Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication and catalytic properties of ordered cyclopalladated diimine monolayer : investigation on catalytic mechanism†
Linhong Wang a,
Pingping Huang
a,
Pingping Huang a,
Jun Yangab,
Tiesheng Li*a,
Luyuan Maob,
Minghua Liu
a,
Jun Yangab,
Tiesheng Li*a,
Luyuan Maob,
Minghua Liu *c and
Yangjie Wu
*c and
Yangjie Wu *a
*a
aCollege of Chemistry and Molecular Engineering, The Key Lab of Chemical Biology and Organic Chemistry of Henan Province, The Key Lab of Nano-information Materials of Zhengzhou, Zhengzhou, 450001, P. R. China. E-mail: lts34@zzu.edu.cn; Fax: +86-371-67766667
bCollege of Materials Science and Engineering, Zhengzhou University, Zhengzhou, 450001, P. R. China
cBeijing National Laboratory for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China
First published on 12th September 2018
Abstract
“Channel-like” self-assembled monolayers having aliphatic and aromatic diimines (denoted as Si@1DIS, Si@2DIS and Si@3DIS) immobilized on substrates and their palladacycle monolayers (Si@1DIS-Pd, Si@2DIS-Pd and Si@3DIS-Pd) were prepared and characterized. Their catalytic performances were investigated using the Suzuki coupling reaction as a model. Si@3DIS-Pd showed the highest catalytic activity in water without ligands, and better recyclability than that of Si@2DIS-Pd and Si@1DIS-Pd. The reason was the carbon in the aliphatic diimine of Si@2DIS-Pd and Si@1DIS-Pd was easily hydrolyzed because of the active hydrogen of α-C, resulting in poor recyclability. Control of the amount of catalyst could be achieved by modulating the diameter of the channel-like structure, which also affected the catalytic activity. The catalytic process and mechanism were investigated systematically and proposed based on the experimental results obtained by the water contact angle, ultraviolet spectroscopy, X-ray photoelectron spectroscopy, cyclic voltammetry and atomic force spectroscopy. Changes in the morphology of monolayer surfaces during the catalytic process with or without stirring presented a clear process from order to disorder, and indicated that the reaction was a heterogeneous catalytic process occurring on the surface of the catalyst monolayer.
1. Introduction
Palladium (Pd)-catalyzed C–C coupling reactions, such as Suzuki, Heck, Sonogashira as well as other reactions, have important roles in organic chemistry1 and have made major progress,2 including use as homogeneous catalysts. Despite their remarkable usefulness and excellent activity, homogeneous catalysts cannot be recycled, especially in the pharmaceutical industry.3 Therefore, heterogeneous catalysts seem to be preferable because they can be removed by simple filtration leaving few metal residues.4Studies on Pd catalysts immobilized on different substrates have been described5–9 and applied to catalyze coupling reactions10 due to their excellent stability and versatility towards functional groups. However, some problems associated with heterogeneous catalyst must be solved, such as high loading, heavily reaction condition, aggregation on the substrate, and repeatability.11 Meanwhile, finding the true mechanism is one of the most striking weaknesses of current studies on heterogeneous catalysis.12
Different mechanisms of coupling reactions catalyzed by heterogeneous Pd catalysts have been investigated intensively, including the homogeneous process by leaching Pd species,13 the direct role of Pd nanoparticles on surface sites in the catalytic cycle,14 and surface-driven coupling.15 Despite these efforts, there is uncertain evidence of surface-catalyzed cross-coupling chemistry and some results have been contradictory. Therefore, research should be focused to elucidate the dynamics and true species type of Pd catalyst. Such research may enable development of a rational design for catalysts. Hence, suitable analytical methods must be employed for research.
The surface properties of Langmuir–Blodgett (LB) films16 and self-assembled films17 immobilized on solid substrates can be characterized, identified and evaluated by various measurement techniques. These techniques provide deep insights into catalytic behaviour at the molecular level, such as in the design of ligands containing certain functional groups (e.g., Schiff-bases), which exhibit excellent performance in heterogeneous catalysis.18,19 Our research team focuses on the fabrication and heterogeneous catalytic properties of Schiff-based cyclopalladated LB films20 and self-assembly films, which show superior catalytic activity.21 LB films can be destroyed in organic solvents, heating or distilling, which affects experimental results. Therefore, their self-assembled monolayers linked with substrates were selected to overcome these disadvantages.20b
Herein, a series of aliphatic and aromatic cyclopalladated diimine catalysts in the form of “channel like” self-assembled monolayers were designed and prepared to evaluate their activity and recyclability in the Suzuki–Miyaura coupling reaction. In this way, we aimed to provide an “ideal” template for investigating the heterogeneous catalytic mechanism.
2. Experimental section
2.1 General
Chemical regents were purchased from commercial and experimental sources. The fabrication of cyclopalladated diimines SAM-monolayer (Si@1DIS-Pd, Si@2DIS-Pd and Si@3DIS-Pd), general procedure for the Suzuki cross-coupling reaction as a model, inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis and the general procedure for control experiments are presented in ESI.†3. Results and discussion
3.1 Preparation of self-assembled monolayers of cyclopalladated diimines (Si@1DIS-Pd, Si@2DIS-Pd and Si@3DIS-Pd)
The channel-like cyclopalladated diimines grafted on substrates, such as silicon, were fabricated as depicted in Scheme 1. The channel diameter could be adjusted by designing the ligands. Characterization of cyclopalladated diimine self-assembled monolayers (Si@1DIS-Pd, Si@2DIS-Pd and Si@3DIS-Pd), including the immobilization process, were carried out and interpreted as shown below.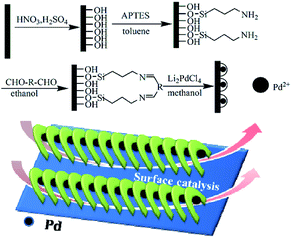 | ||
| Scheme 1 Preparation of cyclopalladated diimine self-assembled monolayers (R = –(CH2)n–: n = 0, Si@1DIS-Pd; n = 3, Si@2DIS-Pd; R = –C6H4–, Si@3DIS-Pd). | ||
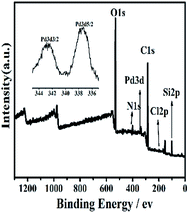
| Element | Pd3d | Si2p | Cl2p | C1s | N1s | O1s |
| Atomic% | 0.46 | 8.70 | 0.60 | 56.66 | 5.16 | 26.85 |
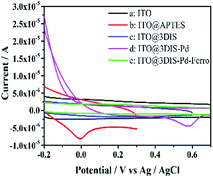 | ||
| Fig. 5 Comparison of cyclic voltammograms of ITO@3DIS-Pd during the preparation. (a) ITO; (b) ITO@APTES; (c) ITO@3DIS; (d) ITO@3DIS-Pd and (e) ITO@3DIS-Pd reacted with 2-ethylamino ferrocene. | ||
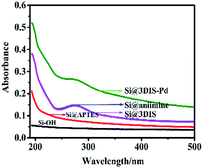
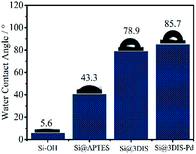
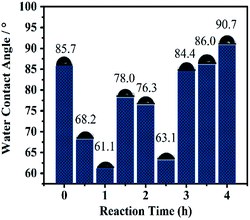
Characterization of the WCA as well as the UV-vis, XPS, AFM and CV data obtained above demonstrated that ordered cyclopalladated diimine self-assembled monolayers were fabricated. Also, the Pd content in cyclopalladated diamine monolayers was 7.7 × 10−10, 45.1 × 10−10 and 188.67 × 10−10 mmol cm−2 for Si@1DIS-Pd, Si@2DIS-Pd and Si@3DIS-Pd, respectively. Therefore, they were used to explore surface chemical reactions using the Suzuki coupling reaction as a model.24
3.2 Catalytic properties and recycling of Si@1DIS-Pd, Si@2DIS-Pd and Si@3DIS-Pd
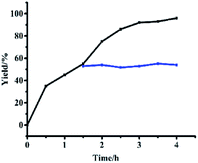
Si@3DIS-Pd had higher activity with less catalyst (Table S4†) compared with that of Si@2DIS-Pd (Table S5†), suggesting that catalytic efficiency is dependent upon the orientation, stability and density of catalysts. Higher yields (97–99%) were obtained in the presence of Si@3DIS-Pd in neat water (Table S4,† entries 1–3 and 5–10), even with electron-rich moieties except for o-OCH3–C6H4–Br due to the steric effect (R = o-OMe, entry 4; o-methyl, entry 11). Especially for 4-chlorobenzaldehyde (Table S4,† entry 12), Si@3DIS-Pd was more catalytically active than that of Si@2DIS-Pd (Table S5,† entry 12) due to the higher stability and ordered orientation of Si@3DIS-Pd. The coupling reactions of derivatives of bromoboronic acids with arylbromides were carried out with Si@3DIS-Pd (Table S4,† entries 14–16) to obtain excellent yields. This difference in catalytic activity was attributed to the easy accessibility of substrates with active sites on monolayer surfaces.
3.3 Investigation of the catalytic mechanism
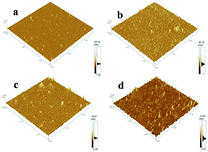
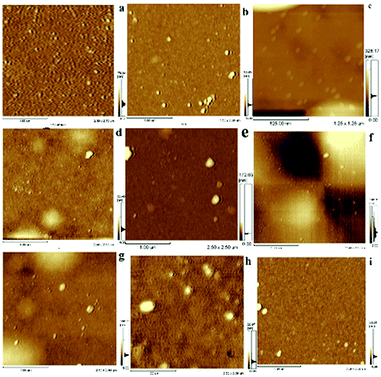
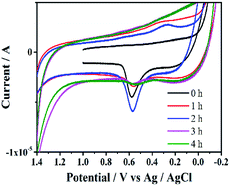
Many efforts have been made to illustrate the changes of active centers and interfaces during catalysis. However, most mechanistic studies have only observed the changes of surfaces and structures before and after the heterogeneous catalytic process.14a Detailed research on the whole process needs to be done. It is necessary to carry out leaching studies,4b kinetic studies, hot filtration tests and poisoning tests to solve the question of heterogeneity.26 Hence, the functional surfaces of monolayer can be designed as “bridges” between homogeneous and heterogeneous systems. Morphologic changes of surfaces during catalysis are important factors for elucidating mechanisms.To elucidate changes on catalytic surfaces related with the catalytic mechanism, the corresponding AFM images as well as Ra and Rms values at different reaction time under stirring (Fig. 8, Table 2) or without stirring (Fig. S10 and S11†) were obtained. AMF (Fig. 8) showed some aggregates of height of 4–5 nm before catalysis (Fig. 8a) but the morphology of the catalyst surface changed dramatically with more aggregates of average height 12 nm in 0.5 h (Fig. 8b), indicating that absorption of substrates occurred on the surface of catalyst monolayers. Round aggregates (30 nm) appeared after 1 h (Fig. 8c), which might have been due to automatic agglomeration of coupling products because the absorption rate was higher than the desorption rate. This phenomenon was the key factor for the catalytic process. It can be observed in Fig. 8d and e that the average height was similar to that at 1 h. Significant morphologic changes on catalyst surfaces were observed during 2.5 h of catalytic processes (Fig. 8f), in which the average height reached ∼120 nm and orientation became irregular. This finding suggested that a violent reaction occurred, accompanied by a sequential adsorption and chemical process (including substrates, intermediates and product), on the surface of the catalyst monolayer. The morphology of the catalyst monolayer turned became more regular during 3–4 h compared with that at 2.5 h, with a height range from 20 nm to 40 nm (Fig. 8g and h). Finally, the ordered catalyst surface could be observed after the used catalyst had been treated by ultrasonic stimulation in water (Fig. 8i). The remarkable changes stated above can be illustrated by active palladacycle on the surface of a catalytic monolayer, including absorption, synergism and adsorption.27 AFM images and Ra/Rms values at different catalytic reaction times without stirring (Fig. S10 and S11†) were also obtained. Results showed that catalytic time must be increased because the covered substrate or product affects the surface catalytic process due to a slower rate of adsorption. This finding was also evidence that stirring could aid surface catalysis (detailed analysis is presented in ESI†).
| Time (h) | 0.0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 4.0 | Ultrasonic |
| Ra (nm) | 4.15 | 1.65 | 16.18 | 3.14 | 3.99 | 25.28 | 6.69 | 1.82 | 1.70 |
| Rms (nm) | 5.86 | 3.56 | 33.49 | 4.99 | 13.38 | 33.19 | 10.27 | 3.46 | 3.29 |
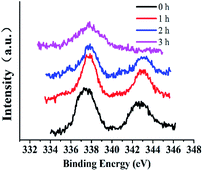 | ||
| Fig. 11 The high-resolution XPS of Pd3d during the catalytic process (Si@3DIS-Pd). (a) 0 h, black line; (b) 1 h, red line; (c) 2 h, blue line; (d) 3 h, pink line. | ||
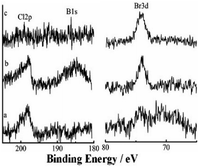 | ||
| Fig. 12 High-resolution XPS of B1s, Cl2p and Br3d during the catalytic process at (a) 1 h, (b) 2 h and (c) 3 h. | ||
Based on the investigations mentioned above, catalytic processes were proposed (Scheme 2). Ph–Br was absorbed and reacted with Pd(0) species on the surface to form oxidation products, followed by insertion with PhB(OH)2 to give an organometallic. Finally, reductive elimination of intermediates yielded the target molecules, accompanied by the generation of Pd(0) species to complete the catalytic cycle.21
4. Conclusions
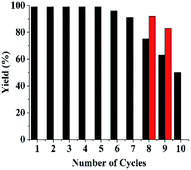
Channel-like self-assembled monolayers with aliphatic and aromatic diimines (denoted as Si@1DIS, Si@2DIS and Si@3DIS) immobilized on substrates and their palladacycle monolayers (Si@1DIS-Pd, Si@2DIS-Pd and Si@3DIS-Pd) were fabricated and characterized. Their catalytic properties for the Suzuki coupling reaction were investigated. Si@3DIS-Pd showed higher catalytic activity in neat water and better recyclability (at least eight times) without Pd leaching. These features can meet the specifications of the pharmaceutical industry better than that of Si@2DIS-Pd and Si@1DIS-Pd because the carbon atom in the aliphatic diimine of Si@2DIS-Pd and Si@1DIS-Pd was easily hydrolyzed due to an active hydrogen of α-C, resulting in poor recyclability. Control of the amount of catalyst immobilized could be achieved by adjusting the channel-like structure, which also enhanced the catalytic activity. The catalytic process and mechanism were investigated systematically and proposed based on experimental results. AFM showed stirring to be an important factor in the catalytic process (particularly in a heterogeneous system) which could improve the reaction rate because of the equilibrium between sorption and desorption on the surface. Results indicated that catalysis was heterogeneous on the surface of the monolayer. These results will allow a rational method to enhance the activity, selectivity and application of heterogeneous catalysts.Conflicts of interest
There are no conflicts.Acknowledgements
The authors gratefully acknowledge the Chinese Academy of Sciences (XDA09030203), Natural Science Foundation of China (20973157, 21172200) and NSFH (142300410223) for their financial support. The authors thank Professor Luyuan Mao for AFM analyses.Notes and references
- (a) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (b) R. Chinchilla and C. Nájera, Chem. Rev., 2014, 114(3), 1783 CrossRef PubMed; (c) M. Tobisu and N. Chatani, Acc. Chem. Res., 2015, 48(6), 1717–1726 CrossRef PubMed; (d) T. Iwasaki and N. Kambe, Top. Curr. Chem., 2016, 374(5), 66 CrossRef PubMed; (e) S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1439 CrossRef PubMed; (f) N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609 CrossRef.
- (a) D. Wang, D. Denux, J. Ruiz and D. Astruc, Adv. Synth. Catal., 2013, 355, 129 CrossRef; (b) X. F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047 CrossRef PubMed; (c) C. Torborga and M. Beller, Adv. Synth. Catal., 2009, 351, 3027 CrossRef; (d) V. Farina, Adv. Synth. Catal., 2004, 364, 1553 CrossRef; (e) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644 CrossRef PubMed.
- (a) J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177–2250 CrossRef PubMed; (b) M. Butters, D. Catterick, A. Craig, A. Curzons, D. Dale, A. Gillmore, S. P. Green, I. Marziano, J.-P. Sherlock and W. White, Chem. Rev., 2006, 106, 3002 CrossRef PubMed.
- (a) Á. Molnár, Chem. Rev., 2011, 111, 2251 CrossRef PubMed; (b) S. Navalona, A. Dhakshinamoorthyb, M. Alvaroa and H. Garciaa, Coord. Chem. Rev., 2016, 312, 99 CrossRef; (c) S. Paul, M. M. Islam and S. M. Islam, RSC Adv., 2015, 5, 42193–42221 RSC; (d) S. Santoro, S. I. Kozhushkov, L. Ackermann and L. Vaccaro, Green Chem., 2016, 18, 3471–3493 RSC; (e) A. Dhakshinamoorthy, M. Opanasenko, J. Čejka and H. Garcia, Adv. Synth. Catal., 2013, 355, 247 Search PubMed.
- (a) J. J. E. Hardy, S. Hubert, D. J Macquarrie and A. J Wilson, Green Chem., 2004, 6, 53 RSC; (b) Y. M. Yamada, S. M. Sarkar and Y. Uozumi, J. Am. Chem. Soc., 2012, 134, 3190 CrossRef PubMed; (c) Y. Li, E. Boone and M. A. El-Sayed, Langmuir, 2002, 18, 4921 CrossRef; (d) P. Boomi, H. G. Prabu and J. Mathiyarasu, Eur. J. Med. Chem., 2014, 72, 18 CrossRef PubMed.
- (a) B. Karimi, D. Elhamifar, J. H. Clark and A. J. Hunt, Org. Biomol. Chem., 2011, 9, 7420 RSC; (b) M. Kim, J. C. Park, A. Kim, K. H. Park and H. Song, Langmuir, 2012, 28, 6441 CrossRef PubMed; (c) Z. Chen, Z.-M. Cui, F. Niu, L. Jiang and W.-G. Song, Chem. Commun., 2010, 46, 6524 RSC; (d) J. Zhi, D. Song, Z. Li, X. Lei and A. Hu, Chem. Commun., 2011, 47, 10707 RSC.
- (a) W. Fang, J. Yang and J. Gong, Adv. Funct. Mater., 2012, 22, 842 CrossRef; (b) K. Shimizu, R. Maruyama, S. Komai, T. Kodama and Y. Kitayama, J. Catal., 2004, 227, 202 CrossRef; (c) M. Mora, C. Jiménez-Sanchidrián and J. R. Ruiz, J. Mol. Catal. A: Chem., 2008, 285, 79 CrossRef; (d) R. M. Mohamed and E. S. Baeissa, Appl. Catal., A, 2013, 464, 218 CrossRef; (e) P. D. Stevens, G. Li, J. Fan, M. Yen and Y. Gao, Chem. Commun., 2005, 4435 RSC.
- (a) M. Lv, W. Xie, S. Sun, G. Wu, L. Zheng, S. Chu, C. Gao and J. Bao, Catal. Sci. Technol., 2015, 5, 2925–2934 RSC; (b) X. Fan, G. Zhanga and F. Zhanga, Chem. Soc. Rev., 2015, 44, 3023–3035 RSC; (c) H. Sakurai, T. Tsukuda and T. Hirao, J. Org. Chem., 2002, 67, 2721 CrossRef PubMed; (d) D. S. Su, S. Perathoner and G. Centi, Chem. Rev., 2013, 113, 5782–5816 CrossRef PubMed.
- (a) M. Opanasenko, P. Štěpnička and J. Čejka, RSC Adv., 2014, 4, 65137–65162 RSC; (b) S. Navalona, A. Dhakshinamoorthyb, M. Alvaroa and H. Garciaa, Coord. Chem. Rev., 2016, 312, 99–148 CrossRef; (c) Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083–2094 RSC; (d) A. Dhakshinamoorthy, A. M. Asiric and H. Garcia, Chem. Soc. Rev., 2015, 44, 1922–1947 RSC; (e) Q. Sun, Z. Dai, X. Meng and F.-S. Xiao, Chem. Soc. Rev., 2015, 44, 6018–6034 RSC; (f) D. S. Su, S. Perathoner and G. Centi, Chem. Rev., 2013, 113, 5782–5816 CrossRef PubMed; (g) H. Fan, Z. Qi, D. Sui, F. Mao, R. Chen and J. Huang, Chin. J. Catal., 2017, 38(3), 589–596 CrossRef.
- (a) R. Franzén and Y. J. Xu, Can. J. Chem., 2005, 83, 266 CrossRef; (b) M. Lamblin, L. Nassar-Hardy, J.-C. Hierso, E. Fouquet and F.-X. Felpin, Adv. Synth. Catal., 2010, 352, 33 CrossRef; (c) J. Wei, J. Jiao, J. Feng, J. Lv, X. Zhang, X. Shi and Z. Chen, J. Org. Chem., 2009, 74, 6283 CrossRef PubMed; (d) S. Ogasawara and S. Kato, J. Am. Chem. Soc., 2010, 132, 4608 CrossRef PubMed; (e) B. Yuan, Y. Pan, Y. Li, B. Yin and H. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4054 CrossRef PubMed.
- L. Jin and J. Liebscher, Chem. Rev., 2007, 107, 13 Search PubMed.
- (a) N. T. S. Phan, M. V. D. Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609 CrossRef; (b) L. Xue and Z. Lin, Chem. Soc. Rev., 2010, 39, 1692 RSC.
- (a) W. He and Y. Li, Chem.–Eur. J., 2012, 18, 9813 CrossRef PubMed; (b) J.-S. Chen, A. N. Vasiliev, A. P. Panarello and J. G. Khinast, Appl. Catal., A, 2007, 325, 76 CrossRef; (c) Z. Niu, Q. Peng, Z. Zhuang and M. Pérez-Lorenzo, J. Phys. Chem. Lett., 2012, 3, 167 CrossRef; (d) S. MacQuarrie, J. H. Horton, J. Barnes, K. McEleney, H.-P. Loock and C. M. Crudden, Angew. Chem., 2008, 120, 3324 CrossRef.
- (a) P. J. Ellis, I. J. S. Fairlamb, S. F. J. Hackett, K. Wilson and A. F. Lee, Angew. Chem., Int. Ed., 2010, 49, 1820 CrossRef PubMed; (b) L. Shao, B. Zhang, W. Zhang, S. Y. Hong, R. Schlçgl and D. S. Su, Angew. Chem., 2013, 125, 2168 (Angew. Chem., Int. Ed., 2013, 52, 2114) CrossRef; (c) B. M. Choudary, S. Madhi, N. S. Chowdari, M. L. Kantam and B. J. Sreedhar, J. Am. Chem. Soc., 2002, 124, 14127 CrossRef PubMed.
- (a) J. J. Davis, K. S. Coleman, K. L. Busuttil and C. B. Bagshaw, J. Am. Chem. Soc., 2005, 127, 13082 CrossRef PubMed; (b) J. J. Davis, C. B. Bagshaw, K. L. Busuttil, Y. Hanyu and K. S. Coleman, J. Am. Chem. Soc., 2006, 128, 14135–14141 CrossRef PubMed.
- A. K. Kakkar, Chem. Rev., 2002, 102, 3579 CrossRef PubMed.
- (a) A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef PubMed; (b) A. K. Kakkar, Chem. Rev., 2002, 102, 3579–3588 CrossRef PubMed; (c) Y. Yang, S. Wang, Y. Wang, X. Wang, Q. Wang and M. Chen, Biotechnol. Adv., 2014, 32(7), 1301–1316 CrossRef PubMed; (d) A. Indra, C. S. Gopinath, S. Bhaduri and G. K. Lahiri, Catal. Sci. Technol., 2013, 3, 1625–1633 RSC; (e) T. Gupta and M. E. van der Boom, J. Am. Chem. Soc., 2006, 128, 8400–8401 CrossRef PubMed; (f) J. Hu, Y. Wang, M. Han, Y. Zhou, X. Jiang and P. Sun, Catal. Sci. Technol., 2012, 2, 2332–2340 RSC; (g) C. Ren, J. Zhang, M. Chen and Z. Yang, Chem. Soc. Rev., 2014, 43(21), 7257–7266 RSC; (h) L. K. Johnson, C. M. Killian and M. Brookhart, J. Am. Chem. Soc., 1995, 117(23), 6414–6415 CrossRef.
- (a) H. P. Mungse, S. Verma, N. Kumar, B. Sain and Om P. Khatri, J. Mater. Chem., 2012, 22, 5427–5433 RSC; (b) D. Domin, D. Benito-Garagorri and K. Mereiter, Organometallics, 2005, 24(16), 3957–3965 CrossRef; (c) P. G. Cozzi, Chem. Soc. Rev., 2004, 33, 410 RSC; (d) D. V. Jawale, E. Gravel, C. Boudet, N. Shah, V. Geertsen, H. Li, I. N. N. Namboothiri and E. Doris, Catal. Sci. Technol., 2015, 5, 2388–2392 RSC; (e) E. F. McCord, S. J. McLain and L. T. J. Nelson, Macromolecules, 2001, 34(3), 362–371 CrossRef; (f) Q. Zhao, B. Chan, W. Zhang, L. Yang, G. Zhang, F. Zhang and X. Fan, Ind. Eng. Chem. Res., 2014, 53, 4232–4238 CrossRef; (g) H. P. Mungse, S. Verma, N. Kumar, B. Sain and O. P. Khatri, J. Mater. Chem., 2012, 22, 5427 RSC; (h) H. Su, S. Wu, Z. Li, Q. Huo, J. Guan and Q. Kan, Appl. Organomet. Chem., 2015, 29, 462 CrossRef; (i) P. K. Khatri, S. Choudhary, R. Singh, S. L. Jain and O. P. Khatri, Dalton Trans., 2014, 43, 8054 RSC; (j) Q. Zhao, C. Bai, W. Zhang, Y. Li, G. Zhang, F. Zhang and X. Fan, Ind. Eng. Chem. Res., 2014, 53, 4232 CrossRef; (k) Z. Li, S. Wu, D. Zheng, J. Liu, H. Liu, H. Lu, Q. Huo, J. Guan and Q. Kan, Appl. Organomet. Chem., 2014, 28, 317 CrossRef.
- (a) M. Rezaeivala and H. Keypour, Coord. Chem. Rev., 2014, 280, 203–245 CrossRef; (b) P. A. Vigato, V. Peruzzo and S. Tamburini, Coord. Chem. Rev., 2012, 256, 953–1114 CrossRef; (c) C. J. Whiteoak, G. Salassa and A. W. Kleij, Chem. Soc. Rev., 2012, 41, 622–661 RSC; (d) G. S. Smith and S. F. Mapolie, J. Mol. Catal. A: Chem., 2004, 213(2), 187–192 CrossRef; (e) E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38(2), 606–631 RSC; (f) A. El-Faham and F. Albericio, Chem. Rev., 2011, 111(11), 6557–6602 CrossRef PubMed; (g) M. Semler, J. Čejka and P. Štěpnička, Catal. Today, 2014, 227, 207–214 CrossRef.
- (a) Y. Zhao, N. Zhao, M. Zou, T. Li, G. Li, J. Li and Y. Wu, Appl. Organomet. Chem., 2016, 30, 540–549 CrossRef; (b) Z. Fu, T. Li, B. Mu, L. Mao, G. Li, W. Xu and Y. Wu, J. Mol. Catal. A: Chem., 2012, 363, 200 CrossRef; (c) N. Zhao, F. Wang, M. Zhou, T. LI, H. Liu, W. Xu and Y. Wu, Chin. J. Catal., 2013, 34(8), 1583–1588 CrossRef; (d) B. Mu, T. Li, C. Li, P. Liu, W. Shang and Y. Wu, Tetrahedron, 2009, 65, 2599 CrossRef.
- (a) H. Liu, T. Li, X. Xue, W. Xu and Y. Wu, Catal. Sci. Technol., 2016, 6, 1667 RSC; (b) N. Zhao and T. Li, ChemCatChem, 2013, 5, 1481 CrossRef; (c) Z. Fu, T. Li, X. He, J. Liu, W. Xu and Y. Wu, J. Mol. Catal. A: Chem., 2014, 395, 293 CrossRef; (d) Y. Li, Z. Fu, L. Rao, L. Wang, R. Li, T. Li and Y. Wu, J. Chin. Chem. Soc., 2014, 61, 397 CrossRef; (e) H. Liu, X. Xue, T. Li, J. Wang, W. Xu, M. Liu, P. Chen and Y. Wu, RSC Adv., 2016, 6, 84815 RSC; (f) K. Zou, H. Liu, T. Li, P. Chen, M. Liu and Y. Wu, RSC Adv., 2015, 5, 16654 RSC; (g) Z. Fu, J. Liu, X. He, T. Li and Y. Wu, RSC Adv., 2014, 4, 26413 RSC; (h) Z. Fu, N. Zhang, T. Li, W. Xu and Y. Wu, J. Colloid Interface Sci., 2013, 394, 409 CrossRef PubMed; (i) Z. Q. Xue, P. P. Huang, T. S. Li, P. L. Chen, M. H. Liu and Y. J. Wu, Nanoscale, 2017, 9, 781–791 RSC.
- (a) J. S. Sharp, D. J. Farmer and J. Kelly, Langmuir, 2011, 27, 9367–9371 CrossRef PubMed; (b) A. W. Adamson and A. P. Gast, The Solid-Liquid Interface—Contact Angle, Academic Press, New York, 1997, pp. 347–383 Search PubMed.
- E. S. Gadelmawla, M. M. Koura and T. M. A. Maksoud, Roughness parameters, J. Mater. Process. Technol., 2002, 123, 133–145 CrossRef.
- (a) B. Yuan, Y. Pan, Y. Li, B. Yin and H. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4054–4058 CrossRef PubMed; (b) S. Ogasawara and S. Kato, J. Am. Chem. Soc., 2010, 132, 4608–4613 CrossRef PubMed.
- (a) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644–4680 CrossRef PubMed; (b) A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef PubMed; (c) J. Wei, J. Jiao, J. Feng, J. Lv, X. Zhang, X. Shi and Z. Chen, J. Org. Chem., 2009, 74, 6283–6286 CrossRef PubMed.
- J. A. Widegren and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 198, 317–324 CrossRef.
- (a) K. Köhler, W. Kleist and S. S. Pröckl, Inorg. Chem., 2007, 46, 1876–1883 CrossRef PubMed; (b) F. Y. Zhao, M. Shirai, Y. Ikushima and M. Arai, J. Mol. Catal. A: Chem., 2002, 180, 211–219 CrossRef.
- (a) Z. Zhang, T. Sun, C. Chen, F. Xiao, Z. Gong and S. Wang, ACS Appl. Mater. Interfaces, 2014, 6(23), 21035–21040 CrossRef PubMed; (b) H. Li, G. A. Grasa and T. J. Colacot, Org. Lett., 2010, 12(15), 3332–3335 CrossRef PubMed.
- M. Choi, D. H. Lee, K. Na, B. W. Yu and R. Ryoo, Angew. Chem., Int. Ed., 2009, 48(20), 3673–3676 CrossRef PubMed.
- J. Zou, S. G. Stewart, C. L. Raston and K. S. Iyera, Chem. Commun., 2011, 47, 1803–1805 RSC.
- (a) C. Röhlich, A. S. Wirth and K. Köhler, Chem.–Eur. J., 2012, 18, 15485–15494 CrossRef PubMed; (b) F. Rajabi and W. R. Thiel, Adv. Synth. Catal., 2014, 356(8), 1873–1877 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06365f |
| This journal is © The Royal Society of Chemistry 2018 |