Open Access Article
Open Access ArticleUltrasonic-assisted enzymatic extraction of a water soluble polysaccharide from dragon fruit peel and its antioxidant activity
Shiquan Qian†
 a,
Xiaohui Fang†b,
Demiao Dan†c,
Enjie Diaoa and
Zhaoxin Lud
a,
Xiaohui Fang†b,
Demiao Dan†c,
Enjie Diaoa and
Zhaoxin Lud
aSchool of Life Science, Huaiyin Normal University, Huaian 223300, China
bSchool of Food Science and Technology, Nanchang University, Nanchang 330047, China
cInstitute of Life Sciences, Jiangsu University, Zhenjiang 212000, China
dCollege of Food Science and Technology, Nanjing Agricultural University, 1 Weigang, Nanjing 210095, China
First published on 18th December 2018
Abstract
A novel water soluble polysaccharide from dragon fruit peel named DFPWSP-1 was isolated and purified and chemical analysis was performed. The results indicated that DFPWSP-1, with an average molecular weight of 2.98 × 102 kDa, mainly contained galacturonic acid, glucose and galactose. Then, a Box–Behnken design (BBD) was employed to optimize the ultrasonic-assisted enzymatic extraction (UAEE) of DFPWSP-1. The optimal extraction conditions for the maximum yield of DFPWSP-1 were a cellulase volume of 104 U, an enzymolysis time of 2.06 h, an ultrasonication power of 105 W and a ratio of solution to sample of 8.5 mL g−1. Under these conditions, the extraction yield of DFPWSP-1 was 20.28%. Furthermore, the polysaccharide DFPWSP-1 exhibited a significant scavenging activity of 2-diphenyl-picrylhydrazyl (DPPH) radical, superoxide anion and hydroxyl radical. DFPWSP-1 may be a potential natural antioxidant in the food industry.
1. Introduction
Dragon fruit (Pitaya) is a native plant that mainly exists in Mexico, Central and South America1 and is also widely found in China.2 Dragon fruit is abundant in edible nutrients and bioactive substances, such as plant polysaccharides,3 pectin4 and oligosaccharides.5 Previous works have reported that plant polysaccharides potentially possess antioxidant activity,6 anticarcinogenic activity7 and immunologic competence.8 A water soluble polysaccharide was purified from dragon fruit pulp and its structure was identified by NMR and GC-MS.9 However, there is no publication on water soluble polysaccharides from dragon fruit peel. It is difficult to isolate polysaccharides from dragon fruit peel because of its heavy red color.10The extraction method is a key factor for isolation of plant polysaccharides. Hot water extraction is a traditional method for extraction of polysaccharide and the cause lies in its simplicity and safety of operation, but it is time-consuming and needs high energy.11 Therefore, various efficient extraction methods have been successfully developed for extraction of active components from plants, such as ultrasonic-assisted extraction (UAE) and enzymatic-assisted extraction (EAE).12 UAE can cause collapse of cavitation bubbles which contributes to elevating the extraction efficiency of target product of plant materials.13–16 EAE can also disrupt plant cells and cause hydrolysis of the cytoderm, and enhance the release of bioactive substance.13,16 To date, however, there has been no report on ultrasound-assisted extraction combined with enzymatic treatment for polysaccharides from dragon fruit peel. In addition, response surface methodology (RSM) has been applied commonly in optimizing the extraction parameters for plant polysaccharides.17–19
In the present study, a water soluble polysaccharide was purified from dragon fruit peel and the chemical analysis was studied. Then, RSM was used to optimize the ultrasonic-assisted enzymatic process (UAEE) for DFPWSP-1 extraction. Moreover, radical scavenging assays of DFPWSP-1 including scavenging activities of 2-diphenyl-picrylhydrazyl (DPPH) radical, superoxide anion and hydroxyl radical were also evaluated.
2. Materials and methods
2.1 Materials
Dragon fruit (Hylocereus undatus) was purchased from local supermarket in Bengbu city of China. The fresh fruits were washed with distilled water and thick skins were peeled off from the fruits manually. Then, the fresh fruits peels were dried at 60 °C and pulverized to an 60-mesh powder. All chemicals used in present study were purchased from Sinopharm Chemical Reagent Co., Ltd, China.2.2 Extraction of polysaccharides
5.0 g of dried dragon fruit peel powder was defatted using 50 mL of petroleum ether for 12 h and mixed with 150 mL of distilled water evenly. Then, the pretreated samples were conducted with cellulase enzymolysis at 55 °C and extracted with ultrasonication treatment. The treated samples were centrifugated at 8000g for 10 min to collect the supernatant. 10 mL of 4% hydrogen peroxide was added to the supernatant and decolorized at 60 °C for 1.5 h. Then the decolourization solution was mixed with 50 mL of 95% ethanol and stirred vigorously. The precipitates were collected by centrifugation at 8000g for 10 min. Proteins of the precipitates were removed by Sevag method and then dialyzed with a 3500 Mw membrane at 4 °C overnight. The precipitates were collected by again centrifugation and lyophilized under vacuum to obtain crude polysaccharides.2.3 Purification of DFPWSP-1
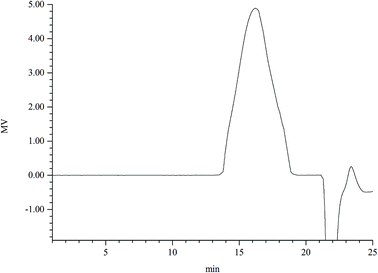
0.2 g of the crude polysaccharide was dissolved in 20 mL deionized water and the supernatant was collected by centrifugation at 8000g for 10 min. Then, the supernatant was added onto an anion-exchange DEAE cellulose column (2.6 cm × 50 cm) and eluted with different concentrations of NaCl solutions (0, 0.1, 0.2, 0.3, 0.4 and 0.5 mol L−1) at the flow rate of 3.0 mL min−1. The polysaccharide fraction was collected by elution with 0.1 mol L−1 NaCl solution and was further purified by gel-filtration (2.0 × 100 cm) on Sephadex G-100 column at a flow rate of 0.4 mL min−1 with deionized water as elution solvent. The main peak was concentrated and lyophilized to obtain the pure polysaccharide named DFPWSP-1.2.4 Analysis of DFPWSP-1
2.5 FT-IR analysis
Fourier transform-infrared (FT-IR) of the polysaccharide DFPWSP-1 was analyzed using the previous method.24 Briefly, the sample was mixed into potassium bromide (KBr) powder and made into 1.0 mm pellet. FT-IR spectra were recorded on a Bruker Vector 22 instrument (Agilent, USA) at the absorbance from 4000 to 400 cm−1.2.6 Experimental design
| Yield of DFPWSP-1 (%) = m/M, |
| Y = α0 + α1X1 + α2X2 + α3X3 + α4X4 + α11X12 + α22X22 + α33X32 + α44X42 + α12X1X2 + α13X1X3 + α14X1X4 + α23X2X3 + α24X2X4 + α34X3X4 |
2.7 Radical scavenging assays
where A0 was the absorbance of the control (DPPH radical solution instead of the DFPWSP-1 solution), and A1 was the absorbance of the samples.
where A0 was the absorbance of the control (distilled water instead of the DFPWSP-1 solution), and A1 was the absorbance of the samples.
where A0 was the absorbance of the control (distilled water instead of the DFPWSP-1 solution), and A1 was the absorbance of the samples.
2.8 Statistical analysis
All experiments were carried out in triplicate. The results were analyzed using SPSS 20.0 (SPSS, IBM, USA) and expressed as mean ± SD. One-way analysis of variance was performed to determine the statistical difference. Statistical significance was evaluated by Duncan's test. Differences at P < 0.05 or P < 0.01 were considered to be significant.3. Results and discussion
3.1 Chemical analysis of the purified polysaccharide DFPWSP-1
| Monosaccharide | Composition (%) |
|---|---|
| Glucose | 12.53 ± 0.25 |
| Fructose | 3.57 ± 0.06 |
| Arabinose | 4.26 ± 0.0.09 |
| Xylose | 1.79 ± 0.05 |
| Galactose | 11.47 ± 0.21 |
| Rhamnose | 9.24 ± 0.18 |
3.2 IR analysis
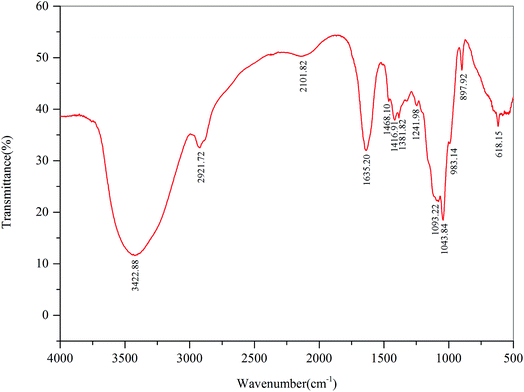
The FT-IR spectra of DFPWSP-1 were depicted in Fig. 2. The strong band at 3422.88 cm−1 was attributed to the –OH stretching vibration, which was indicative of interactions of the polysaccharides chains. The band at 2921.72 cm−1 was due to C–H stretching and bending vibration. The absorption bands at 1635.2 cm−1, associating with ester carbonyl and carboxyl groups, suggested the possible presence of uronic acid in polysaccharides. The absorption peak at 1468.10 and 1416.9 cm−1 were attributed to the deforming vibration of the C–H bond. The absorption bands at 1093.22 and 1043.84 cm−1 suggested the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–C bonds. Moreover, the characteristic absorption bands at 1241.98, 1093.22 and 897.12 cm−1 indicated that the sugar units existed in DFPWSP-1. In addition, the strong extensive absorption peaks in the region of 1000–1200 cm−1 could be caused by the stretching vibrations of C–O–H side groups and the C–O–C glycosidic band vibrations. Overall, these results evidenced that DFPWSP-1 possessed the typical absorption peaks of polysaccharides.
O and C–C bonds. Moreover, the characteristic absorption bands at 1241.98, 1093.22 and 897.12 cm−1 indicated that the sugar units existed in DFPWSP-1. In addition, the strong extensive absorption peaks in the region of 1000–1200 cm−1 could be caused by the stretching vibrations of C–O–H side groups and the C–O–C glycosidic band vibrations. Overall, these results evidenced that DFPWSP-1 possessed the typical absorption peaks of polysaccharides.
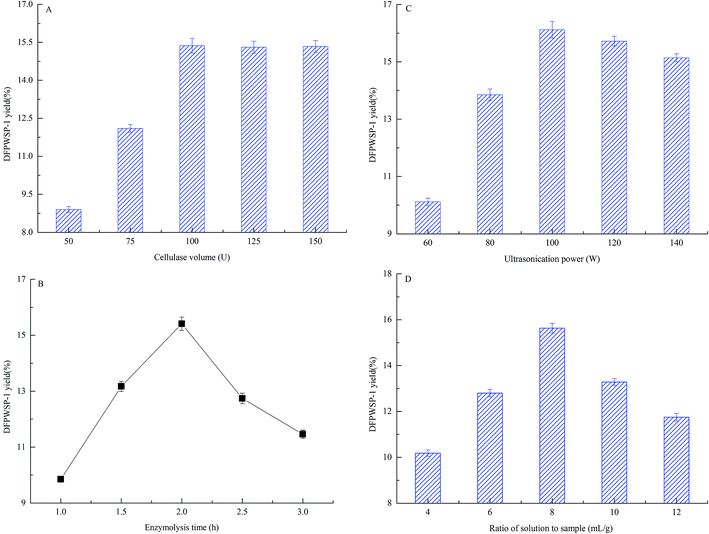
3.3 Effects of enzymolysis-ultrasonic assisted extraction conditions on the yield of DFPWSP-1
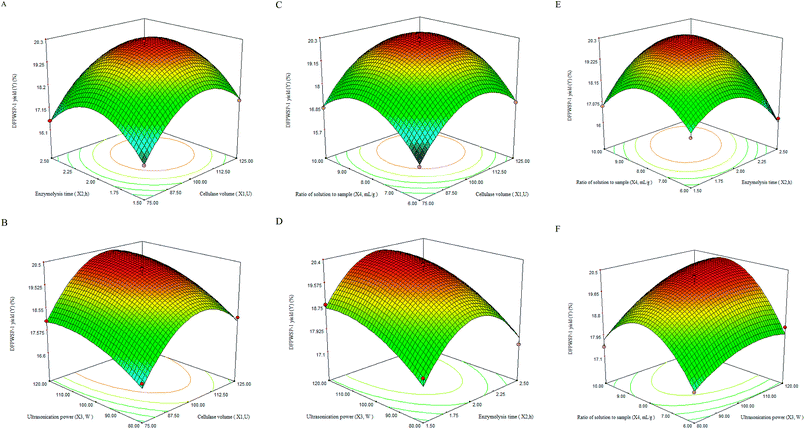
3.4 Optimization of DFPWSP-1 extraction
| Y = −51.50 + 0.49X1 + 17.40X2 + 0.21X3 + 3.64X4 + 4.20 × 10−3X1X2 + 1.35 × 10−4X1X3 + 1.45 × 10−3X1X4 − 2.75 × 10−3X2X3 + 0.76X2X4 + 6.88 × 10−3X3X4 − 2.45 × 10−3X12 − 5.82X22 − 1.20 × 10−4X32 − 0.36X42 |
| Standard run | Cellulase volume (X1, U) | Enzymolysis time (X2, h) | Ultrasonication power (X3, W) | Ratio of solution to sample (X4, mL g−1) | DFPWSP-1 yield (Y) (%) | |
|---|---|---|---|---|---|---|
| Actual | Predicted | |||||
| 1 | −1 (75) | −1 (1.5) | 0 (100) | 0 (8) | 16.21 | 16.26 |
| 2 | 1 (125) | −1 (1.5) | 0 (100) | 0 (8) | 17.51 | 17.59 |
| 3 | −1 (75) | 1 (2.5) | 0 (100) | 0 (8) | 16.53 | 16.48 |
| 4 | 1 (125) | 1 (2.5) | 0 (100) | 0 (8) | 18.04 | 18.02 |
| 5 | 0 (100) | 0 (2) | −1 (80) | −1 (6) | 17.12 | 17.16 |
| 6 | 0 (100) | 0 (2) | 1 (120) | −1 (6) | 18.28 | 18.01 |
| 7 | 0 (100) | 0 (2) | −1 (80) | 1 (10) | 17.48 | 17.78 |
| 8 | 0 (100) | 0 (2) | 1 (120) | 1 (10) | 19.74 | 19.74 |
| 9 | −1 (75) | 0 (2) | 0 (100) | −1 (6) | 15.76 | 15.89 |
| 10 | 1 (125) | 0 (2) | 0 (100) | −1 (6) | 17.12 | 17.18 |
| 11 | −1 (75) | 0 (2) | 0 (100) | 1 (10) | 16.83 | 16.92 |
| 12 | 1 (125) | 0 (2) | 0 (100) | 1 (10) | 18.48 | 18.50 |
| 13 | 0 (100) | −1 (1.5) | −1 (80) | 0 (8) | 17.51 | 17.25 |
| 14 | 0 (100) | 1 (2.5) | −1 (80) | 0 (8) | 17.37 | 17.63 |
| 15 | 0 (100) | −1 (1.5) | 1 (120) | 0 (8) | 18.82 | 18.71 |
| 16 | 0 (100) | 1 (2.5) | 1 (120) | 0 (8) | 18.57 | 18.98 |
| 17 | −1 (75) | 0 (2) | −1 (80) | 0 (8) | 16.90 | 16.71 |
| 18 | 1 (125) | 0 (2) | −1 (80) | 0 (8) | 18.16 | 18.01 |
| 19 | −1 (75) | 0 (2) | 1 (120) | 0 (8) | 18.01 | 17.98 |
| 20 | 1 (125) | 0 (2) | 1 (120) | 0 (8) | 19.54 | 19.55 |
| 21 | 0 (100) | −1 (1.5) | 0 (100) | −1 (6) | 16.97 | 17.20 |
| 22 | 0 (100) | 1 (2.5) | 0 (100) | −1 (6) | 16.21 | 16.02 |
| 23 | 0 (100) | −1 (1.5) | 0 (100) | 1 (10) | 16.86 | 16.87 |
| 24 | 0 (100) | 1 (2.5) | 0 (100) | 1 (10) | 19.12 | 18.71 |
| 25 | 0 (100) | 0 (2) | 0 (100) | 0 (8) | 20.24 | 20.08 |
| 26 | 0 (100) | 0 (2) | 0 (100) | 0 (8) | 19.85 | 20.08 |
| 27 | 0 (100) | 0 (2) | 0 (100) | 0 (8) | 20.23 | 20.08 |
| 28 | 0 (100) | 0 (2) | 0 (100) | 0 (8) | 19.85 | 20.08 |
| 29 | 0 (100) | 0 (2) | 0 (100) | 0 (8) | 20.21 | 20.08 |
| Source | Sum of squares | df | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| a ** Significant (P < 0.05). | |||||
| Model | 49.84 | 14 | 3.56 | 49.17 | <0.0001** |
| X1 | 6.18 | 1 | 6.18 | 85.34 | <0.0001** |
| X2 | 0.32 | 1 | 0.32 | 4.42 | 0.0540 |
| X3 | 5.91 | 1 | 5.91 | 81.61 | <0.0001** |
| X4 | 4.14 | 1 | 4.14 | 57.22 | <0.0001** |
| X1X2 | 0.011 | 1 | 0.011 | 0.15 | 0.7022 |
| X1X3 | 0.018 | 1 | 0.018 | 0.25 | 0.6236 |
| X1X4 | 0.021 | 1 | 0.021 | 0.29 | 0.5984 |
| X2X3 | 3.025 × 10−3 | 1 | 3.025 × 10−3 | 0.042 | 0.8410 |
| X2X4 | 2.28 | 1 | 2.28 | 31.50 | <0.0001** |
| X3X4 | 0.30 | 1 | 0.30 | 4.18 | 0.0602 |
| X12 | 15.24 | 1 | 15.24 | 210.46 | <0.0001** |
| X22 | 13.71 | 1 | 13.71 | 189.39 | <0.0001** |
| X32 | 1.50 | 1 | 1.50 | 20.76 | 0.0004** |
| X42 | 13.13 | 1 | 13.13 | 181.34 | <0.0001** |
| Residual | 1.01 | 14 | 0.072 | ||
| Lack of fit | 0.84 | 10 | 0.084 | 1.97 | 0.2675 |
| Pure error | 0.17 | 4 | 0.043 | ||
| Cor total | 50.85 | 28 | |||
| R2 | 0.9801 | ||||
| RAdj2 | 0.9601 | ||||
| C.V.% | 1.49 | ||||
The P-value of the model was smaller than 0.01, meaning that the model was highly statistically significant. The P-value of the lack of fit was 0.2675, which confirmed the goodness-of-fit and the suitability of regression model. The determination coefficient (R2 = 0.9801) and the adjusted determination coefficient (RAdj2 = 0.9601) indicated that the predicted values was found to fit the observed values well. The coefficient variation value of 1.49 revealed that experimental value of regression model was dependable. Table 3 also demonstrated that the linear coefficients (X1, X3 and X4), the quadratic term coefficients (X12, X22 and X42) and the cross product coefficient (X2X4) were significant (P < 0.01). These results showed that the predicted model could be efficiently used to predict the yield of DFPWSP-1.
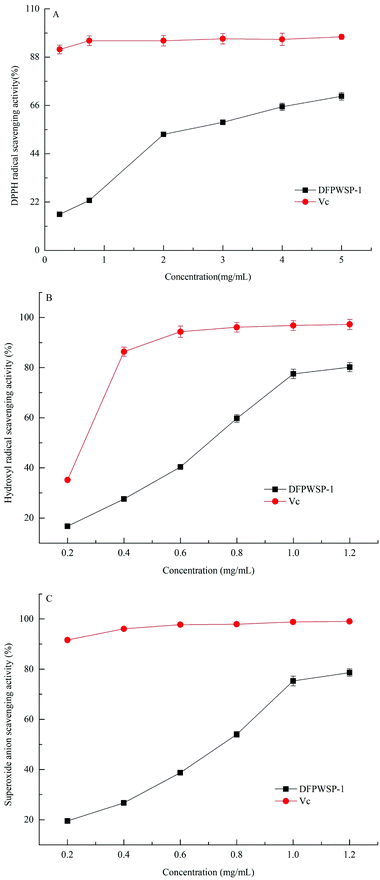
3.5 Radical scavenging assays
4. Conclusions
In the present study, a novel water soluble polysaccharide named DFPWSP-1 was purified from dragon fruit peel. The chemical analysis showed that DFPWSP-1, with an average molecular weight of 2.98 × 102 kDa, mainly contained galacturonic acid, glucose and galactose. FTIR spectrum revealed that DFPWSP-1 had the characteristic absorption bands of polysaccharides. The response surface methodology (RSM) was successfully used for optimizing the ultrasonic-assisted enzymatic extraction (UAEE) of DFPWSP-1. The optimum conditions were determined to be the cellulase volume of 104 U, the enzymolysis time of 2.06 h, the ultrasonication power of 105 W and the ratio of solution to sample of 8.5 mL g−1 and 20.28% of DFPWSP-1 was obtained. Moreover, DFPWSP-1 exhibited significant scavenging activities for 2-diphenyl-picrylhydrazyl (DPPH) radical, hydroxyl radical and superoxide anion. Potentially, DFPWSP-1 can be used a nature antioxidant in food industry.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 31571887) and Young Talents Project for Jiangsu Collaborative Innovation Center of Regional Modern Agriculture and Environmental Protection (HSXT2-312).References
- A. Nerd and Y. Mizrahi, Hortic. Rev., 1997, 18, 321–346 Search PubMed.
- L. C. Wu, H. W. Hsu, Y. C. Chen, C. C. Chiu, Y. I. Lin and J. A. Ho, Food Chem., 2006, 95, 319–327 CrossRef CAS.
- L. Xu, Y. Zhang and L. Wang, Carbohydr. Polym., 2016, 146, 224–230 CrossRef CAS PubMed.
- K. Muhammad, N. I. M. Zahari, S. P. Gannasin, N. M. Adzahan and J. Bakar, Food Hydrocolloids, 2014, 42, 289–297 CrossRef CAS.
- S. Wichienchot, M. Jatupornpipat and R. A. Rastall, Food Chem., 2010, 120, 850–857 CrossRef CAS.
- A. Raza, F. Li, X. Xu and J. Tang, Int. J. Biol. Macromol., 2017, 94, 335–344 CrossRef CAS PubMed.
- X. Jia, C. Zhang, J. Qiu, L. Wang, J. Bao, K. Wang, Y. Zhang, M. Chen, J. Wan and H. Su, Carbohydr. Polym., 2015, 132, 67–71 CrossRef CAS PubMed.
- E. Wang, X. Chen, K. Wang, J. Wang, D. Chen, Y. Geng, W. Lai and X. Wei, Fish Shellfish Immunol., 2016, 59, 196–202 CrossRef CAS PubMed.
- Y. Xu, L. Zhang, Y. Yang, X. Song and Z. Yu, Carbohydr. Polym., 2015, 117, 895–902 CrossRef CAS PubMed.
- K. Kishore, Sci. Hortic., 2016, 213, 294–302 CrossRef.
- H. Wu, J. Zhu, W. Diao and C. Wang, Carbohydr. Polym., 2014, 113, 314–324 CrossRef CAS PubMed.
- N. Liao, J. Zhong, X. Ye, S. Lu, W. Wang, R. Zhang, J. Xu, S. Chen and D. Liu, LWT--Food Sci. Technol., 2015, 60, 1113–1121 CrossRef CAS.
- H. Chen, X. Zhou and J. Zhang, Carbohydr. Polym., 2014, 111, 567–575 CrossRef CAS PubMed.
- A. Hematian Sourki, A. Koocheki and M. Elahi, Int. J. Biol. Macromol., 2017, 95, 462–475 CrossRef CAS PubMed.
- J. Hu, X. Jia, X. Fang, P. Li, C. He and M. Chen, Int. J. Biol. Macromol., 2016, 85, 277–284 CrossRef CAS PubMed.
- S. Varakumar, K. V. Umesh and R. S. Singhal, Food Chem., 2017, 216, 27–36 CrossRef CAS PubMed.
- C. Chen, Y. Shao, Y. Tao and H. Wen, LWT--Food Sci. Technol., 2015, 64, 1263–1269 CrossRef CAS.
- S. Tahmouzi, Carbohydr. Polym., 2014, 106, 238–246 CrossRef CAS PubMed.
- L. Zhang and M. Wang, Int. J. Biol. Macromol., 2017, 95, 675–681 CrossRef CAS PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. T. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- T. Bitter and H. M. Muir, Anal. Biochem., 1962, 4, 330–334 CrossRef CAS PubMed.
- X. Li and W. Lu, Int. J. Biol. Macromol., 2016, 83, 270–276 CrossRef CAS PubMed.
- D. Y. Zhang, Y. Wan, J. Y. Xu, G. H. Wu, L. Li and X. H. Yao, Carbohydr. Polym., 2016, 137, 473–479 CrossRef CAS PubMed.
- Q. Han, Z. Wu, B. Huang, L. Sun, C. Ding, S. Yuan, Z. Zhang, Y. Chen, C. Hu, L. Zhou, J. Liu, Y. Huang, J. Liao and M. Yuan, Int. J. Biol. Macromol., 2016, 92, 116–124 CrossRef CAS PubMed.
- B. Zeng, M. Su, Q. Chen, Q. Chang, W. Wang and H. Li, Carbohydr. Polym., 2016, 153, 391–398 CrossRef CAS PubMed.
- Z. Ye, W. Wang, Q. Yuan, H. Ye, Y. Sun, H. Zhang and X. Zeng, Carbohydr. Polym., 2016, 147, 354–364 CrossRef CAS PubMed.
- Y. Liu, M. Qiang, Z. Sun and Y. Du, Int. J. Biol. Macromol., 2015, 80, 350–357 CrossRef CAS PubMed.
- T. Baran and A. Menteş, Int. J. Biol. Macromol., 2015, 72, 94–103 CrossRef CAS PubMed.
- M. F. Barroso, N. De-Los-Santos-Álvarez, M. J. Lobo-Castañón, A. J. Miranda-Ordieres, C. Delerue-Matos, M. B. P. P. Oliveira and P. Tuñón-Blanco, J. Electroanal. Chem., 2011, 659, 43–49 CrossRef CAS.
- X. Zhou, S. Feng, S. Shen, H. Wang, Y. Ming, L. Jing, H. Yan and C. Ding, Carbohydr. Polym., 2016, 144, 122–130 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |