 1). Whereas other solvents, e.g. acetonitrile and 1,4-dioxane, failed to provide higher yields (Table 1, entries 2 and 3). However, in combination with 1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3
water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the desired product was isolated in 93% yield. On substituting dppf with a PPh3 ligand we observed an inferior result (Table 1, entry 5). Different Pd-catalysts were screened but no fruitful results were obtained (Table 1, entries 6–8). Moderate yields (63–75%) were recorded with other bases; viz: K2CO3 and Na2CO3 (Table 1, entries 9 and 10). Increasing the water content in the reaction solvent did not lead to a satisfactory result (Table 1, entry 11), suggesting that dioxane
1), the desired product was isolated in 93% yield. On substituting dppf with a PPh3 ligand we observed an inferior result (Table 1, entry 5). Different Pd-catalysts were screened but no fruitful results were obtained (Table 1, entries 6–8). Moderate yields (63–75%) were recorded with other bases; viz: K2CO3 and Na2CO3 (Table 1, entries 9 and 10). Increasing the water content in the reaction solvent did not lead to a satisfactory result (Table 1, entry 11), suggesting that dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water in a ratio of 3
water in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is the best solvent combination. The reaction yield decreased to 77% (Table 1, entry 12) using one equivalent base and in the absence of a base the reaction did not work (Table 1, entry 14). The reaction did not proceed at rt (Table 1, entry 15). Thus, we identified Pd(OAc)2 (5 mol%)/dppf (5 mol%)/Cs2CO3 (2 equiv.)/1,4-dioxane
1 is the best solvent combination. The reaction yield decreased to 77% (Table 1, entry 12) using one equivalent base and in the absence of a base the reaction did not work (Table 1, entry 14). The reaction did not proceed at rt (Table 1, entry 15). Thus, we identified Pd(OAc)2 (5 mol%)/dppf (5 mol%)/Cs2CO3 (2 equiv.)/1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3
water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/60 °C as the standard conditions for the coupling reaction (Table 1, entry 4). This reaction is selective for C3-arylation and in no case was any C6-arylation product detected.
1)/60 °C as the standard conditions for the coupling reaction (Table 1, entry 4). This reaction is selective for C3-arylation and in no case was any C6-arylation product detected.
| Entry | Catalyst | Ligand | Base | Solvent | Yieldb |
|---|---|---|---|---|---|
a Reaction conditions: 6-chloro-3-iodo-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine (1.0 equiv.), arylboronic acid (1.0 equiv.), catalyst (5 mol%), dppf (5 mol%), base (2.0 equiv.), 1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5 mL), 60 °C, 1 h, air.b Isolated yields.c 1.0 equiv. base used.d Reaction performed for 4 h.e At room temperature; n.r. = no reaction. 1) (5 mL), 60 °C, 1 h, air.b Isolated yields.c 1.0 equiv. base used.d Reaction performed for 4 h.e At room temperature; n.r. = no reaction. |
|||||
| 1 | Pd(OAc)2 | dppf | Cs2CO3 | THF | 68 |
| 2 | Pd(OAc)2 | dppf | Cs2CO3 | CH3CN | 45 |
| 3 | Pd(OAc)2 | dppf | Cs2CO3 | Dioxane | 62 |
| 4 | Pd(OAc)2 | dppf | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
93 |
| 5 | Pd(OAc)2 | PPh3 | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
69 |
| 6 | PdCl2(PPh3)4 | dppf | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
73 |
| 7 | Pd(PPh3)4 | dppf | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 |
| 8 | PdCl2 | dppf | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
78 |
| 9 | Pd(OAc)2 | dppf | K2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
75 |
| 10 | Pd(OAc)2 | dppf | Na2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
63 |
| 11 | Pd(OAc)2 | dppf | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
82 |
| 12c | Pd(OAc)2 | dppf | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
77 |
| 13d | Pd(OAc)2 | dppf | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
86 |
| 14 | Pd(OAc)2 | dppf | — | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
n.r. |
| 15e | Pd(OAc)2 | dppf | Cs2CO3 | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
n.r. |
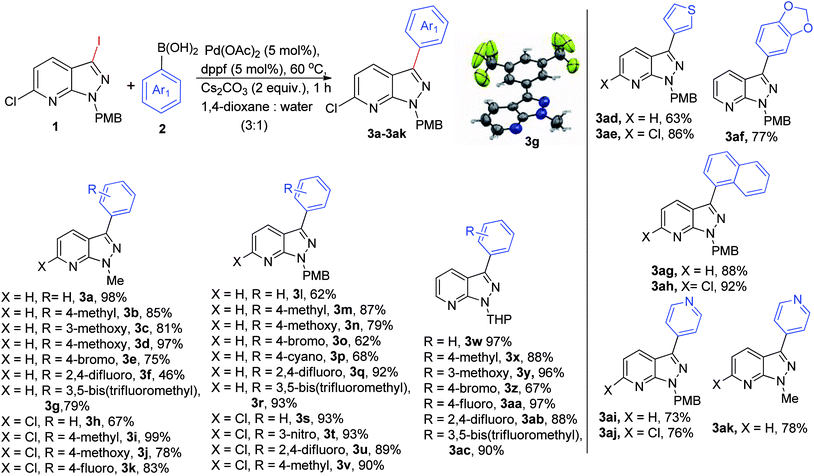
With the optimized protocol in hand, we evaluated the scope of the substrate for coupling reactions (Scheme 1). Interestingly, our standard conditions proved to be efficient for both electron-rich (–Me, –OMe), and electron-deficient (–Br, –F, –CF3, –NO2) boronic acids, leading to the desired product within a range of 62–98% yield with different N-protected pyrazolo[3,4-b]pyridine molecules (Me, PMB, THP) in 1 h. First, we examined coupling of the 3-iodo derivative of N-Me-pyrazolo[3,4-b]pyridine with electronically diverse boronic acids and observed a yield in the range of 75–98% (3a–3g), except for 2,4-difluorophenylboronic acid (3f, 46% yield). Implementation of standard reaction conditions on 6-chloro-3-iodopyrazolopyridines led to the desired product (3h–3k) in excellent yields and to our delight the reaction was chemoselective. A reaction between N-PMB-pyrazolo[3,4-b]pyridines and electron-rich boronic acids gave good yields of 3m-3n, while 3o-3p were obtained in slightly lower yields with electron-deficient partners. The difluoro and bis-(trifluoromethyl)-containing boronic acids proved to be effective in giving 92% and 93% yields for 3q & 3r respectively. High yields (89–93%, 3s–3v) were obtained in the case of 3-iodo-6-chloro-N-PMB-pyrazolo[3,4-b]pyridine and a similar pattern was observed with the single halogenated N-THP-pyrazolo[3,4-b]pyridines (3w–3ac). The thiophene, 3ad–3ae, 63–86% and 6-membered heteroyclic (pyridyl, 3ai-3aj, 73–76%) boronic acids gave desired products in significantly good yields. We were also successful in introducing naphthyl (3ag-3ah) and benzo[d][1,3]dioxole groups (3af) onto the pyrazolo[3,4-b]pyridine core moiety in excellent yields. The crystal structure of 3g was confirmed by X-ray crystallography.
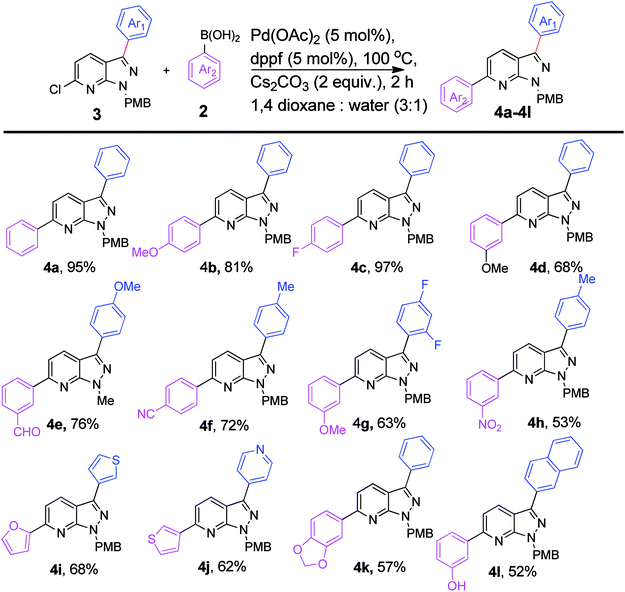
We extended the above findings to C6-arylation on 6-chloro-1-(4-methoxybenzyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (3s) with phenylboronic acid to yield 1-(4-methoxybenzyl)-3,6-diphenyl-1H-pyrazolo[3,4-b]pyridine, 4a (Table 2). Only 54% of the diarylated product was formed on applying the earlier optimized conditions (Table 2, entry 1). Changing the ligand to PPh3 decreased the yield of coupled product to 48% (Table 2, entry 2). Incomplete conversion was observed on using a milder base, such as K2CO3 and Na2CO3 (Table 2, entries 3 and 4). The reaction yield was enhanced to 95% on increasing the temperature to 100 °C. No new spot was formed at rt (Table 2, entry 7). Experimentation showed that the combination of Pd(OAc)2 (5 mol%) and dppf (5 mol%) with 2.0 equiv. of Cs2CO3 in 1,4-dioxane and water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 100 °C for 2 h afforded the C6-arylated product in the best yield (Table 2, entry 6).
1) at 100 °C for 2 h afforded the C6-arylated product in the best yield (Table 2, entry 6).
| Entry | Ligand [5mol%] | Base | T (°C) | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: 6-chloro-1-(4-methoxybenzyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine 3 (1.0 equiv.), arylboronic acid 2 (1.0 equiv.), Pd(OAc)2 (5 mol%), ligand (5 mol%), base (2.0 equiv.), 1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5 mL), 2 h.b Isolated yields.c 15% of starting material recovered; n.r. = no reaction.d 25% of starting material recovered; n.r. = no reaction. 1) (5 mL), 2 h.b Isolated yields.c 15% of starting material recovered; n.r. = no reaction.d 25% of starting material recovered; n.r. = no reaction. |
||||
| 1 | dppf | Cs2CO3 | 60 | 54 |
| 2 | PPh3 | Cs2CO3 | 60 | 48 |
| 3c | dppf | K2CO3 | 60 | 62 |
| 4d | dppf | Na2CO3 | 60 | 55 |
| 5 | dppf | Cs2CO3 | 80 | 78 |
| 6 | dppf | Cs2CO3 | 100 | 95 |
| 7 | dppf | Cs2CO3 | rt | n.r. |
For substrate scope, coupling with 4-fluorophenylboronic acid facilitates excellent reaction yield (97%), when compared to coupling with electron-donating 4-methoxyphenylboronic acid resulted in 81% yield (4b & 4c, Scheme 2). The formyl group functionalized compound, 4e can be utilized for beneficial organic transformations. The reaction of electron-withdrawing (–CN, –NO2) boronic acid also proceeded smoothly with the present starting moiety, giving 72% and 53% of desired products (4f & 4h). The difluorophenyl group containing pyrazolopyridine coupled with 3-methoxyphenylboronic acid produced 4g in 63% yield. The present strategy is efficient enough to couple with heteroaromatic boronic acids producing diarylated product (4i-j) in 62–68% yield. In addition, we successfully arylated the pyrazolo[3,4-b]pyridine with benzo[d][1,3]dioxol-5-ylboronic acid, giving 57% of 4k. The 3-hydroxyphenylboronic acid was quite stable under these conditions, as the coupled product (4l) was isolated in 52% yield.
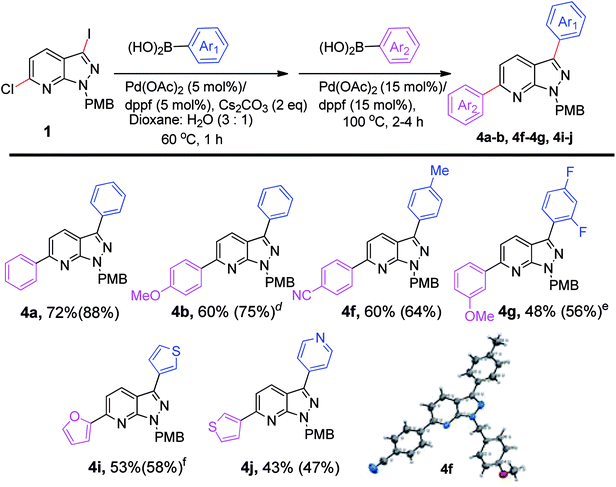
After successful stepwise sequential diarylation, we turned our focus to the one-pot arylation reaction to synthesize 3,6-diarylated pyrazolopyridines (Scheme 3). The combinations of boronic acids that showed the best overall yield in our previous stepwise synthesis of diarylated pyrazolo[3,4-b]pyridines (Scheme 2) were selected for one-pot sequential arylation of 6-chloro-3-iodo-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine (1). The Suzuki cross-coupling was first performed with Pd(OAc)2/dppf as the catalyst in the presence of Cs2CO3 as base and Ar1B(OH)2 at 60 °C. Upon completion of the coupling, a second arylation was accomplished with Ar2B(OH)2 and the same Pd (OAc)2/dppf (15 mol%) catalyst, at 100 °C without purification of the intermediate. The one-pot coupling reaction afforded diaryl-1H-pyrazolo[3,4-b]pyridines (4a-b; 4f-g; 4i-j) in moderate to good yields (43–72%). In a one-pot reaction diphenyl-1H-pyrazolo[3,4-b]pyridine (4a) was isolated in 72% yield. Insertion of 4-methoxyphenylboronic acid as a second arylating agent was successful, as 4b was isolated in 60% yield in 4h. The combination of 4-methylphenylboronic acid as the first arylating agent and 4-cyanophenylboronic acid as the second proved to be successful, with an isolated yield of 60% (4f). The structure of 4f was determined by X-ray crystallography. Further, when 3-methoxyphenylboronic acid reacted with 6-chloro-3-(2,4-difluorophenyl)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine, the product (4g) was isolated in 48% yield in 6 h. The combinations of 5/5 or 5/6 member heterocyclic boronic acids were screened and afforded moderate yields of 53% (4i) and 43% (4j) respectively. The study suggested that the overall yield of biarylated product in the one pot method is comparable to the stepwise sequential method. We have observed that C3 arylation was preferred over C6 arylation, in our reaction conditions. But, we cannot neglect the role of I vs. Cl as one of the governing factors. Thus the one-pot method can be used as a time and cost-effective method for the diarylation of pyrazolopyridines (Scheme 3).
The p-methoxybenzyl protecting group (PMB) was easily cleaved by the action of trifluoroacetic acid (TFA) at 70 °C within 0.25 h.16a We performed a THP de-protection reaction of 3w with methanolic HCl, giving 97% yield of the required product.16b A rapid screening showed that diarylated pyrazolo[3,4-b]pyridines can be de-protected to produce 5a–5d, in excellent yields (Scheme 4). The electron-withdrawing and electron-donating groups do not greatly influence the yield of final compounds. It is important to mention here that the di-heteroarylated product, e.g. a combination of thiophene and furan, remained intact under de-protection conditions to give 5d in 99% yield.
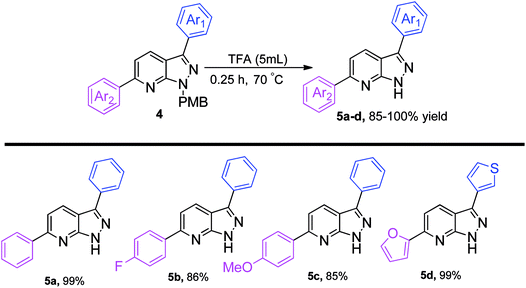 | ||
| Scheme 4 De-protection of the PMB group.a,b aReaction conditions: 3,6-diaryl-1H-pyrazolo[3,4-b]pyridine 4 (1 equiv.), TFA (5 mL), 70 °C, 0.25 h; bisolated yields. | ||
3. Conclusion
In conclusion, we have developed catalytic systems for the stepwise sequential arylation of pyrazolo[3,4-b]pyridines at the C3 and C6 positions via Suzuki–Miyaura reactions. The chemoselective sequential arylation strategy was also performed in one pot without a loss of efficiency when compared to the corresponding stepwise synthesis. This route offers significant flexibility to access heteroaromatic frameworks with challenging substitution patterns. We hope that this new methodology will open access to synthesizing new chemotypes and to discovering functionalized pyrazolo[3,4-b]pyridines with therapeutic potential.4. Experimental
General information
Unless otherwise noted, all reagents were purchased from commercial suppliers and used without purification. All Suzuki–Miyaura reactions were performed in a round bottom flask and monitored through thin layer chromatography (TLC silica gel F254, glass plates) and analysed using 254 nm UV light and iodine, ninhydrin stains. Melting points were recorded on a Büchi Melting Point B-545 instrument and are uncorrected. 1H NMR and 13C NMR spectra were recorded with a 400 MHz (1H = 400 and 13C = 100 MHz) or 500 MHz (1H = 500 and 13C = 125 MHz) spectrometer. Chemical shift values of 1H NMR were recorded in parts per million (ppm, δ) relative to tetramethylsilane (TMS, 0.00 ppm). Multiplicities are indicated as s(singlet), d(doublet), t(triplet), q(quartet), m(multiplet), coupling constants (J) were reported in Hertz (Hz) and integration value. Chemical shift values of 13C NMR were recorded in parts per million (ppm, δ) and calibrated to the residual peak as an internal standard (CDCl3: δ = 77.0 ppm and DMSO: δ = 39.0 ppm). High-resolution mass spectra (HRMS) were obtained using the ESI-TOF method.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3
water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), catalyst and ligand 5 mol% each. Arylboronic acid 2 (0.5 mmol, 1.0 equiv.) and base (2 equiv.), were added to the above after 5 min of stirring and started heating at 60 °C. When the TLC indicated the total consumption of starting material, the reaction mixture was allowed to cool at ambient temperature. The reaction mixture was extracted with ethyl acetate and organic layer was washed with brine. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by column chromatography (silica gel, hexane/EtOAc) to give the desired products (3a–3ak).
1), catalyst and ligand 5 mol% each. Arylboronic acid 2 (0.5 mmol, 1.0 equiv.) and base (2 equiv.), were added to the above after 5 min of stirring and started heating at 60 °C. When the TLC indicated the total consumption of starting material, the reaction mixture was allowed to cool at ambient temperature. The reaction mixture was extracted with ethyl acetate and organic layer was washed with brine. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by column chromatography (silica gel, hexane/EtOAc) to give the desired products (3a–3ak).
1-Methyl-3-phenyl-1H-pyrazolo[3,4-b]pyridine (3a). Light yellow solid; 98% yield (102 mg); mp 86–87 °C; 1H NMR (400 MHz, CDCl3) δ 8.62 (dd, J = 4.5, 1.5 Hz, 1H), 8.38 (dd, J = 7.6, 1.5 Hz, 1H), 8.01–7.90 (m, 2H), 7.56 (t, J = 7.6 Hz, 2H), 7.48–7.45 (m, 1H), 7.22 (dd, J = 8.4, 4.6, 1H), 4.27 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 151.3, 148.6, 142.4, 133.1, 130.4, 128.9, 128.2, 126.9, 116.8, 113.4, 33.9. HRMS (ESI): m/z calcd. for C13H11N3[M + H]+: 210.1031; found: 210.1015.
1-Methyl-3-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine (3b). Light brown solid; 85% yield (94.4 mg) mp 52–53 °C; 1H NMR (400 MHz, CDCl3) δ 8.49 (dd, J = 4.6, 1.4 Hz, 1H), 8.25–8.22 (m, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.25 (d, J = 8.2 Hz, 2H), 7.07 (dd, J = 8.2, 4.6, 1H), 4.14 (s, 3H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 151.2, 148.4, 142.4, 138.0, 130.3, 130.2, 129.5, 126.7, 116.6, 113.8, 33.8, 21.2. HRMS (ESI): m/z calcd. for C14H13N3[M + H]+: 224.1187; found: 224.1182.
3-(3-Methoxyphenyl)-1-methyl-1H-pyrazolo[3,4-b]pyridine (3c). Dusky white solid; 81% yield (96.5 mg); mp 85–86 °C; 1H NMR (400 MHz, CDCl3) δ 8.63–8.61 (m, 1H), 8.40–8.38 (m, 1H), 7.59–7.55 (m, 2H), 7.49–7.45 (m, 1H), 7.24–7.20 (m, 1H), 7.03–7.00 (m, 1H), 4.27 (s, 3H), 3.96 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.0, 151.3, 148.6, 142.3, 134.4, 130.4, 129.9, 119.4, 116.8, 114.1, 113.4, 112.0, 55.3, 33.9. HRMS (ESI): m/z calcd. for C14H13N3O[M + H]+: 240.1136; found: 240.1138.
3-(4-Methoxyphenyl)-1-methyl-1H-pyrazolo[3,4-b]pyridine (3d). Dusky white solid, 97% yield (115.5 mg), mp 76–77 °C; 1H NMR (400 MHz, CDCl3) δ 8.55 (dd, J = 4.6, 0.9 Hz, 1H), 8.29 (dd, J = 8.2, 0.9 Hz, 1H), 7.88–7.86 (m, 2H), 7.16–7.13 (m, 1H), 7.04 (d, J = 8.2, 2H), 4.19 (s, 3H), 3.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.7, 151.3, 148.5, 142.3, 130.4, 128.1, 125.8, 116.5, 114.3, 113.3, 55.3, 33.8. HRMS (ESI): m/z calcd. for C14H13N3O[M + H]+: 240.1136; found: 240.1141.
3-(4-Bromophenyl)-1-methyl-1H-pyrazolo[3,4-b]pyridine (3e). White solid; 75% yield (107.1 mg); mp 104–105 °C; 1H NMR (400 MHz, CDCl3) δ 8.61 (dd, J = 4.6, 0.92 Hz, 1H), 8.32 (dd, J = 8.6, 1.36 Hz, 1H), 7.87–7.85 (m, 2H), 7.67–7.65 (m, 2H), 7.24–7.20 (m, 1H), 4.24 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 151.3, 148.7, 141.2, 132.1, 132.0, 130.0, 128.3, 122.2, 117.0, 113.2, 33.9. HRMS (ESI): m/z calcd. for C13H10BrN3[M + H]+: 288.0136; found: 288.0129.
3-(2,4-Difluorophenyl)-1-methyl-1H-pyrazolo[3,4-b]pyridine (3f). White solid; 46% yield (56.1 mg); mp 92–93 °C; 1H NMR (400 MHz, CDCl3) δ 8.63 (dd, J = 4.6, 1.5 Hz, 1H), 8.25–8.22 (m, 1H), 7.95–7.87 (m, 1H), 7.24–7.20 (m, 1H), 7.10–7.01 (m, 2H), 4.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.0 (dd, J = 247.9, 10.9 Hz, 1C), 159.9 (dd, J = 249.8, 11.4 Hz, 1C), 151.0, 148.9, 137.4, 131.3 (dd, J = 9.5, 5.7 Hz, 1C), 131.1, 131.0, 116.9, 114.1, 112.0 (dd, J = 20.9, 2.86 Hz, 1C), 104.4 (t, J = 25.7 Hz, 1C), 34.0. HRMS (ESI): m/z calcd. for C13H9F2N3[M + H]+: 246.0842; found: 246.0858.
3-(3,5-Bis(trifluoromethyl)phenyl)-1-methyl-1H-pyrazolo[3,4-b]pyridine (3g). Off white solid; 79% yield (135.7 mg); mp 118–119 °C; 1H NMR (400 MHz, CDCl3) δ 8.63 (dd, J = 4.5, 1.5 Hz, 1H), 8.41 (s, 2H), 8.32 (d, J = 8.4 Hz, 1H), 7.89 (s, 1H), 7.26 (dd, J = 8.4, 4.6, 1H), 4.25 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 151.4, 149.2, 139.2, 135.4, 132.2 (q, J = 34.3 Hz, 1C), 129.5, 126.4, 124.6, 121.9, 121.49–121.42 (m, 1C), 117.8, 113.0, 34.2. HRMS (ESI): m/z calcd. for C15H9F6N3[M + H]+: 346.0778; found: 346.0771.
6-Chloro-1-methyl-3-phenyl-1H-pyrazolo[3,4-b]pyridine (3h). Creamy white solid; 67% yield (81.1 mg); mp 75–76 °C; 1H NMR (400 MHz, CDCl3) δ 8.21–8.19 (m, 1H), 7.87 (d, J = 7.8 Hz, 2H), 7.50–7.46 (m, 2H), 7.41–7.39 (m, 1H), 7.14–7.12 (m, 1H), 4.13 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 150.6, 150.4, 142.9, 132.5, 132.5, 128.9, 128.5, 126.8, 117.3, 112.0, 34.1; HRMS (ESI): m/z calcd. for C13H10ClN3[M + H]+: 244.0641; found: 244.0621.
6-Chloro-1-methyl-3-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine (3i). White solid; 99% yield (126.8 mg); mp 141–142 °C; 1H NMR (400 MHz, CDCl3) δ 8.22–8.20 (m, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.30 (d, J = 7.8 Hz, 2H), 7.14 (dd, J = 8.7, 1.3 Hz, 1H), 4.15 (s, 3H), 2.42 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 150.6, 150.3, 143.0, 138.5, 132.5, 129.7, 129.6, 126.7, 117.2, 112.0, 34.0, 21.3; HRMS (ESI): m/z calcd. for C14H12ClN3[M + H]+: 258.0797; found: 258.0796.
6-Chloro-3-(4-methoxyphenyl)-1-methyl-1H-pyrazolo[3,4-b]pyridine (3j). White solid; 78% yield (106.1 mg); mp 137–138 °C; 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.2 Hz, 1H), 7.83 (d, J = 8.2 Hz, 2H), 7.15–7.13 (m, 1H), 7.03 (d, J = 8.2 Hz, 2H), 4.14 (s, 3H), 3.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.9, 150.6, 150.3, 142.8, 132.5, 128.1, 125.2, 117.1, 114.4, 111.9, 55.3, 34.0; HRMS (ESI): m/z calcd. for C14H12ClN3O[M + H]+: 274.0746; found: 274.0745.
6-Chloro-3-(4-fluorophenyl)-1-methyl-1H-pyrazolo[3,4-b]pyridine (3k). White solid; 83% yield (107.9 mg); mp 149–150 °C; 1H NMR (400 MHz, CDCl3) δ 8.19–8.17 (m, 1H), 7.89–7.85 (m, 2H), 7.26–7.15 (m, 3H), 4.15 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.9 (d, J = 246.9 Hz, 1C), 150.6, 142.0, 132.2, 128.8, 128.5 (d, J = 7.6 Hz, 1C), 117.5, 116.1, 115.9, 111.8, 34.1; HRMS (ESI): m/z calcd. for C13H9ClFN3[M + H]+: 262.0541; found: 262.0545.
1-(4-Methoxybenzyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (3l). Dusky brown solid; 62% yield (97.4 mg); mp 115–116 °C; 1H NMR (400 MHz, CDCl3) δ 8.64 (dd, J = 4.6, 1.5 Hz, 1H), 8.39–8.37 (m, 1H), 8.01 (d, J = 7.6 Hz, 2H), 7.55 (t, J = 7.6 Hz, 2H), 7.48–7.44 (m, 3H), 7.24–7.20 (m, 1H), 6.88 (dd, J = 6.8, 2.2 Hz, 2H), 5.78 (s, 2H), 3.80 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 59.0, 151.1, 148.7, 142.8, 133.2, 130.3, 129.3, 129.2, 128.8, 128.2, 127.0, 117.0, 113.8, 113.6, 55.1, 50.2. HRMS (ESI): m/z calcd. for C20H1N3O[M + H]+: 316.1449; found: 316.1468.
1-(4-Methoxybenzyl)-3-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine (3m). Light brown solid; 87% yield (142.7 mg); mp 76–77 °C; 1H NMR (400 MHz, CDCl3) δ 8.52 (d, J = 4.5, 1H), 8.23 (d, J = 7.6 Hz, 1H), 7.84 (d, J = 7.6 Hz, 2H), 7.39 (d, J = 8.4 Hz, 2H), 7.29–7.26 (m, 2H), 7.09–7.06 (m, 1H), 6.85–6.80 (m, 2H), 5.70 (s, 2H), 3.69 (s, 3H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 158.9, 150.9, 148.4, 142.7, 137.9, 130.2, 129.4, 129.2, 128.3, 126.8, 116.7, 113.7, 113.6, 113.4, 54.9, 50.0, 21.1. HRMS (ESI): m/z calcd. for C21H19N3O[M + H]+: 330.1606; found: 330.1607.
1-(4-Methoxybenzyl)-3-(4-methoxyphenyl)-1H-pyrazolo[3,4-b]pyridine (3n). White solid; 79% yield (135.9 mg); mp 125–126 °C; 1H NMR (400 MHz, CDCl3) δ 8.63 (d, J = 4.6, 1H), 8.36 (dd, J = 8.4, 1.5 Hz, 1H), 7.96 (d, J = 9.1 Hz, 2H), 7.45 (d, J = 8.4 Hz, 2H), 7.24–7.20 (m, 1H), 7.10 (d, J = 9.1 Hz, 2H), 6.90 (d, J = 9.1 Hz, 2H), 5.78 (s, 2H), 3.99 (s, 3H), 3.82 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.6, 159.0, 151.0, 148.6, 142.7, 130.3, 129.3, 129.2, 128.3, 125.9, 116.7, 114.2, 113.8, 113.5, 55.3, 55.1, 50.1. HRMS (ESI): m/z calcd. for C21H19N3O[M + H]+: 346.1555; found: 346.1555.
3-(4-Bromophenyl)-1-(4-methoxyphenyl)-1H-pyrazolo[3,4-b]pyridine (3o). Off white solid; 62% yield (121.4 mg); mp 75–76 °C; 1H NMR (400 MHz, CDCl3) δ 8.56 (dd, J = 4.1, 0.9 Hz, 1H), 8.23 (dd, J = 8.2, 1.4 Hz, 1H), 7.81–7.79 (m, 2H), 7.60–7.58 (m, 2H), 7.37–7.32 (m, 2H), 7.15 (d, J = 8.2, 4.6 Hz, 1H), 6.82–6.80 (m, 2H), 5.68 (s, 2H), 3.73 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.1, 151.1, 148.8, 141.6, 132.1, 131.9, 130.0, 129.3, 129.0, 128.4, 122.2, 117.2, 113.8, 113.4, 55.1, 50.3. HRMS (ESI): m/z calcd. for C20H16BrN3O[M + H]+: 394.0554; found: 394.0546.
4-(1-(4-Methoxybenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)benzonitrile (3p). Light yellow solid; 68% yield (115.3 mg); mp 175–176 °C; 1H NMR (400 MHz, CDCl3) δ 8.62 (dd, J = 4.5, 1.8 Hz, 1H), 8.33 (dd, J = 8.2, 1.3 Hz, 1H), 8.09 (dd, J = 6.88, 1.8 Hz, 2H), 7.76 (dd, J = 6.8, 1.8 Hz, 2H), 7.40–7.38 (m, 2H), 7.23 (dd, J = 8.2, 4.6 Hz, 1H), 6.83 (dd, J = 6.88, 2.2 Hz, 2H), 5.72 (s, 2H), 3.75 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.3, 151.2, 149.1, 140.6, 137.7, 132.6, 129.9, 129.5, 128.8, 127.2, 118.8, 117.8, 114.0, 113.5, 111.4, 55.2, 50.5. HRMS (ESI): m/z calcd. for C21H16N4O[M + H]+: 341.1402; found: 341.1391.
3-(2,4-Difluorophenyl)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine (3q). Pale yellow solid; 92% yield (161.0 mg); mp 60–61 °C; 1H NMR (400 MHz, CDCl3) δ 8.65 (dd, J = 4.6, 1.5 Hz, 1H), 8.25–8.22 (m, 1H), 7.93 (q, J = 8.4 Hz, 1H), 7.48–7.45 (m, 2H), 7.21 (dd, J = 8.4, 4.6 Hz, 1H), 7.09–7.02 (m, 2H), 6.92–6.89 (m, 2H), 5.80 (s, 2H), 3.81 (s, 3H); 13C NMR (100 MHz, CDCl3). 13C NMR showed one less carbon, it might be due to one carbon peak got merged at 129.3 ppm. δ 162.9 (dd, J = 248.8, 11.4 Hz, 1C), 159.3 (dd, J = 250.7, 12.4 Hz, 1C), 159.0, 150.7, 148.9, 137.7, 131.5 (dd, J = 9.5, 5.7 Hz, 1C), 130.9 (d, J = 10.4, 1C), 129.3 (b, 1C), 129.0, 117.0, 114.2, 113.8, 111.9 (d, J = 20.9 Hz, 1C), 104.6–104.0 (m, 1C), 55.2–54.9 (m, 1C), 50.2. HRMS (ESI): m/z calcd. for C20H15F2N3O[M + H]+: 352.1261; found: 352.1264.
3-(3,5-Bis(trifluoromethyl)phenyl)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine (3r). White solid; 93% yield (209.1 mg); mp 127–128 °C; 1H NMR (400 MHz, CDCl3) δ 8.63 (dd, J = 4.5, 1.5 Hz, 1H), 8.40 (s, 2H), 8.31–8.29 (m, 1H), 7.87 (s, 1H), 7.40 (d, J = 8.4 Hz, 2H), 7.28–7.26 (m, 1H), 6.84 (dd, J = 6.84, 2.2 Hz, 2H), 5.73 (s, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.3, 151.2, 149.3, 139.6, 135.5, 132.2 (q, J = 32.3 Hz, 1C), 129.5, 128.7, 126.6, 124.6, 121.9, 121.4 (b, 1C), 117.9, 114.0, 113.2, 55.2, 50.6. HRMS (ESI): m/z calcd. for C22H15F6N3O[M + H]+: 452.1197; found: 452.1191.
6-Chloro-1-(4-methoxybenzyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (3s). White solid; 93% yield (162 mg); mp 85–86 °C; 1H NMR (400 MHz, CDCl3) δ 8.22–8.19 (m, 1H), 7.90–7.88 (m, 2H), 7.49–7.45 (m, 2H), 7.41–7.38 (m, 3H), 7.16–7.13 (m, 1H), 6.83 (dd, J = 3.6, 1.4 Hz, 2H), 5.63 (s, 2H), 3.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.1, 150.5, 150.3, 143.2, 132.7, 132.4, 129.5, 128.8, 128.7, 128.5, 127.0, 117.6, 113.8, 112.2, 55.1, 50.3; HRMS (ESI): m/z calcd. for C20H16ClN3O[M + H]+: 350.1059; found: 350.1041.
6-Chloro-1-(4-methoxybenzyl)-3-(3-nitophenyl)-1H-pyrazolo[3,4-b]pyridine (3t). Light yellow solid; 93% yield (182.7 mg); mp 155–156 °C; 1H NMR (400 MHz, CDCl3) δ 8.76–8.75 (m, 1H), 8.27–8.22 (m, 3H), 7.65 (t, J = 7.7 Hz, 1H), 7.42–7.40 (m, 2H), 7.26–7.23 (m, 1H), 6.85 (dd, J = 6.8, 1.8 Hz, 2H), 5.65 (s, 2H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.3, 151.0, 150.3, 148.6, 140.6, 134.5, 132.5, 131.8, 129.9, 129.7, 128.2, 122.9, 121.5, 118.4, 114.0, 111.8, 55.2, 50.6; HRMS (ESI): m/z calcd. for C20H15ClN4O[M + H]+: 395.0910; found: it decompose during mass analysis.
6-Chloro-3-(2,4-difluorophenyl)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine (3u). White solid; 89% yield (170.9 mg); mp 87–88 °C; 1H NMR (500 MHz, CDCl3) δ 8.12 (dd, J = 8.4, 3.2 Hz, 1H), 7.87 (q, J = 8.3 Hz, 1H), 7.42 (d, J = 8.5 Hz, 2H), 7.19 (d, J = 8.4 Hz, 1H), 7.04–6.97 (m, 2H), 6.87 (d, J = 8.5 Hz, 2H), 5.68 (s, 2H), 3.79 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 163.1 (dd, J = 249.2, 32.7 Hz, 1C), 159.9 (dd, J = 249.7, 11.7 Hz, 1C), 159.3, 150.8, 150.1, 138.2, 133.3 (d, J = 10.8 Hz, 1C), 131.5 (dd, J = 9.7, 5.6 Hz, 1C), 129.6, 128.6, 117.8, 116.7 (dd, J = 14.8, 2.5 Hz, 1C), 114.0, 112.9, 112.1 (d, J = 20.8 Hz, 1C), 104.4 (t, J = 25.8 Hz, 1C), 55.2, 50.5; HRMS (ESI): m/z calcd. for C20H14ClF2N3O[M + H]+: 386.0871; found: 386.0859.
6-Chloro-1-(4-methoxybenzyl)-3-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine (3v). White solid; 90% yield (162.9 mg); mp 110–111 °C; 1H NMR (500 MHz, CDCl3) δ 8.23 (d, J = 8.2 Hz, 1H), 7.81 (d, J = 7.2 Hz, 2H), 7.42 (d, J = 7.6 Hz, 2H), 7.33–7.28 (m, 2H), 7.17 (d, J = 8.3 Hz, 1H), 6.86 (d, J = 7.5 Hz, 2H), 5.66 (s, 2H), 3.78 (s, 3H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.2, 150.5, 150.4, 143.4, 138.5, 132.5, 129.9, 129.6, 129.6, 128.9, 126.9, 117.5, 113.9, 112.2, 55.2, 50.3, 21.3; HRMS (ESI): m/z calcd. for C21H18ClN3O[M + H]+: 364.1216; found: 364.1201.
3-Phenyl-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[3,4-b]pyridine (3w). Light brown solid; 97% yield (135 mg); mp 74–75 °C; 1H NMR (400 MHz, CDCl3) δ 8.42 (d, J = 4.6 Hz, 1H), 8.13 (d, J = 7.6 Hz, 1H), 7.86 (d, J = 7.6 Hz, 2H), 7.35 (t, J = 7.6 Hz, 2H), 7.27–7.24 (m, 1H), 7.01–6.98 (m, 1H), 6.09 (dd, J = 10.7, 2.3 Hz, 1H), 4.04–4.01 (m, 1H), 3.74–3.69 (m, 1H), 2.73–2.64 (m, 1H), 2.03 (b, 1H), 1.91–1.88 (m, 1H), 1.70–1.60 (m, 2H), 1.48–1.46 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 151.0, 148.3, 143.3, 132.6, 130.0, 128.4, 128.0, 126.8, 117.3, 113.7, 82.0, 67.8, 29.0, 24.6, 22.7. HRMS (ESI): m/z calcd. for C17H17N3O[M + H]+: 280.1449; found: 280.1442.
1-(Tetrahydro-2H-pyran-2-yl)-3-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine (3x). Brown solid; 88% yield (128.5 mg); mp 76–77 °C; 1H NMR (400 MHz, CDCl3) δ 8.56 (dd, J = 4.6, 1.3 Hz, 1H), 8.32 (dd, J = 8.2, 1.8 Hz, 1H), 7.88 (d, J = 7.7 Hz, 2H), 7.31–7.29 (m, 2H), 7.21–7.18 (m, 1H), 6.19 (dd, J = 10.5, 2.3 Hz, 1H), 4.18–4.15 (m, 1H), 3.89–3.86 (m, 1H), 2.83–2.74 (m, 1H), 2.43–2.42 (m, 3H), 2.18–2.17 (m, 1H), 2.04–2.01 (m, 1H), 1.84–1.83 (m, 2H), 1.65 (b, 1H); 13C NMR (100 MHz, CDCl3) δ 151.4, 148.6 (d, J = 2.8 Hz, 1C), 143.9, 138.3, 130.5 (d, J = 2.8 Hz, 1C), 130.1, 129.4 (d, J = 4.7 Hz, 1C), 127.2, 117.5 (d, J = 6.67 Hz, 1C), 114.2, 82.3 (d, J = 9.5 Hz, 1C), 68.3, 29.5, 25.0, 23.1, 21.3 (d, J = 3.8 Hz, 1C). HRMS (ESI): m/z calcd. for C18H19N3O[M + H]+: 294.1606; found: 294.1605.
3-(3-Methoxyphenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[3,4-b]pyridine (3y). Yellow liquid; 96% yield (147.9 mg); liquid; 1H NMR (400 MHz, CDCl3) δ 8.60 (dd, J = 4.6, 1.4 Hz, 1H), 8.35 (dd, J = 8.2, 1.3 Hz, 1H), 7.59–7.57 (m, 2H), 7.43 (t, J = 8.0 Hz, 1H), 7.21 (d, J = 8.2, 4.6 Hz, 1H), 7.00–6.97 (m, 1H), 6.23 (dd, J = 10.5, 2.2 Hz, 1H), 4.21–4.11 (m, 1H), 3.91 (s, 3H), 3.89–3.85 (m, 1H), 2.88–2.78 (m, 1H), 2.21–2.18 (m, 1H), 2.06–2.03 (m, 1H), 1.86–1.82 (m, 2H), 1.67–1.65 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 159.8, 151.3, 148.6, 143.6, 134.1, 130.4, 129.6, 119.7, 117.6, 114.2, 114.1, 112.4, 82.3, 68.2, 55.2, 29.53, 24.9, 23.0; HRMS (ESI): m/z calcd. for C18H19N3O2[M + H]+: 310.1555; found: 310.1538.
3-(4-Bromophenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[3,4-b]pyridine (3z). Creamy white solid; 67% yield (119.1 mg); mp 112–113 °C; 1H NMR (400 MHz, CDCl3) δ 8.59 (dd, J = 4.6, 1.8 Hz, 1H), 8.28 (dd, J = 8.2, 1.8 Hz, 1H), 7.88–7.85 (m, 2H), 7.63–7.60 (m, 2H), 7.22 (q, J = 4.6 Hz, 1H), 6.20 (dd, J = 10.5, 2.3 Hz, 1H), 4.18–4.14 (m, 1H), 3.89–3.83 (m, 1H), 2.81–2.72 (m, 1H), 2.19–2.16 (m, 1H), 2.03–2.00 (m, 1H), 1.86–1.79 (m, 2H), 1.65–1.63 (m, 1H); 13C NMR (100 MHz, CDCl3). 13C NMR showed one less carbon, it might be due to one carbon peak got merged at 131.9 ppm. δ 151.3, 148.8, 142.7, 131.9, 130.2, 128.7, 122.5, 117.8, 113.9, 82.3, 68.3, 29.9, 24.9, 23.0; HRMS (ESI): m/z calcd. for C18H16BrN3O[M + H]+: 358.0554; found: 358.0547.
3-(4-Fluorophenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[3,4-b]pyridine (3aa). White solid; 97% yield (143.6 mg); mp 92–93 °C; 1H NMR (400 MHz, CDCl3) δ 8.59 (dd, J = 4.6, 1.4 Hz, 1H), 8.28 (dd, J = 8.2, 1.4 Hz, 1H), 7.97–7.93 (m, 2H), 7.23–7.16 (m, 3H), 6.20 (dd, J = 10.5, 2.7 Hz, 1H), 4.18–4.11 (m, 1H), 3.89–3.83 (m, 1H), 2.82–2.72 (m, 1H), 2.19–2.16 (m, 1H), 2.04–2.01 (m, 1H), 1.89–1.79 (m, 2H), 1.68–1.63 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 162.9 (d, J = 246.9 Hz, 1C), 151.4, 148.8 (d, J = 3.8 Hz, 1C), 143.0, 130.2 (d, J = 3.82 Hz, 1C), 129.1 (d, J = 2.86 Hz, 1C), 129.0 (d, J = 8.58 Hz, 1C), 117.7 (d, J = 5.72 Hz, 1C), 115.6 (dd, J = 20.2, 5.7 Hz, 1C), 114.0, 82.3 (d, J = 9.5 Hz, 1C), 68.4, 29.5, 25.0, 23.1; HRMS (ESI): m/z calcd. for C17H16FN3O[M + H]+: 298.1355; found: 298.1324.
3-(2,4-Difluorophenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[3,4-b]pyridine (3ab). Light brown liquid; 88% yield (138.1 mg); liquid; 1H NMR (400 MHz, CDCl3) δ 8.59–8.58 (m, 1H), 8.19–8.17 (m, 1H), 7.96–7.90 (m, 1H), 7.21–7.18 (m, 1H), 7.04–6.95 (m, 2H), 6.22 (dd, J = 10.5, 2.2 Hz, 1H), 4.18–4.11 (m, 1H), 3.89–3.84 (m, 1H), 2.79–2.71 (m, 1H), 2.18 (b, 1H), 2.05–2.01 (m, 1H), 1.85–1.80 (m, 2H), 1.65–1.63 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 163.1 (dd, J = 249.8, 11.4 Hz, 1C), 160.0 (dd, J = 250.7, 12.3 Hz, 1C), 150.9, 148.9, 138.9, 131.7 (q, J = 4.7 Hz, 1C), 131.1 (d, J = 10.4 Hz, 1C), 117.6, 116.8 (dd, J = 14.3, 3.8 Hz, 1C), 114.8, 111.9 (dd, J = 20.9, 2.8 Hz, 1C), 104.2 (t, J = 25.7 Hz, 1C), 82.3, 68.3, 29.4, 24.9, 23.0; HRMS (ESI): m/z calcd. for C17H15F2N3O[M + H]+: 316.1261; found: 316.1259.
3-(3,5-Bis(trifluoromethyl)phenyl)-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[3,4-b]pyridine (3ac). Creamy white solid; 90% yield (186.2 mg); mp 160–161 °C; 1H NMR (400 MHz, CDCl3) δ 8.65 (d, J = 4.5 Hz, 1H), 8.46 (s, 2H), 8.33 (dd, J = 7.3, 0.92 Hz, 1H), 7.91 (s, 1H), 7.33–7.31 (m, 1H), 6.25 (dd, J = 10.5, 2.3 Hz, 1H), 4.20–4.17 (m, 1H), 3.91–3.85 (m, 1H), 2.84–2.75 (m, 1H), 2.22–2.18 (m, 1H), 2.05–2.02 (m, 1H), 1.87–1.82 (m, 2H), 1.68–1.66 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 151.4, 149.4, 140.7, 135.2, 132.2 (q, J = 32.4 Hz, 1C), 129.7, 126.9, 124.6, 121.9–121.7 (m, 1C), 118.5, 113.6, 82.5, 68.6, 29.5, 24.9, 23.0; HRMS (ESI): m/z calcd. for C19H15F6N3O[M + H]+: 416.1197; found: 416.1183.
1-(4-Methoxybenzyl)-3-(thiophen-3-yl)-1H-pyrazolo[3,4-b]pyridine (3ad). Brown solid; 63% yield (100.8 mg); mp 108–109 °C; 1H NMR (400 MHz, CDCl3) δ 8.56 (dd, J = 4.6, 1.3 Hz, 1H), 8.26 (dd, J = 7.8, 1.4 Hz, 1H), 7.76 (dd, J = 3.2, 1.4 Hz, 1H), 7.68 (dd, J = 5.0, 1.4 Hz, 1H), 7.43 (dd, J = 5.0, 3.2 Hz, 1H), 7.36–7.34 (m, 2H), 7.15 (dd, J = 7.8, 4.6 Hz, 1H), 6.81 (dd, J = 6.8, 2.2 Hz, 2H), 5,68 (s, 2H), 3.74 (s, 3H); 13C NMR (100 MHz, CDCl3). 13C NMR showed one less carbon, it might be due to one carbon peak got merged at 129.2 ppm. δ 159.0, 150.8, 148.8, 139.2, 134.3, 130.0, 129.2, 126.4, 126.1, 121.8, 116.9, 113.8, 113.5, 55.1, 50.1; HRMS (ESI): m/z calcd. for C18H15N3OS[M + H]+: 322.1013; found: 322.1001.
6-Chloro-1-(4-methoxybenzyl)-3-(thiophen-3-yl)-1H-pyrazolo[3,4-b]pyridine (3ae). Orange solid; 86% yield (152.2 mg); mp 130–131 °C; 1H NMR (500 MHz, CDCl3) δ 8.17 (d, J = 8.3 Hz, 1H), 7.74–7.74 (m, 1H), 7.65 (d, J = 5.0 Hz, 1H), 7.45–7.44 (m, 1H), 7.38 (d, J = 8.5 Hz, 2H), 7.17 (d, J = 8.3 Hz, 1H), 6.84 (d, J = 8.6 Hz, 2H), 5.62 (s, 2H), 3.76 (s, 3H); 13C NMR (125 MHz, CDCl3). 13C NMR showed one less carbon, it might be due to one carbon peak got merged at 126.3 ppm. δ 159.2, 150.6, 150.1, 139.6, 133.9, 132.1, 129.5, 128.8, 126.3 (b, 1C), 122.2, 117.6, 113.9, 112.1, 55.2, 50.2; HRMS (ESI): m/z calcd. for C18H14ClN3OS[M + H]+: 356.0624; found: it decomposes during mass analysis.
3-(Benzo[d][1,3]dioxo-5-yl)-1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine (3af). White solid; 77% yield (137.8 mg); mp 97–98 °C; 1H NMR (500 MHz, CDCl3) δ 8.50 (s, 1H), 8.20 (s, 1H), 7.37–7.31 (m, 4H), 7.09 (s, 1H), 6.87–6.76 (m, 3H), 5.95 (s, 2H), 5.62 (s, 2H), 3.69 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.1, 151.2, 148.7, 148.2, 147.8, 142.6, 130.2, 129.4, 127.5, 120.9, 116.9, 113.9, 113.5, 108.6, 107.6, 101.2, 55.2, 50.2; HRMS (ESI): m/z calcd. for C21H17N3O3[M + H]+: 360.1347; found: 360.1225.
1-(4-Methoxybenzyl)-3(naphthalen-2-yl)-1H-pyrazolo[3,4-b]pyridine (3ag). Creamy white solid, 88% yield (160.1 mg); mp 96–97 °C; 1H NMR (400 MHz, CDCl3) δ 8.63 (dd, J = 4.5, 1.8 Hz, 1H), 8.46 (dd, J = 8.2, 1.4 Hz, 1H), 8.42 (s, 1H), 8.17 (dd, J = 8.72, 1.8 Hz, 1H), 7.99–7.96 (m, 2H), 7.92–7.89 (m, 1H), 7.56–7.53 (m, 2H), 7.48–7.46 (m, 2H), 7.22–7.20 (m, 1H), 6.89–6.87 (m, 2H), 5.80 (s, 2H), 3.77 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.0, 151.1, 148.7, 142.7, 133.4, 133.0, 130.6, 130.4, 129.3, 129.2, 128.5, 128.1, 127.7, 126.3, 126.1, 125.8, 124.9, 117.0, 113.8, 113.8, 55.1, 50.2; HRMS (ESI): m/z calcd. for C24H19N3O[M + H]+: 366.1606; found: 366.1592.
6-Chloro-1-(4-methoxybenzyl)-3-(naphthalen-2-yl)-1H-pyrazolo[3,4-b]pyridine (3ah). White solid; 92% yield (183.1 mg); mp 143–144 °C; 1H NMR (500 MHz, CDCl3) δ 8.38–8.36 (m, 2H), 8.11–8.07 (m, 1H), 8.02–7.89 (m, 3H), 7.57–7.54 (m, 2H), 7.49–7.45 (m, 2H), 7.23 (d, J = 8.3 Hz, 1H), 6.83 (d, J = 8.5 Hz, 2H), 5.71 (s, 2H), 3.79 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.2, 150.6, 143.2, 133.4, 133.2, 132.6, 130.2, 129.8, 129.6, 128.8, 128.7, 128.2, 127.8, 126.5, 126.4, 126.0, 124.8, 117.7, 114.0, 112.4, 55.2, 50.4; HRMS (ESI): m/z calcd. for C24H18ClN3O[M + H]+: 400.1216; found: 400.1202.
1-(4-Methoxybenzyl)-3-(pyridin-4-yl)-1H-pyrazolo[3,4-b]pyridine (3ai). Brown solid; 73% yield (114.9 mg); mp 88–89 °C; 1H NMR (400 MHz, CDCl3) δ 8.69 (dd, J = 4.5, 1.3 Hz, 2H), 8.46 (dd, J = 4.6, 1.3 Hz, 1H), 8.34 (dd, J = 8.2, 1.3 Hz, 1H), 7.85 (dd, J = 4.1, 1.3 Hz, 2H), 7.38 (d, J = 8.2 Hz, 2H), 7.22 (m, 1H), 6.83–6.81 (m, 2H), 5,71 (s, 2H), 3.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.2, 151.1, 150.3, 149.0, 140.6, 139.8, 129.8, 129.5, 128.7, 121.0, 117.7, 113.9, 113.6, 55.1, 50.5; HRMS (ESI): m/z calcd. for C19H16N4O[M + H]+: 317.1402; found: 317.1405.
6-Chloro-1-(4-methoxybenzyl)-3-(pyridin-4-yl)-1H-pyrazolo[3,4-b]pyridine (3aj). Light brown solid; 76% yield (132.6 mg); mp 149–150 °C; 1H NMR (400 MHz, CDCl3) δ 8.70 (d, J = 5.5 Hz, 2H), 8.26–8.24 (m, 1H), 7.81 (d, J = 5.0 Hz, 2H), 7.40 (d, J = 8.6 Hz, 2H), 7.24–7.22 (m, 1H), 6.84 (d, J = 8.2 Hz, 2H), 5.65 (s, 2H), 3.76 (s, 3H); 13C NMR (100 MHz, CDCl3). 13C NMR showed one less carbon, it might be due to one carbon peak got merged. δ 159.3, 150.9, 150.3, 140.2, 140.0, 131.9, 129.7, 128.2, 120.9, 118.5, 113.9, 112.1, 55.2, 50.6; HRMS (ESI): m/z calcd. for C19H15ClN4O[M + H]+: 351.1012; found: 351.1003.
1-Methyl-3-(pyridin-4-yl)-1H-pyrazolo[3,4-b]pyridine (3ak). Brown solid; 78% yield (81.6 mg); mp 70–71 °C; 1H NMR (400 MHz, CDCl3) δ 8.71 (d, J = 4.1 Hz, 2H), 8.59 (dd, J = 4.1, 1.3 Hz, 1H), 8.34 (dd, J = 8.2, 1.3 Hz, 1H), 7.86–7.85 (m, 2H), 7.23–7.20 (m, 1H), 4.21 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 151.4, 150.2, 148.9, 140.6, 139.3, 129.8, 120.8, 117.6, 113.4, 34.1; HRMS (ESI): m/z calcd. for C12H10N4[M + H]+: 211.0983; found: 211.0981.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3
water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), catalyst and ligand 5 mol% each. Arylboronic acid 2 (0.5 mmol, 1.0 equiv.) and base (2 equiv.) were added to the above after 5 min of stirring and started heating at 100 °C. When the TLC indicated the total consumption of starting material, the reaction mixture was allowed to cool at ambient temperature. The reaction mixture was extracted with ethyl acetate and organic layer was washed with brine and distilled water. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by column chromatography (silica gel, hexane/EtOAc) to give the desired products (4a–4l).
1), catalyst and ligand 5 mol% each. Arylboronic acid 2 (0.5 mmol, 1.0 equiv.) and base (2 equiv.) were added to the above after 5 min of stirring and started heating at 100 °C. When the TLC indicated the total consumption of starting material, the reaction mixture was allowed to cool at ambient temperature. The reaction mixture was extracted with ethyl acetate and organic layer was washed with brine and distilled water. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by column chromatography (silica gel, hexane/EtOAc) to give the desired products (4a–4l).
1-(4-Methoxybenzyl)-3,6-diphenyl-1H-pyrazolo[3,4-b]pyridine (4a). White solid; 95% yield (185.3 mg); mp 118–119 °C; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 8.2 Hz, 1H), 8.18–8.16 (m, 2H), 7.96 (dd, J = 8.7, 1.3 Hz, 2H), 7.64 (d, J = 8.7 Hz, 1H), 7.52–7.45 (m, 7H), 7.40–7.36 (m, 1H), 6.83 (dd, J = 6.6, 0.9 Hz, 2H), 5.76 (s, 2H), 3.74 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.1, 156.2, 151.5, 142.7, 139.2, 133.4, 130.7, 129.6, 126.5, 129.3, 128.8, 128.7, 128.1, 127.4, 127.0, 114.6, 113.8, 112.3, 55.2, 50.2; HRMS (ESI): m/z calcd. for C26H21N3O[M + H]+: 392.1762; found: 392.1746.
1-(4-Methoxybenzyl)-6-(4-methoxyphenyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (4b). White solid; 81% yield (170.1 mg); mp 123–124 °C; 1H NMR (500 MHz, CDCl3) δ 8.31 (d, J = 8.5 Hz, 1H), 8.16 (d, J = 8.8 Hz, 2H), 7.97 (d, J = 7.15 Hz, 2H), 7.60 (d, J = 8.4 Hz, 1H), 7.52–7.47 (m, 4H), 7.40 (t, J = 7.4, 1H), 7.05 (d, J = 8.8 Hz, 2H), 6.85 (d, J = 8.6 Hz, 2H), 5.76 (s, 2H), 3.90 (s, 3H), 3.76 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 160.8, 159.1, 156.0, 151.6, 142.8, 133.5, 131.8, 130.7, 129.6, 128.8 (d, J = 2.7 Hz, 1C), 128.1, 127.0, 116.0, 114.8, 114.2, 114.1, 113.9, 111.9, 55.4, 55.2, 50.2; HRMS (ESI): m/z calcd. for C27H23N3O2[M + H]+: 422.1868; found: 422.1857.
6-(4-Fluorophenyl)-1-(4-methoxyphenyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (4c). White solid; 97% yield (197.9 mg); mp 136–137 °C; 1H NMR (500 MHz, CDCl3) δ 8.37–8.34 (m, 1H), 8.20–8.16 (m, 2H), 7.97 (d, J = 7.2 Hz, 2H), 7.61 (d, J = 8.4 Hz, 1H), 7.51 (t, J = 7.5 Hz, 2H), 7.46 (d, J = 8.6 Hz, 2H), 7.41 (t, J = 7.4 Hz, 1H), 7.22 (t, J = 8.6, 2H), 6.85 (d, J = 8.6 Hz, 2H), 5.76 (s, 2H), 3.76 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 163.8 (d, J = 247.8 Hz, 1C), 159.1, 155.2, 151.5, 142.8, 135.3 (d, J = 2.9 Hz, 1C), 133.3, 130.9, 129.6, 129.5, 129.3 (d, J = 8.3 Hz, 1C), 128.8, 128.2, 127.0, 115.7 (d, J = 21.4 Hz, 1C), 114.3, 113.9, 112.3, 55.2, 50.2; HRMS (ESI): m/z calcd. for C26H20FN3O[M + H]+: 410.1668; found: 410.1681.
1-(4-Methoxybenzyl)-6-(3-methoxyphenyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (4d). White solid; 68% yield (142.8 mg); mp 95.9–96.6 °C; 1H NMR (400 MHz, CDCl3): 8.33 (d, J = 8.2 Hz, 1H), 7.97–7.95 (m, 2H), 7.77–7.76 (m, 1H), 7.72–7.70 (m, 1H), 7.63 (d, J = 8.2, 1H), 7.48–7.38 (m, 6H), 7.02–6.99 (m, 1H), 6.82 (d, J = 8.7 Hz, 2H), 5.75 (s, 2H), 3.92 (s, 3H), 3.73 (s, 3H); 13C NMR (100 MHz, CDCl3): 160.0, 159.0, 157.0, 156.0, 151.4, 142.7, 140.6, 133.3, 130.7, 130.0, 129.7, 129.6, 129.4, 128.8, 128.1, 127.0, 119.9, 114.9, 114.8, 113.8, 113.0, 112.4, 55.1, 50.2; HRMS (ESI): m/z calcd. for C27H23N3O2[M + H]+: 422.1868; found: 422.1841.
3-(3-(4-Methoxyphenyl)-1-methyl-1H-pyrazolo[3,4-b]pyridin-6-yl)benzaldehyde (4e). Creamy white solid; 76% yield (129.9 mg); mp 179–180 °C; 1H NMR (500 MHz, CDCl3) δ 10.17 (s, 1H), 8.67 (s, 1H), 8.46 (d, J = 7.4 Hz, 1H), 8.38–0.36 (m, 1H), 7.98 (d, J = 7.3 Hz, 1H), 7.92 (d, J = 8.0 Hz, 2H), 7.71–7.67 (m, 2H), 7.07 (d, J = 7.9 Hz, 2H), 4.26 (s, 3H), 3.90 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 192.2, 159.8, 154.6, 151.7, 142.4, 140.2, 136.9, 133.2, 131.1, 130.3, 129.5, 128.6, 128.1, 125.8, 114.4, 114.0, 112.4, 55.3, 33.8; HRMS (ESI): m/z calcd. for C21H17N3O2[M + H]+: 344.1398; found: 344.1371.
4-(1-(4-Methoxybenzyl)-3-(p-tolyl)-1H-pyrazolo[3,4-b]pyridin-6-yl)benzonitrile (4f). Light yellow solid; 72% yield (154.5 mg); mp 186–187 °C; 1H NMR (500 MHz, CDCl3) δ 8.38 (d, J = 8.4 Hz, 1H), 8.28 (d, J = 8.2 Hz, 2H), 7.86 (d, J = 8.0 Hz, 2H), 7.81 (d, J = 8.2 Hz, 2H), 7.64 (t, J = 8.4 Hz, 1H), 7.44 (d, J = 8.6 Hz, 2H), 7.32 (d, J = 7.9 Hz, 2H), 6.85 (d, J = 8.7 Hz, 2H), 5.76 (s, 2H), 3.76 (s, 3H), 2.44 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.2, 153.7, 151.1, 143.4, 143.0, 138.3, 132.5, 131.3, 130.2, 129.6, 129.6, 129.3, 127.9, 126.9, 118.8, 114.5, 113.9, 113.1, 112.6, 55.2, 50.3, 21.3; HRMS (ESI): m/z calcd. for C28H22N4O[M + H]+: 431.1871; found: 431.1888.
3-(2,4-Difluorophenyl)-1-(4-methoxybenzyl)-6-(3-methoxyphenyl)-1H-pyrazolo[3,4-b]pyridine (4g). White solid; 63% yield (143.6 mg); mp 100–101 °C; 1H NMR (500 MHz, CDCl3) δ 8.22 (dd, J = 8.4, 3.4 Hz, 1H), 7.89 (q, J = 6.8 Hz, 1H), 7.78 (s, 1H), 7.74 (d, J = 7.2 Hz, 1H), 7.65 (d, J = 8.5 Hz, 1H), 7.49–7.43 (m, 3H), 7.04–6.97 (m, 3H), 6.85 (d, J = 8.5, 2H), 5.78 (s, 2H), 3.94 (s, 3H), 3.77 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 163.05 (dd, J = 248.9, 11.7 Hz, 1C), 160.0, 159.9 (dd, J = 249.8, 11.9 Hz, 1C), 159.2, 156.3, 151.1, 140.7, 137.7, 131.5 (t, J = 2.4 Hz, 1C), 131.5 (t, J = 3.4 Hz, 1C), 129.8, 129.7, 129.3, 120.0, 117.3 (dd, J = 14.7, 3.7 Hz, 1C), 115.0, 114.9, 113.9, 113.2, 113.1, 112.0 (dd, J = 21.1, 3.3 Hz, 1C), 104.4 (t, J = 25.6 Hz, 1C), 55.4, 55.2, 50.4; HRMS (ESI): m/z calcd. for C27H21F2N3O2[M + H]+: 458.1679; found: 458.1664.
1-(4-Methoxybenzyl)-6-(3-nitrophenyl)-3-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine (4h). Yellow solid; 53% yield (118.9 mg); mp 160–161 °C; 1H NMR (500 MHz, CDCl3) δ 9.03 (s, 1H), 8.49 (d, J = 7.4 Hz, 1H), 8.38 (dd, J = 8.3, 2.4 Hz, 1H), 8.30 (d, J = 7.9 Hz, 1H), 7.86 (d, J = 7.8 Hz, 2H), 7.70–7.66 (m, 2H), 7.48 (d, J = 8.2 Hz, 2H), 7.32 (d, J = 7.7 Hz, 2H), 6.87 (d, J = 8.3 Hz, 2H), 5.77 (s, 2H), 3.76 (s, 3H), 2.43 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.2, 153.2, 151.3, 148.8, 143.0, 140.9, 138.3, 133.1, 131.4, 130.2, 129.7, 129.6, 129.2, 126.9, 123.8, 122.4, 114.1, 113.9, 113.2, 55.2, 50.5, 21.3; HRMS (ESI): m/z calcd. for C27H22N4O3[M + H]+: 451.1769; found: 451.1775.
6-(Furan-2-yl)-1-(4-methoxybenzyl)-3-(thiophen-3-yl)-1H-pyrazolo[3,4-b]pyridine (4i). White solid; 68% yield (131.3 mg); mp 163–164 °C; 1H NMR (400 MHz, CDCl3) δ 8.25 (d, J = 8.4 Hz, 1H),7.77 (d, J = 1.5 Hz, 1H), 7.69 (d, J = 4.9 Hz, 1H), 7.63–7.60 (m, 2H), 7.44–7.41 (m, 3H), 7.24 (d, J = 3.1 Hz, 1H), 6.83 (d, J = 8.4 Hz, 2H), 6.60 (s, 1H), 5.70 (s, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, CDCl3). [We found less 1C.] δ 159.1, 153.9, 151.0, 148.2, 143.8, 139.3, 134.5, 130.3, 129.6, 129.5, 126.4, 126.1, 121.8, 113.9, 113.0, 112.3, 109.8, 55.2, 50.1. HRMS (ESI): m/z calcd. for C27H17N3O2S[M + H]+: 388.1119; found: 388.1103.
1-(4-Methoxybenzyl)-3-(pyridine-4-yl)-6-(thiophen-3-yl)-1H-pyrazolo[3,4-b]pyridine (4j). White solid; 62% yield (123.1 mg); mp 150–151 °C; 1H NMR (500 MHz, CDCl3) δ 8.71 (d, J = 5.6 Hz, 2H), 8.31 (d, J = 8.3 Hz, 1H), 8.06 (d, J = 1.7 Hz, 1H), 7.88–7.86 (m, 3H), 7.59 (d, J = 8.2 Hz, 1H), 7.47–7.45 (m, 3H), 6.85 (d, J = 8.6 Hz, 2H), 5.74 (s, 2H), 3.76 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 159.2, 152.6, 151.4, 150.3, 142.0, 140.8, 139.8, 130.2, 129.7, 129.0, 126.7, 126.5, 124.9, 120.9, 115.4, 114.0, 112.1, 55.2, 50.5. HRMS (ESI): m/z calcd. for C23H18N4OS[M + H]+: 399.1279; found: 399.1269.
6-(Benzo[d][1,3]dioxol-5-yl)-1-(4-methoxybenzyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (4k). White solid; 57% yield (123.6 mg); mp 143–144 °C; 1H NMR (500 MHz, CDCl3) δ 8.80 (d, J = 2.6 Hz, 1H), 8.43 (d, J = 2.5 Hz, 1H), 7.99–7.96 (m, 2H), 7.63–7.61 (m, 2H), 7.52–7.47 (m, 4H), 7.43–7.38 (m, 4H), 6.85–6.82 (m, 2H), 5.73 (s, 2H), 3.75 (s, 3H); 13C NMR (125 MHz, CDCl3).13C NMR showed one less carbon, it might be due to one carbon peak got merged. δ 159.3, 150.5, 150.4, 148.2, 148.0, 143.0, 132.4, 129.6, 129.5, 128.8, 127.0, 126.9, 121.7, 120.9, 117.5, 113.9, 112.0, 108.7, 107.5, 101.3, 55.2, 50.3; HRMS (ESI): m/z calcd. for C27H21N3O3[M + H]+: 436.1660; found: 436.1648.
3-(1-(4-Methoxybenzyl)-3-(naphthalen-2-yl)-1H-pyrazolo[3,4-b]pyridin-6-yl)phenol (4l). White solid; 52% yield (118.5 mg); mp 179.6–180.4 °C; 1H NMR (500 MHz, CDCl3): 8.45–8.41 (m, 2H), 8.15 (d, J = 8.5 Hz, 1H), 7.98–7.95 (m, 2H), 7.89 (d, J = 7.1 Hz, 1H), 7.735–7.731 (b, 2H), 7.63 (d, J = 8.4 Hz, 1H), 7.55–7.51 (m, 2H), 7.47 (d, J = 8.5 Hz, 2H), 7.40 (t, J = 8.1 Hz, 1H), 6.96 (dd, J = 7.9, 1.7 Hz, 1H), 6.83 (d, J = 8.5 Hz, 2H), 5.91 (s, 1H), 5.79 (s, 2H), 3.74 (s, 3H); 13C NMR (125 MHz, CDCl3): 159.1, 156.2, 156.0, 151.5, 142.8, 140.6, 133.5, 133.1, 131.0, 130.7, 130.0, 129.6, 129.4, 128.6, 128.2, 127.8, 126.4, 126.2, 125.9, 125.0, 119.9, 116.6, 114.9, 114.4, 113.9, 112.7, 55.2, 50.2. HRMS (ESI): m/z calcd. for C3H23N3O2[M + H]: 458.1868; found: 458.1846.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3
water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), catalyst and ligand 5 mol% each. Arylboronic acid 2 (0.5 mmol, 1.0 equiv.) and base (2 equiv.) were added to the above after 5 min of stirring and started heating at 60 °C. When the TLC indicated the total consumption of starting material, the condenser fitted to the RB, next 15 mol% of catalyst and 15 mol% of ligand were added to the same pot. Arylboronic acid 2 (0.5 mmol, 1 equiv.) was added after 5 min of stirring and stirring continued at 100 °C. After the reaction was completed as monitored by TLC the reaction mixture was allowed to cool at ambient temperature. The reaction mixture was extracted with ethyl acetate and the organic layer was washed with brine and distilled water. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by column chromatography (silica gel, hexane/EtOAc) to give the desired products. The characterisation of 4a-4b, 4f-4g, 4i-4j is given above.
1), catalyst and ligand 5 mol% each. Arylboronic acid 2 (0.5 mmol, 1.0 equiv.) and base (2 equiv.) were added to the above after 5 min of stirring and started heating at 60 °C. When the TLC indicated the total consumption of starting material, the condenser fitted to the RB, next 15 mol% of catalyst and 15 mol% of ligand were added to the same pot. Arylboronic acid 2 (0.5 mmol, 1 equiv.) was added after 5 min of stirring and stirring continued at 100 °C. After the reaction was completed as monitored by TLC the reaction mixture was allowed to cool at ambient temperature. The reaction mixture was extracted with ethyl acetate and the organic layer was washed with brine and distilled water. The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by column chromatography (silica gel, hexane/EtOAc) to give the desired products. The characterisation of 4a-4b, 4f-4g, 4i-4j is given above.3,6-Diphenyl-1H-pyrazolo[3,4-b]pyridine (5a). White solid; 99% yield (25.9 mg); mp 207.7–208.2 °C; 1H NMR (500 MHz, DMSO-d6) δ 13.8 (s, 1H), 8.61 (d, J = 8.4 Hz, 1H), 8.18 (d, J = 7.4 Hz, 2H), 8.05 (d, J = 7.5 Hz, 2H), 7.84 (d, J = 8.5 Hz, 1H), 7.54–7.51 (m, 4H), 7.49–7.41 (m, 2H); 13C NMR (125 MHz, DMSO-d6) δ 155.9, 153.7, 143.0, 139.0, 133.6, 131.7, 129.9, 129.4, 129.3, 128.6, 127.6, 126.9, 115.1, 111.5; HRMS (ESI): m/z calcd. for C18H13N3[M + H]+: 272.1187; found: 272.1167.
6-(4-Fluorophenyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (5b). White solid; 86% yield (24.7 mg); mp 250–251 °C; 1H NMR (500 MHz, DMSO-d6) δ 13.8 (s, 1H), 8.61 (d, J = 8.0 Hz, 1H), 8.24–8.17 (m, 2H), 8.04 (d, J = 7.3 Hz, 2H), 7.83 (d, J = 8.0 Hz, 1H), 7.53 (t, J = 7.2 Hz, 2H), 7.42 (t, J = 7.1 Hz, 1H), 7.35 (t, J = 8.4 Hz, 2H); 1H NMR-deutrated (500 MHz, DMSO-d6) δ 8.45 (d, J = 8.4 Hz, 1H), 8.09–8.06 (m, 2H), 7.89 (d, J = 7.6 Hz, 2H), 7.67 (d, J = 8.4 Hz, 1H), 7.43 (t, J = 7.4 Hz, 2H), 7.32 (t, J = 7.2 Hz, 1H), 7.22 (t, J = 8.6 Hz, 2H); 13C NMR (125 MHz, DMSO-d6) δ 163.5 (d, J = 245.7 Hz, 1C), 162.6, 154.8, 153.5, 143.1, 135.5, 133.6, 131.8, 129.8 (d, J = 33.7 Hz, 1C), 129.4, 128.6, 126.9, 116.2 (d, J = 21.4 Hz, 1C), 114.9, 111.4; HRMS (ESI): m/z calcd. for C18H12FN3[M + H]+: 290.1093; found: 290.1075.
6-(4-Methoxyphenyl)-3-phenyl-1H-pyrazolo[3,4-b]pyridine (5c). White solid; 85% yield (25.5 mg); mp 252–253 °C; 1H NMR (500 MHz, DMSO-d6) δ 13.6 (s, 1H), 8.46 (d, J = 8.5 Hz, 1H), 8.07 (d, J = 8.6 Hz, 2H), 7.96 (d, J = 7.7 Hz, 2H), 7.71 (d, J = 8.5 Hz, 1H), 7.45 (t, J = 7.6 Hz, 2H), 7.34 (t, J = 7.4 Hz, 1H), 6.99 (d, J = 8.7 Hz, 2H), 3.74 (s, 3H); 1H NMR-deutrated (500 MHz, DMSO-d6) δ 8.42 (d, J = 8.2 Hz, 1H), 8.01 (d, J = 8.4 Hz, 2H), 7.89 (d, J = 7.9 Hz, 2H), 7.66 (d, J = 8.4 Hz, 1H), 7.43 (t, J = 7.5 Hz, 2H), 7.33 (t, J = 7.0 Hz, 1H), 6.97 (d, J = 8.4 Hz, 2H), 3.70 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 161.0, 155.6, 153.7, 143.0, 133.7, 131.5, 131.4, 129.4, 129.0, 128.5, 126.8, 114.7, 114.5, 111.0, 55.7; HRMS (ESI): m/z calcd. for C19H15N3O[M + H]+: 302.1293; found: 302.1273.
6-(Furan-2-yl)-3-(thiophen-3-yl)-1H-pyrazolo[3,4-b]pyridine (5d). White solid; 99% yield (25.9 mg); mp 252–253 °C; 1H NMR (500 MHz, DMSO-d6) δ 13.7 (s, 1H), 8.66 (d, J = 8.3 Hz, 1H), 8.23 (s, 1H), 7.93 (s, 1H), 7.74 (d, J = 8.9 Hz, 2H), 7.67 (d, J = 8.4 Hz, 1H), 7.29 (s, 1H), 6.73 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 153.4, 152.9, 147.9, 145.3, 140.0, 134.6, 131.6, 127.4, 126.5, 122.8, 113.2, 113.0, 111.2, 110.6; HRMS (ESI): m/z calcd. for C14H9N3OS[M + H]+: 268.0544; found: 268.0524.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
We gratefully acknowledge the University Grants Commission (UGC) and DBT for financially supporting this work, University of Delhi for the SC-XRD analysis; USIC-CIF University of Delhi, Delhi, India and AIRF, Jawaharlal Nehru University for NMR data and Instrumentation facility, Mass analysis.References
- For selected reviews on Suzuki-Miyaura see: (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef CAS; (c) F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270 RSC; (d) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed; (e) I. Maluenda and O. Navarro, Molecules, 2015, 20, 7528 CrossRef CAS PubMed; (f) A. J. J. Lennox and G. C. Lioyd-Jones, Chem. Soc. Rev., 2014, 43, 412 RSC; (g) A. Chatterjee and T. R. Ward, Catal. Lett., 2016, 146, 820 CrossRef CAS.
- For selected reviews on arylation of heteroaromatics see: (a) S. W. Cho, Ji Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (b) R. Rossi, F. Bellina, M. Lessi and C. Manzini, Adv. Synth. Catal., 2014, 356, 17 CrossRef CAS; (c) S. El-Kazzouli, J. Koubachi, N. El-Brahmi and G. Guillaumet, RSC Adv., 2015, 5, 15292 RSC; (d) S. Suzuki and J. Yamaguchi, Chem. Commun., 2017, 53, 1568 RSC; (e) Y. Yang, J. Lan and J. You, Chem. Rev., 2017, 117, 8787 CrossRef CAS PubMed; (f) V. F. Slagt, A. H. M. Vries, J. G. Vries and R. M. Kellogg, Org. Process Res. Dev., 2010, 14, 30 CrossRef CAS; (g) L. W. Qi, J. H. Mao, J. Zhang and B. Tan, Nat. Chem., 2018, 10, 58 CrossRef CAS PubMed; (h) H. H. Zhang, C. S. Wang, C. Li, G. J. Mei, Y. Li and F. Shi, Angew. Chem., Int. Ed., 2017, 56, 116 CrossRef CAS PubMed; (i) Y. Y. He, X. X. Sun, G. H. Li, G. J. Mei and F. Shi, J. Org. Chem., 2017, 82, 2462 CrossRef CAS PubMed.
- (a) S. Yanagisawa, K. Ueda, H. Sekizawa and K. Itami, J. Am. Chem. Soc., 2009, 131, 14622 CrossRef CAS PubMed; (b) F. Shibahara, E. Yamaguchi and T. Murai, J. Org. Chem., 2011, 76, 2680 CrossRef CAS PubMed; (c) J. M. Joo, B. B. Toure and D. Sames, J. Org. Chem., 2010, 75, 4911 CrossRef CAS PubMed; (d) F. Shibahara, T. Yamauchi, E. Yamaguchi and T. Murai, J. Org. Chem., 2012, 77, 8815 CrossRef CAS PubMed; (e) S. El. Kazzaouli, L. Boussane, M. Khouili and G. Guillaumet, Tetrahedron Lett., 2005, 46, 6163 CrossRef; (f) M. Ye, A. J. F. Edmunds, J. A. Morris, D. Sale, Y. Zhanga and Yu. Jin-Quan, Chem. Sci., 2013, 4, 2374 RSC; (g) P. Kannabodia, K. Anilkumar, S. Aravida, R. A. Vishwakarma and P. Das, Org. Lett., 2013, 15, 5718 CrossRef PubMed; (h) M. P. Heutis and K. A. Fagnou, Org. Lett., 2009, 11, 1357 CrossRef PubMed; (i) V. Gembus, J.-F. Bonfanti, O. Querolle, P. Jubault, V. Levacher and C. Hoarau, Org. Lett., 2012, 14, 6012 CrossRef CAS PubMed.
- (a) J. A. Thynne, D. C. Blakemore, D. C. Pryde and A. C. Spivey, Chem. Sci., 2017, 8, 40 RSC; (b) K. Sato, Z. Omahdi, K. Shibata, K. Sonoda, S. Yamasaki and H. Tanaka, Chem.–Eur. J., 2017, 23, 16374 CrossRef CAS PubMed.
- (a) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451 CrossRef CAS PubMed; (b) L. G. Mercier and M. Leclerc, Acc. Chem. Res., 2013, 41, 1597 CrossRef PubMed; (c) Y. Segawa, T. Maekawa and K. Itami, Angew. Chem., Int. Ed., 2015, 54, 66 CrossRef CAS PubMed.
- (a) J. Witherington, V. Bordas, S. L. Garland, D. M. B. Hickey, R. J. Ife, J. Liddle, M. Saunders, D. G. Smith and R. W. Ward, Bioorg. Med. Chem. Lett., 2003, 13, 1577 CrossRef CAS PubMed; (b) J. Witherington, V. Bordas, A. Gaiba, N. S. Garton, A. Naylor, A. D. Rawlings, B. P. Slingsby, D. G. Smith, A. K. Takle and R. W. Ward, Bioorg. Med. Chem. Lett., 2003, 13, 3055 CrossRef CAS PubMed; (c) S. Huang, R. Lin, Y. Yu, Y. Lu, P. J. Connolly, G. Chiu, S. Li, S. L. Emanuel and S. A. Middleton, Bioorg. Med. Chem. Lett., 2007, 17, 1243 CrossRef CAS PubMed; (d) R. Lin, G. Chiu, Y. Yu, P. J. Connolly, S. Li, Y. Lu, M. Adams, A. R. Fuentes-Pesquera, S. L. Emanuel and L. M. Greenberger, Bioorg. Med. Chem. Lett., 2007, 17, 4557 CrossRef CAS PubMed; (e) P. Czodrowski, A. Mallinger, D. Wienke, C. Esdar, O. Poschke, M. Busch, F. Rohdich, S. A. Eccles, M. Ortiz-Ruiz, R. Schneider, P. A. Raynaud, D. Clarke, D. Musil, T. Schwarz, K. Dale, K. Urbahns, J. Blagg and K. Schiemann, J. Med. Chem., 2016, 59, 9337 CrossRef CAS PubMed.
- F. Manetti, S. Schenone, F. Bondavalli, C. Brullo, O. Bruno, A. Ranise, L. Mosti, G. Menozzi, P. Fossa, M. L. Trincavelli, C. Martini, A. Martenelli, C. Tintori and M. Botta, J. Med. Chem., 2005, 48, 7172 CrossRef CAS PubMed.
- (a) B. Zhao, Y. Li, P. Xu, Y. Dai, C. Luo, Y. Sun, J. Ai, M. Geng and W. Duan, ACS Med. Chem. Lett., 2016, 7, 629 CrossRef CAS PubMed; (b) A. F. Abdel-Magid, ACS Med. Chem. Lett., 2015, 6, 369 CrossRef CAS PubMed.
- Y. Li, H. Cheng, Z. Zhang, X. Zhuang, J. Luo, H. Long, Y. Zhou, Y. Xu, R. Taghipouran, D. Li, A. Patterson, J. Smaill, Z. Tu, D. Wu, X. Ren and K. Ding, ACS Med. Chem. Lett., 2015, 6, 543 CrossRef CAS PubMed.
- M. D. Hill, H. Fang, J. M. Brown, T. Molski, A. Easton, X. Han, R. Miller, M. Hill-Drzewi, L. Gallagher, M. Matchett, M. Gulianello, A. Balakrishnan, R. L. Bertekap, K. S. Santone, V. J. Whiterock, X. Zhuo, J. J. Bronson, J. E. Macor and A. P. Degnan, ACS Med. Chem. Lett., 2016, 7, 1082 CrossRef CAS PubMed.
- J. Jouha, M. Loubidi, J. Bouali, S. Hamri, A. Hafid, F. Suzenet, G. Guillaumet, T. Dagci, M. Khouili, F. Aydin, L. Saso and G. Armagan, Eur. J. Med. Chem., 2017, 129, 41 CrossRef CAS PubMed.
- (a) G. Lavecchia, S. Berteina-Raboin and G. Guillaumet, Tetrahedron Lett., 2004, 45, 2389 CrossRef CAS; (b) S. T. Heller and S. R. Natarajan, Org. Lett., 2007, 9, 4947 CrossRef CAS PubMed; (c) S. Lee and S. B. Park, Org. Lett., 2009, 11, 5214–5217 CrossRef CAS PubMed; (d) G. L. Beutner, J. T. Kuethe, M. M. Kim and N. Yasuda, J. Org. Chem., 2009, 74, 789 CrossRef CAS PubMed.
- (a) S. Faarasse, S. El-Kazzouli, M. Naas, J. Jouha, F. Suznet and G. Guillaumet, J. Org. Chem., 2017, 82, 12300 CrossRef CAS PubMed; (b) H. Lavrard and F. Popowycz, Synthesis, 2018, 50, 998 CrossRef CAS.
- (a) M. Hussain, R. A. Khera, N. T. Hunga and P. Langer, Org. Biomol. Chem., 2011, 9, 370 RSC; (b) S. Reimann, P. Ehlers, A. Petrosyan, S. Kohse, A. Spannenberg, A. E. Surkus, T. V. Ghochikyan, A. S. Saghyan, S. Lochbrunner, O. Khn, R. Ludwig and P. Langer, Adv. Synth. Catal., 2014, 356, 1987 CrossRef CAS; (c) X. Dai, Y. Chen, S. Garrell, H. Liu, Li-K. Zhang, A. Palani, G. Hughes and R. Nargund, J. Org. Chem., 2013, 78, 7758 CrossRef CAS PubMed; (d) S. Reimann, S. Parpart, P. Ehlers, M. Sharif, A. Spannenberg and P. Langer, Org. Biomol. Chem., 2015, 13, 6832 RSC; (e) D. Montoir, A. Tonnerre, M. Duflos and B. Marc-Antoine, Eur. J. Org. Chem., 2014, 7, 1487 CrossRef; (f) Sanfoi and A. Chantal, Patent WO2014/198942, 2014, A1.
- S. Minakata, M. Komatsu and Y. Ohshiro, Synthesis, 1992, 7, 661 CrossRef.
- (a) T. Chan, K. Guckian, T. Jenkins, J. Thomas, J. Vessels, G. Kumaravel, R. Meissner, J. Lyssikatos, B. Lukas, I. Leaf and J. Duffield, Patent WO 2016/011390 A1, 2016, 105; (b) B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Tatchell, Vogel's Textbook of Practical Organic Chemistry, John Wiley & Sons, Inc., New York, 5th edn, 1989 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Spectroscopic data for all compounds. CCDC 1828970 and 1817412. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra07104g |
| This journal is © The Royal Society of Chemistry 2018 |