Open Access Article
Open Access ArticleRespirometric activities of unacclimatized Enterobacter aerogenes and mixed culture bacteria in sequencing batch reactor systems in response to acrylamide and its biodegradation products
Romsan Madmananga,
Zhen He b and
Tongchai Sriwiriyarat
b and
Tongchai Sriwiriyarat *c
*c
aEnvironmental Science Program, Faculty of Science, Burapha University, Chonburi, Thailand
bDepartment of Civil and Environmental Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA
cDepartment of Chemical Engineering, Faculty of Engineering, Burapha University, Chonburi, Thailand. E-mail: sriwiri@buu.ac.th
First published on 11th October 2018
Abstract
The acute effects of acrylamide and its biodegradation products on the respiration activities of microbes during wastewater treatment are not well understood. Herein, unacclimatized mixed culture bacteria and Enterobacter aerogenes from two aerobic treatment systems, Activated Sludge (AS) and Integrated Fixed Film Activated Sludge (IFAS) both of which were sequencing batch reactors (SBR), were studied for their response to acrylamide. Respiration activities and biodegradation rates were determined by both the OxiTop respirometer and batch studies. The experimental results revealed that E. aerogenes in the AS system quickly removed both acrylamide and acrylic acid without the need of an acclimation period, but required two hours for removing acrylic acid in the IFAS system. The mixed culture bacteria in both AS and IFAS systems required 2 hours to acclimatize with acrylamide and 1 hour for acrylic acid, respectively. Acrylic acid was initially polymerized to produce acrylic acid polymer or reacted with ammonia to form acrylamide, resulting in the reduced acrylamide biodegradation rate. Both E. aerogenes and mixed culture bacteria from the AS systems could simultaneously biodegrade acrylamide and acrylic acid whereas only acrylamide was biodegraded by both cultures in the IFAS systems due to the limitation of acrylic acid diffusion. The results also indicated that ammonia inhibited the acrylamide biodegradation by both E. aerogenes and mixed culture bacteria from the AS systems. The unacclimatized E. aerogenes and mixed culture bacteria from the AS systems showed superior performances compared to the ones from the IFAS systems.
1. Introduction
Acrylamide monomer (AM) is primarily used in the production of polyacrylamides (PAMs) for various industrial applications. It is also known as a carcinogenic, neurotoxicant, and hazardous substance.1,2 Acrylamide can be released to the receiving water during its production or use in the PAMs or other polymer productions.2 Even though PAMs are not directly identified as a hazardous compound, their degradation processes including heat and ultraviolet radiation could possibly hydrolyze PAMs to AM, resulting in the releases of AM to the environment.3It has been reported that acrylamide is lethal to most microorganisms due to its inhibitory effect on sulfhydryl proteins.3,4 However, several laboratory studies have reported the success of using several microbial genera including Arthrobacter sp., Nocardia sp., Bacillus sp., Xanthomonas sp., Rhodopseudomonas sp., Ralstonia sp., Geobacillus sp., Pseudomonas sp., Rhodococcus sp., K. georgiana, and E. aerogenes5–9 to degrade acrylamide in aquatic and soil environment. Among them, E. aerogenes was reported to be capable of degrading acrylamide at a concentration as high as 5000 mg AM L−1 in the culture media.5 E. aerogenes could adapt their metabolisms including the evolution of genes for encoding the amidase and other synthesis proteins for the deamination of acrylamide during the acclimation period.4 Some of these studies compared the acrylamide biodegradation efficiencies between microorganisms cultured as free cells and immobilized cells. It was found that the free cells of Pseudomonas aeruginosa began to degrade acrylamide after an incubation period of 24 h, but the immobilized cells removed acrylamide within 24 h.8 Another study found that the immobilized cells of Rhodococcus sp. could degrade 64 mM of acrylamide within 3 h, but free cells needed a longer period than 24 h to remove it.7 On the other hands, Buranasilp and Charoenpanich5 reported that free cells of E. aerogenes degraded acrylamide within 1 h after incubation, but immobilized cells of E. aerogenes took over 6 h after cultivation to degrade acrylamide. It appears that immobilized cells and free cells of bacteria in the culture media required different acclimation time for adapting them with acrylamide and this warrants further investigation.
As a result of acrylamide biodegradation, ammonia and acrylic acid (AA) are produced by the deamination reaction with amidase as a catalyst.6,8–10 For wastewater treatment practices, additional carbon oxidation of acrylic acid and biological nitrogen removal (BNR) of ammonia would be required to meet the effluent standards. Jangkorn et al.11 studied the acrylamide biodegradation by E. aerogenes and mixed culture bacteria in the SBR activated sludge (AS) treatment system. They found that both E. aerogenes and mixed bacteria culture could completely mineralize acrylamide at 200 mg AM L−1. Free ammonia nitrogen (FAN) from the acrylamide biodegradation accumulated in the SBR systems and inhibited the acrylamide biodegradation and nitrification. Acrylic acid from the acrylamide biodegradation is typically considered as a readily biodegradable substrate and can be rapidly oxidized by various microorganisms under an aerobic condition.12 Larson et al.13 reported that mixed culture bacteria that were acclimatized with a mixture of acrylic acid polymers in the AS system degraded completely both acrylic acid monomer and dimer. However, the biodegradability of acrylic acid decreases proportionally with the increase of molecular weight of acrylic acid polymer. These previous findings suggest that both acrylamide biodegradation products including ammonia and acrylic acid are possible to interfere with the acrylamide biodegradation.
To implement the acrylamide biodegradation successfully in the biological wastewater treatment system, it is necessary to increase the capacity of systems and to minimize the amount of ammonia. Integrated Fixed Film Activated Sludge (IFAS), a hybrid system of both suspended growth (free cells) and attached growth (immobilized cells), has widely been accepted as an alternative method to sustain nitrification at low temperatures14,15 and to enhance the capacity and stability of an activated sludge system.16 With effective nitrification, the FAN inhibition can be minimized, resulting in the enhancement of acrylamide biodegradation. Nevertheless, as reported previously, the acclamation periods were required differently between free cells and immobilized cells of unacclimatized microorganisms in the culture media. It is important to investigate the acclimation period of unacclimatized bacteria in both AS and IFAS for acrylamide biodegradation. In this study, the acute effects of acrylamide and its biodegradation products including ammonium and acrylic acid on the unacclimatized E. aerogenes and mixed culture bacteria in the conventional AS and IFAS sequencing batch reactor (SBR) wastewater treatment systems were evaluated with the respirometric activities and biodegradation studies. Specifically, the acclimation periods, respirometric activities and biodegradation rates of both unacclimatized bacteria from both biological wastewater treatment processes were reported.
2. Materials and methods
2.1 Reactor setup and operation
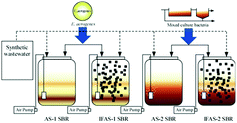
Four bench-scale SBR wastewater treatment systems named as AS-1, AS-2, IFAS-1 and IFAS-2 as illustrated in Fig. 1 were set up and operated in the laboratory of Burapha University (Thailand) at the room temperature of ∼28 °C. Both AS-1 and IFAS-1 were inoculated with pure culture of E. aerogenes cultured from the Department of Biochemistry (Burapha University). The mixed culture bacteria taken from a pilot-scale AS wastewater treatment system operated for biological nitrogen removal (BNR) in the same laboratory were seeded to the AS-2 and IFAS-2 systems. Each SBR system was operated with two cycles (12 h per cycle) per day with a working volume of 10 L. Each operating cycle consisted of five operating periods including 15 min filling, 10 h aerobic reacting, 1 h settling, 15 min decanting, and 30 min idling. The pure culture of E. aerogenes was cultured according to the procedures described by Jangkorn et al.11 Both IFAS-1 and IFAS-2 systems were installed with BioPortz (ENTEX Technologies, Inc., USA) moving media at the filling media fraction of 30% (3 L, 510 BioPortz) resulting in the specific surface area of 1.73 m2. BioPortz is the high-density polyethylene (HDPE) media with the specific surface area of 576 m2 m−3 (ref. 17) and the specific gravity of 0.96. The bulk volume displacement of BioPortz media with biomass and without biomass were 12.75% and 5.10%, respectively. With the decant volume of 5.0 L, all IFAS and AS systems were operated at the nominal hydraulic retention times (HRTs) of 21 and 24 h, respectively. Furthermore, the suspended biomass was wasted directly from the reactor at the end of reacting period to obtain the operating solids retention time (SRT) of 9.0 ± 0.4 days. The dissolved oxygen (DO) was maintained at 6.0–7.0 mg O2 L−1 in each system with air stone diffusers connected with the air pump.All four SBR systems were fed with synthetic wastewater containing total chemical oxygen demand (TCOD) of 445 ± 30 mg COD L−1 and total Kjeldahl nitrogen (TKN) of 42.3 ± 2.5 mg N L−1 resulting in the carbon to nitrogen ratio (C/N ratio) of 10.5. The synthetic wastewater was prepared with 12 g of sucrose (Commercial Grade, Wangkanai, Thailand), 24 g of CH3COONa (Industrial Grade of 58.8%, Sinoway International, China), 2.0 g of K2HPO4 (Food Grade of 99.2%, Young Jin Chemical, South Korea), 4 g of KH2PO4 (ACS Grade, VWR Chemicals, EC), 20 g of NaHCO3 (Food Grade of 99.5%, Tianjin Soda Plant, China), 9 g of NH4Cl (Industrial Grade of 99.5%, Tianjin Soda Plant, China), 2.8 g of MgCl2 (Industrial Grade of 47%, Dead Sea Works, Ltd., Israel), and 1.6 g of CaCl2 (Food Grade of 74.0%, Young Jin Chemical, South Korea) in a 40 L tap water. The total suspended solids (TSS) concentrations and pH values of the synthetic wastewater were 83.8 ± 9.2 mg SS L−1 and 7.2 ± 2.7, respectively.
2.2 Enrichment of E. aerogenes
Freeze-dried E. aerogenes was taken from the Biochemistry department of Burapha University and then resuscitated in the W-minimal medium to develop the pure culture of E. aerogenes.18 E. aerogenes colonies were subsequently transferred to sterile liquid culture of W-minimal medium after the incubation period of 48 h. The medium solution was mixed for 24 h at the temperature of 30 °C and at the mixing speed of 200 rpm. A 0.5 McFarland standard was used to adjust the bacterial suspension.19 The bacterial suspension was enriched in a 3 L reactor fed with synthetic wastewater. Finally, E. aerogenes was transferred to AS-1 and IFAS-1 SBR systems.2.3 Respirometric activities evaluation and biodegradation studies
The respirometric activities of sludge were evaluated by the manometric respirometric BOD OxiTop Control apparatus (OC 110, WTW, Germany). The OxiTop is a highly reliable equipment for measuring a pressure drop in gaseous phase of the closed vessels at a constant temperature.20 Typically, the pressure in the vessel increases during the first 2–3 hours as a result of the temperature difference between sample and incubator.21,22 After the sample has equilibrated with temperature in the incubator, it requires an acclimation period for microorganisms to adapt themselves to new conditions causing nonlinear reduction of pressure. Finally, the pressure decreases linearly due to the steady microbial oxygen consumption.22 The slope of linear pressure drop determined by linear regression was used to indicate the pressure drop per unit time (ΔP/t), which subsequently was used to determine the oxygen uptake rate (OUR). The OUR was derived from the relationship between the number of moles based on the mass of oxygen and pressure drop according to the ideal gas law as follows:
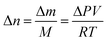 | (1) |
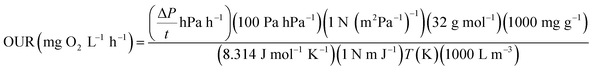 | (2) |
To determine the respirometric activities of E. aerogenes and mixed culture bacteria in response to the synthetic wastewater, acrylamide, and acrylic acid additions, both E. aerogenes and mixed culture bacteria were collected from the AS-1, AS-2, IFAS-1, and IFAS-2 SBR systems at the end of reacting period in each cycle to obtain the total sludge volume of 1 L after achieving the steady state conditions. Each type of sludge was washed three times with distilled water to remove remaining substrates and subsequently was diluted with distilled water to obtain 2 L sludge samples (50% dilution factor). The sludge was transferred into four 1 L OxiTop bottles (0.5 L each). Subsequently, all OxiTop bottles were injected with three different substrates including synthetic wastewater that was fed to the SBR systems, acrylamide (Acrylamide PAGE, GE Healthcare Bio-Sciences, USA), and acrylic acid (ACS Grade 99%, Sigma-Aldrich, Netherlands) at the concentrations of 400 mg COD L−1, 400 mg AM L−1 (5.63 mM), and 400 mg AA L−1 (5.55 mM), respectively. Acrylic acid contained 180–200 ppm of hydroquinone monomethyl either (MEHQ) or methoxyphenol as a polymerization inhibitor. Ammonium at a concentration of about 50 mg N L−1 was added as a nitrogen source for synthetic wastewater and acrylic acid. Two different ammonium concentrations of 50 and 500 mg N L−1 were added with acrylamide to evaluate the inhibitory effects of ammonia on the acrylamide biodegradation as reported by Jangkorn et al.11 Furthermore, 20 BioPortz media containing the sludge were randomly taken from the IFAS-1 and IFAS-2 SBR systems and added into the OxiTop bottles. Other nutrients similar to the synthetic wastewater were also supplemented for acrylamide and acrylic acid. Two mL of N-allylthiourea (C4H8N2S) (98%, Alfa Aesar, UK) at a concentration of 5 g L−1 were added to each bottle to inhibit nitrification; therefore, the effects of ammonia on acrylamide biodegradation could be evaluated. To minimize the difference in temperature between sample and incubator, all chemicals and sludge samples were allowed to acclimatize with the room temperature of ∼28 °C. The OxiTop system was incubated in the incubator controlled at the temperature of 28 °C, which was the same operating temperature of SBR systems, for a time period of 5 days. The pressure data were recorded at 20 min time interval by a handheld remote controller and then were transferred to the personal computer (PC) via a cable and a software called Achat OC (version 2.03).
The biodegradations of acrylamide and its products including ammonia and acrylic acid were also conducted in parallel with the respirometric evaluations in four closed and stirred bottles for each type of microorganisms from the AS-1, AS-2, IFAS-1, and IFAS-2 systems. Each sludge was prepared according to the procedures described above and then transferred to the bottles. The experiments were setup in similar to the OxiTop bottles and were run in the same incubator as the OxiTop system at the temperature of 28 °C. The samples were collected at different time intervals from the bottles for parameter analyses including COD, acrylamide, acrylic acid, ammonium, nitrite, nitrate, pH, MLSS, and MLVSS.
2.4 Measurement and analysis
The SBR systems were operated for over a year to achieve the steady state conditions during which samples were collected periodically for analyzing several parameter including mixed liquor suspended solids (MLSS), mixed liquor volatile suspended solids (MLVSS), TCOD (Closed Reflux, Titrimetric Method), ammonium nitrogen (NH4+–N) (Phenate Method), nitrite nitrogen (NO2−–N) (Colorimetric Method), and nitrate nitrogen (NO3−–N) (Brucine Method), according to Standard Methods for the Examination of Water and Wastewater.23 For soluble COD (SCOD), ammonium, nitrite and nitrate, particulates in the samples were filtered out with 0.45 μm membrane after centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Dissolved oxygen (DO) and pH were measured with DO (Cyberscan DO110, Eutech Instruments) and pH meters (Cyberscan pH510, Eutech Instruments), respectively.
000 rpm for 10 min. Dissolved oxygen (DO) and pH were measured with DO (Cyberscan DO110, Eutech Instruments) and pH meters (Cyberscan pH510, Eutech Instruments), respectively.
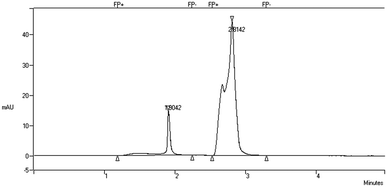
For the determination of attached biomass in the BioPortz media, two BioPortz media were randomly sampling from the IFAS systems. The biomass was removed out from the media with a high-pressurized water jet produced from a syringe into a beaker. After mixing the samples to achieve homogenous liquid, the samples were collected for further MLSS and MLVSS analyses. The MLSS and MLVSS concentrations were calculated to determine the attached biomass per BioPortz media so that the biomass residing in the 510-BioPortz media of IFAS SBR systems could be determined. Equivalent MLSS and MLVSS concentrations of attached biomass were then calculated by dividing the amount of biomass by the volume of reactors. The combination of both suspended and attached biomass indicated the total MLSS and MLVSS in this study. A high performance liquid chromatography (HPLC) (Varian 9050) was used to quantitatively determine acrylamide and acrylic acid. The HPLC was equipped with a UV spectrophotometric detector (JENWAY 6305) operating at 254 nm and a Nova-Pack C18 (4 μm 60 Å) guard pak insert column (Waters, Ireland) in a reversed system using 50% deionized water and 50% acetonitrile as a mobile phase. A 60 μL injection loop of filtered sample was injected into the HPLC system to deliver 20 μL volume to obtain the peak areas as shown in Fig. 2 with a run time of 5 min. at room temperature under a constant flowrate of 1 mL min−1. Acrylamide and acrylic acid standards were used to determine both acrylamide and acrylic acid concentrations. The first and second peaks were identified as acrylic acid and acrylamide, respectively.
3. Results and discussion
3.1 SBR system performances
At the steady state conditions, it was found that MLSS and MLVSS concentrations of the AS-1 and AS-2 SBR systems containing E. aerogenes and mixed culture bacteria, respectively, as listed in Table 1 were considerably lower than those of the IFAS systems. This can be explained that the effective volume of the IFAS system was greatly smaller than that of the AS systems due to the volume replacement by BioPortz media. Integration of BioPortz had enhanced the amount of biomass in the IFAS systems without any increase of SRT. The biofilm densities of the IFAS-1 and IFAS-2 systems were 10.9 and 15.0 g m−2, respectively. It was found that the MLVSS/MLSS ratios were very low for the attached biomass of BioPortz, indicating that inorganic compound was accumulated in the media. The accumulation of calcium carbonate precipitates inside the BioPortz was a result of a hardness concentration of about 120 mg CaCO3 L−1 in the synthetic wastewater at the relative high operating temperature of 28 °C.| System | Inocula | MLSS mg L−1 | MLVSS mg L−1 | Equivalent MLSS mg L−1 | Equivalent MLVSS mg L−1 | Total MLSSmg L−1 | Total MLVSSmg L−1 |
|---|---|---|---|---|---|---|---|
| AS-1 | E. aerogenes | 913 ± 54 | 903 ± 54 | — | — | 913 ± 54 | 903 ± 54 |
| IFAS-1 | E. aerogenes | 1233 ± 60 | 1223 ± 60 | 3188 | 1887 | 4420 ± 60 | 3110 ± 60 |
| AS-2 | Mixed culture | 855 ± 42 | 845 ± 42 | — | — | 855 ± 42 | 845 ± 42 |
| IFAS-2 | Mixed culture | 1668 ± 127 | 1613 ± 97 | 4361 | 2601 | 6028 ± 127 | 4214 ± 97 |
All four SBR systems achieved the similar COD removal efficiencies of about 80% within the reacting period of 10 h, resulting in the effluent COD concentrations of about 80 mg COD L−1. With regarding to nitrification, all four systems could completely nitrify ammonium resulting in the ammonium removal efficiencies of nearly 100%. Nitrite was not detectable in all SBR systems, indicating that nitrite was rapidly converted to nitrate nitrogen. The effluent nitrate concentrations of AS-1, IFAS-1, AS-2, and IFAS-2 were 33.2 ± 8.3, 29.6 ± 7.0, 34.1 ± 5.9, and 29.3 ± 7.4 mg N L−1, respectively. E. aerogenes in both the AS-1 and IFAS-1 systems could heterotrophically nitrify the ammonium nitrogen.11,24 Mixed culture bacteria in the AS-2 and IFAS-2 systems contained nitrifiers that autotrophically removed the ammonium nitrogen.
3.2 Respirometric activities of microbes from the AS systems
The effects of acrylamide and its biodegradation products i.e., ammonia and acrylic acid on the respirometric activities were evaluated by using the OxiTop system (Fig. 3 and 4). Fig. 3(a) illustrates the pressure drops in the OxiTop systems resulted from the biodegradation of synthetic wastewater, acrylamide, and acrylic acid by pure culture of E. aerogenes sampled from the AS-1 system.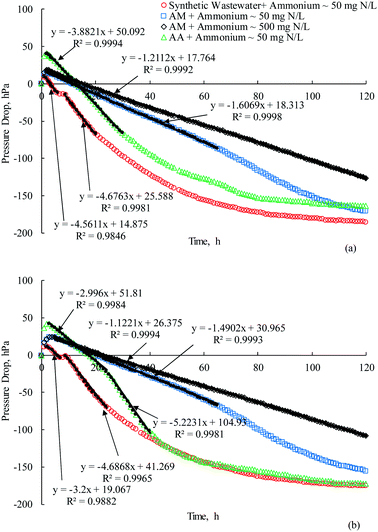 | ||
| Fig. 3 Pressure drops during 5 day incubation period of (a) E. aerogenes (AS-1) and (b) mixed culture bacteria (AS-2). | ||
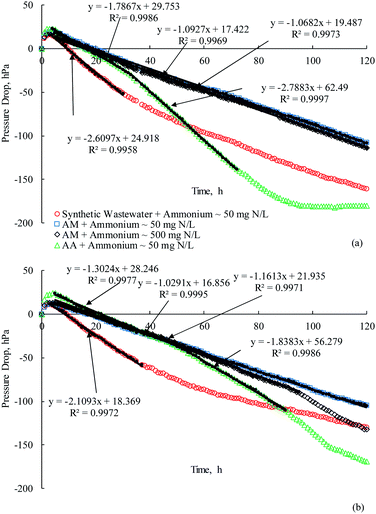 | ||
| Fig. 4 Pressure drops during 5 days incubation period of (a) E. aerogenes (IFAS-1) and (b) mixed culture bacteria from the IFAS-2 systems. | ||
It appears that the pressures in four OxiTop bottles initially increased with time during the first 1–2 hours, reaching different maximum pressures depending on the substrates. The maximum pressure of 9 hPa was observed after the incubation period of 1 h for synthetic wastewater. It is expected that E. aerogenes had already been acclimatized with synthetic wastewater in the AS-1 system because the systems reached the steady state condition; therefore, it is likely that the increase in pressure was resulted from the temperature. After the temperature equilibrium, there was a linear decrease in pressure with time due to the microbial metabolism consuming oxygen and producing carbon dioxide. E. aerogenes required no acclimation period to synthetic wastewater. The results from the linear regression analyses revealed that there were two different slopes on the straight line, likely related to the degradation of acetate and sucrose as carbon sources in the synthetic wastewater. It was observed that the negative pressure of 14 hPa remained constant for one hour during the 7th and 8th hour after the addition of synthetic wastewater, indicating a short lag phase in this period. It is evident that E. aerogenes exhibited the diauxic growth pattern with the presence of two carbon sources.25 After the linear decrease of pressure, it appears that E. aerogenes consumed oxygen at a slower rate, suggesting that less biodegradable substrate was available or endogenous respiration occurred. It is expected that oxygen in the gaseous phase was not depleted; otherwise, the pressure drop must remain constant over a period of time. Table 2 lists the oxygen uptake rates (OURs) and the specific oxygen uptake rates (SOURs) of E. aerogenes for biodegrading the synthetic wastewater.
| Experiments | SBR system | E. aerogenes | Mixed culture bacteria | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OUR mg O2 L−1 h−1 | SOUR mg O2 (g VSS)−1 h−1 | OUR mg O2 L−1 h−1 | SOUR mg O2 (g VSS)−1 h−1 | ||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | ||
| a Ammonium as a nitrogen source at the concentration of about 50 mg N L−1.b Ammonium as a nitrogen source at the concentration of about 500 mg N L−1; 1 and 2 indicated the first and second OUR and SOUR rates. | |||||||||
| Synthetic wastewatera | AS | 5.81 | 5.97 | 10.75 | 11.05 | 4.09 | 5.99 | 7.05 | 10.33 |
| Acrylamidea | 2.05 | — | 3.80 | — | 1.90 | — | 3.28 | — | |
| Acrylamideb | 1.54 | — | 2.86 | — | 1.56 | — | 2.69 | — | |
| Acrylic acida | 4.97 | — | 9.21 | — | 3.83 | 6.68 | 6.60 | 11.51 | |
| Synthetic wastewatera | IFAS | 3.34 | — | 0.94 | — | 2.70 | — | 1.13 | — |
| Acrylamidea | 1.37 | — | 0.38 | — | 1.32 | — | 0.55 | — | |
| Acrylamideb | 1.40 | — | 0.39 | — | 1.48 | — | 0.62 | — | |
| Acrylic acida | 2.28 | 3.56 | 0.64 | 1.00 | 1.66 | 2.35 | 0.70 | 0.98 | |
According to the results in Fig. 3(a), the highest pressure was detected for acrylic acid at 40 hPa during the first two hours of incubation period, which was much higher than the maximum pressure as a result of temperature difference in the OxiTop bottle containing synthetic wastewater (9 hPa). It is noted that acrylic acid can be easily polymerized and then a large amount of heat can be generated due to the exothermic polymerization of acrylic acid. Even though the MEHQ as a polymerization inhibitor was added into acrylic acid to prevent polymerization, some contamination or excessive heat can cause polymerization.26 The results indicate that pressure in the OxiTop bottle containing acrylic acid increased tremendously as compared with synthetic wastewater; therefore, the acrylic acid must have been polymerized. After the temperature equilibrium, the pressures decreased linearly in the similar fashion to the synthetic wastewater. It can be interpreted that E. aerogenes did not require any acclimation time for acrylic acid biodegradation. However, the OUR and SOUR of acrylic acid by E. aerogenes in Table 2 suggest that the rates were slightly lower than the synthetic wastewater.
Acrylamide was reported as a toxic compound to most microorganisms;3,4 therefore, the experiments spiked acrylamide to two OxiTop bottles with different ammonium concentrations for evaluating the respirometric activities. The maximum pressures of 15 hPa and 18 hPa were found at the incubation period of 2 hours for acrylamide with ammonium concentrations of about 50 and 500 (mg N) L−1, respectively. Higher increases in pressure of acrylamide than synthetic wastewater indicate that a certain process occurred in the systems in addition to the temperature difference; otherwise, the increases in pressure must be the same as the synthetic wastewater. It is possible that acrylamide was biodegraded and then acrylic acid was produced and polymerized. A linear reduction in pressure with time was found during a period between 2nd hour and 65th hour as a result of microbial oxygen consumption. It appears that E. aerogenes from the AS-1 system did not require the acclimation period to acrylamide. The calculated OUR and SOUR in Table 2 indicate that the rates were considerably lower than the rates of E. aerogenes for biodegrading the synthetic wastewater or acrylic acid. It was evident that the FAN reduced the OUR and SOUR of E. aerogenes as compared with acrylamide supplemented with lower ammonium concentration. However, the inhibition effects of ammonia on the acclamation period of E. aerogenes was not found in this experiment.
Fig. 3(b) shows the respirometric activities of mixing culture bacteria from the AS-2 system degrading different substrates in the OxiTop bottles. The pressures increased during the initial incubation time of 2–3 hours to 12, 24, 24, and 42 hPa when degrading synthetic wastewater, acrylamide with ammonium concentration of 50 mg N L−1, acrylamide with ammonium concentration of 500 mg N L−1, and acrylic acid, respectively. The pressure dropped immediately after the temperature equilibrium, suggesting that the acclimation period was not required for mixed culture bacteria to degrade synthetic wastewater. The results revealed that the pressures remained constant for 1 hour for the acrylic acid biodegradation and for 2 hours to degrade acrylamide with both ammonium concentrations of 50 and 500 mg N L−1. Thus, the mixed culture bacteria required an acclimation period to biodegrade acrylamide or acrylic acid. The FAN inhibited the acrylamide biodegradation of mixed culture bacteria, resulting in lower biodegradation rates. There was no difference in terms of the inhibition effects of the FAN on the acclimation period. The OUR of mixed culture bacteria for the acrylic acid biodegradation was less than that of E. aerogenes during the first 25 hours, but subsequently the rate greatly increased. It was reported that acclimatized mixed culture bacteria completely biodegraded both acrylic acid monomer and dimer, and the biodegradability of acrylic acid decreased as the molecular weight increased.13 Therefore, it is suggested that the unacclimatized mixed culture bacteria required time to reduce the polymerized acrylic acid.
3.3 Respirometric activities of microbes from the IFAS systems
Investigations were extended to evaluate the effects of acrylamide and its biodegradation products on the respirometric activities of the hybrid process between suspended and attached growth of E. aerogenes from the IFAS SBR systems (Fig. 4). Fig. 4(a) shows that the pressures in the OxiTop systems increased during the first 2–3 hours for the synthetic wastewater and acrylic acid biodegradations; however, the acrylamide biodegradation required 4 hours to reach the temperature equilibrium. In general, E. aerogenes from the IFAS-1 system required longer time to reach temperature equilibrium than the suspended growth of E. aerogenes, possibly as the results of mass transport resistance in the fixed film media. After achieving the temperature equilibrium, the pressures decreased linearly due to the microbial oxygen consumptions in all substrates except for acrylic acid; therefore, it required little acclimation period to the synthetic wastewater and acrylamide. The acclimation period of 2 hours was needed for the removal of acrylic acid, which was in contrast with E. aerogenes from the AS-1 system. It could be explained that acrylic acid was polymerized and the diffusion of polymerized acrylic acid into the fixed film media was limited. It is apparent that the OUR increased considerably after the 33th hour, possibly because that the composition of wastewater was changed. In general, the OURs of E. aerogenes from the IFAS-1 system for biodegrading synthetic wastewater, acrylamide and acrylic acid were lower than those of the AS-1 system (Table 2). The inhibition effect of ammonia on the acrylamide biodegradation by E. aerogenes from the IFAS-1 system was not different from E. aerogenes from the AS-1 system.When the mixed culture bacteria was immobilized in the BioPortz media (IFAS-2 system), the time to reach temperature equilibrium was longer than the suspended growth of mixed culture bacteria (AS-2 system) (Fig. 4b). After reaching temperature equilibrium, the mixed culture bacteria from the IFAS-2 system required 1 hour to acclimatize with acrylic acid and acrylamide with ammonium concentration of 50 mg N L−1. The FAN resulted in longer acclimatization period of 3 hours for the mixed culture bacteria from the IFAS-2 system to biodegrade the acrylamide. It appears from the OURs and SOURs (Table 2) that the inclusion of the attached growth of mixed culture bacteria into the suspended biomass was not superior to biodegrade the synthetic wastewater, acrylamide, and acrylic acid over the suspended growth system.
3.4 Biodegradation of acrylic acid
Acrylic acid produced from the acrylamide biodegradation can possibly be used as carbon and energy sources for microorganisms. It is evident from Fig. 5 that degradation of acrylic acid started quickly in a few minutes after addition. About 31.7% and 26.5% of acrylic acid in the batch reactors containing E. aerogenes from the AS-1 and IFAS-1 systems, respectively, were removed. The mixed culture bacteria from the AS-2 and IFAS-2 systems removed 22.7 and 28.8% of acrylic acid, respectively. It should be noted that the initial acrylic acid concentration used in this study was 400 mg AA L−1 or 5.55 mM [72.06 g AA mol−1 for molecular weight]. Both the pressure increase in the OxiTop systems and the amounts of acrylic acid removed initially suggest that acrylic acid was polymerized. Interestingly, acrylamide appeared after the addition of acrylic acid, likely as a result of the reaction between acrylic acid and ammonia, at the concentrations of 1.88, 1.93, 1.99, and 1.82 mM in the solutions of AS-1, IFAS-1, AS-2, and IFAS-2 batch reactors. Yasuhara et al.27 reported that acrylic acid can react with ammonia to produce acrylamide. The stoichiometric molar ratio of acrylamide to acrylic acid is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1;10 therefore, acrylic acid must be used to produce acrylamide at the same concentrations as those of acrylamide. It appears from Fig. 5 that the acrylamide concentrations were relatively stable. In addition, there was little change in the ammonium concentration during the incubation period of 5 days. Thus, it is possible that acrylic acid was used to form acrylamide only at the beginning of the tests.
1;10 therefore, acrylic acid must be used to produce acrylamide at the same concentrations as those of acrylamide. It appears from Fig. 5 that the acrylamide concentrations were relatively stable. In addition, there was little change in the ammonium concentration during the incubation period of 5 days. Thus, it is possible that acrylic acid was used to form acrylamide only at the beginning of the tests.
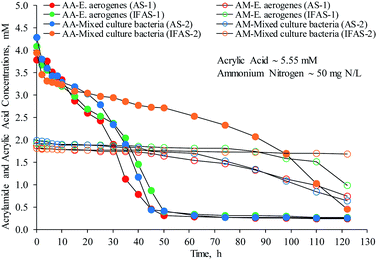 | ||
| Fig. 5 Acrylic acid and acrylamide concentrations in the batch reactors containing E. aerogenes and mixed culture bacteria from the AS and IFAS systems (AA: acrylic acid and AM: acrylamide). | ||
After the initial removal of acrylic acid due to the acrylamide formation, acrylic acid was continuously removed during the incubation period of 50 hours at the biodegradation rates of 5.47 (R2 = 0.99) and 4.54 (R2 = 0.96) mg AA L−1 h−1 by E. aerogenes from the AS-1 and IFAS-1 systems, respectively. The mixed culture bacteria from the AS-2 system had a biodegradation rate of 5.11 (R2 = 0.95) mg AA L−1 h−1 in a similar fashion to the synthetic wastewater, because acrylic acid is a readily biodegradable organic compound and can be rapidly oxidized under an aerobic condition.12 The mixed culture bacteria in the IFAS-2 reactor could not degrade acrylic acid efficiently as compared with other systems, this result is in good agreement with the respirometric activity of mixed culture bacteria from the IFAS-2 system. The OUR of IFAS-2 for acrylic acid biodegradation was much less than other systems (Table 2). As shown in Fig. 5, acrylamide began to decrease at the 50th hour, after the depletion of acrylic acid. In contrast, acrylamide was not consumed by the mixed culture bacteria from the IFAS-2 system because acrylic acid was still available in the solution. Thus, acrylic acid is a preferable substrate than acrylamide for biodegradation by both E. aerogenes and mixed culture bacteria because acrylamide can be toxic to microorganisms3,4 and acrylic acid is a readily biodegradable organic compound.12
3.5 Effects of acrylamide
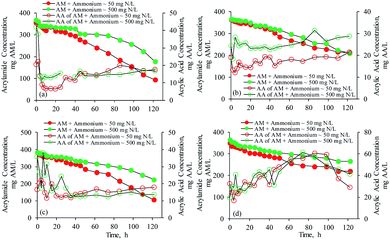
The respirometric activities indicated that acrylamide could be degraded without any acclimation periods by E. aerogenes from the AS-1 and IFAS-1 systems when acrylamide was added as a primary substrate, but the OUR was greatly lower than the synthetic wastewater. The batch studies in Fig. 6(a) reveal that the acrylamide biodegradation by E. aerogenes from the AS-1 system occurred quickly in a few minutes after the acrylamide injection, resulting in acrylic acid and ammonia as biodegradation products. Acrylic acid was produced rapidly; however, its concentration decreased immediately soon after it was produced because acrylic acid was polymerized and the result was in good agreement with the initial increase of pressures in the OxiTop bottle during the first two hours due to the exothermic polymerization. It should be noted that only acrylamide was added as a substrate in this test. Furthermore, ammonium was also produced quickly at the accumulation rate of 0.44 mg N L−1 h−1 (R2 = 0.950) during the incubation period of 5 days, suggesting that acrylamide is a biodegradable substrate for E. aerogenes. According to the respirometric activities as shown in Fig. 3(a), the steady microbial oxygen consumption occurred during the first 65 hours; therefore, the acrylamide biodegradation rate of 1.57 mg AM L−1 h−1 (R2 = 0.942) was determined by the linear regression during this period. The subsequent biodegradation rate increased considerably to 2.54 mg AM L−1 h−1 (R2 = 0.996). It appears from Fig. 7(a) that the SCOD concentration of acrylamide did not decrease while acrylamide was being degraded during first 40 hours of the incubation period. It was found from the measurements in this study that the COD values of acrylamide and acrylic acid were about 1.00 g COD (g AM)−1 and 1.45 g COD (g AA)−1, respectively. The amount of COD oxidized in the purely aerobic system can be determined by the OUR.28 With the information listed above, it is possible to approximate the COD of acrylic acid produced in the solution. According to the stoichiometric molar ratio of acrylamide to acrylic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the acrylamide biodegradation rate of 1.57 mg AM L−1 h−1 would produce acrylic acid at the production rate of 1.59 mg AA L−1 h−1 [(1.57 mg AM L−1 h−1/71.08 mg AM mmol−1) × 72.06 mg AA mmol−1], which was equivalent to 2.31 mg COD L−1 h−1 [1.59 mg AA L−1 h−1 × 1.45 mg COD (mg AA)−1]. The OUR of 2.05 mg O2 L−1 h−1 obtained from the respirometric evaluation indicated that the COD was oxidized at the rate of 2.05 mg COD L−1 h−1. As compared with the acrylamide biodegradation rate of 1.57 mg AM L−1 h−1 (∼1.57 mg COD L−1 h−1), the OUR was greater than the acrylamide biodegradation rate, suggesting that acrylic acid produced from the acrylamide biodegradation was degraded. The remaining acrylic acid caused the COD to sustain in the solution. As discussed in the previous section, acrylamide could be formed from the reaction between acrylic acid and ammonia in the solution. In this experiment, acrylamide was not generated from this reaction because the ammonium increased in the solution.
1), the acrylamide biodegradation rate of 1.57 mg AM L−1 h−1 would produce acrylic acid at the production rate of 1.59 mg AA L−1 h−1 [(1.57 mg AM L−1 h−1/71.08 mg AM mmol−1) × 72.06 mg AA mmol−1], which was equivalent to 2.31 mg COD L−1 h−1 [1.59 mg AA L−1 h−1 × 1.45 mg COD (mg AA)−1]. The OUR of 2.05 mg O2 L−1 h−1 obtained from the respirometric evaluation indicated that the COD was oxidized at the rate of 2.05 mg COD L−1 h−1. As compared with the acrylamide biodegradation rate of 1.57 mg AM L−1 h−1 (∼1.57 mg COD L−1 h−1), the OUR was greater than the acrylamide biodegradation rate, suggesting that acrylic acid produced from the acrylamide biodegradation was degraded. The remaining acrylic acid caused the COD to sustain in the solution. As discussed in the previous section, acrylamide could be formed from the reaction between acrylic acid and ammonia in the solution. In this experiment, acrylamide was not generated from this reaction because the ammonium increased in the solution.
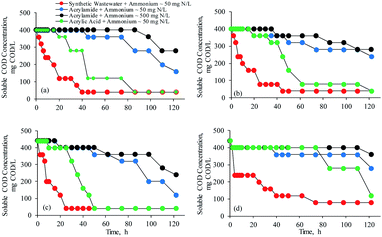 | ||
| Fig. 7 Time courses of residual COD concentrations of (a) E. aerogenes (AS-1), (b) E. aerogenes (IFAS-1), (c) mixed culture bacteria (AS-2), and (d) mixed culture bacteria (IFAS-2). | ||
It is evident from Fig. 6(b) that E. aerogenes immobilized in the BioPortz media did not degrade acrylamide at the same rate as the suspended growth of E. aerogenes. The diffusion of substrates could be limited due to the mass transfer resistance in the media. As shown in Table 3, the acrylamide biodegradation rate and removal efficiency were 1.36 mg AM L−1 h−1 (R2 = 0.981) and 41.6%, respectively. It is obvious that E. aerogenes from the IFAS-1 system did not enhance the acrylamide biodegradation. The OUR obtained from the respirometric evaluation was 1.37 mg O2 L−1 h−1, which was approximately the same as the acrylamide biodegradation rate, suggesting that only acrylamide was degraded. In addition, it was found that about 31.3% of ammonium was removed during the first incubation period of 6 hours, suggesting that acrylic acid produced from the acrylamide biodegradation was used to form acrylamide, resulting in the reduced acrylamide biodegradation rate. As acrylamide was not considerably degraded, less amount of acrylic acid was accumulated in the system; thereby, the acrylic acid concentrations remained constant at the average concentration of 16.9 ± 2.47 mg AA L−1. Fig. 7(b) confirms the explanations, as the SCOD concentrations did not decrease during the first 45 hours of incubation period.
| Experiments | SBR system | NH4+ mg N L−1 | AM removal efficiency (%) | AM biodegradation rate mg AM L−1 h−1 | R2 |
|---|---|---|---|---|---|
| E. aerogenes | AS-1 | 50 | 73.7 | 2.05 | 0.978 |
| Mixed culture bacteria | AS-2 | 72.3 | 2.09 | 0.978 | |
| E. aerogenes | IFAS-1 | 41.6 | 1.36 | 0.981 | |
| Mixed culture bacteria | IFAS-2 | 31.2 | 1.06 | 0.982 | |
| E. aerogenes | AS-1 | 500 | 51.7 | 1.18 | 0.909 |
| Mixed culture bacteria | AS-2 | 41.7 | 1.14 | 0.977 | |
| E. aerogenes | IFAS-1 | 42.5 | 1.28 | 0.988 | |
| Mixed culture bacteria | IFAS-2 | 22.4 | 0.88 | 0.975 |
The unacclimatized mixed culture bacteria from the AS-2 system biodegraded acrylamide slowly during the first incubation period of 15 hours (Fig. 6(c)). The results supported the respirometric evaluation that the suspended growth of mixed culture bacteria required a few hours to acclimatize with acrylamide. The acrylamide biodegradation rate during the first 65 hours was 1.79 mg AM L−1 h−1 (R2 = 0.978), which was approximately equal to the OUR of 1.90 mg O2 L−1 h−1 as listed in Table 2. This implied that minimal acrylic acid was degraded; thus, the SCOD concentrations did not decrease as illustrated by Fig. 7(c). Subsequently, the acrylamide biodegradation rate was increased to 2.85 mg AM L−1 h−1 (R2 = 0.984). Ammonium increased gradually as a result of acrylamide biodegradation; thereby, the acrylamide formation from the reaction between acrylic acid and ammonia did not occur.
Fig. 6(d) shows that acrylamide concentrations decreased linearly with time due to the acrylamide biodegradation by the mixed culture bacteria from the IFAS-2 system with the biodegradation rate of 1.06 mg AM L−1 h−1 (R2 = 0.982). According to the OUR of 1.32 mg O2 L−1 h−1 in Table 2, it is implied that acrylic acid was minimally degraded. A minimal amount of ammonium decreased during the first 4 hours, indicating that acrylamide was formed from the reaction of acrylic acid and ammonia during this period. The respirometric activities indicated that the acrylic acid produced from the acrylamide biodegradation was polymerized. Therefore, acrylic acid from the acrylamide biodegradation in this test was polymerized and used to form acrylamide. Subsequently, ammonium increased in the solution at the production rate of 0.34 mg N L−1 h−1 (R2 = 0.932), indicating that acrylamide was not produced from the reaction of acrylic acid and ammonia. Acrylic acid was slowly degraded by the mixed culture bacteria from the IFAS-2 system as illustrated by Fig. 5; therefore, acrylic acid was accumulated over a period of 86 hours. Thus, the SCOD concentrations as shown in Fig. 6(d) remained constant for a period of about 5 days. Afterward, acrylic acid was gradually biodegraded at the slow biodegradation rate resulting in the reductions of SCOD concentrations.
The experimental results in Fig. 6(a) and (c), the OURs and SOURs in Table 2, and the acrylamide biodegradation rates in Table 3 support the conclusion that the acrylamide biodegradations by E. aerogenes and the mixed culture bacteria from the AS-1 and AS-2 systems were inhibited by the high concentration of ammonium. The experimental results were in good agreement with the previous findings from Jangkorn et al.11 The effects of ammonia inhibition on the acrylamide biodegradation by E. aerogenes and the mixed culture bacteria from the IFAS-1 and IFAS-2 systems were minimized. It is possible that the diffusion of acrylamide and other substrates into the BioPortz media were limited in the IFAS systems as a result of mass transfer resistances.
4. Conclusions
The experiments were conducted to evaluate the acute effects of acrylamide and its biodegradation products on the unacclimatized E. aerogenes and the mixed culture bacteria from the AS systems and the IFAS systems. E. aerogenes from the AS-1 system did not require the acclimation period to degrade acrylamide and acrylic acid, but required the acclimation period for acrylic acid when bacteria were immobilized in the media because of limited substrate diffusion into the biofilm. The mixed culture bacteria from both AS-2 and IFAS-2 systems required 1 hour to acclimatize with acrylic acid and 2 hours for acrylamide. Inhibition effect of ammonia on the acclimation periods of E. aerogenes and the mixed culture bacteria was not observed. However, the respirometric activities and biodegradation studies confirmed that ammonia had inhibited the acrylamide biodegradation. The experiments revealed that acrylic acid in the wastewater or from the acrylamide biodegradation could be initially polymerized or reacted with ammonia nitrogen in the wastewater to form acrylamide, reducing the acrylamide biodegradation rate. Both E. aerogenes and mixed culture bacteria from the AS systems could simultaneously degrade both acrylamide and acrylic acid, whereas both E. aerogenes and mixed culture bacteria from the IFAS systems could remove only acrylamide because the diffusion of acrylic acid into the biofilm was limited.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Research Funds of Burapha University through National Research Council of Thailand [grant number 122/2558] to Tongchai Sriwiriyarat and the Office of the Higher Education Commission (OHEC), Ministry of Education, Thailand through a PhD scholarship to Romsan Madmanang.Notes and references
- G. Junqua, S. Spinelli and C. Gonzalez, Environ. Sci. Pollut. Res., 2015, 22, 6452–6460 CrossRef CAS PubMed.
- R. S. DeWoskin, K. Hogan, D. W. Wohlers, P. R. McClure, J. Rhoades, K. Salinas and J. G. Teeguarden, Toxicological Review of Acrylamide, US. Environmental Protection Agency, 2010 Search PubMed.
- S. J. Joshi and R. M. M. Abed, Environ. Processes, 2017, 4, 463–476 CrossRef CAS.
- J. Charoenpanich and A. Tani, CMU Journal of Natural of Sciences, 2014, 13, 11–22 Search PubMed.
- K. Buranasilp and J. Charoenpanich, J. Environ. Sci., 2011, 23, 396–403 CrossRef CAS.
- J. Charoenpanich, Applied Bioremediation-Active and Passive Approaches, InTech Open Science Online Publishers, 2013 Search PubMed.
- M. S. Nawaz, S. M Billedeau and C. E. Cerniglia, Biodegradation, 1998, 9, 381–387 CrossRef CAS PubMed.
- C. S. Prabu and A. J. Thatheyus, Int. Biodeterior. Biodegrad., 2007, 60, 69–73 CrossRef CAS.
- R. Shanker, C. Ramakrishna and P. K. Seth, Arch. Microbiol., 1990, 154, 192–198 CrossRef CAS PubMed.
- M. S. Nawaz, W. Franklin and C. E. Cerniglia, Can. J. Microbiol., 1993, 39, 207–212 CrossRef CAS PubMed.
- S. Jangkorn, J. Charoenpanich and T. Sriwiriyarat, J. Environ. Eng. Div. (Am. Soc. Civ. Eng.), 2018, 144, 04017112 CrossRef.
- C. A. Staples, S. R. Murphy, J. E. McLaughlin, H. W. Leung, T. C. Cascieri and C. H Farr, Chemosphere, 2000, 40, 29–38 CrossRef CAS PubMed.
- R. J. Larson, E. A. Bookland, R. T. Williams, K. M. Yocom, D. A. Saucy, M. B. Freeman and G. Swift, J. Environ. Polym. Degrad., 1997, 5, 41–48 CAS.
- D. Sen, P. Mitta and C. W. Randall, Water Sci. Technol., 1994, 30, 13–24 CrossRef CAS.
- C. W. Randall and D. Sen, Water Sci. Technol., 1996, 33, 155–161 CrossRef CAS.
- T. Sriwiriyarat, K. Pittayakool, P. Fongsatitkul and S. Chinwetkitvanich, J. Environ. Sci. Heal. A, 2011, 43, 1318–1324 CrossRef PubMed.
- H. Kim, A. J Schuler, C. K Gunsch, R. Pei, J. Gellner, J. P. Boltz, R. G. Freudenberg and R. Dodson, Water Environ. Res., 2011, 83, 627–635 CrossRef CAS PubMed.
- K. Kimbara, T. Hashimoto, M. Fukuda, T. Koana, M. Takagi, M. Oishi and K. Yano, J. Bacteriol., 1989, 171, 2740–2747 CrossRef CAS PubMed.
- J. McFarland, JAMA, J. Am. Med. Assoc., 1907, 14, 1176–1178 CrossRef.
- P. Reuschenbach, U. Pagga and U. Strotmann, Water Res., 2003, 37, 1571–1582 CrossRef CAS PubMed.
- H. K. Ahn, T. L. Richard and T. D. Glanville, Waste Management., 2008, 28, 1411–1416 CrossRef CAS PubMed.
- K. Malińska, Arch. Environ. Prot., 2016, 42, 56–62 Search PubMed.
- L. S. Clesceri, A. E. Greenberg and A. D. Eaton, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, 20th edn, 1998 Search PubMed.
- G. P. Robertson and P. M. Groffman, Soil Microbiology, Ecology and Biochemistry, Academic Press, 2007 Search PubMed.
- D. Chu and D. J. Barnes, Sci. Rep., 2016, 6, 25191 CrossRef CAS PubMed.
- EBAM, Safety Handling and Storage of Acrylic Acid, European Basic Acrylic Monomer Group, 3rd edn, 2013 Search PubMed.
- A. Yasuhara, Y. Tanaka, M. Hengel and T. Shibamoto, J. Agric. Food Chem., 2003, 51, 3999–4003 CrossRef CAS PubMed.
- P. S. Barker and P. L. Dold, Water Res., 1995, 29, 633–643 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |