Open Access Article
Open Access ArticleStructure and reducibility of yttrium-doped cerium dioxide nanoparticles and (111) surface†
Hristiyan A. Aleksandrov *ab,
Iskra Z. Kolevaa,
Konstantin M. Neyman
*ab,
Iskra Z. Kolevaa,
Konstantin M. Neyman bc,
Tatyana T. Tabakova
bc,
Tatyana T. Tabakova d and
Georgi N. Vayssilov
d and
Georgi N. Vayssilov *a
*a
aFaculty of Chemistry and Pharmacy, University of Sofia, 1126 Sofia, Bulgaria
bDepartament de Ciència de Materials i Química Física and Institut de Quimica Teòrica i Computacional, Universitat de Barcelona, 08028 Barcelona, Spain
cICREA (Institució Catalana de Recerca i Estudis Avançats), 08010 Barcelona, Spain
dInstitute of Catalysis, Bulgarian Academy of Sciences, Acad. G. Bonchev Str., bl. 11, Sofia 1113, Bulgaria. E-mail: haa@chem.uni-sofia.bg
First published on 2nd October 2018
Abstract
Using periodic density functional calculations, we studied the local structure and preferred locations of yttrium cations and oxygen vacancies in Y-doped cerium dioxide. We employed three kinds of models – a slab of the CeO2(111) surface and two ceria nanoparticles of different sizes and shapes. In the slab models, which represent the (111) surface of ceria and the corresponding extended terraces on the facets of its nanoparticles, Y3+ cation dopants were calculated to be preferentially located close to each other. They tend to surround a subsurface oxygen vacancy that forms to maintain the charge balance. Such general behavior was not found for the nanoparticle models, in which structural flexibility and the presence of various low-coordinated surface centers seem to be crucial and suppress most of the trends. Configurations with four Y3+ cations were calculated to be particularly stable when they combined two of the most stable configurations with two Y3+ cations. However, no clear trend was found regarding the preferential spatial distribution of the Y3+ pairs – they can be stable both in isolation and close to each other. In general, doping by yttrium does not notably change the reducibility of ceria systems but selectively facilitates the formation of oxygen vacancies at the ceria surface in comparison with pristine ceria. Yttrium cations also slightly increase the basicity of the nearby oxygen centers with respect to a stoichiometric ceria surface.
1. Introduction
In recent years, ceria-based materials have excited tremendous interest from both fundamental scientific and commercial points of view. In particular, ceria has attracted much attention because of its crucial role as a component of catalysts for numerous catalytic applications. The ever-growing interest is demonstrated by the vast numbers of publications starting from the review by Trovarelli in 1996,1 which was followed by two books in 2002 (ref. 2) and 2013 (ref. 3) and many reviews,4–6 as well as a special issue of Catalysis Today in 2015.7Many studies have revealed the applicability of ceria as a support in different catalysts owing to its well-known high oxygen storage capacity (OSC).1–3 It is a reducible oxide, and O vacancies can be created in ceria at elevated temperatures. This process is accompanied by the reduction of two Ce4+ cations to the Ce3+ state for each O vacancy and can be reversible depending on the conditions.1–4
Doping of ceria is a commonly exploited approach for tailoring its redox properties and concomitantly affecting its catalytic behavior.8 The presence of aliovalent dopants9 causes the appearance of extrinsic defects that play a key role in the performance of many catalytic processes. Of particular importance for both technological applications and fundamental research are CeO2 materials doped with rare-earth elements, including yttrium. When CeO2 is doped by an oxide of a trivalent metal, such as Y2O3, the initial structure contains O vacancies that are created in order to compensate the effective negative charge produced by the substitution of Ce4+ with Y3+. Thus, a distortion is induced in the cubic matrix of the resulting Y2O3–CeO2 mixed oxide. The beneficial effect of yttrium doping on the activity of ceria in soot combustion has been demonstrated by Atribak et al.10 Considering the abundance of yttrium resources, She et al. studied the effect of the addition of Y (0–5 wt%) to a CuO/CeO2 catalyst on its activity in the water–gas shift.11 The highest catalytic activity was exhibited by the catalyst doped with 2 wt% Y. The effects of different synthesis routes for the preparation of Y-doped ceria and of various amounts of Y on the performance of the preferential oxidation of CO (PROX) in the presence of excess hydrogen on supported gold catalysts have been discussed by Ilieva et al.12 Irrespective of the preparation method, very similar degrees of conversion of CO were recorded for samples containing 1–5 wt% Y2O3 in the temperature range of 80–120 °C, which is of interest for the operation of fuel cells. The relatively low activity that was observed for doped ceria samples with a Y2O3 content of 7.5 wt% was attributed to the restricted supply of oxygen due to the assumed ordering of surface oxygen vacancies around segregated Y3+. Similarly, no improvement in the PROX activity and stability of gold catalysts supported on Y-doped ceria was reported by Jardim et al.,13 who explained this result by the surface segregation of the large amount of Y2O3 used (30 wt%).
Several theoretical investigations have focused on the understanding of the doping of ceria by yttrium and lanthanide metals. For instance, Dholabhai et al. studied the formation and migration of oxygen vacancies in Pr- and Gd-doped ceria with the help of methods based on density functional theory (DFT).14,15 The local ordering of oxygen vacancies in Ce1−xYxO2−x/2 where x = 0.04, 0.08, 0.11, 0.18, and 0.26 (corresponding to 2, 4, 6, 10, and 15 mol% Y2O3) has been investigated by analyzing data from experimental measurements (impedance spectroscopy and neutron diffraction) and ab initio molecular dynamics simulations.16 It was concluded that the ionic conductivity of Y-doped ceria decreased with an increase in the amount of Y2O3 because of ordering of anion vacancies. The distribution of rare-earth oxide dopants in ceria and the oxygen ion conductivity of these systems were also investigated by Grieshammer et al., who combined DFT and Monte Carlo studies.17 Hu and Metiu18 modelled the doping of the ceria(111) surface by a single Y3+ cation with no charge-compensating O vacancy, which would require the introduction of a second dopant cation. This single dopant lowered the formation energy of an O vacancy by more than 1.2 eV. Surprisingly, in another DFT-based study of an yttrium-doped CeO2(110) surface19 it was reported that a peculiar arrangement of the charge distribution in the form of a structure comprising two Y3+ cations, one O vacancy, one Ce3+ cation and one O− anion was found. This charge distribution was reported to be the most stable but did not provide the energetics of the conventional arrangement containing only Ce4+ and O2− ions. In any case, it will be interesting for catalytic applications and further studies to understand the influence of Y3+ cations on the reducibility of stoichiometric CeO2/Y2O3 systems and to clarify whether nanostructuring changes the behavior of these systems with respect to the surface models.
In the current study, we modeled various structures in which Y3+ cations replace Ce4+ cations on/in the CeO2(111) slab and two models of ceria nanoparticles with different sizes (Ce21O42 and Ce40O80) with low Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce atomic ratios of 3.9%/8.0% for the slab model and 10.5%/23.5% and 5.3%/11.1% for the two nanoparticle models, respectively. The goals of the present study were to clarify whether yttrium dopants prefer to segregate or be distantly distributed among cerium cations and to estimate changes in the reducibility of ceria in the presence of Y3+ cations. It is also interesting to observe the differences between the behavior of the slab, which can represent larger particles, and the nanoparticle models, where irregularities and low-coordinated surface positions (edges and corners) are more abundant.
Ce atomic ratios of 3.9%/8.0% for the slab model and 10.5%/23.5% and 5.3%/11.1% for the two nanoparticle models, respectively. The goals of the present study were to clarify whether yttrium dopants prefer to segregate or be distantly distributed among cerium cations and to estimate changes in the reducibility of ceria in the presence of Y3+ cations. It is also interesting to observe the differences between the behavior of the slab, which can represent larger particles, and the nanoparticle models, where irregularities and low-coordinated surface positions (edges and corners) are more abundant.
2. Computational details and models
The calculations were performed using a periodic plane-wave DFT method with the PW91 (ref. 20) exchange-correlation functional of the generalized gradient approximation (GGA) type as implemented in the VASP program.21–23 Stoichiometric and reduced ceria were described within the so-called GGA+U approach,24,25 in which an on-site coulombic interaction with U = 4 eV (ref. 26) was included. Our test calculations without applying the U correction for systems that do not contain Ce3+ cations showed that the relative stability of the structures remains similar to that determined with U = 4 eV. A plane-wave basis set with a cut-off of 415 eV for the kinetic energy and the projector-augmented wave23 description of core-valence electron interactions were employed. During geometry optimization all atoms were allowed to relax locally until the maximum forces that acted on each atom became less than 0.02 eV Å−1. We performed only Γ-point calculations. Spin polarization was taken into account when O vacancies were modeled because this leads to the formation of Ce3+ cations, which have unpaired electrons. The formation energy of an oxygen vacancy Evac was calculated with respect to half the energy of an O2 molecule in its triplet state as follows:| Evac = E(CexO2x−1) + 1/2 × E(O2) − E(CexO2x) |
| Evac = E(Cex−2Y2O2x−2) + 1/2 × E(O2) − E(Cex−2Y2O2x−1) |
2.1 CeO2(111) surface slab model
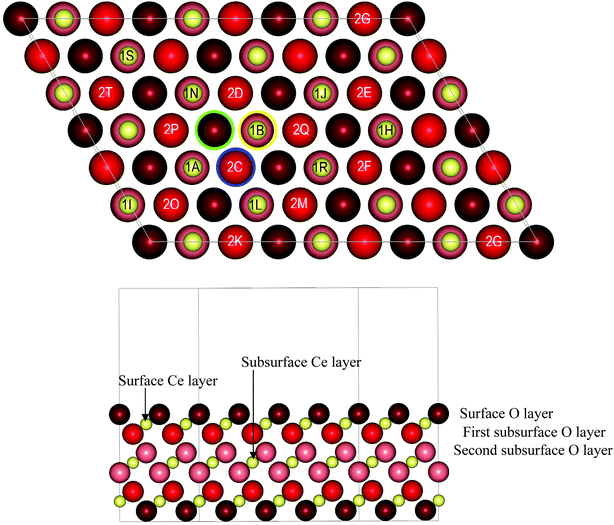
The CeO2(111) surface was modelled with a Ce54O108 slab comprising three CeO2 (nine atomic) layers and a 3 × 6 surface cell with cell parameters of a = 13.224 Å, b = 19.836 Å, c = 19.353 Å, α = β = 90°, and γ = 60°. The lateral dimensions of the cell were fixed according to the experimental bulk geometry (a = 541 pm).27 The notation used for the modelled structures is given in the sections below, where the corresponding structures are discussed.2.2 Ce21O42 and Ce40O80 nanoparticle models
We employed a small Ce21O42 ceria nanoparticle with a diameter of about 1 nm,28–34 which was located in a cubic unit cell with dimensions of 2.0 × 2.0 × 2.0 nm, as well as a larger Ce40O80 nanoparticle with a diameter of about 1.5 nm,30,35–38 which was positioned in a unit cell with a rectangular parallelepiped shape and dimensions of 2.2 × 1.9 × 1.9 nm. In these models, the distance between neighboring images in the periodic unit cells is at least 0.9 nm. The notation used for the modelled structures is given in the sections below, where the corresponding structures are discussed.3. Results and discussion
3.1 Yttrium-doped CeO2(111) surface
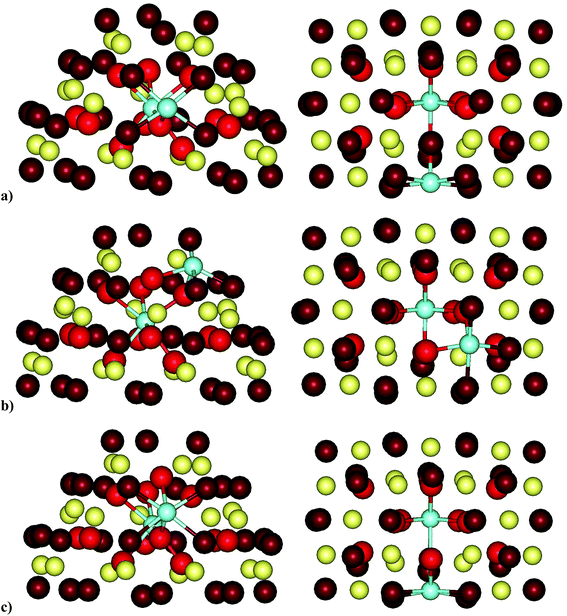
For the regular CeO2(111) surface, we modeled various structures of two types of stoichiometric system in which two and four cerium ions, respectively, were substituted by yttrium ions. In addition, for the former type of model we computed the formation energies of an oxygen vacancy.| Structurea | Erelb | Y–Yc | Y–Ovacd | Ne | Mf |
|---|---|---|---|---|---|
| a Position of Y3+ cations as shown in Fig. 1. Structure names in italics indicate that the Y3+ cations are located in nearest-neighboring positions.b Relative energy with respect to the most stable structure, i.e., OFS(1B,2C).c Distance between two nearest-neighboring Y cations.d Distances between Y atoms and the O vacancy.e Coordination numbers of Y3+ with respect to O atoms.f Number of O atoms bound simultaneously to both Y3+ cations. | |||||
| OSL(1A,1B) | 0.32 | 421 | 260, 263 | 6, 6 | 1 |
| OSL(1B,2C) | 0.33 | 384 | 263, 456 | 6, 8 | 2 |
| OSL(2C,2D) | 0.40 | 375 | 458, 460 | 8, 8 | 2 |
| OSL(2E,2F) | 0.67 | 378 | 893, 894 | 8, 8 | 2 |
| OSL(2E,2G) | 0.68 | 376 | 895, 1041 | 8, 8 | 2 |
| OSL(1H,2G) | 0.79 | 662 | 890, 1042 | 7, 8 | 0 |
| OSL(2F,1H) | 0.83 | 381 | 889, 893 | 8, 7 | 2 |
| OSL(1I,1J) | 0.86 | 1137 | 596, 609 | 7, 7 | 0 |
| OSL(1H,1J) | 1.00 | 375 | 600, 887 | 7, 7 | 2 |
| OFS(1B,2C) | 0.00 | 410 | 252, 256 | 6, 7 | 1 |
| OFS(2C,2D) | 0.02 | 378 | 257, 448 | 7, 8 | 2 |
| OFS(1A,1B) | 0.26 | 409 | 250, 253 | 6, 6 | 1 |
| OFS(2E,2F) | 0.37 | 378 | 706, 801 | 8, 8 | 2 |
| OFS(2E,2G) | 0.40 | 378 | 803, 1038 | 8, 8 | 2 |
| OFS(1H,2G) | 0.51 | 661 | 799, 801 | 7, 8 | 0 |
| OFS(2F,1H) | 0.52 | 380 | 706, 800 | 8, 7 | 2 |
| OFS(1I,1J) | 0.63 | 1156 | 591, 596 | 7, 7 | 0 |
| OFS(1H,1J) | 0.73 | 376 | 595, 799 | 7, 7 | 2 |
| OSS(2C,2D) | 0.02 | 406 | 249, 252 | 7, 7 | 1 |
| OSS(1B,2C) | 0.38 | 420 | 249, 265 | 6, 7 | 1 |
| OSS(2E,2F) | 0.49 | 379 | 593, 594 | 8, 8 | 2 |
| OSS(2E,2G) | 0.55 | 376 | 595, 798 | 8, 8 | 2 |
| OSS(2F,1H) | 0.72 | 377 | 593, 701 | 8, 7 | 2 |
| OSS(1H,2G) | 0.73 | 660 | 703, 799 | 7, 8 | 0 |
| OSS(1A,1B) | 0.83 | 377 | 266, 442 | 7, 6 | 2 |
| OSS(1I,1J) | 0.91 | 1147 | 448, 797 | 7, 7 | 0 |
| OSS(1H,1J) | 0.93 | 381 | 448, 700 | 7, 7 | 2 |
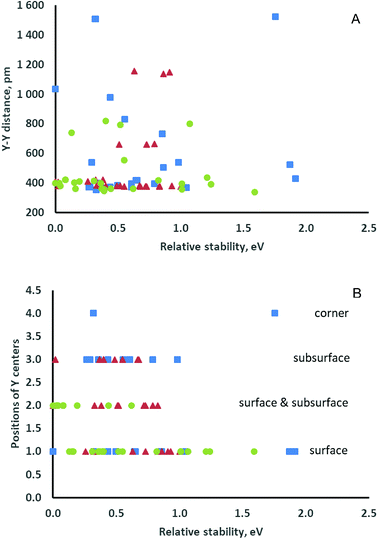
The stability of the modeled structures (Table 1) depends on the positions of both the two yttrium centers and the O vacancy and varies by up to 1.0 eV. The three most stable and essentially isoenergetic structures, namely, OFS(1B,2C), OFS(2C,2D) and OSS(2C,2D), contain at least one subsurface Y3+ cation, and the O vacancy is in either the subsurface or the second subsurface layer. The next two structures, namely, OFS(1A,1B) and OSL(1A,1B), in which both yttrium cations are on the surface, are less stable by ca. 0.3 eV. In all the most stable structures destabilized by up to 0.5 eV the two Y3+ cations are located at neighboring sites separated by up to ca. 420 pm. Note, however, that there are structures that completely or partially have the above features but are notably less stable (see the brown triangles in Fig. 2A). Fig. 2B (brown triangles), which includes all the modeled relative locations of the yttrium centers, also shows the lack of a clear relation between the relative stability and the location of the two dopant cations on the surface, in the subsurface layer or in both positions.
Although the O vacancies in the most stable structures are located at sites that neighbor yttrium centers, a general correlation between the Y–Ovac distances (accounted for in different ways, see Fig. S4 in ESI†) and the relative stability of all the modeled structures cannot be found.
In order to determine whether the space of the missing O atom can be refilled by an O atom, we modeled structures in which an added O atom was put in the position of the missing O atom in the Y-containing models discussed above. The systems were optimized to be spin-polarized and neutral. All the structures with an additional O atom were calculated to be less stable by 0.83, 1.27 and 1.12 eV than the initial structures, namely, OSL(1A,1B), OFS(1B,2C) and OSS(2C,2D), plus half a gas-phase O2 molecule, respectively. Hence, the filling by an oxygen atom of the charge-compensating O vacancy in such surface and subsurface positions is, not unexpectedly, energetically unfavorable up to rather high oxygen pressures.
In order to determine the relative stability of the electronic states of a system reported elsewhere,19 we performed geometry optimization of the OSL(1I,1J) and OSL(1B,2C) structures in the fixed triplet state. In this way, we obtained structures with an electron density distribution analogous to that in ref. 19, with one Ce3+ cation and partially electron-deficient oxygen anions due to the forced transfer of one O 2p electron to a Ce4+ cation. However, in the cases of both OSL(1I,1J) and OSL(1B,2C) this triplet state was less stable by more than 1.4 eV than the aforementioned singlet state. Hence, the spontaneous transfer of an electron from an O2− ion to a Ce4+ ion in the yttrium-doped ceria systems appears to result in an excited state rather than the ground state.
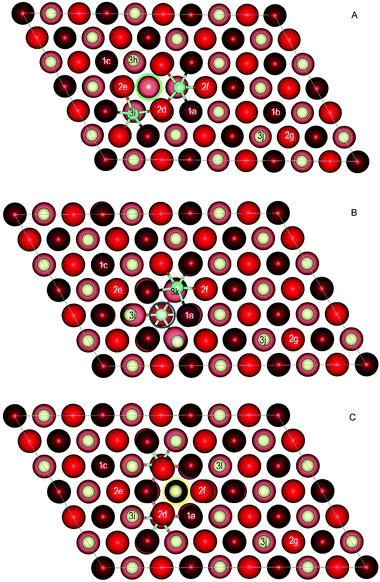 | ||
| Fig. 3 Top view of the initial structures of the doped ceria surface used to model the formation of a second oxygen vacancy: A – OSL(1A,1B) structure, B – OFS(1B,2C) structure, and C – OSS(2C,2D) structure. The first O vacancy is marked as a green, blue or yellow circle; see the caption of Fig. 1. Each position of the second removed O atom is denoted by a number and a lower-case letter. The number indicates the vertical position of the removed O atom: 1 – from the first (surface) anionic layer, 2 – from the second (subsurface) anionic layer and 3 – from the third (directly below a surface cation) anionic layer. Lower-case letters are used to distinguish various surface and subsurface positions. Color coding: Ce – yellow, O in the surface layer – dark red, O in the first subsurface layer – red, O in the second subsurface layer – light red, Y – light blue. For clarity the Y–O bonds are shown. | ||
| Structurea | Erelb | Evacc | Ovac–Ovacd | #Cee |
|---|---|---|---|---|
| a The positions of the second O vacancy in the most stable structures, i.e., OSL(1A,1B), OFS(1B,2C), and OSS(2C,2D) (see Fig. 1 and Table 1), are denoted by lower-case letters, as shown in Fig. 2–4, and numbers. The numbers specify the positions of the removed O atom: 1 – in the first (surface) layer, 2 – in the second (subsurface) layer, and 3 – in the third (below a surface Ce or Y cation) layer.b Relative energy with respect to the most stable structure, i.e., OFS(1B,2C)-2f.c Formation energy of an O vacancy.d Distance between two O vacancies.e Positions of the Ce3+ cations (see numbers in Fig. S1). | ||||
| OSL(1A,1B)-1a | 0.41 | 2.61 | 389 | 39, 40 |
| OSL(1A,1B)-2g | 0.55 | 2.75 | 1179 | 13, 48 |
| OSL(1A,1B)-1b | 0.60 | 2.80 | 656 | 47, 48 |
| OSL(1A,1B)-1c | 0.60 | 2.80 | 389 | 15, 16 |
| OSL(1A,1B)-3j | 0.63 | 2.83 | 1014 | 47, 48 |
| OSL(1A,1B)-2f | 0.75 | 2.94 | 482 | 27, 31 |
| OSL(1A,1B)-3h | 0.90 | 3.10 | 388 | 16, 20 |
| OSL(1A,1B)-2e | 0.92 | 3.12 | 242 | 15, 16 |
| OSL(1A,1B)-2d | 1.02 | 3.21 | 233 | 31, 40 |
| OSL(1A,1B)-3i | 1.05 | 3.25 | 386 | 20, 31 |
| OFS(1B,2C)-2f | 0.00 | 2.52 | 391 | 27, 39 |
| OFS(1B,2C)-1c | 0.14 | 2.65 | 606 | 6, 16 |
| OFS(1B,2C)-2e | 0.15 | 2.67 | 388 | 4, 16 |
| OFS(1B,2C)-1a | 0.25 | 2.76 | 247 | 39, 40 |
| OFS(1B,2C)-3j | 0.29 | 2.80 | 811 | 47, 48 |
| OFS(1B,2C)-2g | 0.30 | 2.82 | 1009 | 13, 47 |
| OFS(1B,2C)-3k | 0.72 | 3.24 | 255 | 1, 30 |
| OFS(1B,2C)-3i | 0.76 | 3.28 | 248 | 4, 32 |
| OSS(2C,2D)-2g | 0.10 | 2.61 | 974 | 13, 48 |
| OSS(2C,2D)-1c | 0.18 | 2.70 | 662 | 6, 15 |
| OSS(2C,2D)-2e | 0.23 | 2.75 | 463 | 4, 16 |
| OSS(2C,2D)-3i | 0.38 | 2.90 | 385 | 4, 20 |
| OSS(2C,2D)-3j | 0.45 | 2.97 | 764 | 42, 48 |
| OSS(2C,2D)-3l | 0.47 | 2.98 | 386 | 18, 27 |
| OSS(2C,2D)-2d | 0.53 | 3.05 | 241 | 3, 4 |
| OSS(2C,2D)-1a | 0.53 | 3.05 | 397 | 39, 40 |
| OSS(2C,2D)-2f | 0.71 | 3.23 | 243 | 26, 27 |
| OSS(2C,2D)-4a | 0.78 | 3.30 | 253 | 30, 35 |
As a reference, we considered the formation of an oxygen vacancy on the surface and in the first and second subsurface layers of the undoped surface (Fig. 1). The most stable structure is that in which the O vacancy is in the first subsurface layer, for which Evac = 2.55 eV, which is close to the value of 2.48 eV from PW91+4 calculations.40 The structures with the vacancy in the second subsurface layer or surface layer are higher in energy by 0.22 and 0.33 eV, respectively.
The lowest formation energy of an O vacancy among the Y-doped structures corresponds to OFS(1B,2C)-2f, in which both missing O atoms are in the first subsurface layer with Evac = 2.52 eV, which is only slightly lower than the value calculated for the pristine CeO2(111) surface. The next four structures have Evac values that are higher by 0.1–0.2 eV and feature O vacancies in different locations – a combination of the surface and first and second subsurface layers. This similarity in the stability of structures with the O vacancy in different locations suggests that various spatial distributions of O vacancies may exist in Y-doped ceria. One should note the OSL(1A,1B)-1a structure, for which Evac = 2.61 eV, where both O vacancies are on the surface. This value is lower by 0.27 eV than the Evac calculated for the removal of an equivalent surface O atom from the pristine CeO2(111) surface. This result implies that the presence of Y3+ can facilitate the removal of surface oxygen atoms in comparison with those on the undoped ceria surface. Thus, the difference between the formation energies of an oxygen vacancy on the surface and in the subsurface layer of the oxide is reduced to only 0.10 eV from the notably larger value of 0.33 eV for the bare ceria surface.
A comparison of the locations of vacancies in different structures suggests that two O vacancies may be either close to or far from each other, but it is not favorable to remove an O atom located between two Y3+ cations.
In summary, doping by Y does not influence the formation energy of an O vacancy in the subsurface region. However, the formation energy of a surface vacancy is reduced in comparison with that for the undoped surface and becomes close to the formation energy of a subsurface vacancy.
This finding agrees with the results of a recent experimental study of relationships between the structural features and activity in the water-gas shift (WGS) of gold catalysts supported on Y-doped ceria (1.0, 2.5, 5.0 and 7.5 wt% Y2O3).41 Temperature-programmed reduction measurements revealed that the doping of ceria with Y2O3 affected the reduction behavior. The effect was stronger when the doped ceria supports were prepared by impregnation rather than co-precipitation. The improved reducibility of the gold catalysts on ceria with predominant surface modification (impregnated samples) was closely correlated with better WGS performance in comparison with the gold samples on supports prepared by co-precipitation with prevailing bulk modification by Y. A nanoscale 3D electron microscopy study of La-doped ceria by Collins et al. also showed that reduction occurred predominantly at the surface of nanoparticles, as estimated from the locations of Ce3+ ions.9
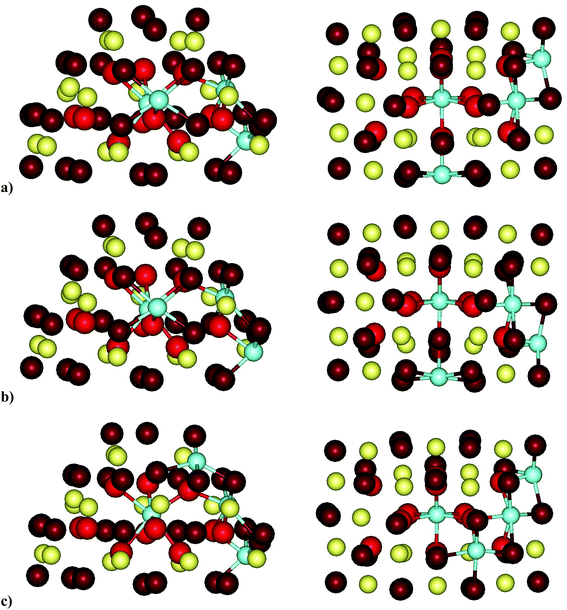
| Structurea | Erelb | Y–Yc | ΔY–Yd |
|---|---|---|---|
| a Positions of Y3+ cations as shown in Fig. 1. Structure names in italics indicate that both Y3+ pairs are located close to each other.b Relative energy with respect to the most stable structure, i.e., OFS(1B,2C)/OFS(1H,2F).c Distances between two nearest Y cations.d Difference between the Y–Y distances in the structure with four Y3+ cations and the structures with two Y3+ cations, i.e., OFS(1B,2C) (410 pm) and OSS(2C,2D) (406 pm), respectively. | |||
| OFS(1B,2C)/OSL(1A,1N) | 0.64 | 414; 421 | 4 |
| OFS(1B,2C)/OFS(1A,1N) | 0.19 | 407; 399 | −3 |
| OFS(1B,2C)/OSS(2D,2Q) | 0.51 | 461; 402 | 51 |
| OFS(1B,2C)/OSL(1A,1L) | 0.80 | 408; 455 | −2 |
| OFS(1B,2C)/OFS(1H,2F) | 0.00 | 408; 410 | −2 |
| OFS(1B,2C)/OFS(1R,2M) | 0.10 | 408; 409 | −2 |
| OFS(1B,2C)/OFS(1S,2T) | 0.14 | 410; 408 | 0 |
| OSS(2C,2D)/OSS(1N,1B) | 0.64 | 404; 417 | −2 |
| OSS(2C,2D)/OSS(1L,2K) | 0.37 | 396; 421 | −10 |
| OSS(2C,2D)/OSS(2K,2M) | 0.05 | 396; 407 | −10 |
| OSS(2C,2D)/OSS(2E,2F) | 0.01 | 405; 406 | −1 |
| OSS(2C,2D)/OSS(2M,2Q) | 0.10 | 408; 399 | 2 |
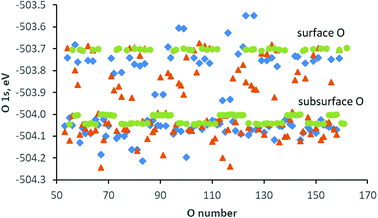
Oxygen atoms in pristine CeO2(111) feature a very distinct separation of the O 1s core levels – the surface and subsurface oxygen centers have E(O 1s) values of around −503.7 and −504.0 eV, respectively (see the green bullets in Fig. 4). The presence of yttrium cations and an oxygen vacancy modifies the O 1s energy values (see the brown triangles and blue rhombuses in Fig. 4 and S3A in ESI†), and the E(O 1s) values extend over a somewhat wider interval from −503.5 to −504.2 eV. For most of the oxygen centers, the O 1s core level is stabilized by only up to 0.1 eV (Fig. S3A in ESI†). Oxygen centers close to yttrium ions and oxygen vacancies exhibit a more specific behavior. In the structure with both yttrium cations and the oxygen vacancy in the surface layer, the O 1s level of the oxygen centers between two cations (in the first subsurface layer) is destabilized by 0.4 eV, and the other oxygen centers around each of the dopant cations that do not neighbor an oxygen vacancy are destabilized by 0.2 eV. The most basic oxygen centers in the structure are shown in indigo in Fig. S3B in ESI;† they have E(O 1s) values that are lower in magnitude by 0.2–0.4 eV than those of the surface oxygen centers in pristine ceria and are thus somewhat more basic. On the other hand, the O 1s core levels of the oxygen centers around oxygen vacancies (shown in orange in Fig. S3B†) are stabilized by 0.2 eV with respect to the corresponding centers in the pristine ceria surface.
3.2 Yttrium-doped Ce21O42 nanoparticles
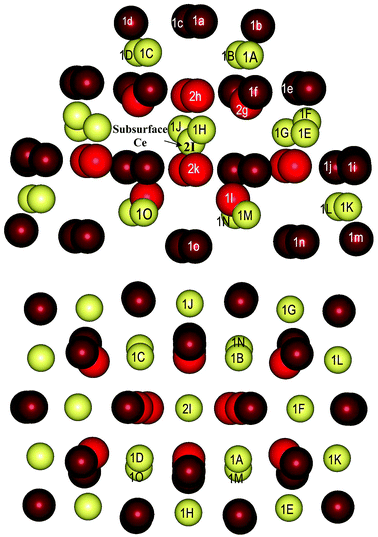
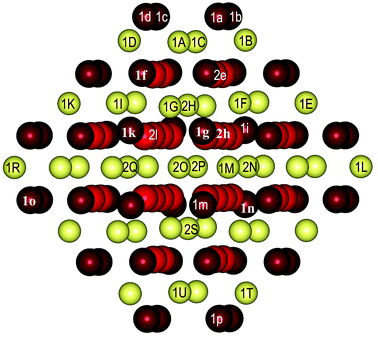
As well as the CeO2(111) surface slab model, we modeled structures with two and four Y3+ cations by replacing two and four Ce4+ cations, respectively, using a smaller Ce21O42 nanoparticle (Fig. 5), from which one O atom was removed to maintain charge balance for each pair of Y3+ cations.There are two- and three-coordinated O centers on the nanoparticle surface, whereas the subsurface O centers are four-coordinated. The two-coordinated oxygen ions 1a, 1b, 1c and 1d shown in Fig. 5 form a small (100) facet. The corresponding Ce4+ cations, which form part of this facet, are denoted as 1A, 1B, 1C and 1D. The other two-coordinated O centers (1i, 1j, 1m, 1n, and 1o) are located at the corners of the nanoparticle. The three-coordinated O centers are denoted as 1e, 1f, and 1l, whereas the subsurface centers (four-coordinated) are denoted as 2g, 2h and 2k. Owing to the small size of the nanoparticle, there is only one subsurface Ce4+ cation, which is denoted as 2I.
| Structurea | Erelb | Y–Yc | Y–Ovacd | Ne | Mf |
|---|---|---|---|---|---|
| a Position of Y3+ cations as shown in Fig. 5. Structure names in italics indicate that the Y3+ cations are located in nearest-neighboring positions.b Relative energy with respect to the most stable structure, i.e., O4c(2I,1H)-2h.c Distance between two nearest Y cations.d Distances between Y atoms and the O vacancy.e Coordination numbers of Y3+ with respect to O atoms.f Number of O atoms bound simultaneously to both Y3+ cations. | |||||
| O2c(1A,1B)-1b | 0.31 | 412 | 215, 214 | 5, 5 | 1 |
| O2c(1A,1E)-1a | 0.39 | 345 | 215, 540 | 5, 5 | 2 |
| O2c(1A,1H)-1a | 0.16 | 359 | 215, 404 | 5, 6 | 2 |
| O2c(1A,1C)-1a | 0.15 | 402 | 215, 215 | 5, 5 | 1 |
| O2c(1A,1C)-1m | 1.59 | 339 | 771, 1005 | 5, 5 | 2 |
| O2c(1D,1E)-1d | 0.40 | 817 | 214, 778 | 5, 5 | 0 |
| O2c(1D,1E)-1o | 1.07 | 800 | 817, 553 | 6, 5 | 0 |
| O2c(1E,1F)-1b | 0.39 | 358 | 533, 373 | 5, 6 | 2 |
| O2c(1E,1F)-1i | 1.01 | 357 | 211, 411 | 4, 6 | 2 |
| O2c(1E,1H)-1a | 0.37 | 362 | 540, 404 | 5, 6 | 1 |
| O2c(1K,1L)-1m | 0.36 | 398 | 215, 215 | 4, 4 | 1 |
| O2c(1K,1E)-1i | 1.21 | 436 | 223, 211 | 4, 4 | 1 |
| O2c(1K,1M)-1n | 0.82 | 419 | 211, 216 | 4, 5 | 1 |
| O2c(1J,1H)-1a | 0.13 | 740 | 714, 404 | 6, 6 | 0 |
| O2c(2I,1A)-1a | 0.03 | 397 | 477, 215 | 7, 5 | 1 |
| O2c(2I,1F)-1b | 0.08 | 420 | 465, 373 | 7, 6 | 0 |
| O2c(2I,1H)-1a | 0.04 | 381 | 477, 404 | 7, 6 | 1 |
| O2c(2I,1M)-1a | 0.44 | 361 | 477, 690 | 7, 6 | 1 |
| O2c(2I,1M)-1o | 0.62 | 360 | 440, 215 | 8, 5 | 2 |
| O3c(1A,1E)-1f | 1.24 | 392 | 239, 228 | 5, 4 | 1 |
| O3c(1A,1H)-1f | 1.01 | 394 | 239, 228 | 5, 5 | 1 |
| O3c(1M,1N)-1l | 0.55 | 555 | 223, 223 | 5, 5 | 0 |
| O4c(2I,1H)-2h | 0.00 | 399 | 244, 228 | 7, 5 | 1 |
| O4c(2I,1A)-2h | 0.19 | 410 | 244, 230 | 7, 5 | 1 |
| O4c(1D,1E)-2h | 0.52 | 793 | 421, 433 | 6, 5 | 0 |
| O4c(2I,1F)-2g | 0.08 | 420 | 236, 221 | 7, 6 | 0 |
The calculated energies of the other 14 modeled structures are higher by 0.36–1.21 eV with respect to the most stable structure, i.e., O4c(2I,1H)-2h. In these cases, yttrium dopants are present in all three possible cation layers of the smaller nanoparticle. In the least stable structures, of which the relative energies exceed 0.80 eV, both Y3+ cations are in surface positions (see the green bullets in Fig. 2B).
In general, the energetic stability of the doped structures under consideration is not related to the coordination number of the removed O center. The mutual location of two Y3+ cations does not determine the stability either. Hence, there is no clear correlation between the stability of a given structure and the corresponding Y–Y distance (see the green bullets in Fig. 2A).
The distances between the oxygen center that is removed and the Y3+ cations are not crucial for the stability of a given Ce19Y2O41 structure (Table 4), which is partly due to the geometric flexibility of the nanoparticle model. For instance, in structures that are almost the most stable, namely, O2c(2I,1A)-1a and O2c(2I,1H)-1a, the oxygen vacancy neighbors none or only one of the Y3+ cations, whereas in the O2c(1A,1B)-1b, O2c(1K,1M)-1n, and O2c(1K,1E)-1i structures, which are destabilized in comparison with the most stable structure, i.e., O4c(2I,1H)-2h, by 0.31, 0.82 and 1.21 eV, respectively, the oxygen vacancy neighbors both Y3+ ions.
| Structurea | Erelb | Evacc | Ovac–Ovacd | #Cee |
|---|---|---|---|---|
| a The letters indicate the positions of the Y3+ cations and removed O centers shown in Fig. 5, whereas the numbers indicate whether the Y3+ cation or removed O center is located in the first (surface) layer, 1, or the second (subsurface) layer, 2. In parentheses is the structure with two Y3+ cations and one Ovac from which the O center is removed.b Relative energy with respect to the most stable structure, i.e., O2c(O2c(2I,1A)-1a)-1c.c Formation energy of an O vacancy.d Distance between two O vacancies.e Positions of the Ce3+ cations (see numbers in Fig. S2). | ||||
| O2c(O2c(2I,1A)-1a)-1c | 0.00 | 1.87 | 459 | 1, 4 |
| O2c(O2c(2I,1A)-1a)-1b | 0.46 | 2.33 | 319 | 1, 19 |
| O2c(O2c(2I,1A)-1a)-1d | 0.42 | 2.29 | 319 | 4, 13 |
| O2c(O2c(2I,1A)-1a)-1i | 0.21 | 2.08 | 742 | 2, 10 |
| O2c(O4c(2I,1H)-2h)-1a | 0.97 | 2.85 | 262 | 13, 18 |
| O2c(O4c(2I,1H)-2h)-1b | 0.38 | 2.25 | 379 | 1, 18 |
| O2c(O4c(2I,1H)-2h)-1c | 0.16 | 2.03 | 486 | 1, 4 |
| O2c(O4c(2I,1H)-2h)-1d | 0.30 | 2.18 | 379 | 4, 13 |
| O2c(O4c(2I,1H)-2h)-1o | 0.85 | 2.72 | 527 | 7, 11 |
| O2c(O4c(2I,1H)-2h)-1i | 0.14 | 2.01 | 613 | 2, 10 |
The most stable structure is O2c(O2c(2I,1A)-1a)-1c, in which the two vacancies are located opposite each other in the (100) facet with Evac = 1.87 eV. The latter value is slightly lower than 2.04 eV, which we calculated for the pristine Ce21O42 nanoparticle with the same locations of the O vacancy and Ce3+ cations. Note, however, that the lowest formation energy of an O vacancy in the Ce21O42 nanoparticle determined with the same computational setup, namely, 1.67 eV,28,29 indicates that the presence of Y3+ does not facilitate the formation of an O vacancy. The formation energies of an O vacancy in the other positions in the (100) facet of the pristine Ce21O42 nanoparticle are similar, namely, 2.07 eV (1a), 2.07 eV (1b) and 2.20 eV (1d). For the removal of an O center from the 1b, 1d and 1i positions of the O2c(2I,1A)-1a structure, the Evac values are at least 0.2 eV higher than the value for O2c(O2c(2I,1A)-1a)-1c.
For the removal of an O center from the O2c(2I,1H)-2h structure, the Evac values vary from 2.01 eV to 2.85 eV. In a similar way to the removal of an O center from O2c(2I,1A)-1a, the lowest Evac values were also calculated for the removal of an O center from the 1i and 1c positions and are 2.01 and 2.03 eV, respectively. Notably, we were unable to find any correlation between the distances between two O vacancies (Ovac–Ovac in Table 5) and the Evac values.
| Structurea | Erelb | Y–Yc | ΔY–Yd |
|---|---|---|---|
| a The positions of Y3+ and O2− ions are shown in Fig. 5. Structure names in italics indicate that both Y3+ pairs are located close to each other.b Relative energy with respect to the most stable structure, i.e., O2c(1L,1F)-1m/O4c(2I,1H)-2h.c Distances between two nearest Y cations.d Difference between the Y–Y distances in the structures with two Y3+ cations (O4c(2I,1H)-2h: 399 pm; O2c(2I,1A)-1a: 397 pm; and O2c(1A,1C)-1a: 402 pm) and the structure with four Y3+ cations. | |||
| O2c(1B,1A)-1b/O4c(2I,1H)-2h | 0.40 | 397, 408 | 9 |
| O2c(1B,1D)-1c/O4c(2I,1H)-2h | 0.19 | 400, 395 | −4 |
| O2c(1E,1F)-1b/O4c(2I,1H)-2h | 0.66 | 356, 407 | 8 |
| O2c(1K,1L)-1b/O4c(2I,1H)-2h | 1.88 | 338, 408 | 9 |
| O2c(1K,1L)-1m/O4c(2I,1H)-2h | 0.23 | 400, 400 | 1 |
| O2c(1K,1F)-1m/O4c(2I,1H)-2h | 0.04 | 354, 399 | 0 |
| O2c(1L,1F)-1m/O4c(2I,1H)-2h | 0.00 | 354, 401 | 2 |
| O2c(1L,1F)-1j/O4c(2I,1H)-2h | 0.67 | 382, 401 | 2 |
| O2c(1L,1G)-1j/O4c(2I,1H)-2h | 0.86 | 437, 401 | 2 |
| O2c(1M,1O)-1o/O4c(2I,1H)-2h | 0.95 | 393, 396 | −3 |
| O4c(1M,1O)-2k/O4c(2I,1H)-2h | 2.60 | 369, 432 | 33 |
| O2c(1B,1J)-1b/O2c(2I,1A)-1a | 0.88 | 348, 443 | 46 |
| O2c(1B,1J)-1c/O2c(2I,1A)-1a | 0.39 | 364, 383 | −14 |
| O2c(1G,1J)-1b/O2c(2I,1A)-1a | 1.11 | 355, 449 | 52 |
| O2c(1G,1J)-1c/O2c(2I,1A)-1a | 0.47 | 361, 376 | −21 |
| O2c(1K,1E)-1i/O2c(2I,1A)-1a | 1.01 | 439, 398 | 1 |
| O2c(1K,1L)-1m/O2c(2I,1A)-1a | 0.18 | 404, 398 | 1 |
| O2c(1L,1G)-1j/O2c(2I,1A)-1a | 0.92 | 440, 398 | 1 |
| O2c(1L,1F)-1m/O2c(2I,1A)-1a | 0.04 | 352, 393 | −4 |
| O2c(1G,1F)-1b/O2c(2I,1A)-1a | 1.06 | 356, 417 | 20 |
| O3c(1G,1F)-1e/O2c(2I,1A)-1a | 1.26 | 397, 390 | −7 |
| O2c(1B,1D)-1c/O2c(1A,1C)-1a | 0.38 | 403, 403 | 1 |
| O2c(1K,1L)-1m/O2c(1A,1C)-1a | 0.29 | 402, 403 | 1 |
| O2c(1G,1L)-1j/O2c(1A,1C)-1a | 1.09 | 442, 410 | 8 |
| O2c(1G,1J)-1j/O2c(1A,1C)-1a | 0.88 | 346, 410 | 8 |
There are six modeled structures with relative energies within a range of 0.23 eV, and in all of these one of the yttrium ions is located in the subsurface position denoted as 2I (see Fig. 7). The relative energy of the other studied structures ranges between 0.29 and 2.60 eV. Note that in several of these destabilized structures a Y3+ cation also occupies the subsurface 2I position, which hence does not seem to be a decisive factor for the stabilization of the Ce17Y4O40 structures.
The least stable structure is O4c(1M,1O)-2k/O4c(2I,1H)-2h, in which the four-coordinated O center between the Y3+ ions in the 2I and 1H cation positions is removed. As a comparison, the O2c(1M,1O)-1o/O4c(2I,1H)-2h structure, which has the same four Y3+ positions but instead a two-coordinated O center (1o) is removed, is more stable by ca. 1.7 eV than O4c(1M,1O)-2k/O4c(2I,1H)-2h. In the latter, four Y3+ cations form a triangular pyramid, inside which the 2k center is located. The distance between a Y3+ cation in the pyramid base and the top Y3+ cation is greater by 35 pm in comparison with that in O4c(1M,1O)-1o/O4c(2I,1H)-2h as a consequence of the removal of the 2k center.
The O2c(1B,1D)-1c/O2c(1A,1C)-1a structure, in which all four Y3+ cations are in the (100) facet and the two removed O centers were located opposite each other in the same facet, is less stable by 0.38 eV than the most stable structure, namely, O2c(1L,1F)-1m/O4c(2I,1H)-2h.
Table 6 also shows the differences between the Y–Y distances in the parent structures (with two Y3+ cations and one O vacancy) and the corresponding resulting structures with four Y3+ cations and two O vacancies, denoted as ΔY–Y. In some structures (including three of the most stable structures) this distance is essentially identical or only changes by up to 8 pm. However, when all the modeled structures are considered no clear correlation between the stability and the variations in the Y–Y distance can be observed.
In summary, the stability of the Y-doped structures based on the Ce21O42 nanoparticle is not clearly determined by only one factor, such as the coordination of the removed O centers or the Y–Y and Y–Ovac distances. In general, it can be seen that the structures in which one of the Y3+ cations is located in a subsurface position are among the most stable structures.
3.3 Yttrium-doped Ce40O80 larger nanoparticles
| Structurea | Erelb | Y–Yc | Y–Ovacd | Ne | Mf |
|---|---|---|---|---|---|
| a Positions of Y3+ cations as shown in Fig. 8. Structure names in italics indicate that the Y3+ cations are located in nearest-neighboring positions.b Relative energy with respect to the most stable structure, i.e., O2c(1E,1K)-1a.c Distance between two nearest Y cations.d Distances between Y cations and the O vacancy.e Coordination numbers of Y3+ with respect to O atoms.f Number of O atoms bound simultaneously to both Y3+ cations. | |||||
| O2c(1A,1B)-1a | 0.65 | 419 | 219; 214 | 5, 5 | 1 |
| O2c(1A,2H)-1a | 0.44 | 370 | 219; 446 | 5, 8 | 2 |
| O2c(1A,1L)-1a | 0.44 | 976 | 219; 900 | 5, 4 | 0 |
| O2c(1A,2S)-1a | 0.56 | 829 | 219; 945 | 5, 8 | 0 |
| O2c(1A,1G)-1a | 1.05 | 369 | 219; 567 | 5, 5 | 2 |
| O2c(1E,1K)-1a | 0.00 | 1034 | 545; 789 | 6, 6 | 0 |
| O2c(1B,1E)-1a | 0.32 | 354 | 214; 545 | 5, 6 | 2 |
| O2c(1F,1M)-1g | 0.50 | 383 | 408; 214 | 7, 5 | 2 |
| O2c(1F,2H)-1a | 0.28 | 375 | 408; 446 | 7, 8 | 2 |
| O2c(2H,2O)-1a | 0.28 | 372 | 446; 680 | 8, 8 | 2 |
| O2c(2H,2S)-1a | 0.29 | 540 | 446; 945 | 8, 8 | 0 |
| O2c(1L,1M)-1a | 0.85 | 731 | 900; 214 | 6, 4 | 0 |
| O2c(1L,1R)-1a | 0.32 | 1509 | 900; 1134 | 4, 4 | 0 |
| O2c(1B,1D)-1a | 1.87 | 522 | 214; 453 | 5, 6 | 0 |
| O2c(1B,1D)-1p | 0.86 | 505 | 1212; 1275 | 6, 6 | 0 |
| O3c(1L,1R)-1o | 1.75 | 1522 | 1437; 213 | 3, 4 | 0 |
| O3c(1A,1G)-1f | 1.92 | 429 | 242; 241 | 5, 5 | 2 |
| O4c(1L,1R)-2e | 0.32 | 1509 | 752; 983 | 4, 4 | 0 |
| O4c(1A,1B)-2e | 0.64 | 419 | 232; 232 | 5, 5 | 1 |
| O4c(1F,2H)-2e | 0.36 | 400 | 232; 230 | 6, 7 | 1 |
| O4c(2H,2O)-2h | 0.79 | 395 | 233; 233 | 8, 8 | 1 |
| O4c(2H,2O)-2e | 0.27 | 372 | 232; 376 | 8, 9 | 2 |
| O4c(2H,2S)-2h | 0.98 | 537 | 233; 446 | 8, 8 | 0 |
| O4c(2O,2Q)-2l | 0.61 | 372 | 233; 229 | 7, 7 | 1 |
| O4c(2O,2P)-2l | 0.98 | 537 | 233; 445 | 8, 7 | 0 |
| O4c(2O,2N)-2h | 0.61 | 395 | 233; 229 | 7, 7 | 1 |
As a general trend, structures with O vacancies created by removing three-(1o and 1f) or four-coordinated (2h and 2l) centers are unstable, and such structures become stable only when the vacancy is created in the position of a two-coordinated O center (1a). For instance: (i) the O2c(2H,2O)-1a structure is more stable by 0.51 eV than O2c(2H,2O)-2h; (ii) the O2c(2H,2S)-1a structure is more stable by 0.69 eV than O2c(2H,2S)-2h; and (iii) the O2c(1L,1R)-1a structure is more stable by 1.43 eV than O2c(1L,1R)-1o.
In a similar way to the abovementioned models of the ceria(111) surface and the smaller ceria nanoparticle, no clear dependence of the structural stability on the distances between two yttrium cations (blue squares in Fig. 2A) or the location of the dopant cations in surface or subsurface positions (blue squares in Fig. 2B) was found. We also examined conceivable correlations between the relative stability and other characteristics of the models – the influence of the calculated electrostatic potentials at the cerium centers substituted by yttrium and the oxygen centers that were removed (Fig. S5A–C, ESI†) and the average number of oxygen centers surrounding two Y3+ cations (Fig. S5D, ESI†). However, no obvious trends were found.
| Structurea | Erelb | Evacc | Ovac–Ovacd | #Cee |
|---|---|---|---|---|
| a The positions of Y3+ cations and removed O centers are shown in Fig. 8, whereas the numbers indicate whether the Y3+ cation or removed O center is located in the surface region, 1, or the subsurface region, 2. In parentheses is the structure with two Y3+ cations and one Ovac from which the O center is removed.b Relative energy with respect to the most stable structure, i.e., O2c((1E,1K)-1a)-1c.c Formation energy of an O vacancy.d Distance between two O vacancies.e Positions of the Ce3+ cations (see numbers in Fig. S2). | ||||
| CeO2-1a | 0.76 | 9, 12 | ||
| CeO2-1b | 1.51 | 2, 3 | ||
| CeO2-1c | 1.52 | 3, 4 | ||
| CeO2-1d | 1.53 | 1, 4 | ||
| CeO2-1k | 1.60 | 7, 10 | ||
| CeO2-1p | 1.53 | 14, 15 | ||
| CeO2-2h | 1.81 | 5, 12 | ||
| O2c((1E,1K)-1a)-1b | 0.66 | 1.61 | 319 | 5, 3 |
| O2c((1E,1K)-1a)-1c | 0.00 | 0.95 | 449 | 3, 4 |
| O2c((1E,1K)-1a)-1d | 0.65 | 1.60 | 315 | 1, 4 |
| O2c((1E,1K)-1a)-1k | 0.40 | 1.35 | 765 | 7, 10 |
| O2c((1E,1K)-1a)-1p | 0.08 | 1.03 | 1304 | 13, 14 |
| O2c((2H,2O)-1a)-1b | 0.94 | 1.89 | 319 | 3, 5 |
| O2c((2H,2O)-1a)-1c | 0.36 | 1.31 | 449 | 3, 8 |
| O2c((2H,2O)-1a)-1d | 0.99 | 1.94 | 315 | 1, 8 |
| O2c((2H,2O)-1a)-1k | 0.29 | 1.24 | 765 | 7, 11 |
| O2c((2H,2O)-1a)-1p | 0.06 | 1.01 | 1304 | 9, 14 |
| O4c((2H,2O)-1a)-2h | 1.00 | 1.95 | 517 | 5, 6 |
| O2c((1F,2H)-1a)-1b | 0.92 | 1.87 | 319 | 2, 3 |
| O2c((1F,2H)-1a)-1c | 0.40 | 1.35 | 449 | 3, 8 |
| O2c((1F,2H)-1a)-1d | 1.02 | 1.97 | 315 | 1, 8 |
| O2c((1F,2H)-1a)-1k | 0.68 | 1.63 | 765 | 7, 10 |
| O2c((1F,2H)-1a)-1p | 0.34 | 1.29 | 1304 | 13, 14 |
| O2c((1F,2H)-1a)-2e | 1.50 | 2.45 | 255 | 2, 5 |
The lowest formation energy of an O vacancy in the latter structure is 0.9 eV, which is only slightly higher than the value calculated for the pristine Ce40O80 nanoparticle, namely, 0.8 eV.28,29 The next most stable structure, namely, O2c((2H,2O)-1a)-1p, is characterized by the fact that both Y3+ cations are located in neighboring subsurface positions, whereas the positions of both oxygen vacancies are the same as in O2c((1E,1K)-1a)-1p. The former structure is less stable by only 0.06 eV than the O2c((1E,1K)-1a)-1c structure and has a similar formation energy of an O vacancy. The other structures that were considered are less stable by at least ca. 0.3 eV than O2c((1E,1K)-1a)-1c.
In the O2c((1E,1K))-1a)-1p, O2c((2H,2O)-1a)-1k, O2c((2H,2O)-1a)-1p, O4c((2H,2O)-1a)-2h, O2c((1F,2H)-1a)-1p and O2c((1F,2H)-1a)-2e structures one of the Ce3+ cations is located next to the created O vacancy, whereas the other Ce3+ cation is far from the vacancy. In all the other structures that were considered both Ce3+ cations are in the vicinity of the created O vacancy.
| Structurea | Erelb | Y–Yc | ΔY–Yd |
|---|---|---|---|
| a The positions denoted by the corresponding letters are shown in Fig. 8. Structure names in italics indicate that both Y3+ pairs are located close to each other.b Relative energy with respect to the most stable structure, i.e., O2c(1E,1K)-1a/O2c(2H,2S)-1p.c Distances between two nearest Y cations.d Difference between the Y–Y distances in the structure with two Y3+ cations (O4c(2I,1H)-2h: 399 pm; O2c(2I,1A)-1a: 397 pm; and O2c(1A,1C)-1a: 402 pm) and the structure with four Y3+ cations. | |||
| O2c(1E,1K)-1a/O2c(1F,2H)-1p | 0.34 | 1034; 369 | 0 |
| O2c(1E,1K)-1a/O2c(2H,2O)-1p | 0.21 | 1034; 371 | 0 |
| O2c(1E,1K)-1a/O2c(2H,2S)-1p | 0.00 | 1034; 531 | 0 |
| O2c(1E,1K)-1a/O2c(1L,1R)-1p | 0.38 | 1041; 1512 | 7 |
| O2c(1E,1K)-1a/O2c(1B,1D)-1p | 1.17 | 1032; 529 | −2 |
| O2c(1F,2H)-1a/O2c(1L,1R)-1p | 0.36 | 372; 1512 | −3 |
| O2c(1F,2H)-1a/O2c(2Q,2N)-1p | 0.28 | 372; 521 | −3 |
| O2c(1F,2H)-1a/O2c(2O,2N)-1p | 0.52 | 367; 368 | −8 |
| O2c(1L,1R)-1a/O2c(2H,2S)-1p | 0.15 | 1512; 531 | 3 |
| O2c(2H,2O)-1a/O2c(1G,1M)-1g | 0.78 | 373; 412 | 1 |
| O2c(2H,2O)-1a/O2c(1L,1R)-1p | 0.29 | 372; 1512 | 0 |
| O2c(2H,2O)-1a/O2c(1L,1R)-1b | 0.44 | 379; 1513 | 7 |
| O2c(2H,2O)-1a/O2c(2Q,2N)-1c | 0.52 | 374; 518 | 2 |
| O2c(2H,2O)-1a/O2c(2Q,2N)-1p | 0.32 | 368; 519 | −4 |
| O2c(2H,2O)-1a/O2c(2S,2P)-1n | 0.59 | 370; 370 | −2 |
| O2c(2H,2O)-1a/O2c(2S,2P)-1p | 0.51 | 373; 372 | 1 |
| O2c(1A,1G)-1a/O2c(1I,1F)-1p | 1.95 | 351; 528 | −18 |
| O2c(1A,1B)-1a/O2c(1C,1D)-1c | 1.30 | 422; 423 | 3 |
| O2c(1A,1B)-1a/O2c(1C,1D)-1p | 3.23 | 418; 347 | −1 |
| O2c(1A,1B)-1a/O2c(1R,1L)-1c | 0.50 | 426; 1510 | 7 |
| O2c(1A,1B)-1a/O2c(1R,1L)-1d | 1.24 | 420; 1510 | 1 |
| O2c(1A,1B)-1a/O2c(1R,1L)-1p | 0.80 | 418; 1510 | −1 |
| O2c(1A,1B)-1a/O2c(1U,1T)-1p | 1.14 | 417; 416 | −2 |
When both O centers are removed from one small (100) facet, it is not most favorable to remove them from two neighboring O sites. It is more favorable to remove two O centers that are located diagonally with respect to each other or are from two opposite (100) facets.
4. Summary and conclusions
We performed periodic density functional calculations on a CeO2(111) slab as well as models of one smaller (Ce21O42) and one larger (Ce40O80) nanoparticle doped by two and four Y3+ cations. This substitution induces the formation of one and two O vacancies, respectively, for charge compensation.4.1 CeO2(111) slab model
The most stable structures on the CeO2(111) surface are calculated to be those in which Y3+ cations are located at neighboring sites close to an oxygen vacancy created in the first or second subsurface layer.The presence of Y3+ does not noticeably change the reducibility of the ceria slab model in the case of the most stable structures when the vacancy is in the first subsurface layer. However, when the oxygen vacancy is created in the surface or second subsurface layer, the formation energies of an O vacancy decrease by 0.2–0.3 eV in the presence of Y3+ cations in comparison with the corresponding values calculated for the pristine ceria system. This result is important from a practical point of view, because yttrium can increase the mobility of surface oxygen centers and in this way facilitate the oxidation of adsorbates with their participation.
Our CeO2(111) slab model with four Y3+ cations showed that there is no clear preference regarding whether both Y3+ pairs stay close to each other or separate. We also found that by combining two stable configurations of a Y3+ pair and an O vacancy one obtains a stable structure.
When the dopant ions are in the surface layer, the basicity of surface oxygen centers around the dopant ions is slightly higher than that of the corresponding O centers on the surface of pristine ceria, as estimated from the shifts in the O 1s core level. In contrast, oxygen centers near the oxygen vacancy experience stabilization of their O 1s levels, i.e., lower basicity.
4.2 Models of smaller Ce21O42 nanoparticle
Our previous calculations showed that in pristine ceria nanoparticles of this kind O centers are most easily removed from low-(two-)coordinated sites.28,29 However, we were unable to confirm this observation in the case of the doped Ce19Y2O41 structures, in which one O center was removed for charge compensation upon the introduction of the two Y3+ cations. In the most stable structures containing yttrium dopants, oxygen centers are removed from the top two O layers: two-coordinated O centers from the (100) facet and four-coordinated O centers from the layer below. In contrast to the doped ceria slab models, in these models of ceria nanoparticle it does not seem to be obligatory that both Y3+ cations should be in neighboring positions near the O vacancy, which is probably due to the higher structural flexibility of the nanoparticles. Our calculations showed that in the most stable structures Y3+ cations replace Ce4+ cations from the (100) facet or the layers beneath it, including the only subsurface cation site.4.3 Models of larger Ce40O80 nanoparticle
In the most stable structures O vacancies are created in the small (100) facets, where O centers are low-(two-)coordinated. It is not favorable to remove four-coordinated O centers from subsurface regions. In the latter case the energy of the structure is high, or a surface O center moves to the subsurface vacancy during geometry optimization and fills it. A Y3+ cation is stable when it replaces a Ce4+ cation in the same coordination environment; it is not necessary to remove an O center from around the Y3+ cation. The presence of Y3+ dopants can increase the formation energy of an O vacancy to an insignificant extent.When four Y3+ cations are introduced into the nanoparticle it is not favorable that both O vacancies should be created in one (100) facet. In the most stable structures oxygen vacancies are located in different facets of the nanoparticle. Combinations of two stable positions of the Y3+ cations lead to a stable structure. There is no clear preference regarding whether Y3+ cations should be close to each other or well apart. This is also the case for the distance from Y3+ ions to O vacancies.
4.4 General conclusions
Our calculations demonstrate that the smaller is an yttrium-doped ceria system the fewer rules and trends can be elucidated regarding the energetic and structural features of the most stable locations of the dopant ions with respect to each other and to the created charge-compensating O vacancies. In the slab models one can observe some trends in the preferred locations of the Y3+ cations and the O vacancy. It is most stable when the Y3+ cations stay close to each other and the created O vacancy is in their immediate vicinity, both preferably in the subsurface region. In contrast, the smaller Ce21O42 nanoparticle, which has various exposed low-coordinated corner and edge sites and a very flexible structure, does not exhibit a clear tendency either for the locations of Y3+ cations or for the position of the O vacancy. A similar statement can be made for the results of computational modeling we obtained for the doped larger Ce40O80 nanoparticles. However, one trend was found in the latter case regarding the location of the O vacancies, which are preferentially formed on the small (100) facets, where less strongly bound two-coordinated O centers are available for removal.In addition, we found that in the structures with four Y3+ cations and two O vacancies the most stable structures can be obtained by combining the positions in the most stable structures with two Y3+ cations and one O vacancy.
Hence, the main effect of yttrium doping on the ceria slab model, which is representative of large ceria particles, is the lowering of the formation energy of an oxygen vacancy for surface oxygen centers by ca. 0.3 eV, and it thus becomes almost the same as the energy required for the formation of a subsurface vacancy. In general, the doping of ceria with yttrium has a negligible effect on the reducibility of ceria surfaces and nanoparticles. One reason may be the very similar ionic radii of Y3+ and Ce4+ ions, which differ by only 0.03 and 0.05 Å for 6- and 8-coordinated ions, respectively.43
On the other hand, the lack of preference regarding the positions of yttrium cations and oxygen vacancies in the doped ceria nanoparticles, in combination with their flexibility, would facilitate local rearrangements between structures of similar stability. This may also be related to the migration of oxygen in these systems, which can enable the dedicated use of CeO2–Y2O3 nanocomposites in catalytic applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Support by the Bulgarian National Science Fund (project DN-09/5), the Horizon2020 program of the European Commission (project Materials Networking, grant agreement 692146), the European Regional Development Fund and the Operational Program “Science and Education for Smart Growth” under contract UNITe no. BG05M2OP001-1.001-0004-C01 (2018–2023) is gratefully acknowledged. HAA is grateful to the Operational Program “Science and Education for Smart Growth” project BG05M2OP001-2.009-0028. KMN acknowledges support by the Spanish MINECO-FEDER grant CTQ2015-64618-R and by the grant 2017SGR13 from the Generalitat de Catalunya. The authors thank the Red Española de Supercomputación for the computer resources and the technical support.References
- A. Trovarelli, Catal. Rev.: Sci. Eng., 1996, 38, 439–520 CrossRef CAS.
- Catalysis by Ceria and Related Materials, ed. A. Trovarelli, Imperial College Press, London, 2002 Search PubMed.
- Catalysis by Ceria and Related Materials, ed. A. Trovarelli and P. Fonasiero, 2nd edn, Imperial College Press, London, 2013 Search PubMed.
- J. Paier, C. Penschke and J. Sauer, Chem. Rev., 2013, 113, 3949–3985 CrossRef CAS PubMed.
- D. R. Mullins, Surf. Sci. Rep., 2015, 70, 42–85 CrossRef CAS.
- T. Montini, M. Melchionna, M. Monai and P. Fornasiero, Chem. Rev., 2016, 116, 5987–6041 CrossRef CAS PubMed.
- P. Fornasiero and A. Trovarelli, Catal. Today, 2015, 253, 1–218 CrossRef CAS.
- E. W. McFarland and H. Metiu, Chem. Rev., 2013, 113, 4391–4427 CrossRef CAS PubMed.
- S. M. Collins, S. Fernandez-Garcia, J. J. Calvino and P. A. Midgley, Sci. Rep., 2017, 7, 5406 CrossRef PubMed.
- I. Atribak, A. Bueno-López and A. García-García, J. Mol. Catal. A: Chem., 2009, 300, 103–110 CrossRef CAS.
- Y. She, L. Li, Y. Zhan, X. Lin, Q. Zheng and K. Wei, J. Rare Earths, 2009, 27, 411–417 CrossRef.
- L. Ilieva, P. Petrova, G. Pantaleo, R. Zanella, L. F. Liotta, V. Georgiev, S. Boghosian, Z. Kaszkur, J. W. Sobczak, W. Lisowski, A. M. Venezia and T. Tabakova, Appl. Catal., B, 2016, 188, 154–168 CrossRef CAS.
- E. O. Jardim, S. Rico-Francés, F. Coloma, E. V. Ramos-Fernández, J. Silvestre-Albero and A. Sepúlveda-Escribano, Appl. Catal., A, 2014, 487, 119–129 CrossRef CAS.
- P. P. Dholabhai, J. B. Adams, P. Crozier and R. Sharma, J. Chem. Phys., 2010, 132, 094104 CrossRef PubMed.
- P. P. Dholabhai, J. B. Adams, P. Crozier and R. Sharma, Phys. Chem. Chem. Phys., 2010, 12, 7904–7910 RSC.
- M. Burbano, S. T. Norberg, S. Hull, S. G. Eriksson, D. Marrocchelli, P. A. Madden and G. W. Watson, Chem. Mater., 2012, 24, 222–229 CrossRef CAS.
- S. Grieshammer, B. O. H. Grope, J. Koettgen and M. Martin, Phys. Chem. Chem. Phys., 2014, 16, 9974–9986 RSC.
- Z. Hu and H. Metiu, J. Phys. Chem. C, 2011, 115, 17898–17909 CrossRef CAS.
- M. Nolan, J. Phys. Chem. C, 2011, 115, 6671–6681 CrossRef CAS.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6671–6687 (ibid, 1993, 48, 4978) CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS.
- Version VASP.5.3; http://cms.mpi.univie.ac.at/vasp/ Search PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- V. I. Anisimov, F. Aryasetiawan and A. I. Lichtenstein, J. Phys.: Condens. Matter, 1997, 9, 767–808 CrossRef CAS.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505–1509 CrossRef CAS.
- C. Loschen, J. Carrasco, K. M. Neyman and F. Illas, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 035115 CrossRef.
- E. A. Kümmerle and G. Heger, J. Solid State Chem., 1999, 147, 485–500 CrossRef.
- A. Migani, G. N. Vayssilov, S. T. Bromley, F. Illas and K. M. Neyman, Chem. Commun., 2010, 46, 5936–5938 RSC.
- A. Migani, G. N. Vayssilov, S. T. Bromley, F. Illas and K. M. Neyman, J. Mater. Chem., 2010, 20, 10535–10546 RSC.
- G. N. Vayssilov, Y. Lykhach, A. Migani, T. Staudt, G. P. Petrova, N. Tsud, T. Skála, A. Bruix, F. Illas, K. C. Prince, V. Matolín, K. M. Neyman and J. Libuda, Nat. Mater., 2011, 10, 310–315 CrossRef CAS PubMed.
- H. A. Aleksandrov, K. M. Neyman and G. N. Vayssilov, Phys. Chem. Chem. Phys., 2015, 17, 14551–14560 RSC.
- H. A. Aleksandrov, K. M. Neyman, K. I. Hadjiivanov and G. N. Vayssilov, Phys. Chem. Chem. Phys., 2016, 18, 22108–22121 RSC.
- I. Z. Koleva, H. A. Aleksandrov and G. N. Vayssilov, Catal. Sci. Technol., 2017, 7, 734–742 RSC.
- M. Y. Mihaylov, E. Z. Ivanova, H. A. Aleksandrov, P. St. Petkov, G. N. Vayssilov and K. I. Hadjiivanov, Mol. Catal., 2018, 451, 114–124 CrossRef CAS.
- A. Figueroba, A. Bruix, G. Kovács and K. M. Neyman, Phys. Chem. Chem. Phys., 2017, 19, 21729–21738 RSC.
- Y. Lykhach, A. Bruix, S. Fabris, V. Potin, I. Matolínová, V. Matolín, J. Libuda and K. M. Neyman, Catal. Sci. Technol., 2017, 7, 4315–4345 RSC.
- A. Bruix, Y. Lykhach, I. Matolínová, A. Neitzel, T. Skála, N. Tsud, M. Vorokhta, V. Stetsovych, K. Ševčíková, J. Mysliveček, R. Fiala, M. Václavů, K. C. Prince, S. Bruyère, V. Potin, F. Illas, V. Matolín, J. Libuda and K. M. Neyman, Angew. Chem., Int. Ed., 2014, 53, 10525–10530 CrossRef CAS PubMed.
- A. Figueroba, G. Kovács, A. Bruix and K. M. Neyman, Catal. Sci. Technol., 2016, 6, 6806–6813 RSC.
- M. V. Ganduglia-Pirovano, J. L. F. Da Silva and J. Sauer, Phys. Rev. Lett., 2009, 102, 026101 CrossRef PubMed.
- S. M. Kozlov and K. M. Neyman, Phys. Chem. Chem. Phys., 2014, 16, 7823–7829 RSC.
- T. Tabakova, L. Ilieva, I. Ivanov, M. Manzoli, R. Zanella, P. Petrova and Z. Kaszkur, J. Rare Earths, 2018 Search PubMed.
- G. N. Vayssilov and N. Rösch, J. Catal., 1999, 186, 423–432 CrossRef CAS.
- WebElements, https://www.webelements.com, accessed September 2018.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07014h |
| This journal is © The Royal Society of Chemistry 2018 |