Open Access Article
Open Access ArticleSilencing TRIM37 inhibits the proliferation and migration of non-small cell lung cancer cells
Yi Ding,
Yi Lu,
Xinjie Xie,
Bo Sheng and
Zuopei Wang *
*
Department of Thoracic Surgery, Shanghai Pudong New District People's Hospital, No. 490 of Chuanhuan South Road, Shanghai, 201299, China. E-mail: wang_zuopei1@163.com; Tel: +86-021-20509000 ext. 2346
First published on 31st October 2018
Abstract
Tripartite motif containing 37 (TRIM37), a member of the tripartite motif (TRIM) family, has been involved in the development and progression of several tumors. However, its role in non-small cell lung cancer (NSCLC) is still unclear. Therefore, the aim of this study was to investigate the expression pattern and role of TRIM37 in NSCLC. Our results showed that TRIM37 was highly expressed in human NSCLC cell lines. Knockdown of TRIM37 obviously inhibited the proliferation in vitro and xenografted tumor growth in vivo. Furthermore, knockdown of TRIM37 suppressed NSCLC cell migration and invasion by inhibiting the epithelial–mesenchymal transition (EMT) phenotype. Lastly, knockdown of TRIM37 greatly down-regulated the protein expression levels of β-catenin, cyclinD1 and c-myc in A549 cells. In conclusion, the present study revealed that TRIM37 plays an important role in the development and progression of NSCLC. Thus, TRIM37 may act a potential therapeutic target for treating NSCLC.
1 Introduction
Lung cancer is the most common malignant tumor, and it has become the first cause of death in China's urban population. The incidence of lung cancer increases rapidly as the air quality continues to decline in developing countries, especially China.1 Non-small cell lung cancer (NSCLC) accounts for about 80–85% of all lung cancer cases.2 Despite recent advances in the treatment of NSCLC, including surgery, radiotherapy and adjuvant chemotherapy, the 5 year survival rate of patients with NSCLC is very low.3–5 Thus, the identification of the molecular mechanism during NSCLC progression may provide patients with novel diagnostic and therapeutic strategies.The family of tripartite motif (TRIM)-containing proteins plays important roles in regulating cell cycle regulation, autophagy, innate immunity and cell migration.6–8 Tripartite motif containing 37 (TRIM37) is a member of TRIM protein family and has been found to be expressed in dorsal root, trigeminal ganglia and liver.9,10
TRIM37, an E3 ligase, was reported to be related with many signaling pathways. Jiang et al. showed that TRIM37 promoted the epithelial–mesenchymal transition (EMT) in hepatocellular carcinoma cells through the activation of Wnt/β-catenin signaling.11 Another study reported that knockdown of TRIM37 inhibited the proliferation, migration and invasion of glioma cells through the inactivation of PI3K/Akt signaling pathway.12
Several studies indicated that TRIM37 plays a critical role in development and progression of tumors.13–15 One study reported that the expression of TRIM37 was up-regulated in human breast cancer cell lines, and knockdown of TRIM37 substantially decreases tumor growth in mouse xenografts in human breast cancer cells.16 However, its role in NSCLC is still unclear. Therefore, the aim of this study was to investigate the expression pattern and role of TRIM37 in NSCLC. Our results demonstrated that knockdown of TRIM37 inhibited the proliferation and tumorigenesis in NSCLC cells through suppressing the Wnt/beta-catenin signaling pathway.
2 Materials and methods
2.1 Cell culture
Three NSCLC cell lines (A549, PC9 and NCI-H1993) and a normal human bronchial epithelial cell line (BEAS-2B) were provided from the American Type Culture Collection (ATCC, Manassas, VA, USA). They were cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Gibco, Rockville, MD) at 37 °C with 5% CO2 in an incubator (Life Technologies, Baltimore, MD, USA).2.2 Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from NSCLC cells using TRIzol reagent (Dongsheng Biological Co., Ltd., China). The first-strand cDNA was synthesized from 1 μg of total RNA using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). The following primers were used to perform PCR: TRIM37, forward 5′-TCAGGAGGTAGAACATCAG-3′ and reverse 5′-GGACAGGTGTGGTGACAAAGGATGC-3′; GAPDH forward 5′-CTGCACCACCAACTGCTTAG-3′ and reverse 5′-AGGTCCACCACTGACACGTT-3′. We analyzed the relative quantity of mRNA using the 2−ΔΔCt method17 and the internal control to GAPDH.2.3 Western blot
Proteins were extracted from NSCLC cells using RIPA lysis buffer (Beyotime, Nantong, China). Aliquots (about 30 μg per lane) were separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA, USA). The membranes were blocked with 5% fat-free milk for 1 h and incubated with primary antibodies overnight at 4 °C. The primary antibodies were anti-TRIM37, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000; anti-E-cadherin, 1
1000; anti-E-cadherin, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500, anti-N-cadherin, 1
1500, anti-N-cadherin, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500; anti-vimentin, 1
1500; anti-vimentin, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000; anti-β-catenin 1
2000; anti-β-catenin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000; anti-cyclinD1, 1
3000; anti-cyclinD1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000; anti-c-myc, 1
3000; anti-c-myc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 and anti-GAPDH, 1
3000 and anti-GAPDH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies (1
2500 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Santa Cruz Biotechnology) for 1 h at room temperature. The blots were visualized by ECL reagent (Bio-Rad, Hercules, CA, USA). All these used unified GAPDH as the internal control.
000; Santa Cruz Biotechnology) for 1 h at room temperature. The blots were visualized by ECL reagent (Bio-Rad, Hercules, CA, USA). All these used unified GAPDH as the internal control.
2.4 RNA interference and transfection
The specific shRNA against human TRIM37 (sh-TRIM37) and its negative control (mock) were synthesized by Invitrogen (Carlsbad, CA, USA). A549 cells at a density of 5 × 104 cells per well were seeded in six-well culture plate. After 24 h, A549 cells were transfected with sh-TRIM37 or mock using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocols.2.5 Cell proliferation assay
Cell proliferation was evaluated by 3-(4.5-methylthiozol-2yl)-2.5-diphenyltetrazolium bromide (MTT) assay. Briefly, A549 cells at density of 1 × 104 cells per well were seeded in 96-well culture plates and incubated for 1, 2, 3 and 4 days. After that, MTT solution (0.5 mg ml−1; Sigma, St. Louis, MO, USA) was added to each well and the plate was incubated at 37 °C in 5% CO2 atmosphere, and incubated for another 4 h. Then, DMSO (Sigma) was added to terminate the reaction. The cell proliferation was assessed by measuring the optical density (OD) at 570 nm.2.6 Cell migration and invasion assays
Cell invasion assay was performed using Transwell chamber coated with matrigel.18 Before the insert, cells were seeded in 24-well plates in a humidified at 37 °C and under conditions of 5% CO2. After transfection 24 h, the cells were transferred to the upper chamber in serum-free culture medium. 500 μl DMEM with 10% FBS was added at the lower compartment. After incubation at 37 °C 48 h, and non-invasive cells scraped off with a cotton swab. Cells located on the lower surface of the chamber were fixed and stained with crystal violet. Invading cells counted under a microscope. The migration assay was done by the same procedure, except that the inserts of the chambers were not coated with Matrigel.2.7 In vivo xenograft tumor assay
A549 cells (1 × 106 cells/0.1 ml) transfected with sh-TRIM37 or mock re-suspended in PBS and injected subcutaneously into the left flank of each mouse. Tumor size was measured every 7 days using a caliper, and calculated using the formula: volume = length × width2 × π/6. About 4 weeks after inoculation, mice were euthanized by subcutaneous injection with sodium pentobarbital (40 mg kg−1) and the tumors were weighed. All surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. This assay was performed in triplicate.2.8 Statistical analysis
The statistical analysis was performed using Prism 5. Statistical analysis was performed using one-way ANOVA analysis. Statistical significance was determined at the level of P < 0.05.3 Results
3.1 TRIM37 is highly expressed in human NSCLC cell lines
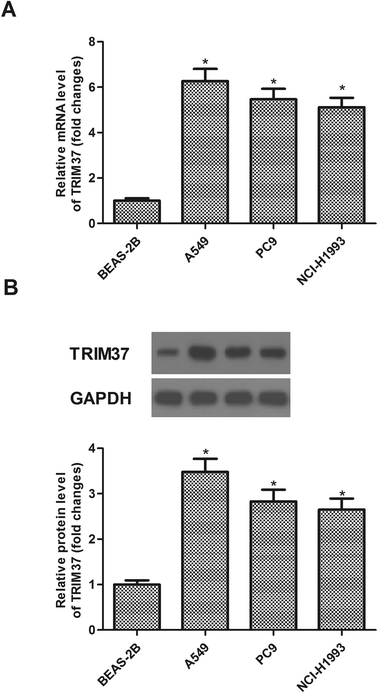
We examined TRIM37 expression in three NSCLC cell lines (A549, PC9 and NCI-H1993) and normal cell line BEAS-2B by qRT-PCR and western blot. The expression of TRIM37 at the mRNA level was significantly increased in three NSCLC cell lines in comparison with that in the normal cell line BEAS-2B (Fig. 1A). Consistently, western blot analysis showed that the protein expression of TRIM37 was also increased in NSCLC cell lines (Fig. 1B).3.2 Knockdown of TRIM37 inhibits NSCLC cell proliferation in vitro
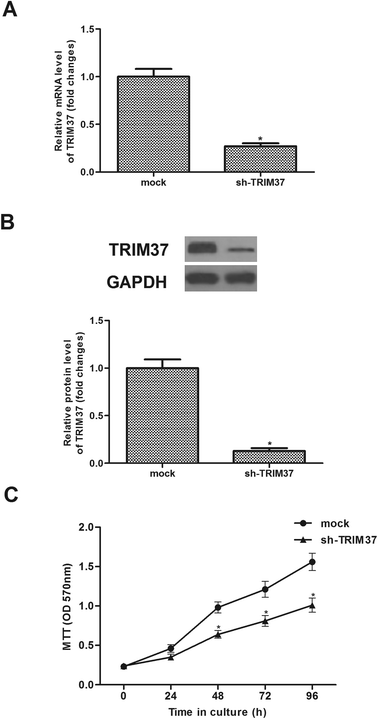
Sh-TRIM37 was transfected into A549 cells for 24 h. As indicated in Fig. 2A, sh-TRIM37 indeed downregulated the mRNA levels of TRIM37. Then, we found that the TRIM37 protein was lower than that of control group (Fig. 2B). The effect of sh-TRIM37 on the proliferation of A549 cells was detected by the MTT assay. The experiment showed that the knockdown of TRIM37 significantly inhibited the proliferation of A549 cells (Fig. 2C).3.3 Knockdown of TRIM37 inhibits NSCLC cell migration and invasion in vitro
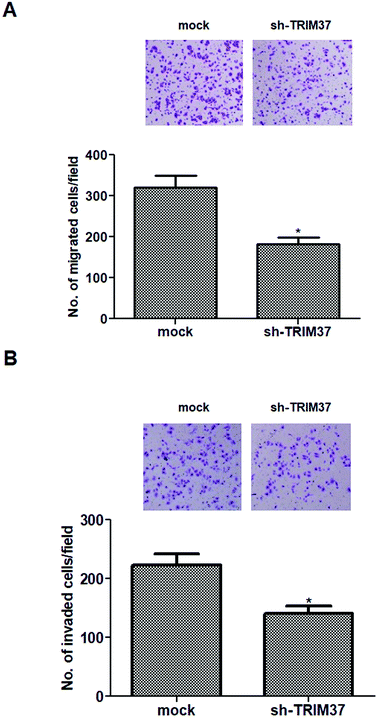
To evaluate the effects of TRIM37 on tumor metastasis, the Transwell migration and invasion assays were performed. As shown in Fig. 3A, the Transwell migration assay showed that the number of migrated cells was significantly lower in the knockdown of TRIM37 group than in the mock group. Similarly, knockdown of TRIM37 also inhibited the invasion of A549 cells (Fig. 3B).3.4 Knockdown of TRIM37 inhibits EMT phenotype in NSCLC cells
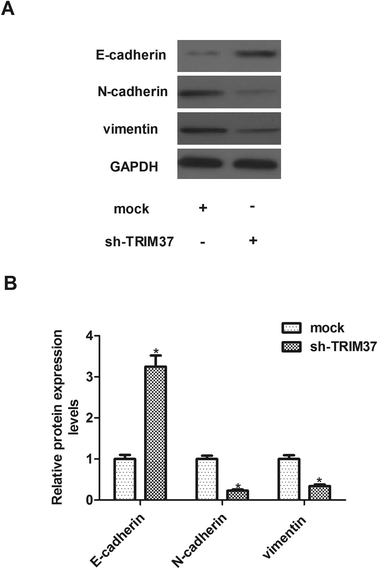
EMT plays an important role in cancer metastasis and is characterized by repression of epithelial markers, such as E-cadherin, and induction of mesenchymal markers, such as N-cadherin and vimentin. Therefore, we investigated the effect of TRIM37 on EMT-related markers expression in A549 cells. Compared to the mock group, the E-cadherin protein level was significantly increased in sh-TRIM37-transfected A549 cells. In contrast, the protein levels of N-cadherin and vimentin were significantly lower in sh-TRIM37-transfected cells than that of in the mock group (Fig. 4).3.5 Knockdown of TRIM37 inhibits the growth of NSCLC in vivo
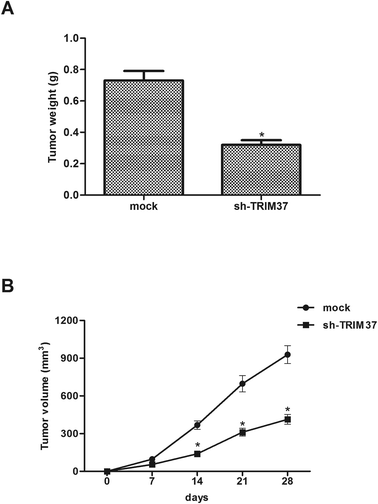
In order to examine the level of TRIM37 on NSCLC growth in vivo, A549 cells transfected with sh-TRIM37 or mock were injected into the flanks of nude mice. As shown in Fig. 5A, compared to control mice, knockdown of TRIM37 significantly reduced the weight of tumors. In addition, the tumor volume was remarkably decreased in the TRIM37-silenced tumor-bearing mice in comparison to the control group (Fig. 5B).3.6 Knockdown of TRIM37 inhibits the activation of the Wnt/beta-catenin pathway in NSCLC cells
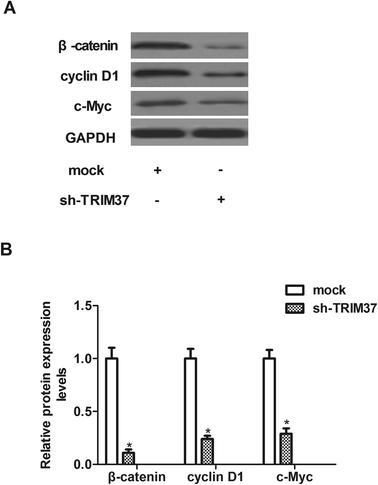
Wnt/beta-catenin signaling pathway is an important signal pathway in cancer development. Therefore, we examined the effect of TRIM37 on the activation of Wnt/beta-catenin signaling pathway in NSCLC cells using western blot assay. As shown in Fig. 6, knockdown of TRIM37 efficiently inhibited the expression of β-catenin, cyclinD1 and c-myc in A549 cells, as compared with the mock group.4 Discussion
In the present study, we found that TRIM37 was highly expressed in human NSCLC cell lines. Knockdown of TRIM37 significantly inhibited the proliferation in vitro and xenografted tumor growth in vivo. Furthermore, knockdown of TRIM37 suppressed NSCLC cell migration and invasion by inhibiting the EMT phenotype. Lastly, knockdown of TRIM37 greatly down-regulated the protein levels of β-catenin, cyclinD1 and c-myc in A549 cells.In recent years, TRIM37 has been involved in many types of tumor development and progression. A study reported that the expression levels of TRIM37 pancreatic tumor tissue were significantly higher than the adjacent normal tissues; and overexpression of TRIM37 promoted the growth and migration of pancreatic cancer cells.19 Another study showed that TRIM37 expression was notably up-regulated in hepatocellular carcinoma samples and was associated with advanced stage and tumor volume. Consistent with the above results, herein, we observed that TRIM37 was highly expressed in human NSCLC cell lines, and knockdown of TRIM37 inhibited NSCLC cell proliferation in vitro and tumor growth in vivo. These results suggest that TRIM37 may act as an oncogene in the development and progression of NSCLC.
The invasion and metastasis of cancer cells is one of the most important features of malignant cell behavior.20–22 Previous studies showed that TRIM37 can regulate tumor cell metastasis. In pancreatic cancer cells, knockdown TRIM37 reduced the ability of metastasis in pancreatic cancer cells.19 Jiang et al. reported that TRIM37 induced the EMT process, thereby promoting metastasis of hepatocellular carcinoma.23 Similarly, herein, we observed that knockdown of TRIM37 inhibited the migration and invasion of NSCLC cells. EMT is a dynamic process mediating cancer metastasis. During EMT procedure, epithelial cells downregulate the expression of epithelial markers including E-cadherin, dissolve cell–cell junctions, lose their apical-basal polarity and acquire migratory and invasive abilities.24 In the current study, we observed that knockdown of TRIM37 up-regulated the protein expression level of E-cadherin, and down-regulated the protein expression levels of N-cadherin and vimentin in A549 cells. These results imply that knockdown of TRIM37 suppressed NSCLC cell migration and invasion by inhibiting the EMT phenotype.
Growing body of evidence suggests that Wnt/beta-catenin signaling pathway plays an important role in regulating cell proliferation, migration, invasion, and apoptosis.25 This pathway is highly active in the progression of NSCLC, leading to proliferation and metastasis of lung cancer cells.26,27 β-catenin is a key regulator in the Wnt signaling pathway. Activated β-catenin induced the EMT process and invasion in NSCLC.28 In addition, β-catenin can induce the transcription of target genes, including c-myc, cyclinD1 and MMP7, resulting in tumor cells proliferation and metastasis.29 Thus, preventing Wnt/beta-catenin signaling pathway might be a promising strategy for the treatment of NSCLC. In this study, we found that knockdown of TRIM37 inhibited the protein expression levels of β-catenin, cyclinD1 and c-myc in NSCLC cells. These data suggest that knockdown of TRIM37 inhibited the proliferation and tumorigenesis in NSCLC cells, at least partially, through suppressing the Wnt/beta-catenin signaling pathway.
5 Conclusion
In conclusion, these findings indicated that knockdown of TRIM37 inhibited the proliferation, invasion and migration of NSCLC cells both in vivo and in vitro. Therefore, TRIM37 may be a potential therapeutic target for treating NSCLC.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
None.References
- Q. F. Liu, Z. F. Zhang, G. J. Hou, G. Y. Yang and Y. He, Lung, 2016, 194, 393–400 CrossRef CAS PubMed.
- W. Chen, J. Wang, S. Liu, S. Wang, Y. Cheng, W. Zhou, C. Duan and C. Zhang, J. Exp. Clin. Cancer Res., 2016, 35, 76 CrossRef PubMed.
- S. E. Schild, P. J. Stella, S. M. Geyer, J. A. Bonner, W. L. Mcginnis, J. A. Mailliard, J. Brindle, A. Jatoi and J. R. Jett, J. Clin. Oncol., 2003, 21, 3201–3206 CrossRef CAS PubMed.
- R. Pirker, Transl. Lung Cancer Res., 2014, 3, 305–310 CAS.
- T. Wang, R. A. Nelson, A. Bogardus and G. F. Jr, Cancer, 2010, 116, 1518–1525 CrossRef PubMed.
- K. Avela, M. Lipsanen-Nyman, N. Idanheimo, E. Seemanova, S. Rosengren, T. P. Makela, J. Perheentupa, A. D. Chapelle and A. E. Lehesjoki, Nat. Genet., 2000, 25, 298–301 CrossRef CAS PubMed.
- S. Karlberg, M. Lipsanen-Nyman, H. Lassus, J. Kallijarvi, A. E. Lehesjoki and R. Butzow, Mod. Pathol., 2009, 22, 570–578 CrossRef CAS PubMed.
- K. Ozato, D. M. Shin, T. H. Chang and H. C. M. Iii, Nat. Rev. Immunol., 2008, 8, 849 CrossRef CAS PubMed.
- A. E. Lehesjoki, V. A. Reed, R. Mark Gardiner and N. D. Greene, Mech. Dev., 2001, 108, 221–225 CrossRef CAS PubMed.
- J. Kallijarvi, K. Avela, M. Lipsanen-Nyman, I. Ulmanen and A. E. Lehesjoki, Am. J. Hum. Genet., 2002, 70, 1215–1228 CrossRef CAS PubMed.
- J. Jiang, C. Yu, M. Chen, S. Tian and C. Sun, Biochem. Biophys. Res. Commun., 2015, 464, 1120–1127 CrossRef CAS PubMed.
- S. L. Tang, Y. L. Gao and W. Z. Hu, Biomed. Pharmacother., 2018, 99, 59–64 CrossRef CAS PubMed.
- J. Kallijarvi, R. H. Hamalainen, N. Karlberg, K. Sainio and A. E. Lehesjoki, Histochem. Cell Biol., 2006, 126, 325–334 CrossRef PubMed.
- J. Jiang, S. Tian, C. Yu, M. Chen and C. Sun, Tumor Biol., 2016, 37, 2629–2634 CrossRef CAS PubMed.
- S. Bhatnagar and M. R. Green, Cell Cycle, 2015, 14, 1345–1350 CrossRef CAS PubMed.
- S. Bhatnagar, C. Gazin, L. Chamberlain, J. Ou, X. Zhu, J. S. Tushir, C. M. Virbasius, L. Lin, L. J. Zhu and N. Wajapeyee, Nature, 2014, 516, 116–120 CAS.
- K. J. Livak and T. D. Schmittgen, Methods, 2001, 25, 402–408 CrossRef CAS PubMed.
- T. Tian, X. Li, Z. Hua, J. Ma, X. Wu, Z. Liu, H. Chen and Z. Cui, Oncotarget, 2017, 8, 24964–24977 Search PubMed.
- J. Jiang, S. Tian, C. Yu, M. Chen and C. Sun, Tumour Biol., 2016, 37, 2629–2634 CrossRef CAS PubMed.
- A. Hammer and M. Diakonova, BMC Cell Biol., 2016, 17, 31 CrossRef PubMed.
- J. X. Pereira, M. C. Azeredo, F. S. Martins, R. Chammas, F. L. Oliveira, S. N. Santos, E. S. Bernardes and M. C. El-Cheikh, BMC Cancer, 2016, 16, 636 CrossRef PubMed.
- P. Zhou, N. Jiang, G. X. Zhang and Q. Sun, Acta Biochim. Biophys. Sin., 2016, 48, 696–703 CrossRef CAS PubMed.
- J. Jiang, C. Yu, M. Chen, S. Tian and C. Sun, Biochem. Biophys. Res. Commun., 2015, 464, 1120–1127 CrossRef CAS PubMed.
- M. Yilmaz and G. Christofori, Cancer Metastasis Rev., 2009, 28, 15–33 CrossRef PubMed.
- N. Barker, Methods Mol. Biol., 2008, 468, 5–15 CrossRef CAS PubMed.
- Y. Peng, J. Cao, X. Y. Yao, J. X. Wang, M. Z. Zhong, P. P. Gan and J. H. Li, Oncotarget, 2017, 8, 52960–52974 Search PubMed.
- S. Yang, L. Yi, M. Y. Li, C. S. H. Ng, S. L. Yang, S. Wang, Z. Chang, Y. Dong, D. Jing and L. Xiang, Mol. Cancer, 2017, 16, 124 CrossRef PubMed.
- X. Niu, S. Liu, L. Jia and J. Chen, Cell. Physiol. Biochem., 2015, 37, 1527–1536 CrossRef CAS PubMed.
- T. Valenta, G. Hausmann and K. Basler, EMBO J., 2012, 31, 2714–2736 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |