Open Access Article
Open Access ArticlePhotoluminescence properties of novel Ba2Lu5B5O17:Eu3+ red emitting phosphors with high color purity for near-UV excited white light emitting diodes
G. Annadurai,
Balaji Devakumar,
Heng Guo,
Bin Li,
Liangling Sun and
Xiaoyong Huang *
*
Key Lab of Advanced Transducers and Intelligent Control System, Ministry of Education and Shanxi Province, College of Physics and Optoelectronics, Taiyuan University of Technology, Taiyuan 030024, P. R. China. E-mail: huangxy04@126.com
First published on 28th August 2018
Abstract
A series of new red-emitting Ba2Lu4.98−xEuxLa0.02B5O17 (0.1 ≤ x ≤ 1.0) phosphors were synthesized via the high-temperature solid-state reaction method. The phase formation of the as-synthesized Ba2Lu4.48Eu0.5La0.02B5O17 phosphor was confirmed by powder X-ray diffraction analysis. It was found that La3+ doping resulted in the reduction of LuBO3 impurities and thus pure phase Ba2Lu5B5O17 was realised. The morphology of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors was studied by field emission scanning electron microscopy (FE-SEM). As a function of Eu3+ concentration the photoluminescence spectra and decay lifetimes were investigated in detail. Under excitation at 396 nm, a dominant red emission peak located at 616 nm (5D0 → 7F2) indicated that Eu3+ ions mainly occupied low symmetry sites with a non-inversion center in Ba2Lu4.48Eu0.5La0.02B5O17. The optimal Eu3+ ion concentration was found to be x = 0.5 and the critical distance of Eu3+ was determined to be 6.55 Å. In addition, the concentration quenching takes place via dipole–dipole interactions. The phosphors exhibited good CIE (Commission International de I'Eclairage) color coordinates (x = 0.643, y = 0.356) situated in the red region and a high color purity of 97.8%. Furthermore, the internal quantum efficiency and the thermal stability of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors were also investigated systematically. The results suggest that Ba2Lu4.48Eu0.5La0.02B5O17 may be a potential red phosphor for white light-emitting diodes.
1. Introduction
The next generation light source of solid state lighting (SSL) technology would be the phosphor-converted white light-emitting diodes (pc-WLEDs) which might solve the critical issues of energy saving and reduced carbon dioxide (CO2) emission. Compared to conventional lighting sources such as incandescent and fluorescent lamps, WLEDs have attracted great attention owing to their numerous advantages such as small volume, low power consumption, high brightness, better flexible design, reliability, long persistence and eco-friendly nature.1–9 There exists a deficiency of red components in the commercially available WLEDs composed of blue emitting InGaN LED chips coated with a yellow Y3Al5O12:Ce3+ phosphor.10,11 Nevertheless, some factors that are involved in reducing the quality of white light include a low color rendering index (CRI), high correlated color temperature (CCT), thermal quenching, blue-halo effect and current dependence of chromaticity.10,12–14 An effective solution to overcome these disadvantages would be to attain a high-quality white light by combining a near-ultraviolet (near-UV) LED chip with three primary (red, green and blue) colored phosphors.13,15–17The efficiency of commercial green (ZnS:Cu+,Al3+) and blue (BaMgAl10O17:Eu2+) phosphors is much higher than that of commercial red (CaS:Eu2+, Y2O2S:Eu3+ and Y2O3:Eu3+) phosphors.15,18,19 However, the sulfide and oxysulfide based red phosphors have poor chemical stability and high toxicity, and decompose at high temperature.18,20 The other commercial red phosphors like Eu2+ activated nitride compounds (CaAlSiN3 and Sr2Si5N8) have several drawbacks such as complex preparations and high cost.18 Henceforth, the current research focus is to explore novel red phosphors that can be effectively excited by near-UV with improved stability and most importantly with enhanced efficiency.
In addition, it is also well known that Eu3+ ion serves as an effective activator in various inorganic host lattices, because they can give rise to bright red emission due to the 4f–4f transitions. Generally, Eu3+ emission originates from the transitions of 5D0 → 7FJ (J = 0, 1, 2, 3, and 4).21–23 Presently, many red phosphors such as rare-earth ions (Eu3+) activated oxides have been investigated, including tungstates, molybdates, silicates, borates, phosphates and vanadates. Among these, a number of researches have been carried out on the investigation of borates, because these compounds are found to express high transmittance in the UV region, large birefringence, and nonlinear optical properties.24,25 As a vital factor for inorganic phosphors, the host lattice must have good chemical and thermal stability.26 Borate compounds have been explored extensively to serve as distinctive luminescent host lattices for phosphors owing to their high chemical stability, large bandgap, high optical damage threshold, high luminescence efficiency, low synthesis temperature.27 Borate-based phosphors are found to have numerous applications in many fields such as optical data storage, flat panel display devices, lasers and nonlinear optics.24 In recent years, various research groups have reported Eu3+ ions activated borate compounds such as GdB5O9:Eu3+, Sr2ScLi(B2O5):Eu3+, Sr3Bi2(BO3)4:Eu3+, Ca3(BO3)2:Eu3+.28–31
Recently, the efficient blue (Ba2Lu5B5O17:Ce3+) and green (Ba2Lu5B5O17:Ce3+, Tb3+) phosphors was proposed by Xiao et al.32,33 In our present work, we report a novel Eu3+ ion activated Ba2Lu5B5O17 red phosphors synthesized by solid-state reaction. A small amount of La3+ ion doped into Ba2Lu5B5O17 enabled to reduce the LuBO3 impurities and thus realize pure phase of Ba2Lu5B5O17. To our knowledge, there is no reported literature available for the detailed photoluminescence properties of Ba2Lu4.98−xEuxLa0.02B5O17. The luminescence properties of excitation and emission spectra and concentration quenching mechanism were investigated in detail. The decay curves have also been discussed. Further, the activation energy for the thermal quenching was determined from the temperature-dependent luminescence intensities. The obtained results suggest that the as-synthesized Ba2Lu4.48Eu0.5La0.02B5O17 would serve as a novel red emitting phosphor with potential application for WLEDs.
2. Experimental
2.1. Materials and synthesis
The Ba2Lu4.98−xEuxLa0.02B5O17 (x = 0.1, 0.3, 0.5, 0.6, 0.7, 0.9, and 1.0) phosphors were prepared via high-temperature solid-state reaction. High purity of raw materials were BaCO3 (analytical reagent; A.R.), Lu2O3 (99.99%), La2O3 (99.99%), H3BO3 (A.R.) and Eu2O3 (99.99%). The stoichiometric amounts of starting materials were thoroughly mixed in an agate mortar. Subsequently, the homogeneous mixture was pre-heated at 470 °C for 4 h. They were then reground and sintered at 1190 °C for 12 h in an air atmosphere. Finally, the as-synthesized samples were cooled down to room temperature naturally and ground again into a fine powder for further characterization.2.2. Characterization
The phase formations of as-synthesized phosphors were analyzed by powder X-ray diffraction (XRD) measurements using Bruker D8 advance powder diffractometer. The diffraction patterns were scanned within the range of 15° ≤ 2θ ≤ 65° operating at 40 kV and 40 mA (step size 0.02°) with CuKα radiation (λ = 1.5406 Å). The morphology and particle size of the as-synthesized samples were characterized by field-emission scanning electron microscopy (FE-SEM; MAIA3 TESCAN). The photoluminescence (PL), PL excitation (PLE) spectra as well as PL decay curves of phosphors were recorded on an Edinburgh FS5 spectrofluorometer equipped with both a continuous-wavelength and a pulsed (150 W) xenon lamp. The internal quantum efficiency (IQE) was also obtained using Edinburgh FS5 spectrofluorometer with an integrating sphere attachment. The PL spectra at different temperatures (303–483 K) were recorded using the same instrument equipped with a temperature controller.3. Results and discussion
Phase purity of as-synthesized phosphors was analyzed using powder X-ray diffraction. Fig. 1 illustrates the XRD patterns of Ba2Lu5B5O17, Ba2Lu4.98La0.02B5O17 and Ba2Lu4.98−xEuxLa0.02B5O17 (x = 0.1, 0.5 and 1.0) phosphors. In previous studies, Xiao et al. reported that it was difficult to obtain pure phase of Ba2Lu5B5O17 due to the smaller ionic radius of Lu3+ compared to Y3+.32,33 Furthermore, in order to stabilize the crystal structure of Ba2Lu5B5O17, La2O3 was substituted for Lu3+ owing to its large ionic radius. From the XRD profiles, it can be seen that the LuBO3 impurities were reduced after a small amount of La2O3 substituting for Lu2O3.32,33 Apparently, all the diffraction peaks of as-synthesized samples are consistent with the previously reported data of Ba2Lu5B5O17.32,33 The crystal structure and the cation coordination environments were investigated by Xiao et al.32,33 In addition, the previously reported results reveal the orthorhombic structure of Ba2Lu5B5O17 with space group Pbcn(60); cell parameters a = 17.2144 Å, b = 6.5990 Å, c = 12.9587 Å; and cell volume (V) = 1472.09 Å3.33 | ||
| Fig. 1 XRD profiles of the Ba2Lu5B5O17, Ba2Lu4.98La0.02B5O17 and Ba2Lu4.98−xEuxLa0.02B5O17 (x = 0.1, 0.5 and 1.0) phosphors. | ||
Fig. 2(a) demonstrates the FE-SEM micrograph of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors prepared by solid state reaction method. In general, the synthesized samples by high temperature solid state reaction exhibit some degree of particle agglomeration with different size distribution.34 As can be seen from the Fig. 2(a), the as-obtained micrograph shows that the shape of the sample consists of an irregular morphology, which was ascribed to the fundamental characteristics of high temperature solid state reaction method.35,36 The particles are agglomerated due to the sustained sintering time and intermediate grindings. The above results clearly suggest that the Ba2Lu4.48Eu0.5La0.02B5O17 phosphors could be useful for potential application in WLEDs. To confirm the homogeneity of elements in the compounds, we carried out elemental mapping. The elemental area profiles with different elements in various colors were shown in Fig. 2(b–h). The elemental mapping results of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors disclosed that Ba, Lu, La, B, O and Eu elements were homogeneously distributed throughout the entire particles.
 | ||
| Fig. 2 (a) SEM micrograph of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors. (b–h) Elemental mapping profiles of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors. | ||
Fig. 3(a) shows the PLE and PL spectra of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors at room temperature. It can be easily seen that the PLE spectrum monitored at 616 nm comprised of a broad band ranging from ∼200 to 300 nm and a series of sharp peaks located in the range of ∼300–550 nm. The broad band centered at 282 nm can be assigned to the charge transfer band (CTB) of O2− → Eu3+.15,37 The electron transfer from the 2p6 orbital of O2− ions to the 4f orbital of Eu3+ ions.38,39 The sharp excitation peak belonged to the intra-configurational 4f–4f electronic transitions of Eu3+ ions, in which the peaks situated at 321, 364, 382, 396, 411, 467, 524 nm correspond to the transitions from 7F0 to 5H6, 5D4, 5L7, 5L6, 5D3, 5D2, and 5D1 levels, respectively.15,40–42 The dominant peak centered at 396 nm suggests that the phosphor can be efficiently excited by near-UV LED chip.15
Fig. 3(a) depicts the PL spectrum of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors (λex = 396 nm). A series of sharp peaks situated at 574, 588, 616, 655 and 707 nm correspond to the transitions from the 5D0 excited state to the 7FJ (J = 0, 1, 2, 3 and 4) ground states of Eu3+ ions.15,40 Generally, when the Eu3+ ions are situated at the crystallographic sites with inversion symmetry, the magnetic dipole (MD) 5D0 → 7F1 transition would be dominant; while Eu3+ ions occupy in a site without inversion symmetry, the electric dipole (ED) 5D0 → 7F2 transition will be dominant.43 From this spectrum, the PL intensity of (ED) 5D0 → 7F2 transition at around 616 nm was stronger than that of the (MD) 5D0 → 7F1 transition at around 588 nm. The above result suggested that the local symmetry of Eu3+ site belonged to the non-centrosymmetric site in Ba2Lu4.48Eu0.5La0.02B5O17 host lattice with beneficial color purity.44
The PL spectra of Ba2Lu4.98−xEuxLa0.02B5O17 (x = 0.1, 0.3, 0.5, 0.6, 0.7, 0.9 and 1.0) phosphors with different Eu3+ concentration under 396 nm excitation were shown in Fig. 3(b). All the PL spectra profiles and peak positions were very similar except for their PL intensity. The dependence of Eu3+ concentration on PL intensity of 5D0 → 7F2 (616 nm) transition was shown in the inset of Fig. 3(b). It can be clearly observed that the PL intensity enhanced with increasing the Eu3+ concentration until it reached a maximum at x = 0.5. Herein, the optimum concentration of Eu3+ was determined to be x = 0.5 in Ba2Lu4.98−xEuxLa0.02B5O17 host. Beyond the optimum concentration, the PL intensity dramatically decreased due to the concentration quenching effect.
The concentration quenching mechanism is often attributed to energy migration between Eu3+ ions. In concentration quenching phenomenon, the critical distance Rc among activator is a significant parameter. When Rc is less than 5 Å, the concentration quenching is dealt to be an exchange interaction. In other cases, the Rc value is greater than 5 Å, electric multipolar interaction will be the dominant mechanism for the concentration quenching. The value of Rc can be estimated via the following equation proposed by Blasse:45
 | (1) |
 | (2) |
 represents the emission intensity per activator concentration, k and β are constants for the given host at the same excitation conditions; θ stands for the electric multipolar interaction, when the value of θ is 3, 6, 8 or 10 corresponding to the exchange interaction, dipole–dipole (d–d), dipole–quadrupole (d–q) or quadrupole–quadrupole (q–q) interactions, respectively.47 The eqn (2) can be simplified by assuming β(x) ≫ 1:48,49
represents the emission intensity per activator concentration, k and β are constants for the given host at the same excitation conditions; θ stands for the electric multipolar interaction, when the value of θ is 3, 6, 8 or 10 corresponding to the exchange interaction, dipole–dipole (d–d), dipole–quadrupole (d–q) or quadrupole–quadrupole (q–q) interactions, respectively.47 The eqn (2) can be simplified by assuming β(x) ≫ 1:48,49
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k − log
k − log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β. Fig. 4 shows the dependence of
β. Fig. 4 shows the dependence of  on log(x). As can be seen from this figure, the fitting result was linear and the slope of the straight line was −1.931. Therefore, the value of θ can be calculated as ∼5.79, which is mostly close to 6. This result proposed that the d–d interaction was mainly responsible for the energy transfer between Eu3+ ions in Ba2Lu4.98−xEuxLa0.02B5O17 phosphors.
on log(x). As can be seen from this figure, the fitting result was linear and the slope of the straight line was −1.931. Therefore, the value of θ can be calculated as ∼5.79, which is mostly close to 6. This result proposed that the d–d interaction was mainly responsible for the energy transfer between Eu3+ ions in Ba2Lu4.98−xEuxLa0.02B5O17 phosphors.
The integrated PL intensity ratio of 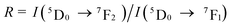 transitions, also known as asymmetry ratio can be used as an index to assess the site symmetry around the Eu3+ ions.40,50–52 The (ED) 5D0 → 7F2 transition is a hypersensitive transition as this type of transition is really sensitive to the local environment, while the (MD) 5D0 → 7F1 transition is insensitive to the local environment at Eu3+ site.53,54 The calculated R value for Ba2Lu4.48Eu0.5La0.02B5O17 phosphor was determined to be 2.88. The results distinctly suggest the lack of an inversion center, and thus the Eu3+ ion is favorable to achieve a bright red emission with high color purity.37,55
transitions, also known as asymmetry ratio can be used as an index to assess the site symmetry around the Eu3+ ions.40,50–52 The (ED) 5D0 → 7F2 transition is a hypersensitive transition as this type of transition is really sensitive to the local environment, while the (MD) 5D0 → 7F1 transition is insensitive to the local environment at Eu3+ site.53,54 The calculated R value for Ba2Lu4.48Eu0.5La0.02B5O17 phosphor was determined to be 2.88. The results distinctly suggest the lack of an inversion center, and thus the Eu3+ ion is favorable to achieve a bright red emission with high color purity.37,55
The PL decay curves of Ba2Lu4.98−xEuxLa0.02B5O17 (x = 0.1, 0.3, 0.5, 0.6, 0.7, 0.9 and 1.0) phosphors monitored at 616 nm under excitation at 396 nm were shown in Fig. 5. All the decay curves were almost overlapping with each other. The corresponding PL decay curves can be well fitted with single exponential function by the following equation:56
 | (4) |
 | ||
| Fig. 5 PL decay curves of Ba2Lu4.98−xEuxLa0.02B5O17 (x = 0.1, 0.3, 0.5, 0.6, 0.7, 0.9 and 1.0) under excited at 396 nm and monitored at 616 nm. | ||
Fig. 6 depicts the CIE chromaticity diagram of optimized Ba2Lu4.48Eu0.5La0.02B5O17 phosphors under excitation at 396 nm. The corresponding CIE chromaticity coordinates were found as (0.643, 0.356), and it is located in the red region. Furthermore, the CIE chromaticity coordinates of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors were nearly close to the National Television Standard Committee (NTSC) system [CIE: (0.670, 0.330)] with superior characteristics as compared to those required for commercial Y2O2S:Eu3+ red phosphors [CIE: (0.637, 0.327)].56 The inset of Fig. 6 shows the digital photograph of Ba2Lu4.48Eu0.5La0.02B5O17 sample under 365 nm UV lamp. The color purity of as-synthesized Ba2Lu4.48Eu0.5La0.02B5O17 phosphors is an essential factor for WLEDs application. Therefore the color purity can be calculated through the following expression:63
 | (5) |
 | ||
| Fig. 6 CIE chromaticity diagram for Ba2Lu4.48Eu0.5La0.02B5O17 red phosphors. Inset shows the digital photograph of optimal Ba2Lu4.48Eu0.5La0.02B5O17 phosphors under 365 nm UV lamp. | ||
These results suggested that the Ba2Lu4.48Eu0.5La0.02B5O17 phosphors demonstrated good CIE chromaticity coordinates with high color purity. Additionally, the IQE of phosphors are a vital parameter for their potential application in near-UV excited WLEDs application. Hence, the IQE value for Ba2Lu4.48Eu0.5La0.02B5O17 phosphors under 396 nm excitation was determined as 27.1%, which is not highly sufficient. To achieve better performance of Ba2Lu4.48Eu0.5La0.02B5O17 phosphor, the obtained IQE value could further be enhanced by suitable experimental conditions, controlling the particle size, size distribution, morphology and optimization of the chemical compositions.35,64,65
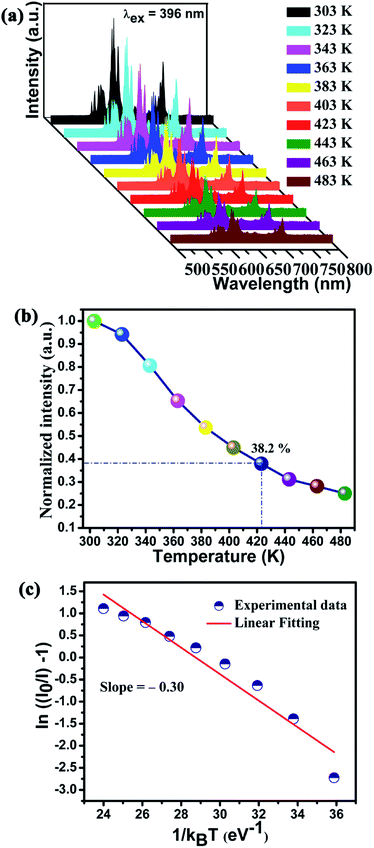
In the action of high power WLEDs application, thermal stability of phosphor is one of the important factors. Fig. 7(a) shows the temperature dependent PL spectra of Ba2Lu4.48Eu0.5La0.02B5O17 phosphors from 303 to 483 K under excitation at 396 nm. As seen in Fig. 7(b), the normalized PL intensities of 5D0 → 7F2 transition gradually decreased as the temperature increased from 303 to 483 K. The thermal quenching due to the non-radiative transition from the excited luminescence center was thermally activated by the crossing point between the excited and ground states.40,66 The PL spectra profile slightly changed due to a decrease in the peak intensities as the temperature was raised above 403 K. It can be noted that the normalized PL intensities at 423 K still remained 38.2% of their initial intensities at 303 K. This implied that the thermal stability of Ba2Lu4.48Eu0.5La0.02B5O17 phosphor further needs to be enhanced. In order to further investigate the relationship between PL intensity and the temperature, the activation energy (ΔEa) for thermal quenching can be described via the Arrhenius equation as follows:67,68
 | (6) |
 | (7) |
Fig. 7(c) depicts the relationship of  versus
versus  . From the slope of linear fitting, the value of ΔEa was deduced to be ∼0.30 eV for Ba2Lu4.48Eu0.5La0.02B5O17 phosphors. These results suggest necessity to enhance the PL intensity of as-synthesized phosphors for improved thermal stability which could be obtain with the optimization of experimental conditions and material compositions.71
. From the slope of linear fitting, the value of ΔEa was deduced to be ∼0.30 eV for Ba2Lu4.48Eu0.5La0.02B5O17 phosphors. These results suggest necessity to enhance the PL intensity of as-synthesized phosphors for improved thermal stability which could be obtain with the optimization of experimental conditions and material compositions.71
4. Conclusions
In summary, the Ba2Lu4.98−xEuxLa0.02B5O17 (0.1 ≤ x ≤ 1.0) red emitting phosphors were successfully synthesized by a traditional solid-state reaction method. The Ba2Lu4.48Eu0.5La0.02B5O17 phosphors can be effectively excited at 396 nm, which matched well with the near-UV chip. Under the excitation at 396 nm, the PL spectrum showed an intense red emission situated at 616 nm, which was certified to the Eu3+ ions occupied at low symmetry sites with non-inversion center. The optimum concentration of Eu3+ for Ba2Lu4.98−xEuxLa0.02B5O17 phosphors was determined as x = 0.5. Also the concentration quenching took place via the dipole–dipole interaction. The decay lifetime of Ba2Lu4.48Eu0.5La0.02B5O17 phosphor was calculated to be 1.409 ms. As a result, the Ba2Lu4.48Eu0.5La0.02B5O17 phosphor exhibited significant red emission intensity, excellent color purity (97.8%), good color coordinates (0.643, 0.356) and the IQE of 27.1%. In addition, the activation energy for the thermal quenching (ΔEa) was calculated to be 0.30 eV. The obtained results indicate that the Ba2Lu4.48Eu0.5La0.02B5O17 red emitting phosphors may be considered as a potential candidate for WLEDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research work was supported by the National Natural Science Foundation of China (No. 51502190), the Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi, and the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology, No. 2017-skllmd-01).References
- L. Zhao, F. Fan, X. Chen, Y. Wang, Y. Li and B. Deng, J. Mater. Sci.: Mater. Electron., 2018, 29, 5975–5981 CrossRef
.
- G. Feng, W. Jiang, J. Liu, C. Li and Q. Zhang, Luminescence, 2018, 33, 455–460 CrossRef PubMed
.
- P. Du, X. Huang and J. S. Yu, Chem. Eng. J., 2018, 337, 91–100 CrossRef
.
- X. Huang and H. Guo, RSC Adv., 2018, 8, 17132–17138 RSC
.
- X. Huang, B. Li and H. Guo, J. Alloys Compd., 2017, 695, 2773–2780 CrossRef
.
- X. Huang and H. Guo, Dyes Pigm., 2018, 152, 36–42 CrossRef
.
- X. Huang, J. Alloys Compd., 2017, 690, 356–359 CrossRef
.
- P. Du, L. Luo, X. Huang and J. S. Yu, J. Colloid Interface Sci., 2018, 514, 172–181 CrossRef PubMed
.
- P. Du, X. Huang and J. S. Yu, Inorg. Chem. Front., 2017, 4, 1987–1995 RSC
.
- J. S. Kim, P. E. Jeon, Y. H. Park, J. C. Choi and H. L. Park, J. Electrochem. Soc., 2005, 152, H29–H32 CrossRef
.
- X. Huang, Nat. Photonics, 2014, 8, 748–749 CrossRef
.
- G. Zhu, Y. Wang, Z. Ci, B. Liu, Y. Shi and S. Xin, J. Electrochem. Soc., 2011, 158, J236–J242 CrossRef
.
- M. Xia, Z. Ju, H. Yang, Z. Wang, X. Gao, F. Pan and W. Liu, J. Alloys Compd., 2018, 739, 439–446 CrossRef
.
- B. Li, X. Huang, H. Guo and Y. Zeng, Dyes Pigm., 2018, 150, 67–72 CrossRef
.
- J. Lu, Z. Mu, D. Zhu, Q. Wang and F. Wu, J. Lumin., 2018, 196, 50–56 CrossRef
.
- X. Huang, B. Li, H. Guo and D. Chen, Dyes Pigm., 2017, 143, 86–94 CrossRef
.
- S. H. Lee, Y. Cha, H. Kim, S. Lee and J. S. Yu, RSC Adv., 2018, 8, 11207–11215 RSC
.
- T. Hasegawa, S. W. Kim, T. Ueda, T. Ishigaki, K. Uematsu, H. Takaba, K. Toda and M. Sato, J. Mater. Chem. C, 2017, 5, 9472–9478 RSC
.
- H. Y. Jiao and Y. Wang, Phys. B, 2012, 407, 2729–2733 CrossRef
.
- L. L. Ying, S. S. Zheng, J. H. Zheng, L. H. Cai and C. Chen, Adv. Mater. Res., 2014, 1003, 7–10 Search PubMed
.
- L. L. Devi and C. Jayasankar, Ceram. Int., 2018, 44, 14063–14069 CrossRef
.
- X. Huang, H. Guo and B. Li, J. Alloys Compd., 2017, 720, 29–38 CrossRef
.
- X. Huang, S. Wang, B. Li, Q. Sun and H. Guo, Opt. Lett., 2018, 43, 1307–1310 CrossRef PubMed
.
- A. A. Reddy, S. Das, S. Ahmad, S. S. Babu, J. M. Ferreira and G. V. Prakash, RSC Adv., 2012, 2, 8768–8776 RSC
.
- M. He, G. Huang, H. Tao and Z. Zhang, Phys. B, 2012, 407, 2725–2728 CrossRef
.
- X. Zhang, Y. Chen, S. Zeng, L. Zhou, J. Shi and M. Gong, Ceram. Int., 2014, 40, 14537–14541 CrossRef
.
- F. Xiong, J. Luo, H. Lin, X. Meng, Y. Wang, H. Shen and W. Zhu, Optik, 2018, 156, 31–38 CrossRef
.
- A. Kruopyte, R. Giraitis, R. Juskenas, D. Enseling, T. Jüstel and A. Katelnikovas, J. Lumin., 2017, 192, 520–526 CrossRef
.
- B. Bondzior and P. Dereń, New J. Chem., 2017, 41, 7662–7666 RSC
.
- A. Shablinskii, R. Bubnova, I. Kolesnikov, M. Krzhizhanovskaya, A. Povolotskiy, V. Ugolkov and S. Filatov, Solid State Sci., 2017, 70, 93–100 CrossRef
.
- H. Huang, X. Feng, T. Chen, Y. Xiong and F. Zhang, Luminescence, 2018, 33, 692–697 CrossRef PubMed
.
- Y. Xiao, Z. Hao, L. Zhang, H. Wu, G.-H. Pan, X. Zhang and J. Zhang, Dyes Pigm., 2018, 154, 121–127 CrossRef
.
- Y. Xiao, Z. Hao, L. Zhang, X. Zhang, G.-H. Pan, H. Wu, H. Wu, Y. Luo and J. Zhang, J. Mater. Chem. C, 2018, 6, 5984–5991 RSC
.
- J. Zhang, M. Chen and Y. Gao, Displays, 2017, 49, 35–39 CrossRef
.
- Y. Wang, X. Liu, P. Niu, L. Jing and W. Zhao, J. Lumin., 2017, 184, 1–6 CrossRef
.
- H. Guo and X. Huang, J. Alloys Compd., 2018, 764, 809–814 CrossRef
.
- J. Qiao, L. Wang, Y. Liu, P. Huang, Q. Shi and Y. Tian, J. Alloys Compd., 2016, 686, 601–607 CrossRef
.
- B. G. Vats, S. K. Gupta, M. Keskar, R. Phatak, S. Mukherjee and S. Kannan, New J. Chem., 2016, 40, 1799–1806 RSC
.
- W. Zhang, X. Yin, Y. Liu, N. Zhang, G. Zhao, J. Hou and Y. Fang, J. Mater. Sci.: Mater. Electron., 2016, 27, 5357–5361 CrossRef
.
- J. Chen, W. Zhao, J. Wang and N. Wang, J. Mater. Sci.: Mater. Electron., 2016, 27, 237–244 CrossRef
.
- J. Liang, P. Du, H. Guo, L. Sun, B. Li and X. Huang, Dyes Pigm., 2018, 157, 40–46 CrossRef
.
- L. Sun, H. Guo, J. Liang, B. Li and X. Huang, J. Lumin., 2018, 202, 403–408 CrossRef
.
- X. Huang, B. Li and H. Guo, Ceram. Int., 2017, 43, 10566–10571 CrossRef
.
- K. Singh and S. Vaidyanathan, ChemistrySelect, 2016, 1, 5448–5462 CrossRef
.
- G. Blasse, J. Solid State Chem., 1986, 62, 207–211 CrossRef
.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836–850 CrossRef
.
- L. Van Uitert, J. Electrochem. Soc., 1967, 114, 1048–1053 CrossRef
.
- S. Xin, Y. Wang, Z. Wang, F. Zhang, Y. Wen and G. Zhu, ECS Solid State Lett., 2011, 14, H438–H441 CrossRef
.
- D. Dexter and J. H. Schulman, J. Chem. Phys., 1954, 22, 1063–1070 CrossRef
.
- J. Zhu, Z. Xia, Y. Zhang, M. S. Molokeev and Q. Liu, Dalton Trans., 2015, 44, 18536–18543 RSC
.
- Z.-w. Zhang, L. Liu, R. Liu, X.-y. Zhang, X.-g. Peng, C.-h. Wang and D.-j. Wang, Mater. Lett., 2016, 167, 250–253 CrossRef
.
- J. Llanos, D. Espinoza and R. Castillo, RSC Adv., 2017, 7, 14974–14980 RSC
.
- J. Liao, B. Qiu, H.-R. Wen, Y. Li, R. Hong and H. You, J. Mater. Sci., 2011, 46, 1184–1189 CrossRef
.
- P. Du, Y. Guo, S. H. Lee and J. S. Yu, RSC Adv., 2017, 7, 3170–3178 RSC
.
- H. Guo, H. Zhang, R. Wei, M. Zheng and L. Zhang, Opt. Express, 2011, 19, A201–A206 CrossRef PubMed
.
- H. Guo, X. Huang and Y. Zeng, J. Alloys Compd., 2018, 741, 300–306 CrossRef
.
- Y. Zhang, W. Gong and G. Ning, New J. Chem., 2016, 40, 10136–10143 RSC
.
- A. Fu, A. Guan, F. Gao, X. Zhang, L. Zhou, Y. Meng and H. Pan, Opt. Laser Technol., 2017, 96, 43–49 CrossRef
.
- J. Grigorjevaite and A. Katelnikovas, ACS Appl. Mater. Interfaces, 2016, 8, 31772–31782 CrossRef PubMed
.
- L. Mengting and J. Baoxiang, J. Rare Earths, 2015, 33, 231–238 CrossRef
.
- G. Annadurai, B. Devakumar, H. Guo, R. Vijayakumar, B. Li, L. Sun, X. Huang, K. Wang and X. W. Sun, RSC Adv., 2018, 8, 23323–23331 RSC
.
- R. Vijayakumar, B. Devakumar, G. Annadurai, H. Guo and X. Huang, Opt. Mater., 2018, 84, 312–317 CrossRef
.
- Y. Wu, K. Qiu, Q. Tang, W. Zhang and J. Wang, Ceram. Int., 2018, 44, 8190–8195 CrossRef
.
- X. Zhang, Z.-C. Wu, Y. Li, J. Xu and L. Tian, Dyes Pigm., 2017, 144, 94–101 CrossRef
.
- X. Chen, Q. Wang, F. Lv, P. K. Chu and Y. Zhang, RSC Adv., 2016, 6, 11211–11217 RSC
.
- K. Li, S. Liang, M. Shang, H. Lian and J. Lin, Inorg. Chem., 2016, 55, 7593–7604 CrossRef PubMed
.
- M. Zhou, H. Ming, J. Peng, Y. Qiang and X. Ye, ECS J. Solid State Sci. Technol., 2017, 6, R175–R182 CrossRef
.
- J. Zhong, D. Chen, H. Xu, W. Zhao, J. Sun and Z. Ji, J. Alloys Compd., 2017, 695, 311–318 CrossRef
.
- W. Guo, Y. Tian, P. Huang, L. Wang and Q. Shi, Ceram. Int., 2016, 42, 5427–5432 CrossRef
.
- Q. Liu, X. Li, B. Zhang, L. Wang, Q. Zhang and L. Zhang, Ceram. Int., 2016, 42, 15294–15300 CrossRef
.
- X. Wang, Z. Zhao, Q. Wu, Y. Li and Y. Wang, Inorg. Chem., 2016, 55, 11072–11077 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2018 |