Open Access Article
Open Access ArticleConstruction of flower-like MoS2/Fe3O4/rGO composite with enhanced photo-Fenton like catalyst performance†
Dongzhao Mu‡
,
Zhe Chen‡ *,
Hongfei Shi* and
Naidi Tan*
*,
Hongfei Shi* and
Naidi Tan*
School of Science, Jilin Institute of Chemical Technology, Jilin 132022, PR China. E-mail: chenzhecz999@163.com
First published on 30th October 2018
Abstract
High-performance and recyclable photocatalysts have attracted considerable amounts of attention for use in wastewater treatment. In this paper, a MoS2/Fe3O4/rGO (0.1 wt%) composite was synthesized by an environmentally-friendly and facile strategy, and showed high potential for recyclability. The nanocomposite exhibited high photocatalytic activity in the presence of H2O2 and rGO (reduced graphene oxide) under visible-light irradiation. Notably, when 3 mg of MoS2/Fe3O4/rGO (0.1 wt%) was added to rhodamine B (RhB, 30 mg L−1) solution, the degradation rate was almost 100% within 40 min at neutral pH under visible-light irradiation. This rate was four times more rapid than that of MoS2 and double that of MoS2/Fe3O4. The results indicate that rGO plays an important role in photocatalysis by suppressing the recombination of photogenerated electron–hole pairs and enhancing the absorption capability of visible-light and organic dyes. Finally, the photocatalytic and stability mechanisms of MoS2/Fe3O4/rGO (0.1 wt%) are proposed. This work further helps our understanding of the photo-Fenton mechanism. Furthermore, the synthesis of this composite has potential for application in energy storage devices.
Introduction
Environmental pollution and energy shortages are becoming increasingly serious problems of modern society.1–4 In recent years, water pollution has been recognized as an important contributor to environmental pollution. Advanced oxidation processes (AOPs) are considered promising technologies for achieving environmental remediation.5–10 Among different AOPs, Fenton oxidation technology has received much attention because of its simple technological requirements, cheap catalysts and effective removal of environmental pollutants.11,12 However, the traditional homogenous Fenton process has the problems of secondary treatment, catalyst loss in effluent, and low pH (pH < 3.0) requirements, which greatly restrict its practical applications. Compared with the traditional Fenton reaction, “Fenton-like” reactions are now considered to be one of the most effective methods, as they can overcome the above-mentioned drawbacks.13–20 Accordingly, the key issue is obtaining semiconducting, heterogeneous, Fenton-like catalysts with high stability, excellent activity, wide pH range and reusability.Molybdenum sulfide (MoS2), as an n-type semiconductor, has been widely studied in the fields of photocatalysis,21,22 electrochemistry,23 sensors,24 supercapacitors,25 and drug delivery26 due to its high abundance, good crystallization, low cost, good conductivity, and large specific surface area. Therefore, much research effort has been devoted to constructing MoS2-based materials through chemical vapor deposition, gas-phase synthesis, heterostructural engineering, hydrothermal methods, and thermal decomposition. These methods have produced materials such as MoS2/Bi2WO6,27 MoS2/TiO2,28 CdS/MoS2,29,30 graphene/MoS2,31,32 MoS2/C3N4,33 MoS2/Ag3PO4,34 and MoS2/Co3O4.35 There is currently very few studies on preparing ternary heterostructure nanocomposite photocatalysts.
Recently, magnetite (Fe3O4) has been widely investigated and extensively applied in biomedicine, photocatalysis, therapeutics, and lithium-ion batteries36–43 owing to its good conductivity, easy collectability, strong adsorption, high stability, and high theoretical capacity. Many studies have reported that it shows promise for application in Fenton/photo-Fenton reactions due to containing ferrous Fe(II) and ferric Fe(III), and producing efficient Fenton reagents under illumination. Therefore, superparamagnetic ferric oxide (Fe3O4) is considered to be an ideal candidate for a magnetic catalyst. In addition, Fe3O4 has good electrical conductivity, which can rapidly improve the efficiency of electron/hole separation. Thus, it has recently been found that the performance of photocatalysis can be improved. Meanwhile, by introducing external magnetics, the recycling of catalysts has been improved at the same time, which is beneficial for practical applications. However, some results demonstrate that magnetite materials usually operate in acidic conditions; furthermore, because of the low surface area of this material, Fe ions can leach into the reaction solution. Hence, combinations with other visible-light-driven semiconductor photocatalytic materials have attracted considerable attention, because they can enhance stability, improve photocatalytic activity, and allow easy separation and recycling. Han et al. prepared a MoS2/Fe3O4 nanocomposite by a hydrothermal method.44 Xu et al. developed Fe3O4/g-C3N4 composites with good photocatalytic activity through a simple electrostatic self-assembly method.45 However, the preparation of these ternary photocatalysts usually requires a multistep process and relatively harsh reaction conditions.
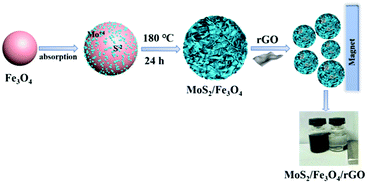
In this study, we report, for the first time, a facile and cost-effective approach to the large-scale synthesis of the ternary photocatalyst MoS2/Fe3O4/rGO (0.1 wt%) under simple hydrothermal conditions. Compared with MoS2/Fe3O4 and pure MoS2, the MoS2/Fe3O4/rGO (0.1 wt%) composite exhibits both a large specific surface area and good photocatalytic activity in the photo-degradation of rhodamine (B) in the presence of H2O2 and rGO under visible-light irradiation. In addition, the experiment reveals that, on the one hand, the rGO creates an MoS2/Fe3O4 hybrid, which can accelerate the separation and transfer of photogenerated electron–hole pairs, which improves photocatalytic activity; on the other hand, hydrogen peroxide (H2O2) produces strong oxidative capacity, whereby ·OH and ·O2−radicals enhance the degradation efficiency of rhodamine (RhB) at neutral pH. The stability and possible mechanisms of the as-prepared MoS2/Fe3O4/rGO are also explored. Our research opens a new door for the practical utilization of photo-degradation. Furthermore, this magnetic material may have potential practical use in sensors and for energy storage.
Experimental
Synthesis and characterization
Ferric chloride hexahydrate (FeCl3), sodium acetate (NaAc), polyvinylpyrrolidone (PVP k30), sodium molybdate (Na2MoO4) and L-cysteine were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used without further purification. Distilled water was used in all experiments. Reduced graphene oxide nanosheets were purchased from Nanjing XFNANO Materials Technology Corporation Ltd., Nanjing, China. Monodispersed Fe3O4 nanoparticles were prepared according to previous literature.46 In brief, monodispersed Fe3O4 (10 mg) nanoparticles were dispersed in 60 mL of distilled water under ultrasonic radiation for 20 min. Afterward, 0.121 g Na2MoO4 and 0.121 g L-cysteine were added slowly into the above solution under mechanical stirring at room temperature. After 30 min of stirring, a certain amount of rGO (0.25 mg mL−1) suspension was added dropwise under stirring. The mixture was transferred into an 80 mL Teflon-lined stainless-steel autoclave and heated to 180 °C for 24 h. The autoclave was allowed to cool to room temperature, the black precipitate was separated and washed by distilled water several times, and then washed with ethanol. Finally, the resulting products were dried at 60 °C overnight.X-ray diffraction (XRD) measurements were carried out by an X-ray powder diffractometer (Rigaku, Japan) equipped with a CuKα source (λ = 0.15418 nm). X-ray photoelectron spectroscopy (XPS) was performed using a JEOL2010F instrument. The morphologies and microstructures of the samples were observed by scanning electron microscopy (SEM; S-4800, Hitachi), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM; JEM-2100, Japan). The Brunauer–Emmett–Teller (BET) specific surface areas (SBET) of the samples were measured through nitrogen adsorption by Micromeritics (ASAP 2020M, USA). Fourier transform infrared (FTIR) spectra of the as-prepared samples were measured on a PerkinElmer Spectrum One spectrometer in the range 4000–400 cm−1 with the KBr tabletting method.
Photocatalytic performance measurements
The photocatalytic efficiencies of the obtained samples were evaluated by the degradation of RhB in the presence of H2O2 under visible-light irradiation supplied by an 800 W Xe-lamp (Phchem III, Beijing NBET Technology Co., Ltd, λ > 420 nm). In a typical photocatalytic degradation experiment, 3 mg of MoS2/Fe3O4/rGO was dispersed in 50 mL RhB (30 mg L−1) solution under magnetic stirring for 10 min at room temperature. Before irradiation, the suspensions were magnetically stirred for another 50 min in darkness to ensure the establishment of an adsorption–desorption equilibrium, and then 30 wt% of H2O2 was added to the above suspensions. During the photocatalytic tests, 3 mL of the suspensions were taken out at given time intervals and magnetically separated to remove the catalyst completely. The concentration of RhB was measured by a UV-Vis spectrophotometer (UV-3600, Shimadzu) at λmax = 554 nm. The catalytic activities were calculated by the formula E = C0 − Ct/C0 × 100%, where C0 and Ct represent the adsorption equilibrium absorbance of RhB and the desired time intervals, respectively. To detect the major active species in the photo Fenton-like process, radical-trapping experiments were conducted. The hydroxyl radical (·OH) scavenger, superoxide anion radical (·O2−), and hole (h+) scavenger can usually be trapped by isopropanol (IPA), 4-hydroxy-TEMPO, and triethanolamine (TEOA), respectively. Typically, different scavengers were dispersed into the suspension solutions before the photocatalytic tests, and the following procedure was similar to the RhB degradation process.Results and discussion
Materials characterizations
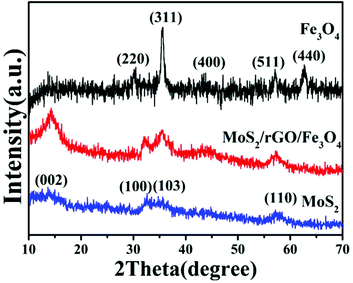
To confirm the crystallographic phases of the as-prepared samples, X-ray diffraction patterns of the precursors were obtained (Fig. 1). The pattern of pure Fe3O4 exhibited six typical peaks, located at 30.1, 35.5, 43.1, 53.4, 57.0 and 62.6°, which can be ascribed to the {220}, {311}, {400}, {422}, {511} and {440} facets of cubic Fe3O4 (JCPDS card no. 17-0320), respectively. For the pattern of pure MoS2, only three characteristic peaks appeared at about 2θ = 14.4° {002}, 32.7° {100}, 39.5° {103} and 58.3° {110}, respectively, which can be assigned to the database of the hexagonal phase of MoS2 (JCPDS card no. 37-1492). Compared with pure MoS2 and Fe3O4, the dominant peaks of MoS2 could be indexed in the XRD spectrum of MoS2/Fe3O4/rGO, while there was low diffraction intensity of Fe3O4, and no peaks for rGO were detected due to the small amounts of Fe3O4 (12 wt%) and rGO (0.5 wt%). Therefore, this suggests that no other impurity-related diffraction peaks were present, indicating that the desired MoS2/Fe3O4/rGO (0.1 wt%) composites were successfully prepared and closely combined using the hydrothermal method.To further test the purity and elemental composition of the MoS2/Fe3O4/rGO (0.1 wt%) material, XPS analysis was carried out. From Fig. 2a, it can be seen that the elements C, N, O, Mo, Fe, and S coexist in the sample, which is consistent with the XRD results. The peaks obtained at 284.8, 286.0, 287.5 and 288.9 eV are associated with the binding energies of sp2 C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O
O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O bonds, respectively (Fig. 2b).47,48 Fig. 2c shows the high-resolution XPS spectrum of Fe 2p. The Fe 2p3/2 and Fe 2p1/2 peak positions at 707.5 eV and 719.6 eV are characteristic of unoxidized Fe in the sample, while the peaks at 711.2 eV (Fe 2p3/2) and 724.6 eV (Fe 2p1/2) correspond to Fe3+, as reported in the literature.49,50 The peaks of the O 1s (Fig. 2d) XPS spectrum at 530.3, 531.2 and 532.2 eV are in accordance with Fe–O (O2−), OH− and H2O.51 The peak of Mo3d (Fig. 2e) can be fitted by three peaks corresponding to 228.6 eV (Mo 3d5/2), 231.7 (Mo 3d3/2) and 226.1 eV (Mo–S bond).52 This suggests that elemental Mo exists in the composite as Mo4+. Additionally, three high-resolution spectral peaks of S 2p with shake-up satellites were detected, which are characteristic of S 2p3/2 (161.5 eV) and S 2p1/2 (162.6 eV), while another peak at 164.1 eV indicated the presence of S–C bonds. These results are close to those reported in previous literature.53–55
C–O bonds, respectively (Fig. 2b).47,48 Fig. 2c shows the high-resolution XPS spectrum of Fe 2p. The Fe 2p3/2 and Fe 2p1/2 peak positions at 707.5 eV and 719.6 eV are characteristic of unoxidized Fe in the sample, while the peaks at 711.2 eV (Fe 2p3/2) and 724.6 eV (Fe 2p1/2) correspond to Fe3+, as reported in the literature.49,50 The peaks of the O 1s (Fig. 2d) XPS spectrum at 530.3, 531.2 and 532.2 eV are in accordance with Fe–O (O2−), OH− and H2O.51 The peak of Mo3d (Fig. 2e) can be fitted by three peaks corresponding to 228.6 eV (Mo 3d5/2), 231.7 (Mo 3d3/2) and 226.1 eV (Mo–S bond).52 This suggests that elemental Mo exists in the composite as Mo4+. Additionally, three high-resolution spectral peaks of S 2p with shake-up satellites were detected, which are characteristic of S 2p3/2 (161.5 eV) and S 2p1/2 (162.6 eV), while another peak at 164.1 eV indicated the presence of S–C bonds. These results are close to those reported in previous literature.53–55
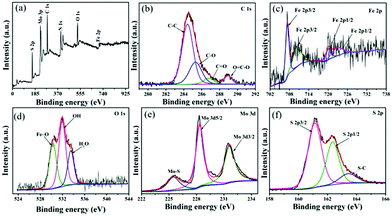 | ||
| Fig. 2 XPS spectra of MoS2/Fe3O4/rGO (1 wt%): (a) the full survey; (b) C 1s (c) Fe 2p (d) O 1s (e) Mo 3d; (f) S 2p. | ||
The morphology and crystal structures of the as-prepared samples were investigated by SEM and TEM (Fig. 3). Fig. 3a and b are typical low- and high-magnification SEM images of the as-prepared samples. The MoS2/Fe3O4/rGO (0.1 wt%) material exhibited hierarchical flower-like microspheres with mean diameters in the range 1–1.5 μm. Further observation showed that the as-prepared sample still had uniformly dispersed flower-like microspheres composed of intercrossed nanosheets with thicknesses of 2–5 nm. A TEM image was made to confirm the morphology and microstructure of the MoS2/Fe3O4/rGO (0.1 wt%) composites. As shown in Fig. 3c, the MoS2/Fe3O4/rGO (0.1 wt%) composites were highly uniform flower-like spheres with mean diameters of 1–1.5 μm, which agrees well with that observed by SEM. To investigate the crystalline structure, HRTEM images were recorded (Fig. 3d). The HRTEM image recorded on the edge of a single MoS2/Fe3O4/rGO (0.1 wt%) nanoflower shows lattice distances of 0.62 and 0.26 nm, which corresponds to the d-spacing of (002) and (100) crystal planes. In addition, the selected area electron diffraction (SAED) pattern indicates the polycrystalline nature of the MoS2/Fe3O4/rGO material.
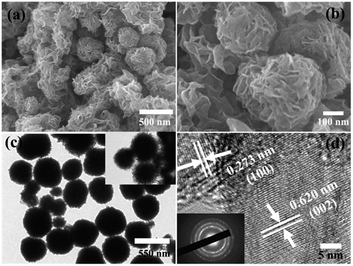 | ||
| Fig. 3 SEM images (a and b), and TEM images low and high magnifications (c and d) of MoS2/Fe3O4/rGO (0.1 wt%). | ||
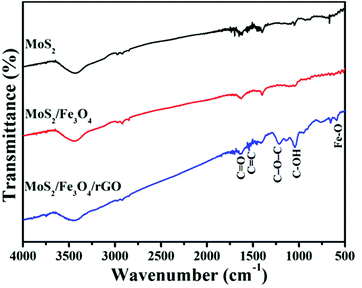
The FTIR spectra of as-prepared photocatalyst samples in the range of 500–4000 cm−1 are shown in Fig. 4. It can be clearly seen that the OH groups were observed and located broadly around 3400 cm−1 in the FTIR spectra, which can be attributed to the stretching and bending vibrations of water molecules adsorbed on the samples. Compare with MoS2 and MoS2/Fe3O4, the characteristic peaks at 1642 cm−1 and 1566 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of stretching COOH groups and aromatic C
O of stretching COOH groups and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively, while the absorption peaks at 1219 cm−1 and 1044 cm−1 accord with epoxy groups (C–O–C group vibrations) and alkoxy groups (C–OH), respectively.56 In addition, the intensive band at 588 cm−1 in the MoS2/Fe3O4/rGO (0.1 wt%) spectrum can also be attributed to the Fe–O stretching vibration mode.57 All of these results indicate the presence of rGO in the composites.
C bonds, respectively, while the absorption peaks at 1219 cm−1 and 1044 cm−1 accord with epoxy groups (C–O–C group vibrations) and alkoxy groups (C–OH), respectively.56 In addition, the intensive band at 588 cm−1 in the MoS2/Fe3O4/rGO (0.1 wt%) spectrum can also be attributed to the Fe–O stretching vibration mode.57 All of these results indicate the presence of rGO in the composites.
To investigate the physicochemical properties of different samples, the as-prepared heterogeneous samples were subject to N2 adsorption–desorption analysis. Fig. 5 shows the N2 adsorption/desorption isotherm and the pore-size distribution (inset) of as-prepared samples, which reveals that all of the samples were type-IV isotherms with an H3-type hysteresis loop, and exhibited mesoporous distribution. Compared to MoS2 and MoS2/Fe3O4, the MoS2/Fe3O4/rGO (0.1 wt%) sample had a specific surface area of 72.23 m2 g−1 and a pore volume of 0.185 cm3 g−1, which are in agreement with the SEM and TEM results (Table 1). In summary, the high specific surface area provides more active sites, which may lead to the enhancement of photocatalytic performance.
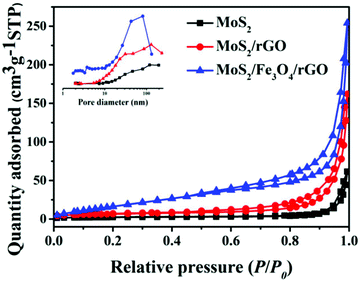 | ||
| Fig. 5 N2 adsorption–desorption isotherms and pore-size distribution curves of the obtained samples. | ||
| Sample | Average crystal size [nm] (standard deviation) | SBET [m2 g−1] | Pore volume [cm3 g−1] |
|---|---|---|---|
| MoS2 | 18.22 | 8.16 | 0.04 |
| MoS2/Fe3O4 | 21.32 | 22.59 | 0.12 |
| MoS2/Fe3O4/rGO | 10.27 | 72.23 | 0.185 |
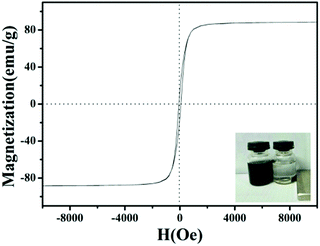
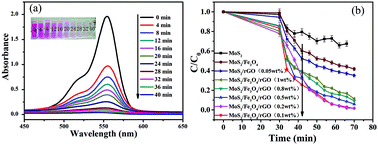
The magnetic properties of MoS2/Fe3O4/rGO (0.1 wt%) were analyzed at room temperature, as shown in Fig. 6. The saturation magnetization (Ms) value of 87 emu g−1 demonstrates its excellent magnetic properties, allowing it to be easily separated from the treated samples by an external magnet within 10 min. The time-dependent degradation intensity change of MoS2/Fe3O4/rGO (0.1 wt%) is shown in Fig. 7a. It can be seen that the main absorption peak of MoS2/Fe3O4/rGO (0.1 wt%) located at 554 nm rapidly diminished in the presence of H2O2 under visible-light irradiation for 40 min. Notably, this result was also consistent with a color change of the sample, from pink to colorless, as shown in the inset in Fig. 7a. In order to investigate the photocatalytic behavior of MoS2/Fe3O4/rGO (0.1 wt%), a series of comparative experiments were carried out, as shown in Fig. 7b and S1.† It shows that before visible-light irradiation, the mixed suspension containing the catalyst and RhB was continually stirred in darkness for 30 min to ensure that the rhodamine B was attached to the surface of the catalyst until reaching adsorption equilibrium. Due to its variable surface areas, the adsorption capacity of RhB onto the catalyst surface (in darkness) ranged from 2.7% to 22.1% of C/C0. Compared with MoS2/Fe3O4 and pure MoS2, the MoS2/Fe3O4/rGO (0.1 wt%) material exhibited the best photocatalytic activity in terms of the photo-degradation of rhodamine (B) in the presence of H2O2 under visible-light irradiation. This was almost four times higher than that of MoS2 and double that of MoS2/Fe3O4. These observations demonstrate that rGO plays an important role in enhancing catalytic activity because it can provide a larger specific surface area, abundant active sites, high visible-light absorption, and improves the separation efficiency of photogenerated electron–hole pairs. However, the catalyst degradation rate decreased with increases in the concentration of graphene from 0.2 wt% to 1 wt%, which was attributed to its shielding of light absorption and decreases of active sites.
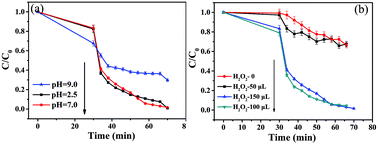
In order to investigate the effects of pH and H2O2 volume on the degradation of RhB in the photo-Fenton reaction, a series of comparative experiments were performed under visible-light irradiation while holding other conditions constant. Fig. 8a shows the effect of pH on RhB degradation resulting from experiments conducted in acidic, neutral and alkaline media. The pH values were adjusted by adding NaOH (1 mol L−1) and HCl (1 mol L−1) to the solutions. As the pH of the solutions increased from 2.5 to 7, it was easily found that the RhB degradation efficiency reached almost 100% in the presence of H2O2 under visible-light irradiation. It is well known that the traditional homogenous Fenton process suffers from secondary treatment, catalyst loss in effluent, and the requirement for low pH (<3.0) conditions; however, the photocatalytic rate did not decrease when using the neutral media in our experiment. This suggests that this photocatalytic material may have potential practical use for industrial wastewater treatment systems. In contrast, the degradation rate decreased as the pH of the solution increased to 9, which indicates that ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(IV)
Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species or high-valent iron species of
O species or high-valent iron species of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeIV
FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were formed, which further reduced the degradation activity of the reaction at alkaline pH values.58 The results of these experiments indicate that pH has a significant influence on the photocatalytic degradation of RhB.
O were formed, which further reduced the degradation activity of the reaction at alkaline pH values.58 The results of these experiments indicate that pH has a significant influence on the photocatalytic degradation of RhB.
In addition, to further explore whether H2O2 can affect the efficiency of photocatalytic degradation, the experiment was performed with different volumes of H2O2 at pH 7 under visible-light irradiation (Fig. 8b). With the addition of various volumes of H2O2 (30%), from 0 to 150 μL, into the solution, the degradation rates were calculated to be 32.5%, 33.6%, 99.8% and 95.6%, respectively. The degradation rate increased with enhancing volume of H2O2. Therefore, introduction of H2O2 is credited with enhancing the RhB degradation rate, which produced more and more ·OH which then reacted with catalyst. In addition, it can be seen that the photocatalytic activity slightly decreased with H2O2 (30%) additions of up to 150 μL into the solution, which was possibly due to the quenching of ·OH by competing reactions between H2O2 and ·OOH.59
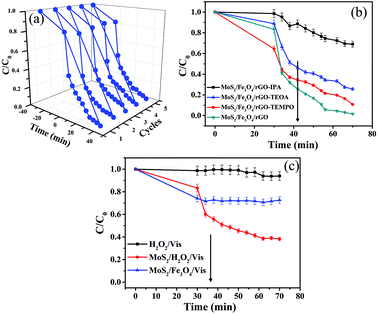
To consider the practical application of these photocatalysts, recycling tests were conducted under visible-light irradiation, as shown in Fig. 9a. Under the same conditions as the photo Fenton-like reaction, an RhB degradation rate of >90% was observed after five successive cycles using the same experimental conditions. This indicates that the MoS2/Fe3O4/rGO (0.1 wt%) catalyst has high stability and reusability. Furthermore, the catalyst can be easily separated for reuse with an external magnet, which is especially important for practical applications such as industrial wastewater treatment. In order to confirm this photo Fenton-like reaction, free-radical and hole-trapping experiments were conducted in the presence of H2O2 (Fig. 9b). The degradation efficiency of RhB decreased to 31.02% when IPA was added, implying that ·OH species have an obvious role in the degradation of RhB. Compared with IPA, a slight decrease in the degradation rate was observed when TEOA and 4-hydroxy-TEMPO were added to the photocatalytic reaction system. Hence, it was also apparent that the photocatalytic activity of MoS2/Fe3O4/rGO (0.1 wt%) was promoted, which was ascribed to the elimination/active decomposition of H2O2, namely, by the generation of active ·OH radicals. It was identified that ·OH radicals play a major role in the photo Fenton-like reaction. To further confirm the effects of the photo Fenton-like reaction, experiments were performed where RhB was degraded under various conditions (Fig. 9c). Notably, it is believed that both H2O2 and Fe3O4 are crucial for enhancing the degradation of RhB in photo Fenton-like reactions.
Next, a possible photocatalytic mechanism is proposed for understanding photo Fenton-like reactions in the presence of H2O2 under visible-light irradiation. Firstly, the degradation products are quickly absorbed onto the catalyst due to graphene having a high surface area and active adsorption sites. Meanwhile, MoS2/Fe3O4/rGO (0.1 wt%) composites are photo-excitable and generate electron–hole pairs. The photo-generated e− rapidly react with Fe3+ to form Fe2+. Secondly, the photogenerated electrons can be directly trapped by O2/H2O2 to form·O2−/·OH. Simultaneously, Fe2+ can react quickly with H2O2 to generate ·OH species for organic degradation, forming the photo-Fenton system. Likewise, photogenerated holes can directly react with RhB. As a result, ·OH/h+/·O2− reactive species would be used for the photodegradation of RhB via a photo Fenton-like system (Fig. 10).
| MoS2/Fe3O4/rGO + hυ → e−+ h+ | (1) |
| Fe3+ + e− → Fe2+ | (2) |
| H2O2+ Fe2+ → Fe3+ + ·OH + OH− | (3) |
| ·OH + RhB = H2O + CO2 | (4) |
Conclusions
In summary, this is the first report of a facile and cost-effective approach to the large-scale synthesis of ternary photocatalysts made from MoS2/Fe3O4/rGO (0.1 wt%) under simple hydrothermal conditions. Their superior performance is attributed to a photo Fenton-like system. We found that rGO played an important role in the experiments, as it suppressed the recombination of photogenerated electron–hole pairs and enhanced the absorption capability of visible-light and organic dyes. Additionally, hydroxyl radicals (·OH) and photogenerated holes exhibited strong abilities to degrade organic contaminants under the photo Fenton-like system. Finally, the photocatalyst can be effectively separated for reuse by simply applying an external magnetic field, which is especially important for industrial wastewater treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Science Technology Development Planning of Jilin Province (20170520069JH) and Education Department Project of Jilin Province (JJKH20180556KJ).References
- E. M. Dias and C. Petit, J. Mater. Chem. A, 2015, 3, 22484–22506 RSC.
- C. Cao, L. Xiao, C. Chen and Q. Cao, Appl. Surf. Sci., 2015, 333, 110–118 CrossRef CAS.
- L. Liang, Q. Zhu, T. Wang, F. Wang, J. Ma, L. Jing and J. Sun, Microporous Mesoporous Mater., 2014, 197, 221–228 CrossRef CAS.
- J. S. Kim, B. Kim, H. Kim and K. Kang, Adv. Energy Mater., 2018, 8, 1702774 CrossRef.
- J. Y. Choe, J. Y. Byun and S. H. Kim, Appl. Catal., B, 2018, 233, 272–280 CrossRef.
- G. X. Zong, H. Chen, R. J. Qu, C. H. Wang and N. Y. Ji, J. Hazard. Mater., 2011, 186, 614–621 CrossRef CAS PubMed.
- D. F. Xu, B. Cheng, S. W. Cao and J. G. Yu, Appl. Catal., B, 2015, 164, 380–388 CrossRef CAS.
- P. F. Xia, B. C. Zhu, B. Cheng, J. G. Yu and J. S. Xu, ACS Sustainable Chem. Eng., 2018, 6, 965–973 CrossRef CAS.
- X. Liu, H. Chen, C. H. Wang, R. J. Qu, C. N. Ji, C. M. Sun and Y. Zhang, J. Hazard. Mater., 2010, 175, 1014–1021 CrossRef CAS PubMed.
- S. W. Cao, F. Tao, Y. Tang, Y. T. Li and J. G. Yu, Chem. Soc. Rev., 2016, 45, 4747–4765 RSC.
- M. A. Oturan and J. J. Aaron, A Review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2577–2641 CrossRef CAS.
- A. M. Khan, A. Mehmood, M. Sayed, M. F. Nazar, B. Ismail, R. A. Khan, H. Ullah, H. M. Abdur Rehman, A. Y. Khan and A. R. Khan, J. Mol. Liq., 2017, 236, 395–403 CrossRef CAS.
- L. Clarizia, D. Russo, I. Di Somma, R. Marotta and R. Andreozzi, Appl. Catal., B, 2017, 209, 358–371 CrossRef CAS.
- X. X. Hua, R. Li, S. Y. Zhao and Y. J. Xing, Appl. Surf. Sci., 2017, 396, 1393–1402 CrossRef.
- X. J. Guo, K. B. Wang, D. Li and J. B. Qin, Appl. Surf. Sci., 2017, 420, 792–801 CrossRef CAS.
- Y. Y. Liu, W. Jin, Y. P. Zhao, G. S. Zhang and W. Zhang, Appl. Catal., B, 2017, 206, 642–652 CrossRef CAS.
- H. F. Wang, Y. G. Xu, L. Q. Jing, S. Q. Huang, Y. Zhao, M. Q. He, H. Xu and H. M. Li, J. Alloys Compd., 2017, 710, 510–518 CrossRef CAS.
- G. Ayoub and A. Ghauch, Chem. Eng. J., 2014, 256, 280–292 CrossRef CAS.
- A. Ghauch, G. Ayoub and S. Naim, Chem. Eng. J., 2013, 228, 1168–1181 CrossRef CAS.
- A. Ghauch and H. Baydoun, Chem. Eng. J., 2011, 172, 18–27 CrossRef CAS.
- W. Z. Fu, X. W. Xu, W. B. Wang, J. F. Shen and M. X. Ye, ACS Sustainable Chem. Eng., 2018, 6, 8935–8944 CrossRef CAS.
- M. Li, D. G. Wang, J. H. Li, Z. D. Pan, H. J. Ma, Y. X. Jiang, Z. J. Tian and A. H. Lu, Chin. J. Catal., 2017, 38, 597–606 CrossRef CAS.
- J. Yan, Z. G. Chen, H. Y. Ji, Z. Liu, X. Wang, Y. G. Xu, X. J. She, L. Y. Huang, L. Xu, H. Xu and H. M. Li, Chem.–Eur. J., 2016, 22, 4764–4773 CrossRef CAS PubMed.
- M. Park, Y. J. Park, X. Chen, Y. K. Park, M. S. Kim and J. H. Ahn, Adv. Mater., 2016, 28, 2556–2562 CrossRef CAS PubMed.
- S. C. Zhang, R. R. Hu, P. Dai, X. X. Yu, Z. L. Ding, M. Z. Wu, G. Li, Y. Q. Ma and C. J. Tu, Appl. Surf. Sci., 2017, 396, 994–999 CrossRef CAS.
- W. Yin, L. Yan, J. Yu, G. Tian, L. Zhou, X. Zheng, X. Zhang, Y. Yong, J. Li, Z. Gu and Y. Zhao, ACS Nano, 2014, 8, 6922–6933 CrossRef CAS PubMed.
- Y. M. Liu, Y. F. Cao, H. Lv, S. Li and H. Zhang, Mater. Lett., 2017, 188, 99–102 CrossRef CAS.
- W. Zhou, Z. Yin, Y. Du, X. Huang, Z. Zeng, Z. Fan, H. Liu, J. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef CAS PubMed.
- D. A. Reddy, J. Choi, S. Lee, Y. J. Kim, S. Hong, D. P. Kumara and T. K. Kim, Catal. Sci. Technol., 2016, 6, 6197–6206 RSC.
- Y. Min, G. He, Q. Xu and Y. Chen, J. Mater. Chem. A, 2014, 2, 2578–2584 RSC.
- C. Zhao, X. Wang, J. Kong, J. M. Ang, P. S. Lee, Z. Liu and X. Lu, ACS Appl. Mater. Interfaces, 2016, 8, 2372–2379 CrossRef CAS PubMed.
- H. Zhao, H. Zeng, Y. Wu, S. Zhang, B. Li and Y. Huang, J. Mater. Chem. A, 2015, 3, 10466–10470 RSC.
- J. Yan, Z. G. Chen, H. Y. Ji, Z. Liu, X. Wang, Y. G. Xu, X. J. She, L. Y. Huang, L. Xu, H. Xu and H. M. Li, Chem.–Eur. J., 2016, 22, 4764–4773 CrossRef CAS PubMed.
- Y. H. Song, Y. C. Lei, H. Xu, C. Wang, J. Yan, H. Z. Zhao, Y. G. Xu, J. X. Xia, S. Yin and H. M. Li, Dalton Trans., 2015, 44, 3057–3066 RSC.
- D. Z. Zhang, C. X. Jiang, P. Li and Y. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 6462–6471 CrossRef CAS PubMed.
- C. Y. Wang, L. Y. Zhang, S. N. Li, M. J. Zhang, T. T. Wang, L. Li, C. G. Wang and Z. G. Su, J. Mater. Chem. B, 2016, 4, 5809–5813 RSC.
- D. Lin, X. Feng, Y. N. Wu, B. D. Ding, T. Lu, Y. B. Liu, X. B. Chen, D. Chen and C. H. Yang, Appl. Surf. Sci., 2018, 436, 140–146 CrossRef.
- K. Y. Li, Y. Q. Zhao, M. J. Janik, C. S. Song and X. W. Guo, Appl. Surf. Sci., 2017, 396, 1383–1392 CrossRef CAS.
- S. Valizadeh, M. H. Rasoulifard and M. S. SeyedDorraji, Appl. Surf. Sci., 2014, 319, 358–366 CrossRef CAS.
- F. Chai, K. Li, C. Song and X. Guo, J. Colloid Interface Sci., 2016, 475, 119–125 CrossRef CAS PubMed.
- D. Du, W. Shi, L. Wang and J. Zhang, Appl. Catal., B, 2017, 200, 484–492 CrossRef CAS.
- L. L. Chen, L. Li, T. T. Wang, L. Y. Zhang, S. X. Xing, C. G. Wang and Z. M. Su, Nanoscale, 2014, 6, 6603–6608 RSC.
- B. Q. Liu, Q. Zhang, Z. S. Jin, L. Y. Zhang, L. Li, Z. G. Gao, C. G. Wang, H. M. Xie and Z. M. Su, Adv. Energy Mater., 2017, 1702347 Search PubMed.
- C. L. Han, G. R. Huang, D. J. Zhu and K. H. Hu, Mater. Chem. Phys., 2017, 200, 16–22 CrossRef CAS.
- S. Sahar, A. Zeb, Y. N. Liu, N. Ullah and A. W. Xu, Chin. J. Catal., 2017, 38, 2110–2119 CrossRef CAS.
- H. Deng, X. Li, Q. Peng, X. Wang, J. Chen and D. Y. Li, Angew. Chem., Int. Ed., 2005, 117, 2842–2845 CrossRef.
- E. Vishnyakova, B. E. Brinson, L. B. Alemany, M. Verma and W. E. Billups, Chem.–Eur. J., 2016, 22, 1452–1460 CrossRef CAS PubMed.
- Y. Li, H. Zhang, Y. Wang, P. Liu, H. Yang, X. Yao, D. Wang, Z. Tang and H. Zhao, Energy Environ. Sci., 2014, 7, 3720–3726 RSC.
- S. W. Li, Y. C. Wang, S. J. Peng, L. J. Zhang, A. M. Al-Enizi, H. Zhang, X. H. Sun and G. F. Zheng, Adv. Energy Mater., 2016, 6, 1501661 CrossRef.
- R. H. Huang, Z. Q. Fang, X. M. Yan and W. Cheng, Chem. Eng. J., 2012, 197, 242–249 CrossRef CAS.
- K. Fan, Y. F. Ji, H. Y. Zou, J. F. Zhang, B. C. Zhu, H. Chen, Q. Daniel, Y. Luo, J. G. Yu and L. C. Sun, Angew. Chem., Int. Ed., 2017, 56, 1–6 CrossRef.
- H. B. Geng, J. Yang, Z. F. Dai, Y. Zhang, Y. Zheng, H. Yu, H. W. Wang, Z. Z. Luo, Y. Y. Guo, Y. F. Zhang, H. S. Fan, X. L. Wu, J. W. Zheng, Y. G. Yang, Q. Y. Yan and H. W. Gu, Small, 2017, 1603490 CrossRef PubMed.
- B. B. Wang, Y. Zhang, J. Zhang, R. Y. Xia, Y. L. Chu, J. C. Zhou, X. W. Yang and J. Huang, ACS Appl. Mater. Interfaces, 2017, 9, 12907–12913 CrossRef CAS PubMed.
- M. Choi, S. K. Koppala, D. Yoon, J. H. wang, S. M. Kim and J. Kim, J. Power Sources, 2016, 309, 202–211 CrossRef CAS.
- W. S. V. Lee, E. Peng, T. A. J. Loh, X. Huang and J. M. Xue, Nanoscale, 2016, 8, 8042–8047 RSC.
- J. F. Shen, Y. Z. Hu, M. Shi, N. Li, H. W. Ma and M. X. Ye, J. Phys. Chem. C, 2010, 114, 1498–1503 CrossRef CAS.
- X. Yang, X. Zhang, Y. Ma, Y. Huang, Y. Wang and Y. Chen, J. Mater. Chem., 2009, 19, 2710 RSC.
- L. Ren, S. Y. Lu, J. Z. Fang, Y. Wu, D. Z. Chen, L. Y. Huang, Y. F. Chen, C. Cheng, Y. Liang and Z. Q. Fang, Catal. Today, 2017, 281, 656–661 CrossRef CAS.
- F. X. Wang, Y. L. Chen, R. S. Zhu and J. M. Sun, Dalton Trans., 2017, 47, 11306–11317 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: The k values of different samples in the photocatalytic process. See DOI: 10.1039/c8ra06537c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2018 |