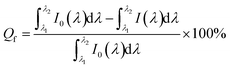Open Access Article
Open Access ArticleEnhanced upconversion fluorescent probe of single NaYF4:Yb3+/Er3+/Zn2+ nanoparticles for copper ion detection†
Baobao Zhang ,
Jiajia Meng,
Xiaohu Mi,
Changjian Zhang,
Zhenglong Zhang* and
Hairong Zheng*
,
Jiajia Meng,
Xiaohu Mi,
Changjian Zhang,
Zhenglong Zhang* and
Hairong Zheng*
School of Physics and Information Technology, Shaanxi Normal University, Xi'an 710119, P. R. China. E-mail: hrzheng@snnu.edu.cn; zlzhang@snnu.edu.cn
First published on 8th November 2018
Abstract
Surface modified NaYF4:Yb3+/Er3+/Zn2+ upconversion nanoparticles were obtained by using branched polyethylenimine (PEI). Strong fluorescence emission was observed and the influence of copper ions on the fluorescence emission of the PEI-modified NaYF4:Yb3+/Er3+/Zn2+ nanoparticles was investigated. It was found that the fluorescence emission can be quenched through luminescence resonance energy transfer from the particle to the copper ions. The results show that the PEI modified NaYF4:Yb3+/Er3+/Zn2+ nanoparticle can be used as a fluorescent probe for highly sensitive and selective detection of copper ions.
1. Introduction
Rare-earth doped up-conversion nanoparticles (UCNPs) have attracted much attention since 1990s with their excellent up-conversion luminescence properties such as large anti-Stokes shift, luminescence with rich color, narrowband emission and good optical stability.1–6 Up to now lots of research on the basic issues of synthesis and luminescence properties of UCNPs has been carried out.7–11 Meanwhile, the application of UCNPs in bioimaging and detection, photothermal therapy, contaminant detection, solar cells, anti-counterfeiting technology, three-dimensional displays and so on have been conducted.12–16 In the development of its application, the detection of heavy metal ions by fluorescent UCNP probes has become a very promising subject due to its advantages such as convenient operation, low cost, highly sensitive and selective.17–19 Considering the important role of copper ion in biological systems and their widespread distribution, the development of fluorescent probe for highly sensitive detection of copper ion is of great significance for environmental protection and life science research.Currently, most methods for detecting heavy metal ions by fluorescent probes are based on liquid phase. With this method, the sample is easy to prepare, but it needs to consume much more fluorescent probe material during the detection process, and the fluorescence emission intensity is easy to fluctuate significantly due to the possible uneven distribution of the fluorescent probes in the solution. While the strategy with single particle probe can avoid these problems and can further improve detection sensitivity.20 However, compared with the powder samples of upconversion nanoparticles, the luminescence intensity of the single nanoparticle is usually very weak, which results in the difficulty of the single nanoparticle fluorescent probe for metal ion detection. In order to improve the luminescence intensity and detecting efficiency, researchers have proposed a variety of methods, such as ions co-doping, the introduction of transition metal ions, core–shell coating, plasmon resonance enhancement and so on.21–25 Many studies have been reported on the introduction of smaller radius ions (Li+, Mn2+, Mg2+, etc.)26–28 in UCNPs to enhance the upconversion emission, but few studies on the introduction of Zn2+ ions into the rare-earth doped hexagonal phase NaYF4 nanoparticles are presented.29
In this paper, NaYF4:Yb3+(20%)/Er3+(2%)/Zn2+(7%) nanoparticles with enhanced luminescence were successfully obtained by introducing Zn2+ ions in the hydrothermal synthesis process to the UCNPs. A method for high-sensitivity detection of copper ion by single NaYF4:Yb3+/Er3+/Zn2+ nanoparticle fluorescent probe has been developed and reported. The linear detection range and the limit of single nanoparticle fluorescent probe for the copper ion detection were studied experimentally.
2. Experimental
2.1 Materials
Er(NO3)3·5H2O (99.9%), Yb(NO3)3·5H2O (99.9%), PEI (99.0%) and Y(NO3)3·6H2O (99.8%) were purchased from Sigma Aldrich. Zn(NO3)2·6H2O (99.0%), CuCl2·2H2O (99.0%), NaNO3 (99.0%), NaF (98.0%), C2H5OH (99.7%), C6H5Na3O7·2H2O (99.0%), FeCl3 (97%), SnCl2·2H2O (98%), KCl (99.5%), MgCl2·6H2O (98%), NaCl (99.5%), NH4Cl (99.5%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (China).All the chemicals were used without further purification.
2.2 Characterization
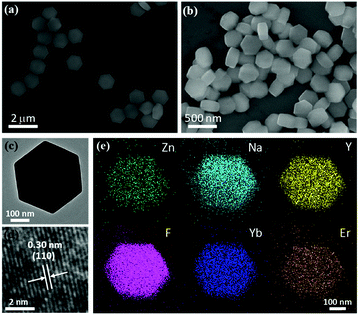
Morphologies of NaYF4:Yb3+/Er3+ and NaYF4:Yb3+/Er3+/Zn2+ particles were characterized by scanning electron microscope (SEM, FEI-Nova-450) and transmission electron microscopy (TEM, FEI-Tecnai G2 F20). Crystalline phase of the particle was studied by the powder X-ray diffraction (XRD, D8 Advance). The PEI modified NaYF4:Yb3+/Er3+/Zn2+ nanoparticles were examined by Fourier transform infrared spectra (FTIR, TENSOR II). These samples were excited by a femtosecond laser at 980 nm with power of 100 mW (Coherent Mira 900-F). The spectral data and microscopic images were collected by the micro-spectroscopy system which consists of a spectrometer (Princeton Instruments SP2750i) equipped with charge coupled device (CCD, ACTON PIXIS 100) and microscopy (OLYMPUS BX51) equipped with camera (Thorlabs 4070C-USB).2.3 Sample characterization
In order to obtain UCNPs with high luminescence efficiency and high surface to volume ratio so that a suitable single nanoparticle fluorescent probe can be designed,30–32 the hexagonal NaYF4:Yb3+/Er3+ microdisks and NaYF4:Yb3+/Er3+/Zn2+ nanoparticles were synthesized by hydrothermal method.33 As shown by scanning electron microscope (SEM) images (Fig. 1(a and b)) and size distribution graphs (Fig. S1(a and b)†), the morphologies of particles are uniform hexagonal disks. According to X-ray diffraction (XRD) shown in Fig. S2,† the crystallization of particles matches to the hexagonal phase crystal. Comparing with NaYF4:Yb3+/Er3+ microdisks, we found that the diffraction peak position and intensity of the NaYF4:Yb3+/Er3+/Zn2+ nanoparticles did not change significantly, which indicates the low content of Zn2+ ions in nanoparticles and less significant change of the crystal structure. Fig. 1(c and d) are typical morphology of the NaYF4:Yb3+/Er3+/Zn2+ nanoparticle, which was further provided by TEM and HR-TEM. The spatial distribution of the Na, Y, F, Yb, Er, and Zn in the nanoparticle are shown by elemental mapping images (Fig. 1(e)) of the single NaYF4:Yb3+/Er3+/Zn2+ nanoparticle, directly indicating that Zn2+ icons have been successfully incorporated into the nanoparticle.3. Results and discussion
3.1 Property of luminescence emission
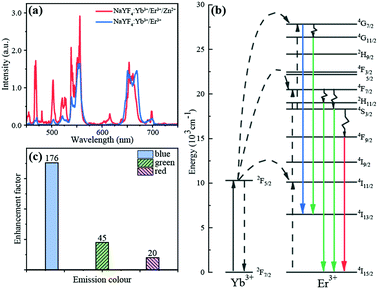
To study the effect of doped zinc ions on the emission property of the single UCNP, the luminescence emission spectra from single NaYF4:Yb3+/Er3+/Zn2+ nanoparticle and single NaYF4:Yb3+/Er3+ microdisk were measured under the 980 nm laser excitation, which are shown in Fig. 2(a). Five dominant emission peaks at around 468 nm, 503 nm, 521 nm, 556 nm and 654 nm are displayed by both particles, which correspond to transitions of 4G7/2 → 4I13/2, 4G11/2 → 4I13/2, 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2, respectively. In the obtained emission spectra, the red emission intensity was similar for both particle of NaYF4:Yb3+/Er3+ and NaYF4:Yb3+/Er3+/Zn2+ nanoparticle, while the green and blue light emission were enhanced for NaYF4:Yb3+/Er3+/Zn2+ nanoparticle comparing with that from NaYF4:Yb3+/Er3+ microdisk. Taking into account the difference in the size of NaYF4:Yb3+/Er3+/Zn2+ nanoparticle and NaYF4:Yb3+/Er3+ microdisk, the enhancement factors for blue, green, and red light emission per unit volume of the particle were 176, 45, and 20, respectively. Although such an estimation ignored the influence of absorption difference of particles and covered area of the excitation light spot etc., the enhancement factor 101 to 102 was sufficient to show that the effect of Zn2+ ions to the enhancement of upconversion luminescence from the rare-earth doped single NaYF4 particle was very significant. Our study on the enhancement mechanism and related reports suggest that the observed enhancement could be due to the local symmetry decrease of the crystal field around Er3+ ion by the introduction of Zn2+ ions into the particle.29,34,353.2 Fluorescent probe of single UCNP for Cu2+ detection
Since UCNP doped with zinc ions has much stronger luminescence emission and larger surface-to-volume ratio, we choose PEI-modified NaYF4:Yb3+/Er3+/Zn2+ as single-particle fluorescent probe. In order to investigate the detection ability and detection limit of the particle probe for detecting Cu2+ ions, we designed the single particle detecting system that is shown by Fig. 3. The luminescence of PEI-modified NaYF4:Yb3+/Er3+/Zn2+ nanoparticle might be quenched by copper ions and the concentration of copper ions can be calculated based on the degree of luminescence quenching.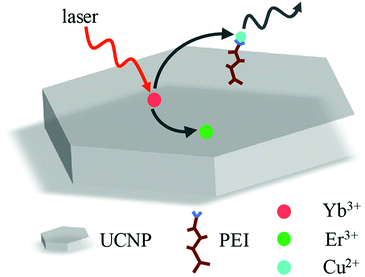 | ||
| Fig. 3 Schematic of the single particle probe for detecting the concentration of copper ion solution. | ||
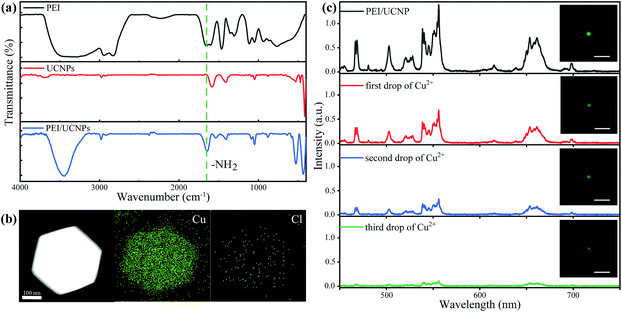
In order to conduct the selective detection of copper ions, we modified the surface of the UCNPs with PEI by physical adsorption. As shown in Fig. 4(a), the PEI and PEI-modified UCNPs both exhibited characteristic absorption peak at 1648 cm−1 that was assigned to the bending vibration of NH2 bond, since the absorption peak did not be observed from the unmodified UCNPs, this result indicates that PEI has been successfully modified on the particle for PEI-modified UCNPs. To examine the application possibility of PEI-modified UCNPs and the selection on the ions in the solution, the elemental distribution was characterized after adding 10 μL CuCl2 solution that contains both Cu2+ and Cl− ions to PEI-modified nanoparticle. Fig. 4(b) shows that a large amount of Cu2+ ions were adsorbed on the nanoparticle while chloride ions were seldomly detected. This indicates that the amino group contained in PEI can selectively bind with Cu2+ ions forming a complex with copper ions. Therefore, the modified nanoparticle can be a good candidate of single-particle fluorescent probe.
To illustrate the application of single PEI modified UCNP for detecting copper ions in solution, we conducted the experimental measurement with following procedure. First, the luminescence intensity of single-particle fluorescent probe was measured and the corresponding image was taken too. After that, 10 μL solution of 20 nM copper ions was completely added to the particle dropwise in situ, luminescence spectrum and microscopic image were recorded accordingly. Fig. 4(c) shows the luminescence spectra and corresponding images of the single particle probe before and after the addition of copper ion solution drop by drop. Obviously, after the copper ion solution was added, the luminescence intensity of the particle probe was significantly reduced. By integrating the luminescence intensity of the emission in the green region, we found that the degree of fluorescence quenching caused by copper ion solution was 52%, 77%, 93%, respectively. The degree of fluorescence quenching is estimated based on the formula of
To examine the influence of water solvent on the luminescence particle probe, we also carried out the spectral measurement of the probe particle by adding pure water solvent to it. It was found that the luminescence intensity of the single-particle fluorescent probe was also quenched obviously after water solvent added (see Fig. S4†). But the degree of luminescence quenching caused by water solvent was much less than that caused by copper ions. It was also found that the luminescence intensity of the bare NaYF4:Yb3+/Er3+/Zn2+ particle (unmodified with PEI) did not present obvious change after adding copper ion solution or water solvent to it. Therefore, it is reasonable to use PEI modified single NaYF4:Yb3+/Er3+/Zn2+ particle as a fluorescent probe for Cu2+ ion detection.
3.3 Sensitivity and selectivity of the single particle probe
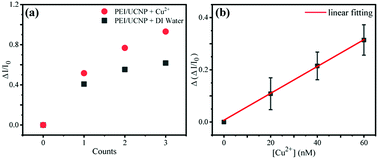
Considering that the copper ion solution is added dropwise and assuming that the copper ions are evenly distributed in the solution, we suppose that the number of copper ions accumulated on a single particle is basically the same for each droplet. With this assumption, the number of Cu2+ ions detected by the single particle probe can be considered equally increase with increase of the droplet number, which is equivalent to the increase of the Cu2+ concentration by one droplet.Since the luminescence intensity of the single-particle probe can also be influenced by water solvent, one need to deduct the luminescence emission contribution resulted by water solvent during the Cu2+ ion detection. Based on this consideration, experimental data obtained from the system with pure water solvent and Cu2+ ion water solution were plotted separately in the Fig. 5(a). After the contribution of water removed, the degree of luminescence quenching on the probe was plotted and fitted in Fig. 5(b). It shows that the single-particle fluorescent probe has a linear detection in the range of 20–60 nM, and the detection limit is about 11 nM that is comparable to the limit detected by existing methods.17,36,37
In addition, the selectivity of the single particle fluorescent probe for copper ion detection was investigated. As shown in Fig. S5,† the single particle probe exhibited significantly different fluorescence quenching responses to 60 nM Cu2+ solution and 600 nM other ions (Fe3+, Na+, K+, Mg2+, Sn2+, NH4+) solution. It was found that the single particle probe exhibited high selectivity towards Cu2+, which can be used for copper ion detection of the tap water from the Water Supply Company. As shown in Fig. S6,† the concentration of Cu2+ in the tap water was between 0.3–0.6 μM according to fitting result in Fig. 5(b), which was similar to the concentration (<0.4 μM) detected by Water Supply Company.
4. Conclusions
NaYF4:Yb3+/Er3+/Zn2+ nanoparticles with enhanced luminescence emission were synthesized and surface modification of the particles was performed successfully by using PEI. It was found that the PEI modified NaYF4:Yb3+/Er3+/Zn2+ particle can be a good fluorescent probe for Cu2+ ion detection. By studying the luminescence quenching effect of the single PEI-modified NaYF4:Yb3+/Er3+/Zn2+ particle, we obtained the linear detection range of 20–60 nM for the single particle probe, and the detection limit was around 11 nM. The current work offered an ion detection system with high sensitivity and selectivity, it also suggested that more single-particle fluorescent probe can be constructed for the detection of heavy metal ions and biomolecules.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 11574190, 11504224), the Fundamental Research Funds for Central Universities (GK201701008).References
- G. Y. Chen, T. Y. Ohulchanskyy, R. Kumar, H. Agren and P. N. Prasad, ACS Nano, 2010, 4, 3163–3168 CrossRef CAS PubMed.
- D. J. Gargas, E. M. Chan, A. D. Ostrowski, S. Aloni, M. V. P. Altoe, E. S. Barnard, B. Sanii, J. J. Urban, D. J. Milliron, B. E. Cohen and P. J. Schuck, Nat. Nanotechnol., 2014, 9, 300–305 CrossRef CAS PubMed.
- C. S. Ma, X. X. Xu, F. Wang, Z. G. Zhou, D. M. Liu, J. B. Zhao, M. Guan, C. I. Lang and D. Y. Jin, Nano Lett., 2017, 17, 2858–2864 CrossRef CAS PubMed.
- A. D. Ostrowski, E. M. Chan, D. J. Gargas, E. M. Katz, G. Han, P. J. Schuck, D. J. Milliron and B. E. Cohen, ACS Nano, 2012, 6, 2686–2692 CrossRef CAS PubMed.
- S. W. Wu, G. Han, D. J. Milliron, S. Aloni, V. Altoe, D. V. Talapin, B. E. Cohen and P. J. Schuck, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10917–10921 CrossRef CAS PubMed.
- D. F. Peng, Q. Ju, X. Chen, R. H. Ma, B. Chen, G. X. Bai, J. H. Hao, X. S. Qiao, X. P. Fan and F. Wang, Chem. Mater., 2015, 27, 3115–3120 CrossRef CAS.
- H. Chen, P. Zhang, H. Cui, W. Qin and D. Zhao, Nanoscale Res. Lett., 2017, 12, 548 CrossRef PubMed.
- S. Fischer, J. K. Swabeck and A. P. Alivisatos, J. Am. Chem. Soc., 2017, 139, 12325–12332 CrossRef CAS PubMed.
- F. Guzzetta, A. Roig and B. Julián-López, J. Phys. Chem. Lett., 2017, 8, 5730–5735 CrossRef CAS PubMed.
- F. Wang, Y. Han, C. S. Lim, Y. H. Lu, J. Wang, J. Xu, H. Y. Chen, C. Zhang, M. H. Hong and X. G. Liu, Nature, 2010, 463, 1061–1065 CrossRef CAS PubMed.
- M. W. Pin, E. J. Park, S. Choi, Y. I. Kim, C. H. Jeon, T. H. Ha and Y. H. Kim, Sci. Rep., 2018, 8, 10 CrossRef PubMed.
- J. J. Peng, A. Samanta, X. Zeng, S. Y. Han, L. Wang, D. D. Su, D. T. B. Loong, N. Y. Kang, S. J. Park, A. H. All, W. X. Jiang, L. Yuan, X. G. Liu and Y. T. Chang, Angew. Chem., Int. Ed., 2017, 56, 4165–4169 CrossRef CAS PubMed.
- A. Xia, Y. Y. Deng, H. Shi, J. Hu, J. Zhang, S. S. Wu, Q. Chen, X. H. Huang and J. Shen, ACS Appl. Mater. Interfaces, 2014, 6, 18329–18336 CrossRef CAS PubMed.
- S. H. Nam, Y. M. Bae, Y. I. Park, J. H. Kim, H. M. Kim, J. S. Choi, K. T. Lee, T. Hyeon and Y. D. Suh, Angew. Chem., Int. Ed., 2011, 50, 6093–6097 CrossRef CAS PubMed.
- P. Hu, X. F. Wu, S. G. Hu, Z. H. Chen, H. Y. Yan, Z. F. Xi, Y. Yu, G. T. Dai and Y. X. Liu, Photochem. Photobiol. Sci., 2016, 15, 260–265 RSC.
- Y. D. Han, H. Y. Li, Y. B. Wang, Y. Pan, L. Huang, F. Song and W. Huang, Sci. Rep., 2017, 7, 1320 CrossRef PubMed.
- F. Wang, C. Zhang, Q. Xue, H. Li and Y. Xian, Biosens. Bioelectron., 2017, 95, 21–26 CrossRef CAS PubMed.
- C. X. Li, J. L. Liu, S. Alonso, F. Y. Li and Y. Zhang, Nanoscale, 2012, 4, 6065–6071 RSC.
- Z. W. Cui, W. B. Bu, W. P. Fan, J. W. Zhang, D. L. Ni, Y. Y. Liu, J. Wang, J. N. Liu, Z. W. Yao and J. L. Shi, Biomaterials, 2016, 104, 158–167 CrossRef CAS PubMed.
- Y. Choi, Y. Park, T. Kang and L. P. Lee, Nat. Nanotechnol., 2009, 4, 742–746 CrossRef CAS PubMed.
- Y. Xue, C. Ding, Y. Rong, Q. Ma, C. Pan, E. Wu, B. Wu and H. Zeng, Small, 2017, 13, 1701155 CrossRef PubMed.
- Y. Li, X. Liu, X. Yang, H. Lei, Y. Zhang and B. Li, ACS Nano, 2017, 11, 10672–10680 CrossRef CAS PubMed.
- J. Zuo, Q. Q. Li, B. Xue, C. X. Li, Y. L. Chang, Y. L. Zhang, X. M. Liu, L. P. Tu, H. Zhang and X. G. Kong, Nanoscale, 2017, 9, 7941–7946 RSC.
- S. Fischer, N. D. Bronstein, J. K. Swabeck, E. M. Chan and A. P. Alivisatos, Nano Lett., 2016, 16, 7241–7247 CrossRef CAS PubMed.
- A. Dubey, A. K. Soni, A. Kumari, R. Dey and V. K. Rai, J. Alloys Compd., 2017, 693, 194–200 CrossRef CAS.
- B. Zhou, B. Xu, H. M. He, Z. J. Gu, B. Tang, Y. Ma and T. Y. Zhai, Nanoscale, 2018, 10, 2834–2840 RSC.
- M. Ding, Y. Ni, Y. Song, X. Liu, T. Cui, D. Chen, Z. Ji, F. Xu, C. Lu and Z. Xu, J. Alloys Compd., 2015, 623, 42–48 CrossRef CAS.
- R. Dey, A. Kumari, A. K. Soni and V. K. Rai, Sens. Actuators, B, 2015, 210, 581–588 CrossRef CAS.
- T. Cong, Y. D. Ding, J. P. Liu, H. Y. Zhao and X. Hong, Mater. Lett., 2016, 165, 59–62 CrossRef CAS.
- Z. H. Li, H. Yuan, W. Yuan, Q. Q. Su and F. Y. Li, Coord. Chem. Rev., 2018, 354, 155–168 CrossRef CAS.
- M. Y. Hossan, A. Hor, Q. Luu, S. J. Smith, P. S. May and M. T. Berry, J. Phys. Chem. C, 2017, 121, 16592–16606 CrossRef CAS.
- A. Pliss, T. Y. Ohulchanskyy, G. Chen, J. Damasco, C. E. Bass and P. N. Prasad, ACS Photonics, 2017, 4, 806–814 CrossRef CAS.
- Q. Y. Han, C. Y. Zhang, C. Wang, Z. J. Wang, C. X. Li, W. Gao, J. Dong, E. J. He, Z. L. Zhang and H. R. Zheng, Sci. Rep., 2017, 7, 5371 CrossRef PubMed.
- Y. Kuang, J. T. Xu, C. Wang, C. Q. Wang, H. Shao, D. Yang, S. L. Gai, F. He and P. P. Yang, CrystEngComm, 2018, 20, 2663–2668 RSC.
- P. Du, E. J. Kim and J. S. Yu, Curr. Appl. Phys., 2018, 18, 310–316 CrossRef.
- H. Shao, D. Xu, Y. Ding, X. Hong and Y. Liu, Microchim. Acta, 2018, 185, 211 CrossRef PubMed.
- Q. Yan, Z.-H. Chen, S.-F. Xue, X.-Y. Han, Z.-Y. Lin, S. Zhang, G. Shi and M. Zhang, Sens. Actuators, B, 2018, 268, 108–114 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07103a |
| This journal is © The Royal Society of Chemistry 2018 |