Open Access Article
Open Access ArticleSynthesis and anticancer studies of Michael adducts and Heck arylation products of sesquiterpene lactones, zaluzanin D and zaluzanin C from Vernonia arborea†
Tushar R. Valkute‡
a,
Eswar K. Aratikatla‡ ab,
Neha A. Gupta‡c,
S. Gangac,
Manas K. Santra*c and
Asish K. Bhattacharya
ab,
Neha A. Gupta‡c,
S. Gangac,
Manas K. Santra*c and
Asish K. Bhattacharya *ab
*ab
aDivision of Organic Chemistry, CSIR-National Chemical Laboratory (CSIR-NCL), Dr Homi Bhabha Road, Pune-41108, India. E-mail: ak.bhattacharya@ncl.res.in
bAcademy of Scientific and Innovative Research (AcSIR), CSIR-NCL, Pune-411 008, India
cBiology of Cancer and Chronic Diseases, National Centre for Cell Sciences, Ganeshkhind Road, Pune-411 007, India
First published on 14th November 2018
Abstract
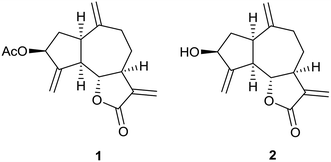
Sesquiterpene lactones containing α-methylene-γ-lactones, zaluzanin D 1 and zaluzanin C 2 were isolated from the leaves of Vernonia arborea. Several diverse Michael adducts (3–22) and Heck arylation analogs (23–34) of 1 have been synthesized by reacting with various amines and aryl iodides, respectively and were assayed for their in vitro anticancer activities against human breast cancer cell lines MCF7 and MDA-MB-231. Among all the synthesized analogs, Michael adducts 9 and 10 showed better anticancer activities as compared to 1. However, among these compounds, only 10 has minimal cytotoxic effect on normal breast epithelial MCF10A cells. Our detailed mechanistic studies reveal that compounds 9 and 10 execute their antiproliferative activity through induction of apoptosis and thereby inhibit the cancer cells proliferation and compound 10 could be a lead compound for designing potential anti-cancer compound.
Introduction
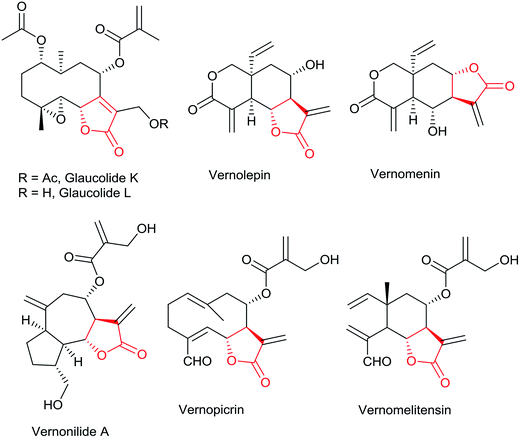
The generation of diverse chemical libraries using natural products as scaffolds is considered as one of the effective methods for drug discovery.1 Sesquiterpenes bearing α,β-unsaturated γ-lactones are ubiquitous in nature and have been reported to be isolated from various genera of the family Asteraceae but also occur sporadically in other angiosperm families, Apiaceae, Magnoliaceae and even in some liverworts. They exhibit diverse biological activities such as anti-microbial, anti-inflammatory, antiulcer, antiviral, anticancer and antimalarial activities.2 The genus Vernonia (family Asteraceae) consists of approximately 1000 species of herbs and shrubs.3 Several research groups have isolated structurally diverse sesquiterpene lactones from various species of genus Vernonia possessing various biological properties (Fig. 1).4,5 Sesquiterpene lactones react with nucleophilic sulfhydryl groups present in enzymes, proteins, and glutathione.6 The use of sesquiterpene lactones as therapeutic agents is limited due to their poor water solubility. To overcome this, amino-adducts of sesquiterpene lactones have been prepared by adding different amines to the α-methylene-γ-lactone substructure to enhance the water solubility of the parent molecules and to retain their biological activity.7,8 Regeneration of parent α,β-unsaturated γ-lactone occurs by the retro-Michael reaction, potentially through bioactivation at the site of action. This prodrug approach has transformed several sesquiterpene lactones such as alantolactone, ambrosin, arglabin, costunolide, helenalin, parthenolide and ivangustin, into successful clinical candidates (Fig. 1).8Continuing our interest in naturally occurring sesquiterpene lactones9 and other bioactive secondary metabolites, we wished to take up the chemical examination of V. arborea leaves for the isolation of bioactive secondary metabolites, zaluzanin C and zaluzanin D. In the present study, several structurally diverse Michael adducts of zaluzanin D have been synthesized with the formation of one or two new C–N bonds. Analogs of zaluzanin D with a new C–C bond formation have also been synthesized by using Pd catalyzed Heck coupling reaction. The in vitro anticancer activities of all the analogs were tested against human breast cancer cell lines MCF7 and MDA-MB-231.
Results and discussion
A portion of the petroleum ether extract (5.3 g) was flash chromatographed on CombiFlash Companion, Isco Teledyne Inc., USA using RediSep® column (SiO2, 2 × 12 g) and elution was carried out isocratically with ethyl acetate–petroleum ether (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96) to furnish a colorless solid. It was identified as zaluzanin D 1, a sesquiterpene lactone having guaianolide skeleton (α-methylene-γ-lactone) based on its NMR and HRMS spectra and comparison with an authentic sample10 (Fig. 2). Further flash chromatography with ethyl acetate–petroleum ether (6
96) to furnish a colorless solid. It was identified as zaluzanin D 1, a sesquiterpene lactone having guaianolide skeleton (α-methylene-γ-lactone) based on its NMR and HRMS spectra and comparison with an authentic sample10 (Fig. 2). Further flash chromatography with ethyl acetate–petroleum ether (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
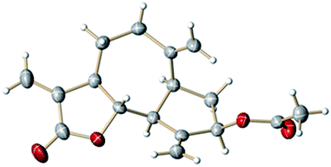
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94) furnished zaluzanin C 2. Finally, we proved the structure of zaluzanin D 1 by its single crystal X-ray analysis (Fig. 3).11
94) furnished zaluzanin C 2. Finally, we proved the structure of zaluzanin D 1 by its single crystal X-ray analysis (Fig. 3).11
Both these compounds were assayed against human breast cancer cell line, MCF7 and zaluzanin D 1 exhibited an IC50 value of 53.7 μM whereas zaluzanin C 2 was found to be inactive (Table 2).
Synthesis of Michael adducts of zaluzanin D using different chiral/achiral amines
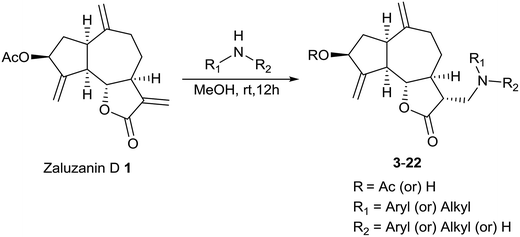
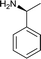
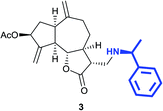
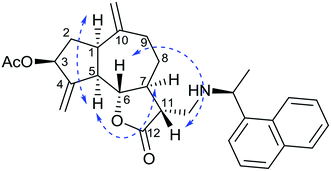
Since Michael addition of primary or secondary amines to α,β-unsaturated lactones resulted in the compounds possessing higher anticancer activities compared to the original compound,7,8 we wished to synthesize a library of amino adducts of zaluzanin D with one or more new C–N bonds. The amino derivatives of zaluzanin D 1 were synthesized via Michael addition of different amines to the α,β-unsaturated γ-lactone functionality present in zaluzanin D. As shown in Scheme 1, a methanolic solution of chiral/achiral amine and zaluzanin D underwent Michael addition at room temperature to furnish various Michael adducts of zaluzanin D, 3–22 (Table 1). Michael addition of chiral amine (S)-(−)-α-methylbenzylamine to zaluzanin D furnished a mixture of two products, compound 3 and its corresponding deacetylated product 4 (entry 1, Table 1) which were isolated by flash chromatography. The formation of deacetylated product 4 may be due to the in situ formation of zaluzanin C, which in turn formed due to the basicity of amine, used in the Michael addition.Addition of the amine to zaluzanin D was found to be diastereoselective, and the stereochemistry of C-11 in compound 3 was assigned12 as α by its NOESY experiment. In the NOESY spectra of compound 3, correlations were observed between 6β-H and 11β-H. Also, 5α-H showed a correlation with 7α-H (Fig. 4).
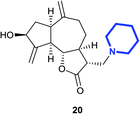
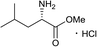
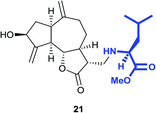
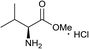
Similarly, the reaction of zaluzanin D 1 with (R)-(+)-α-methylbenzylamine resulted in the formation of compounds 5 and 6. The reaction of zaluzanin D 1 with (S)- and (R)-1-(1-naphthyl) ethylamine also furnished the compounds 7, 8 and 9, 10 respectively (entries 3 and 4, Table 1). However, reaction with (R)- and (S)-1-cyclohexylethylamine furnished the deacetylated product only (compounds 11 and 12, entries 5 and 6, Table 1). Next, we carried out the reaction of zaluzanin D 1 with various six and five-membered cyclic amines, which furnished corresponding C–N derivatives of zaluzanin D 1 (entries 7–11, Table 1). 4-Hydroxypiperidine and morpholine on reaction with zaluzanin D 1 yielded acetylated products (13 and 17) and deacetylated products (14 and 18), respectively (entries 7 and 9, respectively, Table 1). An interesting result was obtained in case of piperazine (entry 9, Table 1), which furnished acetylated dimer 15 and mono deacetylated dimer 16 with two new C–N bonds on both sides of piperazine. However, pyrrolidine and piperidine resulted in the formation of deacetylated products only (19 and 20, respectively) (entries 10 and 11, Table 1). Further, we tried to form a C–N bond of zaluzanin D 1 with amino acid methyl ester hydrochlorides (entries 12 and 13, Table 1). In this case, K2CO3 was used as a base for in situ generation of the free amine, which on reaction with zaluzanin D 1 furnished the deacetylated product 21. However, in case of valine methyl ester hydrochloride, excess use of K2CO3 resulted in the C–O bond formed product 22.
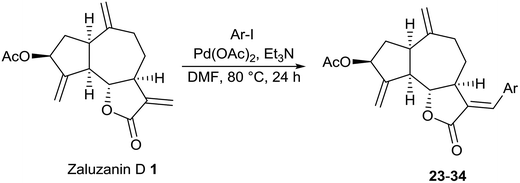
Synthesis of Heck arylated analogs of zaluzanin D using different aryl iodides (23–34)
Although several structural modifications such as Michael addition, reduction, cyclopropanation of the double bond, oxidation of hydroxyl group of sesquiterpene lactones have been reported in literature, transition metal catalyzed cross coupling reactions of sesquiterpene lactones were less explored.13 We presumed that Heck arylation of α,β-unsaturated γ-lactone core of zaluzanin D would provide additional information on structure–activity data of zaluzanin D. As shown in Scheme 2, Heck arylation analogs of zaluzanin D were synthesized using the standard Heck coupling conditions (5 mol% Pd(OAc)2, Et3N in DMF at 80 °C) and readily available aryl iodides.It is pertinent to mention here that arylation preferred to take place at the exo-methylene of α,β-unsaturated γ-lactone substructure over the other two isolated exo-methylene groups present in zaluzanin D 1 which resulted in the exclusive formation of E-olefin14 containing products only (Table 2).
In vitro anticancer activities of Michael adducts of zaluzanin D (3–22)
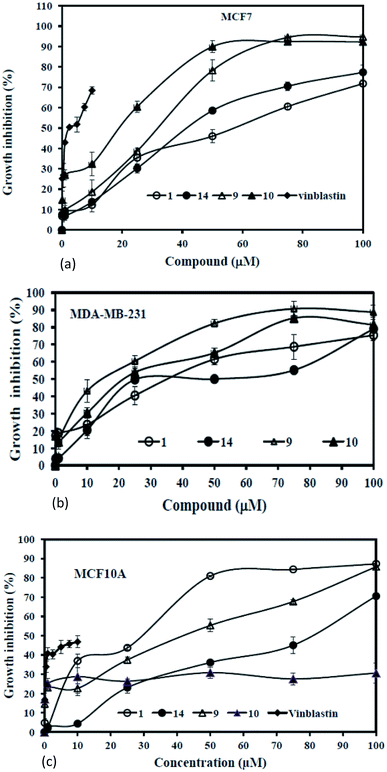
Since zaluzanin D 1 had exhibited anticancer activity against human breast cancer cell line (Table 3), we screened all the synthesized compounds for their antiproliferative activity against breast cancer cell line MCF7 and selected compound in MDA-MB-231. Cells were grown in the presence of different concentrations (0–100 μM) of the synthesized compounds for 48 h and then MTT assay was performed as described in the experimental section. Among these compounds 1, 9, 10 and 14 showed activity (IC50 < 50 μM) against MCF7 and MDA-MB-231 (Table 3 and Fig. 5). Among these four compounds, (R)-1-(1-naphthyl)ethylamine adducts of zaluzanin D and C (i.e. 9 and 10) were found to possess most potent antiproliferative effect (IC50 30 μM and 18.8 μM, respectively) against MCF7 cells as compared to other synthesized compounds. Interestingly, compound 9 was found to possess more potent antiproliferative activity in highly metastatic MDA-MB-231 cell line (IC50 13.33 μM) as compared to MCF7 (IC50 30 μM) suggesting that 9 could be more effective in inducing cell death in higher grades of breast cancer. However, it also has potent cytotoxic effect on the normal breast epithelial MCF10A cells suggesting that compound 9 could not be useful for therapeutic purpose (Fig. 5c and Table 3). Interestingly, compound 10 has the minimal cytotoxic effect against the MCF10A suggesting that it could be a better candidate compound for further designing the small molecule to chemotherapy (Fig. 5c and Table 3). The in vitro anticancer activities of all the synthesized Michael adducts are depicted in Table 3.| Compound | IC50 in μM | ||
|---|---|---|---|
| MCF7 | MDA-MB-231 | MCF10A | |
| 1 (zaluzanin D) | 53.7 ± 4.11 | 34.17 ± 4.48 | 29.6 ± 0.84 |
| 2 (zaluzanin C) | >100 | Not tested | Not tested |
| 3 | 71.2 ± 0.9 | Not tested | Not tested |
| 4 | >100 | Not tested | Not tested |
| 5 | >100 | Not tested | Not tested |
| 6 | >100 | Not tested | Not tested |
| 7 | >100 | Not tested | Not tested |
| 8 | 75.8 ± 3 | Not tested | Not tested |
| 9 | 30 ± 0.7 | 13.33 ± 1.53 | 42 ± 4.1 |
| 10 | 18.83 ± 1.7 | 23 ± 1 | >100 |
| 11 | >100 | Not tested | Not tested |
| 12 | >100 | Not tested | Not tested |
| 13 | 56.1 ± 0.9 | Not tested | Not tested |
| 14 | 40 ± 2.16 | 25 ± 2.3 | >50 |
| 15 | >100 | Not tested | Not tested |
| 16 | >100 | Not tested | Not tested |
| 17 | >100 | Not tested | Not tested |
| 18 | >100 | Not tested | Not tested |
| 19 | >100 | Not tested | Not tested |
| 20 | >100 | Not tested | Not tested |
| 21 | >100 | Not tested | Not tested |
| 22 | >100 | Not tested | Not tested |
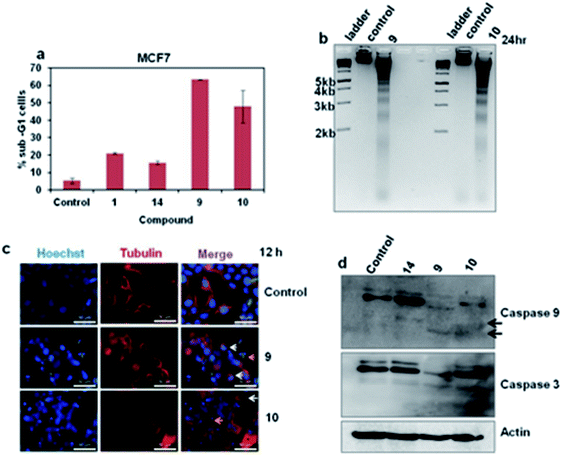
To understand the inhibition of proliferation of cancer cells by these compounds, we have performed the FACS as described in the experimental section. Results showed that treatment of MCF7 cells with 9 and 10 significantly increased the apoptotic populations while compound 1 and 14 induced apoptosis to a lesser extent indicating that compounds 9 and 10 execute their antiproliferative activity through induction of apoptosis (Fig. 6a). To validate the apoptotic induction by these two compounds (9 and 10), we then checked fragmentation profile of genomic DNA following their treatment.15 Results demonstrated that the presence of substantial fragmented DNA ladder following treatment of MCF7 cells with these compounds (Fig. 6b). Similar results were also observed in vivo upon staining the DNA with Hoechst (Fig. 6c). Results taken together revealed that compound 9 and 10 are the potent inducer of apoptosis and thereby inhibits the cancer cells proliferation. We then checked the apoptotic pathway and found that 9 and 10 induced apoptosis through the intrinsic pathway of apoptosis through cleavage of caspase 9 (Fig. 6d).16,17 Collectively these results suggest that 9 and 10 may function as anticancer agents through induction of intrinsic apoptosis.
In vitro anticancer activities of Heck analogs of zaluzanin D (23–34)
All the synthesized Heck analogs 23–34 were assayed for their antiproliferative activity against breast cancer cell line MCF7 as described in experimental section. Among all the synthesized Heck analogs, two analogs, 31 and 32 exhibited good antiproliferative activity with IC50 values of 37 and 36.5 μM, respectively. Compound 29 showed moderate activity with IC50 value of 74 μM. The in vitro anticancer activities of all the synthesized Heck analogs are shown in Table 4.| Compound | IC50 in μM |
|---|---|
| MCF7 | |
| 23 | >100 |
| 24 | >100 |
| 25 | >100 |
| 26 | >100 |
| 27 | >100 |
| 28 | >100 |
| 29 | 74 |
| 30 | >100 |
| 31 | 37 |
| 32 | 36.5 |
| 33 | >100 |
| 34 | >100 |
Conclusions
In summary, we have isolated two guaianolide class of sesquiterpene lactones, zaluzanin D 1 and zaluzanin C 2 from the leaves of V. arborea. A library of several diverse Michael adducts (3–22) and Heck arylation analogs (23–34) of zaluzanin D 1 have been synthesized by reacting with various amines and aryl iodides, respectively. All the new functionalized molecules were assayed for their in vitro anticancer activities against human breast cancer cell lines MCF7 and MDA-MB-231 and selected compounds were checked in MCF10A. Isolated zaluzanin D 1 exhibited IC50 values of 53.7 and 34.17 μM against MCF7 and MDA-MB-231 cell lines whereas zaluzanin C 2 was inactive to both the cell lines. Four Michael analogs (9, 10, 13 and 14) were found to possess potent anti-cancer activity as compared to other synthesized compounds. Out of these four compounds, compound 9 and 10 were found to exhibit potent antiproliferative effect against MCF7 cells. Compound 9 exhibited IC50 values of 30 μM and 13.33 μM, whereas compound 10 exhibited IC50 values of 18.83 μM and 23 μM against MCF7 and MDA-MB-231 cell lines, respectively. However, compound 10 has minimal cytotoxic effect against the normal breast MCF10A cells indicating that compound 10 could be a potential compound for the development of superior anticancer therapeutic compound. Further, amongst all the synthesized Heck analogs 23–34, two analogs 31 and 32 exhibited good antiproliferative activity with IC50 values of 37 and 36.5 μM, respectively.Experimental section
General
All melting points were recorded on a Büchi melting point apparatus in open capillaries and are uncorrected. Commercially available reagents and solvents were used as such received. Dry MeOH was prepared following the standard procedures. All dry reactions were carried out under an argon atmosphere, and flash chromatography was performed with CombiFlash Rf 200i with UV/VIS and ELSD, Isco Teledyne Inc., USA using RediSep® column (SiO2). 1H NMR spectra were recorded on Bruker 500 or 400 or 200 MHz spectrometers, and 13C NMR spectra were recorded at 125 or 100 or 50 MHz, respectively. Chemical shifts are reported as δ values (ppm) relative to internal standard tetramethylsilane in CDCl3. HRMS (ESI) were recorded on an Orbitrap (quadrupole plus ion trap) and TOF mass analyzer. FT-IR spectra were recorded on an FT-IR-8300 Shimadzu spectrometer. Optical rotations were recorded on a JASCO P-1020 polarimeter.Plant material
The aerial parts of V. arborea were collected from the Kolli Hills (Perumakkai Shola), Tamilnadu, India during March 2013. The plant was identified by Prof. Dr N. Parthasarathy, Department of Ecology and Environmental Sciences, Pondicherry University, India where a voucher specimen (no. 5318) is being maintained.Extraction and isolation
Air-dried and grounded leaves (2.8 kg) of V. arborea were extracted with petroleum ether (5 × 5.0 L) at room temperature for five days. After completion of the extraction, the solvent was evaporated under reduced pressure to afford the petroleum ether extract (105 g). The remaining plant material was further extracted with MeOH (5 × 5.0 L) at room temperature for five days. After completion of the extraction, the solvent was evaporated under reduced pressure to afford the MeOH extract (224.15 g).A portion of the petroleum ether extract (5.3 g) was flash chromatographed on CombiFlash Companion, Isco Teledyne Inc., USA using RediSep® column (SiO2, 2 × 12 g, stacked together) and isocratic elution was done with ethyl acetate–petroleum ether (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96) to furnish the pure compound (66.3 mg, 0.04%) which was identified as zaluzanin D 1 on the basis of its spectral data, comparison of spectral data with reported data10 and co-TLC with an authentic sample. Further flash chromatography with ethyl acetate–petroleum ether (6
96) to furnish the pure compound (66.3 mg, 0.04%) which was identified as zaluzanin D 1 on the basis of its spectral data, comparison of spectral data with reported data10 and co-TLC with an authentic sample. Further flash chromatography with ethyl acetate–petroleum ether (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94) furnished a pure compound, which was identified as zaluzanin C 2 (16.3 mg, 0.0098%) on the basis of its spectral data and comparison of spectral data with reported data.10
94) furnished a pure compound, which was identified as zaluzanin C 2 (16.3 mg, 0.0098%) on the basis of its spectral data and comparison of spectral data with reported data.10
Zaluzanin D (1)
Colourless solid; 105–106 °C [reported10 103–104 °C]; Rf 0.35 (DCM); [α]25D +23.5 (c 1, CHCl3) [reported10 +21.43 (c 0.28, CHCl3)]; 1H NMR (200 MHz, CDCl3) δH 6.22 (d, J = 3.5 Hz, 1H), 5.63–5.45 (m, 3H), 5.30 (t, J = 2.0 Hz, 1H), 4.96 (s, 2H), 4.07 (t, J = 9.2 Hz, 1H), 3.04–2.78 (m, 3H), 2.58–2.35 (m, 2H), 2.34–2.15 (m, 2H), 2.11 (s, 3H), 1.90–1.72 (m, 1H), 1.58–1.45 (m, 1H); 13C NMR (50 MHz, CDCl3) δC 170.8, 170.0, 148.1, 147.8, 139.6, 120.4, 114.4, 113.6, 83.8, 74.7, 50.3, 45.3, 44.6, 36.5, 34.6, 30.6, 21.3; LC-MS (ESI): m/z at 311.06 (M + Na)+; HRMS (ESI) calcd for C17H21O4 [M + H]+ 289.1434, found 289.1433.Zaluzanin C (2)
Brown viscous liquid; Rf 0.30 (MeOH–DCM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19); [α]26D +45.4 (c 1, CHCl3) [reported10 +50 (c 0.1, CHCl3)]; 1H NMR (400 MHz, CDCl3) δH 6.22 (d, J = 3.7 Hz, 1H), 5.50 (d, J = 3.1 Hz, 1H), 5.46 (s, 1H), 5.33 (s, 1H), 5.01 (s, 1H), 4.95 (s, 1H), 4.58 (t, J = 7.3 Hz, 1H), 4.11 (t, J = 9.2 Hz, 1H), 2.96–2.88 (m, 1H), 2.87–2.81 (m, 1H), 2.54–2.46 (m, 1H), 2.37–2.21 (m, 3H), 2.22–2.12 (m, 1H), 1.82–1.73 (m, 1H), 1.52–1.41 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 170.1, 153.0, 148.0, 139.7, 120.3, 114.4, 111.3, 83.9, 73.6, 49.9, 45.6, 44.2, 39.0, 34.2, 30.6; HRMS (ESI) calcd for C15H19O3 [M + H]+ 247.1329, found 247.1329.
19); [α]26D +45.4 (c 1, CHCl3) [reported10 +50 (c 0.1, CHCl3)]; 1H NMR (400 MHz, CDCl3) δH 6.22 (d, J = 3.7 Hz, 1H), 5.50 (d, J = 3.1 Hz, 1H), 5.46 (s, 1H), 5.33 (s, 1H), 5.01 (s, 1H), 4.95 (s, 1H), 4.58 (t, J = 7.3 Hz, 1H), 4.11 (t, J = 9.2 Hz, 1H), 2.96–2.88 (m, 1H), 2.87–2.81 (m, 1H), 2.54–2.46 (m, 1H), 2.37–2.21 (m, 3H), 2.22–2.12 (m, 1H), 1.82–1.73 (m, 1H), 1.52–1.41 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 170.1, 153.0, 148.0, 139.7, 120.3, 114.4, 111.3, 83.9, 73.6, 49.9, 45.6, 44.2, 39.0, 34.2, 30.6; HRMS (ESI) calcd for C15H19O3 [M + H]+ 247.1329, found 247.1329.
General procedure for the synthesis of amino derivatives of zaluzanin D 1 (3–22)
Zaluzanin D 1 (1 equiv.) was dissolved in dry MeOH (5 mL) and then amine (1.5 equiv.) was added to it and stirred at rt for overnight under argon. After completion of the reaction (TLC), the reaction mixture was evaporated to dryness and the residue was purified using CombiFlash Rf 200i with UV/VIS and ELSD, Isco Teledyne Inc. using RediSep® column (12 g, SiO2) and eluted with EtOAc–petroleum ether (0 → 70%, gradient) to furnish the pure amino derivatives 3–22.General procedure for the synthesis of Heck arylated analogs of zaluzanin D 1 (23–34)
To a mixture of aryl halide (3 equiv.) and palladium(II) acetate (5 mol%) in DMF was added zaluzanin D (1 equiv.) and stirred at room temperature for 10 min and then triethylamine (7 equiv.) was added to the reaction mixture and heated at 80 °C under air for 24 h. After completion of the reaction (TLC), the reaction mixture was allowed to cool to room temperature, water (2 mL) was added, and the resultant mixture was extracted with EtOAc (5 mL ×3). The organic layers were dried over Na2SO4 and concentrated under reduced pressure and then purified using CombiFlash Rf 200i with UV/VIS and ELSD, Isco Teledyne Inc. using RediSep® column (12 g, SiO2) and eluted with EtOAc–petroleum ether (0 → 70%, gradient) which furnished the pure aryl derivatives of zaluzanin D.(3R,3aS,6aR,8S,9aR,9bS)-6,9-Dimethylene-2-oxo-3-((((S)-1-phenyl ethyl)amino)methyl)dodecahydroazuleno[4,5-b]furan-8-yl-acetate (3)
Dark brown viscous liquid (62%); Rf 0.30 (EtOAc–petroleum ether, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]25D +25.3 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 7.36–7.32 (m, 1H), 7.32–7.28 (m, 3H), 7.27–7.22 (m, 1H), 5.57–5.51 (m, 1H), 5.39 (t, J = 2.1 Hz, 1H), 5.26 (t, J = 2.1 Hz, 1H), 4.90 (s, 2H), 4.01 (t, J = 9.6 Hz, 1H), 3.96 (s, 1H), 3.77 (q, J = 6.4 Hz, 1H), 2.93–2.85 (m, 2H), 2.84–2.77 (m, 1H), 2.49–2.36 (m, 4H), 2.30–2.20 (m, 1H), 2.10 (s, 3H), 2.04 (s, 1H), 1.97–1.88 (m, 1H), 1.86–1.74 (m, 2H), 1.36 (d, J = 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δC 177.8, 170.8, 148.6, 148.5, 144.9, 128.6, 127.2, 126.8, 113.6, 113.2, 84.3, 74.8, 58.4, 50.0, 47.3, 45.0, 44.5, 44.1, 36.4, 36.1, 32.1, 29.8, 24.5, 21.3; LCMS (ESI): m/z 432.05 (M + Na)+; HRMS (ESI) calcd for C25H32O4N [M + H]+ 410.2326, found 410.2328.
3); [α]25D +25.3 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 7.36–7.32 (m, 1H), 7.32–7.28 (m, 3H), 7.27–7.22 (m, 1H), 5.57–5.51 (m, 1H), 5.39 (t, J = 2.1 Hz, 1H), 5.26 (t, J = 2.1 Hz, 1H), 4.90 (s, 2H), 4.01 (t, J = 9.6 Hz, 1H), 3.96 (s, 1H), 3.77 (q, J = 6.4 Hz, 1H), 2.93–2.85 (m, 2H), 2.84–2.77 (m, 1H), 2.49–2.36 (m, 4H), 2.30–2.20 (m, 1H), 2.10 (s, 3H), 2.04 (s, 1H), 1.97–1.88 (m, 1H), 1.86–1.74 (m, 2H), 1.36 (d, J = 6.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δC 177.8, 170.8, 148.6, 148.5, 144.9, 128.6, 127.2, 126.8, 113.6, 113.2, 84.3, 74.8, 58.4, 50.0, 47.3, 45.0, 44.5, 44.1, 36.4, 36.1, 32.1, 29.8, 24.5, 21.3; LCMS (ESI): m/z 432.05 (M + Na)+; HRMS (ESI) calcd for C25H32O4N [M + H]+ 410.2326, found 410.2328.
(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-6,9-dimethylene-3-((((S)-1-phenylethyl)amino)methyl)decahydroazuleno[4,5-b]furan-2(3H)-one (4)
Dark brown viscous liquid (32%); Rf 0.30 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]26D +21.1 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 7.36–7.35 (m, 2H), 7.34 (s, 1H), 7.32–7.30 (m, 2H), 5.36 (t, J = 1.8 Hz, 1H), 5.29 (t, J = 2.3 Hz, 1H), 4.54 (tt, J = 7.3, 1.8 Hz, 1H), 4.18 (q, J = 6.8 Hz, 1H), 4.04 (t, J = 9.6 Hz, 1H), 3.77 (q, J = 6.6 Hz, 1H), 2.93–2.75 (m, 3H), 2.48–2.35 (m, 3H), 2.34–2.27 (m, 1H), 2.26–2.16 (m, 1H), 1.96–1.89 (m, 1H), 1.89–1.85 (m, 4H), 1.84–1.77 (m, 1H), 1.78–1.68 (m, 1H), 1.36 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δC 177.9, 153.4, 148.8, 144.9, 128.8, 128.6, 127.8, 126.8, 126.2, 113.6, 110.9, 84.4, 73.5, 58.4, 51.1, 47.2, 45.4, 44.5, 43.7, 38.9, 35.9, 29.8, 24.5, 23.3; LCMS (ESI): m/z 390.05 (M + Na)+; HRMS (ESI) calcd for C23H30O3N [M + H]+ 368.2220, found 368.2220.
3); [α]26D +21.1 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 7.36–7.35 (m, 2H), 7.34 (s, 1H), 7.32–7.30 (m, 2H), 5.36 (t, J = 1.8 Hz, 1H), 5.29 (t, J = 2.3 Hz, 1H), 4.54 (tt, J = 7.3, 1.8 Hz, 1H), 4.18 (q, J = 6.8 Hz, 1H), 4.04 (t, J = 9.6 Hz, 1H), 3.77 (q, J = 6.6 Hz, 1H), 2.93–2.75 (m, 3H), 2.48–2.35 (m, 3H), 2.34–2.27 (m, 1H), 2.26–2.16 (m, 1H), 1.96–1.89 (m, 1H), 1.89–1.85 (m, 4H), 1.84–1.77 (m, 1H), 1.78–1.68 (m, 1H), 1.36 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δC 177.9, 153.4, 148.8, 144.9, 128.8, 128.6, 127.8, 126.8, 126.2, 113.6, 110.9, 84.4, 73.5, 58.4, 51.1, 47.2, 45.4, 44.5, 43.7, 38.9, 35.9, 29.8, 24.5, 23.3; LCMS (ESI): m/z 390.05 (M + Na)+; HRMS (ESI) calcd for C23H30O3N [M + H]+ 368.2220, found 368.2220.
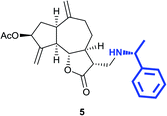
(3R,3aS,6aR,8S,9aR,9bS)-6,9-Dimethylene-2-oxo-3-((((R)-1-phenyl ethyl)amino)methyl)dodecahydroazuleno[4,5-b]furan-8-yl-acetate (5)
Brown viscous liquid (66%); Rf 0.30 (EtOAc–petroleum ether, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]26D +34.4 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 7.37–7.22 (m, 5H), 5.59–548 (m, 1H), 5.40 (t, J = 2.0 Hz, 1H), 5.26 (t, J = 2.0 Hz, 1H), 4.90 (s, 2H), 4.00 (t, J = 9.2 Hz, 1H), 3.75 (q, J = 6.6 Hz, 1H), 2.96–2.75 (m, 2H), 2.70 (s, 1H), 2.67 (s, 1H), 2.55–2.28 (m, 3H), 2.26–2.14 (m, 1H), 2.10 (s, 3H), 2.05–1.96 (m, 3H), 1.79 (td, J = 14.2, 6.4 Hz, 1H), 1.56 (d, J = 6.3 Hz, 1H), 1.35 (d, J = 6.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δC 177.6, 170.8, 148.5, 148.3, 144.3, 128.6, 127.2, 126.7, 113.6, 113.2, 84.2, 74.7, 58.7, 49.9, 47.2, 45.9, 45.7, 44.0, 36.3, 36.1, 32.4, 29.7, 23.9, 21.3; LCMS (ESI): m/z 432.05 (M + Na)+; HRMS (ESI) calcd for C25H32O4N [M + H]+ 410.2326, found 410.2329.
3); [α]26D +34.4 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 7.37–7.22 (m, 5H), 5.59–548 (m, 1H), 5.40 (t, J = 2.0 Hz, 1H), 5.26 (t, J = 2.0 Hz, 1H), 4.90 (s, 2H), 4.00 (t, J = 9.2 Hz, 1H), 3.75 (q, J = 6.6 Hz, 1H), 2.96–2.75 (m, 2H), 2.70 (s, 1H), 2.67 (s, 1H), 2.55–2.28 (m, 3H), 2.26–2.14 (m, 1H), 2.10 (s, 3H), 2.05–1.96 (m, 3H), 1.79 (td, J = 14.2, 6.4 Hz, 1H), 1.56 (d, J = 6.3 Hz, 1H), 1.35 (d, J = 6.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δC 177.6, 170.8, 148.5, 148.3, 144.3, 128.6, 127.2, 126.7, 113.6, 113.2, 84.2, 74.7, 58.7, 49.9, 47.2, 45.9, 45.7, 44.0, 36.3, 36.1, 32.4, 29.7, 23.9, 21.3; LCMS (ESI): m/z 432.05 (M + Na)+; HRMS (ESI) calcd for C25H32O4N [M + H]+ 410.2326, found 410.2329.
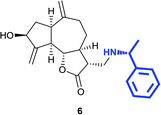
(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-6,9-dimethylene-3-((((R)-1-phenylethyl)amino)methyl)decahydroazuleno[4,5-b]furan-2(3H)-one (6)
Brown viscous liquid (28%); Rf 0.30 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]26D +33.1 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 7.36–7.26 (m, 5H), 5.37 (t, J = 1.9 Hz, 1H), 5.29 (t, J = 1.8 Hz, 1H), 4.96 (s, 1H), 4.91 (s, 1H), 4.59–4.48 (m, 1H), 4.03 (t, J = 9.4 Hz, 1H), 3.75 (q, J = 6.6 Hz, 1H), 2.93–2.75 (m, 2H), 2.69 (s, 1H), 2.66 (s, 1H), 2.51–2.27 (m, 3H), 2.00–1.96 (m, 6H), 1.80–1.66 (m, 1H), 1.62–1.47 (m, 1H), 1.35 (d, J = 6.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δC 177.8, 153.3, 148.7, 144.6, 128.6, 127.2, 126.7, 113.7, 110.9, 84.3, 73.5, 58.7, 49.6, 47.3, 46.3, 45.8, 43.6, 38.8, 35.9, 32.4, 29.7, 24.0; LCMS (ESI): m/z 390.07 (M + Na)+; HRMS (ESI) calcd for C23H30O3N [M + H]+ 368.2220, found 368.2208.
3); [α]26D +33.1 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 7.36–7.26 (m, 5H), 5.37 (t, J = 1.9 Hz, 1H), 5.29 (t, J = 1.8 Hz, 1H), 4.96 (s, 1H), 4.91 (s, 1H), 4.59–4.48 (m, 1H), 4.03 (t, J = 9.4 Hz, 1H), 3.75 (q, J = 6.6 Hz, 1H), 2.93–2.75 (m, 2H), 2.69 (s, 1H), 2.66 (s, 1H), 2.51–2.27 (m, 3H), 2.00–1.96 (m, 6H), 1.80–1.66 (m, 1H), 1.62–1.47 (m, 1H), 1.35 (d, J = 6.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δC 177.8, 153.3, 148.7, 144.6, 128.6, 127.2, 126.7, 113.7, 110.9, 84.3, 73.5, 58.7, 49.6, 47.3, 46.3, 45.8, 43.6, 38.8, 35.9, 32.4, 29.7, 24.0; LCMS (ESI): m/z 390.07 (M + Na)+; HRMS (ESI) calcd for C23H30O3N [M + H]+ 368.2220, found 368.2208.
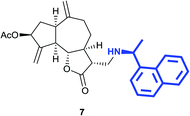
(3R,3aS,6aR,8S,9aR,9bS)-6,9-Dimethylene-3-((((S)-1-(naphthalen-1-yl)ethyl)amino)methyl)-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (7)
Dark brown viscous liquid (52%); Rf 0.60 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +20.4 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 8.29 (d, J = 7.6 Hz, 1H), 7.94–7.83 (m, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 7.0 Hz, 1H), 7.55–7.42 (m, 3H), 5.54 (t, J = 7.0 Hz, 1H), 5.40 (s, 1H), 5.26 (s, 1H), 4.86 (s, 2H), 4.72–4.52 (m, 1H), 3.98 (t, J = 8.2 Hz, 1H), 3.03 (d, J = 11.2 Hz, 2H), 2.84–2.72 (m, 2H), 2.60–2.33 (m, 4H), 2.10 (s, 3H), 1.85–1.66 (m, 3H), 1.53 (d, J = 6.4 Hz, 3H), 1.18–1.04 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 177.8, 170.9, 148.8, 148.5, 143.2, 140.5, 134.0, 130.8, 129.1, 127.4, 126.1, 125.7, 125.5, 123.0, 121.5, 113.4, 113.0, 84.3, 74.8, 53.9, 49.9, 48.0, 46.6, 45.5, 45.0, 43.9, 36.4, 32.5, 24.8, 23.3, 21.4; LCMS (ESI): m/z 482.09 (M + Na)+; HRMS (ESI) calcd for C29H34O4N [M + H]+ 460.2482, found 460.2487.
1); [α]25D +20.4 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 8.29 (d, J = 7.6 Hz, 1H), 7.94–7.83 (m, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 7.0 Hz, 1H), 7.55–7.42 (m, 3H), 5.54 (t, J = 7.0 Hz, 1H), 5.40 (s, 1H), 5.26 (s, 1H), 4.86 (s, 2H), 4.72–4.52 (m, 1H), 3.98 (t, J = 8.2 Hz, 1H), 3.03 (d, J = 11.2 Hz, 2H), 2.84–2.72 (m, 2H), 2.60–2.33 (m, 4H), 2.10 (s, 3H), 1.85–1.66 (m, 3H), 1.53 (d, J = 6.4 Hz, 3H), 1.18–1.04 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 177.8, 170.9, 148.8, 148.5, 143.2, 140.5, 134.0, 130.8, 129.1, 127.4, 126.1, 125.7, 125.5, 123.0, 121.5, 113.4, 113.0, 84.3, 74.8, 53.9, 49.9, 48.0, 46.6, 45.5, 45.0, 43.9, 36.4, 32.5, 24.8, 23.3, 21.4; LCMS (ESI): m/z 482.09 (M + Na)+; HRMS (ESI) calcd for C29H34O4N [M + H]+ 460.2482, found 460.2487.
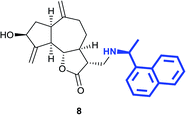
(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-6,9-dimethylene-3-((((S)-1-(naphthalene-1-yl)ethyl)amino)methyl)decahydroazuleno[4,5-b] furan-2(3H)-one (8)
Dark brown viscous liquid (27%); Rf 0.30 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +35.1 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 8.22 (d, J = 8.2 Hz, 1H), 7.90–7.84 (m, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.64 (d, J = 6.9 Hz, 1H), 7.54–7.45 (m, 3H), 5.35 (s, 1H), 5.28 (s, 1H), 4.92 (s, 1H), 4.86 (s, 1H), 4.65 (q, J = 6.4 Hz, 1H), 4.52 (t, J = 7.8 Hz, 1H), 4.05–3.95 (m, 1H), 2.85 (dd, J = 12.4, 4.1, Hz, 1H), 2.77–2.65 (m, 3H), 2.43–2.22 (m, 4H), 2.21–2.13 (m, 1H), 2.11–2.01 (m, 1H), 1.91–1.82 (m, 1H), 1.83–1.65 (m, 3H), 1.52 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δC 177.9, 153.4, 148.9, 140.4, 134.1, 131.5, 129.0, 127.4, 125.8, 125.7, 125.4, 123.4, 123.0, 113.5, 110.8, 84.3, 73.6, 53.9, 49.6, 47.9, 46.0, 45.2, 43.5, 38.8, 36.1, 32.5, 23.3; LCMS (ESI): m/z 440.14 (M + Na)+; HRMS (ESI) calcd for C27H32O3N [M + H]+ 418.2377, found 418.2373.
1); [α]25D +35.1 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 8.22 (d, J = 8.2 Hz, 1H), 7.90–7.84 (m, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.64 (d, J = 6.9 Hz, 1H), 7.54–7.45 (m, 3H), 5.35 (s, 1H), 5.28 (s, 1H), 4.92 (s, 1H), 4.86 (s, 1H), 4.65 (q, J = 6.4 Hz, 1H), 4.52 (t, J = 7.8 Hz, 1H), 4.05–3.95 (m, 1H), 2.85 (dd, J = 12.4, 4.1, Hz, 1H), 2.77–2.65 (m, 3H), 2.43–2.22 (m, 4H), 2.21–2.13 (m, 1H), 2.11–2.01 (m, 1H), 1.91–1.82 (m, 1H), 1.83–1.65 (m, 3H), 1.52 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δC 177.9, 153.4, 148.9, 140.4, 134.1, 131.5, 129.0, 127.4, 125.8, 125.7, 125.4, 123.4, 123.0, 113.5, 110.8, 84.3, 73.6, 53.9, 49.6, 47.9, 46.0, 45.2, 43.5, 38.8, 36.1, 32.5, 23.3; LCMS (ESI): m/z 440.14 (M + Na)+; HRMS (ESI) calcd for C27H32O3N [M + H]+ 418.2377, found 418.2373.
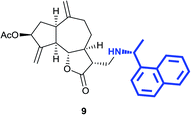
(3R,3aS,6aR,8S,9aR,9bS)-6,9-Dimethylene-3-((((R)-1-(naphthalen-1-yl)ethyl)amino)methyl)-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (9)
Pale yellow viscous liquid (57%); Rf 0.70 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]25D +30.9 (c 1, CHCl3); 1H NMR (500 MHz, CDCl3) δH 8.29 (d, J = 7.9 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.75 (d, J = 7.9 Hz, 1H), 7.61 (d, J = 7.0 Hz, 1H), 7.55–7.40 (m, 3H), 5.54 (t, J = 7.0 Hz, 1H), 5.40 (s, 1H), 5.27 (s, 1H), 4.88–4.85 (m, 2H), 4.60 (q, J = 6.3 Hz, 1H), 3.98 (t, J = 8.7 Hz, 1H), 3.02 (dd, J = 12.2, 2.8 Hz, 1H), 2.87–2.69 (m, 2H), 2.52 (dd, J = 12.2, 5.5 Hz, 1H), 2.48–2.40 (m, 1H), 2.40–2.25 (m, 3H), 2.10 (s, 3H), 1.84–1.62 (m, 3H), 1.52 (d, J = 6.7 Hz, 3H), 1.19–1.07 (m, 2H); 13C NMR (125 MHz, CDCl3) δC 177.7, 170.8, 148.6, 148.4, 143.1, 140.3, 133.9, 130.7, 129.0, 127.2, 126.0, 125.6, 125.4, 122.9, 121.4, 113.3, 112.9, 84.1, 74.7, 53.8, 49.8, 47.9, 46.4, 45.4, 44.9, 43.8, 36.3, 32.3, 24.7, 23.1, 21.2; LCMS (ESI): m/z 482.05 (M + Na)+; HRMS (ESI) calcd for C29H34O4N [M + H]+ 460.2482, found 460.2487.
3); [α]25D +30.9 (c 1, CHCl3); 1H NMR (500 MHz, CDCl3) δH 8.29 (d, J = 7.9 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.75 (d, J = 7.9 Hz, 1H), 7.61 (d, J = 7.0 Hz, 1H), 7.55–7.40 (m, 3H), 5.54 (t, J = 7.0 Hz, 1H), 5.40 (s, 1H), 5.27 (s, 1H), 4.88–4.85 (m, 2H), 4.60 (q, J = 6.3 Hz, 1H), 3.98 (t, J = 8.7 Hz, 1H), 3.02 (dd, J = 12.2, 2.8 Hz, 1H), 2.87–2.69 (m, 2H), 2.52 (dd, J = 12.2, 5.5 Hz, 1H), 2.48–2.40 (m, 1H), 2.40–2.25 (m, 3H), 2.10 (s, 3H), 1.84–1.62 (m, 3H), 1.52 (d, J = 6.7 Hz, 3H), 1.19–1.07 (m, 2H); 13C NMR (125 MHz, CDCl3) δC 177.7, 170.8, 148.6, 148.4, 143.1, 140.3, 133.9, 130.7, 129.0, 127.2, 126.0, 125.6, 125.4, 122.9, 121.4, 113.3, 112.9, 84.1, 74.7, 53.8, 49.8, 47.9, 46.4, 45.4, 44.9, 43.8, 36.3, 32.3, 24.7, 23.1, 21.2; LCMS (ESI): m/z 482.05 (M + Na)+; HRMS (ESI) calcd for C29H34O4N [M + H]+ 460.2482, found 460.2487.
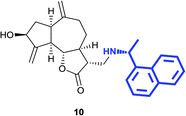
(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-6,9-dimethylene-3-((((R)-1-(naphthalen-1-yl)ethyl)amino)methyl)decahydroazuleno[4,5-b] furan-2(3H)-one (10)
Pale yellow viscous liquid (34%); Rf 0.40 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]25D +24.6 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 8.24 (d, J = 8.2 Hz, 1H), 7.89–7.85 (m, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.64 (d, J = 6.9 Hz, 1H), 7.53–7.46 (m, 3H), 5.35 (s, 1H), 5.28 (s, 1H), 4.92 (s, 1H), 4.86 (s, 1H), 4.66 (q, J = 6.4 Hz, 1H), 4.53 (t, J = 7.8 Hz, 1H), 4.04–3.97 (m, 1H), 2.87 (dd, J = 12.4, 4.1 Hz, 1H), 2.76–2.67 (m, 3H), 2.43–2.26 (m, 4H), 2.20–2.14 (m, 1H), 2.06 (dq, J = 11.9, 3.7 Hz, 1H), 1.90–1.82 (m, 1H), 1.83–1.66 (m, 3H), 1.53 (d, J = 6.4 Hz, 3H), 1.30–1.16 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 177.8, 153.3, 148.7, 140.3, 134.0, 131.4, 128.9, 127.3, 125.7, 125.6, 125.3, 123.2, 122.9, 113.4, 110.6, 84.2, 76.7, 73.5, 53.8, 49.5, 47.8, 45.9, 45.0, 43.4, 38.7, 36.0, 32.3, 23.2; LCMS (ESI): m/z 440.02 (M + Na)+; HRMS (ESI) calcd for C27H32O3N [M + H]+ 418.2377, found 418.2379.
3); [α]25D +24.6 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 8.24 (d, J = 8.2 Hz, 1H), 7.89–7.85 (m, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.64 (d, J = 6.9 Hz, 1H), 7.53–7.46 (m, 3H), 5.35 (s, 1H), 5.28 (s, 1H), 4.92 (s, 1H), 4.86 (s, 1H), 4.66 (q, J = 6.4 Hz, 1H), 4.53 (t, J = 7.8 Hz, 1H), 4.04–3.97 (m, 1H), 2.87 (dd, J = 12.4, 4.1 Hz, 1H), 2.76–2.67 (m, 3H), 2.43–2.26 (m, 4H), 2.20–2.14 (m, 1H), 2.06 (dq, J = 11.9, 3.7 Hz, 1H), 1.90–1.82 (m, 1H), 1.83–1.66 (m, 3H), 1.53 (d, J = 6.4 Hz, 3H), 1.30–1.16 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 177.8, 153.3, 148.7, 140.3, 134.0, 131.4, 128.9, 127.3, 125.7, 125.6, 125.3, 123.2, 122.9, 113.4, 110.6, 84.2, 76.7, 73.5, 53.8, 49.5, 47.8, 45.9, 45.0, 43.4, 38.7, 36.0, 32.3, 23.2; LCMS (ESI): m/z 440.02 (M + Na)+; HRMS (ESI) calcd for C27H32O3N [M + H]+ 418.2377, found 418.2379.
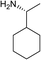
(3R,3aS,6aR,8S,9aR,9bS)-3-((((R)-1-Cyclohexylethyl)amino)methyl)-8-hydroxy-6,9-dimethylenedecahydroazuleno[4,5-b]furan-2(3H)-one (11)
Brown viscous liquid (88%); Rf 0.40 (EtOAc); [α]24D +17.5 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.34 (s, 1H), 5.31 (s, 1H), 4.99–4.89 (m, 2H), 4.54 (t, J = 7.3 Hz, 1H), 4.23–4.08 (m, 1H), 3.42 (dd, J = 11.2, 3.4 Hz, 1H), 2.97–2.73 (m, 4H), 2.55–2.45 (m, 1H), 2.36–2.27 (m, 1H), 2.23–2.07 (m, 2H), 2.04–2.01 (m, 5H), 1.84–1.65 (m, 6H), 1.65–1.51 (m, 2H), 1.49–1.34 (m, 2H), 1.20 (d, J = 6.9 Hz, 3H), 1.10–1.01 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 178.3, 176.1, 153.0, 148.2, 114.0, 111.1, 85.1, 73.4, 59.7, 49.4, 46.5, 45.0, 44.5, 43.6, 40.5, 38.7, 35.3, 31.5, 29.7, 29.5, 29.3, 27.2, 26.2, 26.1, 25.9, 21.7, 14.0; LCMS (ESI): m/z 396.15 (M + Na)+; HRMS (ESI) calcd for C23H36O3N [M + H]+ 374.2690, found 374.2685.(3R,3aS,6aR,8S,9aR,9bS)-3-((((S)-1-Cyclohexylethyl)amino)methyl)-8-hydroxy-6,9-dimethylenedecahydroazuleno[4,5-b]furan-2(3H)-one (12)
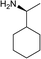
Dark brown viscous liquid (86%); Rf 0.30 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]26D +25.9 (c 1, CHCl3); 1H NMR (500 MHz, CDCl3) δH 5.38–5.26 (m, 2H), 4.9–4.91 (m, 2H), 4.55 (t, J = 7.6 Hz, 1H), 4.15 (t, J = 9.5 Hz, 1H), 3.19–3.15 (m, 1H), 2.97–2.74 (m, 4H), 2.54–2.47 (m, 1H), 2.38–2.28 (m, 1H), 2.23–2.10 (m, 2H), 2.05–1.96 (m, 5H), 1.80–1.65 (m, 6H), 1.59–1.52 (m, 1H), 1.47–1.36 (m, 2H), 1.30–1.27 (m, 1H), 1.14 (d, J = 6.5 Hz, 3H), 1.09–1.00 (m, 2H); 13C NMR (125 MHz, CDCl3) δC 177.8, 176.0, 153.1, 148.4, 113.9, 111.0, 84.9, 77.3, 77.0, 76.8, 73.5, 58.0, 49.4, 46.3, 45.0, 43.6, 43.3, 40.9, 38.7, 35.6, 31.9, 29.7, 27.3, 26.3, 26.2, 26.0, 21.7, 14.3; LCMS (ESI): m/z 396.13 (M + Na)+; HRMS (ESI) calcd for C23H36O3N [M + H]+ 374.2690, found 374.2693.
3); [α]26D +25.9 (c 1, CHCl3); 1H NMR (500 MHz, CDCl3) δH 5.38–5.26 (m, 2H), 4.9–4.91 (m, 2H), 4.55 (t, J = 7.6 Hz, 1H), 4.15 (t, J = 9.5 Hz, 1H), 3.19–3.15 (m, 1H), 2.97–2.74 (m, 4H), 2.54–2.47 (m, 1H), 2.38–2.28 (m, 1H), 2.23–2.10 (m, 2H), 2.05–1.96 (m, 5H), 1.80–1.65 (m, 6H), 1.59–1.52 (m, 1H), 1.47–1.36 (m, 2H), 1.30–1.27 (m, 1H), 1.14 (d, J = 6.5 Hz, 3H), 1.09–1.00 (m, 2H); 13C NMR (125 MHz, CDCl3) δC 177.8, 176.0, 153.1, 148.4, 113.9, 111.0, 84.9, 77.3, 77.0, 76.8, 73.5, 58.0, 49.4, 46.3, 45.0, 43.6, 43.3, 40.9, 38.7, 35.6, 31.9, 29.7, 27.3, 26.3, 26.2, 26.0, 21.7, 14.3; LCMS (ESI): m/z 396.13 (M + Na)+; HRMS (ESI) calcd for C23H36O3N [M + H]+ 374.2690, found 374.2693.
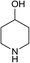
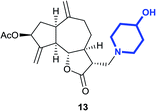
(3R,3aS,6aR,8S,9aR,9bS)-3-((4-Hydroxypiperidin-1-yl)methyl)-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b]furan-8-yl-acetate (13)
Pale yellow viscous liquid (73%); Rf 0.50 (MeOH–DCM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9); [α]25D +63.2 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.54 (t, J = 7.3 Hz, 1H), 5.40 (m, 1H), 5.27 (m, 1H), 4.93–4.89 (m, 2H), 4.01 (t, J = 9.4 Hz, 1H), 3.74–3.65 (m, 1H), 2.93 (q, J = 7.9 Hz, 1H), 2.84–2.74 (m, 3H), 2.74–2.66 (m, 1H), 2.64–2.56 (m, 1H), 2.52–2.31 (m, 4H), 2.31–2.15 (m, 3H), 2.11 (s, 3H), 2.09–2.02 (m, 1H), 1.93–1.76 (m, 4H), 1.64–1.48 (m, 2H), 1.42–1.32 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 177.5, 170.9, 148.8, 148.4, 113.5, 113.3, 83.9, 74.8, 67.9, 57.6, 52.2, 50.9, 50.2, 47.9, 45.3, 44.2, 36.4, 36.3, 34.6, 34.3, 32.8, 21.4; LCMS (ESI): m/z 412.07 (M + Na)+; HRMS (ESI) calcd for C22H32O5N [M + H]+ 390.2275, found 390.2272.
9); [α]25D +63.2 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.54 (t, J = 7.3 Hz, 1H), 5.40 (m, 1H), 5.27 (m, 1H), 4.93–4.89 (m, 2H), 4.01 (t, J = 9.4 Hz, 1H), 3.74–3.65 (m, 1H), 2.93 (q, J = 7.9 Hz, 1H), 2.84–2.74 (m, 3H), 2.74–2.66 (m, 1H), 2.64–2.56 (m, 1H), 2.52–2.31 (m, 4H), 2.31–2.15 (m, 3H), 2.11 (s, 3H), 2.09–2.02 (m, 1H), 1.93–1.76 (m, 4H), 1.64–1.48 (m, 2H), 1.42–1.32 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 177.5, 170.9, 148.8, 148.4, 113.5, 113.3, 83.9, 74.8, 67.9, 57.6, 52.2, 50.9, 50.2, 47.9, 45.3, 44.2, 36.4, 36.3, 34.6, 34.3, 32.8, 21.4; LCMS (ESI): m/z 412.07 (M + Na)+; HRMS (ESI) calcd for C22H32O5N [M + H]+ 390.2275, found 390.2272.
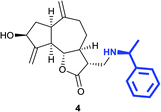
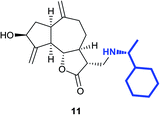
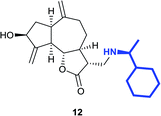
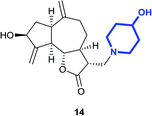
(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-3-((4-hydroxypiperidin-1-yl) methyl)-6,9-di-methylenedecahydroazuleno[4,5-b]furan-2(3H)-one (14)
Pale yellow viscous liquid (20%); Rf 0.40 (MeOH–DCM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9); [α]25D +61.8 (c 1, CHCl3); 1H NMR (400 MHz, CD3OD) δH 5.28–5.23 (m, 2H), 5.01–4.88 (m, 1H), 4.56–4.45 (m, 1H), 4.17–4.06 (m, 1H), 3.66–3.53 (m, 1H), 3.20–3.10 (m, 1H), 3.00–2.88 (m, 2H), 2.86–2.79 (m, 2H), 2.77–2.70 (m, 2H), 2.63–2.53 (m, 2H), 2.52–2.46 (m, 1H), 2.31–2.20 (m, 4H), 2.17–2.03 (m, 2H), 1.96–1.78 (m, 3H), 1.73–1.66 (m, 1H), 1.58–1.49 (m, 2H), 1.45–1.37 (m, 1H); 13C NMR (100 MHz, CDCl3) δ δC 178.7, 153.3, 149.4, 112.0, 108.2, 84.2, 72.4, 66.9, 56.9, 51.8, 50.8, 48.9, 44.8, 42.9, 38.0, 35.5, 33.6, 33.5, 32.2; LCMS (ESI): m/z 370.12 (M + Na)+; HRMS (ESI) calcd for C20H30O4N [M + H]+ 348.2169, found 348.2156.
9); [α]25D +61.8 (c 1, CHCl3); 1H NMR (400 MHz, CD3OD) δH 5.28–5.23 (m, 2H), 5.01–4.88 (m, 1H), 4.56–4.45 (m, 1H), 4.17–4.06 (m, 1H), 3.66–3.53 (m, 1H), 3.20–3.10 (m, 1H), 3.00–2.88 (m, 2H), 2.86–2.79 (m, 2H), 2.77–2.70 (m, 2H), 2.63–2.53 (m, 2H), 2.52–2.46 (m, 1H), 2.31–2.20 (m, 4H), 2.17–2.03 (m, 2H), 1.96–1.78 (m, 3H), 1.73–1.66 (m, 1H), 1.58–1.49 (m, 2H), 1.45–1.37 (m, 1H); 13C NMR (100 MHz, CDCl3) δ δC 178.7, 153.3, 149.4, 112.0, 108.2, 84.2, 72.4, 66.9, 56.9, 51.8, 50.8, 48.9, 44.8, 42.9, 38.0, 35.5, 33.6, 33.5, 32.2; LCMS (ESI): m/z 370.12 (M + Na)+; HRMS (ESI) calcd for C20H30O4N [M + H]+ 348.2169, found 348.2156.
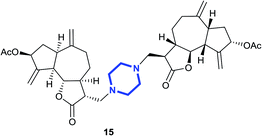
(3R,3aS,3′R,3a′S,6aR,6a′R,8S,8′S,9aR,9bS,9a′R,9b′S)-(Piperazine-1,4 diylbis(methylene))bis(6,9-dimethylene-2-oxododecahydro azuleno[4,5-b]furan-3,8-diyl)diacetate (15)
Pale yellow viscous liquid (26%); Rf 0.40 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]26D +67.7 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 5.61–5.49 (m, 2H), 5.72–5.39 (m, 2H), 5.29–5.26 (m, 2H), 4.95–4.90 (m, 4H), 4.01 (t, J = 9.5 Hz, 2H), 2.96–2.74 (m, 6H), 2.67–2.58 (m, 2H), 2.56–2.38 (m, 14H), 2.33–2.27 (m, 2H), 2.26–2.16 (m, 2H), 2.11 (s, 6H), 2.04–1.96 (m, 2H), 1.88–1.74 (m, 2H), 1.46–1.38 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 177.4, 170.9, 148.8, 148.4, 113.5, 113.4, 83.9, 74.8, 57.6, 57.6, 53.5, 53.5, 50.1, 47.9, 47.9, 45.2, 44.2, 36.4, 36.3, 32.8, 21.4; LCMS (ESI): m/z 685.39 (M + Na)+; HRMS (ESI) calcd for C38H51O8N2 [M + H]+ 663.3640, found 663.3637.
3); [α]26D +67.7 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 5.61–5.49 (m, 2H), 5.72–5.39 (m, 2H), 5.29–5.26 (m, 2H), 4.95–4.90 (m, 4H), 4.01 (t, J = 9.5 Hz, 2H), 2.96–2.74 (m, 6H), 2.67–2.58 (m, 2H), 2.56–2.38 (m, 14H), 2.33–2.27 (m, 2H), 2.26–2.16 (m, 2H), 2.11 (s, 6H), 2.04–1.96 (m, 2H), 1.88–1.74 (m, 2H), 1.46–1.38 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 177.4, 170.9, 148.8, 148.4, 113.5, 113.4, 83.9, 74.8, 57.6, 57.6, 53.5, 53.5, 50.1, 47.9, 47.9, 45.2, 44.2, 36.4, 36.3, 32.8, 21.4; LCMS (ESI): m/z 685.39 (M + Na)+; HRMS (ESI) calcd for C38H51O8N2 [M + H]+ 663.3640, found 663.3637.
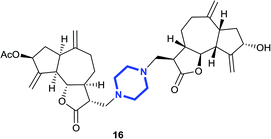
(3R,3aS,6aR,8S,9aR,9bS)-3-((4-(((3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-6,9-dimethyl-ene-2-oxododecahydroazuleno[4,5-b]furan-3-yl)methyl)piperazin-1-yl)methyl)-6,9-dimethyl-ene-2-oxododeca hydroazuleno[4,5-b]furan-8-yl acetate (16)
Pale yellow viscous liquid (15%); Rf 0.30 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]26D +69.0 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 5.58–5.52 (m, 1H), 5.42–5.37 (m, 2H), 5.32–5.26 (m, 2H), 4.97 (s, 1H), 4.94–4.91 (m, 2H), 4.56 (t, J = 7.6 Hz, 1H), 4.08–3.96 (m, 2H), 3.48–3.40 (m, 3H), 2.97–2.85 (m, 2H), 2.83–2.76 (m, 3H), 2.64–2.56 (m, 2H), 2.54–2.45 (m, 6H), 2.44–2.35 (m, 4H), 2.35–2.30 (m, 2H), 2.27–2.14 (m, 2H), 2.11 (s, 3H), 2.09–2.01 (m, 2H), 1.84–1.71 (m, 2H), 1.39–1.33 (m, 6H); 13C NMR (100 MHz, CDCl3) δC 177.4, 177.3, 170.8, 153.4, 148.9, 148.8, 148.4, 113.5, 113.5, 113.3, 111.0, 83.9, 83.8, 74.8, 73.6, 58.6, 57.6, 57.5, 53.5, 50.1, 49.8, 48.2, 47.8, 45.2, 44.1, 43.8, 38.9, 36.4, 36.2, 36.0, 32.8, 21.3, 8.3; LCMS (ESI): m/z 643.38 (M + Na)+; HRMS (ESI) calcd for C36H49O7N2 [M + H]+ 621.3534, found 621.3508.
3); [α]26D +69.0 (c 1, CHCl3); 1H NMR (200 MHz, CDCl3) δH 5.58–5.52 (m, 1H), 5.42–5.37 (m, 2H), 5.32–5.26 (m, 2H), 4.97 (s, 1H), 4.94–4.91 (m, 2H), 4.56 (t, J = 7.6 Hz, 1H), 4.08–3.96 (m, 2H), 3.48–3.40 (m, 3H), 2.97–2.85 (m, 2H), 2.83–2.76 (m, 3H), 2.64–2.56 (m, 2H), 2.54–2.45 (m, 6H), 2.44–2.35 (m, 4H), 2.35–2.30 (m, 2H), 2.27–2.14 (m, 2H), 2.11 (s, 3H), 2.09–2.01 (m, 2H), 1.84–1.71 (m, 2H), 1.39–1.33 (m, 6H); 13C NMR (100 MHz, CDCl3) δC 177.4, 177.3, 170.8, 153.4, 148.9, 148.8, 148.4, 113.5, 113.5, 113.3, 111.0, 83.9, 83.8, 74.8, 73.6, 58.6, 57.6, 57.5, 53.5, 50.1, 49.8, 48.2, 47.8, 45.2, 44.1, 43.8, 38.9, 36.4, 36.2, 36.0, 32.8, 21.3, 8.3; LCMS (ESI): m/z 643.38 (M + Na)+; HRMS (ESI) calcd for C36H49O7N2 [M + H]+ 621.3534, found 621.3508.
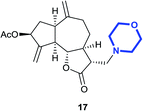
(3R,3aS,6aR,8S,9aR,9bS)-6,9-Dimethylene-3-(morpholinomethyl)-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (17)
Brown viscous liquid (84%); Rf 0.50 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]26D +69.7 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.59–5.52 (m, 1H), 5.41 (m, 1H), 5.28 (m, 1H), 4.94–4.90 (m, 2H), 4.02 (t, J = 9.8 Hz, 1H), 3.73–3.64 (m, 4H), 2.97–2.89 (m, 1H), 2.84–2.76 (m, 2H), 2.65–2.58 (m, 1H), 2.53–2.40 (m, 7H), 2.39–2.32 (m, 1H), 2.23 (dq, J = 11.5, 3.4 Hz 1H), 2.11 (s, 3H), 2.08–2.03 (m, 1H), 1.81 (quint, J = 6.9 Hz, 1H), 1.40–1.29 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 177.1, 170.8, 148.6, 148.3, 113.5, 113.4, 83.7, 74.7, 66.9, 58.0, 54.0, 50.1, 47.8, 44.9, 44.1, 36.3, 36.1, 32.7, 21.3; LCMS (ESI): m/z 398.07 (M + Na)+; HRMS (ESI) calcd for C21H30O5N [M + H]+ 376.2118, found 376.2115.
3); [α]26D +69.7 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.59–5.52 (m, 1H), 5.41 (m, 1H), 5.28 (m, 1H), 4.94–4.90 (m, 2H), 4.02 (t, J = 9.8 Hz, 1H), 3.73–3.64 (m, 4H), 2.97–2.89 (m, 1H), 2.84–2.76 (m, 2H), 2.65–2.58 (m, 1H), 2.53–2.40 (m, 7H), 2.39–2.32 (m, 1H), 2.23 (dq, J = 11.5, 3.4 Hz 1H), 2.11 (s, 3H), 2.08–2.03 (m, 1H), 1.81 (quint, J = 6.9 Hz, 1H), 1.40–1.29 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 177.1, 170.8, 148.6, 148.3, 113.5, 113.4, 83.7, 74.7, 66.9, 58.0, 54.0, 50.1, 47.8, 44.9, 44.1, 36.3, 36.1, 32.7, 21.3; LCMS (ESI): m/z 398.07 (M + Na)+; HRMS (ESI) calcd for C21H30O5N [M + H]+ 376.2118, found 376.2115.
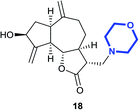
(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-6,9-dimethylene-3-(morpholinomethyl)decahydroazuleno[4,5-b]furan-2(3H)-one (18)
Brown viscous liquid (14%); Rf 0.40 (EtOAc–petroleum ether, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]26D +66.1 (c 1, CHCl3); 1H NMR (500 MHz, CDCl3) δH 5.39 (s, 1H), 5.31 (s, 1H), 4.97 (s, 1H), 4.93 (s, 1H), 4.56 (t, J = 7.3 Hz, 1H), 4.05 (t, J = 9.5 Hz, 1H), 3.71–3.65 (m, 4H), 3.45 (q, J = 7.0 Hz, 1H), 2.88 (q, J = 8.2 Hz, 1H), 2.83–2.76 (m, 2H), 2.64–2.57 (m, 1H), 2.53–2.41 (m, 5H), 2.37–2.32 (m, 2H), 2.24–2.18 (m, 1H), 2.08–2.02 (m, 1H), 1.81–1.71 (m, 1H), 1.38–1.32 (m, 2H); 13C NMR (125 MHz, CDCl3) δC; 177.3, 153.3, 148.8, 113.5, 111.1, 83.8, 73.6, 66.9, 58.0, 54.1, 49.7, 48.2, 45.0, 43.7, 38.8, 35.9, 32.8; LCMS (ESI): m/z 356.06 (M + Na)+; HRMS (ESI) calcd for C19H28O4N [M + H]+ 334.2013, found 334.2013.
3); [α]26D +66.1 (c 1, CHCl3); 1H NMR (500 MHz, CDCl3) δH 5.39 (s, 1H), 5.31 (s, 1H), 4.97 (s, 1H), 4.93 (s, 1H), 4.56 (t, J = 7.3 Hz, 1H), 4.05 (t, J = 9.5 Hz, 1H), 3.71–3.65 (m, 4H), 3.45 (q, J = 7.0 Hz, 1H), 2.88 (q, J = 8.2 Hz, 1H), 2.83–2.76 (m, 2H), 2.64–2.57 (m, 1H), 2.53–2.41 (m, 5H), 2.37–2.32 (m, 2H), 2.24–2.18 (m, 1H), 2.08–2.02 (m, 1H), 1.81–1.71 (m, 1H), 1.38–1.32 (m, 2H); 13C NMR (125 MHz, CDCl3) δC; 177.3, 153.3, 148.8, 113.5, 111.1, 83.8, 73.6, 66.9, 58.0, 54.1, 49.7, 48.2, 45.0, 43.7, 38.8, 35.9, 32.8; LCMS (ESI): m/z 356.06 (M + Na)+; HRMS (ESI) calcd for C19H28O4N [M + H]+ 334.2013, found 334.2013.
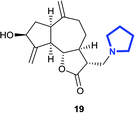
(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-6,9-dimethylene-3-(pyrrolidin-1-ylmethyl)decahydroazuleno[4,5-b]furan-2(3H)-one (19)
Brown viscous liquid (78%); Rf 0.20 (EtOAc); [α]26D +42.0 (c 1, CHCl3); 1H NMR (500 MHz, CDCl3) δH 5.39–5.26 (m, 2H), 5.00–4.88 (m, 2H), 4.14–3.95 (m, 1H), 3.55–3.36 (m, 1H), 2.93–2.78 (m, 4H), 2.76–2.68 (m, 1H), 2.66–2.59 (m, 2H), 2.54–2.44 (m, 2H), 2.38–2.21 (m, 3H), 2.09–1.99 (m, 2H), 1.99–1.92 (m, 1H), 1.91–1.84 (m, 1H), 1.82–1.77 (m, 2H), 1.76–1.70 (m, 1H), 1.52 (d, J = 6.8 Hz, 1H), 1.42–1.30 (m, 1H); 13C NMR (125 MHz, CDCl3) δC 177.3, 153.3, 148.9, 113.4, 110.8, 83.9, 73.5, 54.5, 54.4, 49.6, 47.7, 46.3, 43.5, 38.8, 36.2, 32.6, 23.5; LCMS (ESI): m/z 318.18 (M + H)+; HRMS (ESI) calcd for C19H28O3N [M + H]+ 318.2064, found 318.2060.(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-6,9-dimethylene-3-(piperidin-1-ylmethyl)decahydroazuleno[4,5-b]furan-2(3H)-one (20)
Dark brown viscous liquid (66%); Rf 0.30 (EtOAc); [α]26D +46.1 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.37 (d, J = 1.8 Hz, 1H), 5.30 (d, J = 1.8 Hz, 1H), 4.96 (s, 1H), 4.93 (s, 1H), 4.55 (dt, J = 7.3, 1.4, Hz, 1H), 4.06 (dt, J = 9.6, 1.8 Hz, 1H), 2.92–2.75 (m, 3H), 2.70–2.63 (m, 1H), 2.61–2.53 (m, 3H), 2.52–2.46 (m, 3H), 2.38–2.29 (m, 2H), 2.22–2.10 (m, 1H), 2.08–1.98 (m, 2H), 1.79–1.71 (m, 1H), 1.65–1.57 (m, 4H), 1.50–1.42 (m, 2H), 1.38–1.32 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 177.6, 153.4, 149.0, 113.5, 110.9, 84.0, 73.6, 57.7, 54.6, 49.7, 48.3, 44.7, 43.6, 38.8, 36.1, 32.5, 29.8, 25.3, 23.8; LCMS (ESI): m/z 354.14 (M + Na)+; HRMS (ESI) calcd for C20H30O3N [M + H]+ 332.2220, found 332.2219.Methyl(((3R,3aS,6aR,8S,9aR,9bS)-8-hydroxy-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b]furan-3-yl)methyl)-L-leucinate (21)
Yellow viscous liquid (82%); Rf 0.30 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +29.8 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.37 (s, 1H), 5.30 (s, 1H), 4.97 (s, 1H), 4.93 (s, 1H), 4.55 (t, J = 7.8 Hz, 1H), 4.05 (t, J = 9.6 Hz, 1H), 3.72 (s, 3H), 3.30 (t, J = 7.3 Hz, 1H), 2.91–2.84 (m, 2H), 2.84–2.77 (m, 1H), 2.72 (dd, J = 11.9, 4.6 Hz, 1H), 2.50 (td, J = 13.3, 4.6 Hz, 1H), 2.44–2.26 (m, 3H), 2.26–2.11 (m, 2H), 2.06–1.98 (m, 1H), 1.79–1.67 (m, 2H), 1.43–1.28 (m, 2H), 1.53–1.43 (m, 2H), 0.94–0.88 (m, 6H); 13C NMR (100 MHz, CDCl3) δC 177.4, 176.1, 153.4, 148.8, 113.7, 111.1, 84.1, 73.6, 60.7, 51.8, 49.7, 48.0, 46.1, 46.0, 43.7, 42.6, 38.9, 35.9, 32.5, 25.0, 22.8, 22.3; LCMS (ESI): m/z 414.27 (M + Na)+; HRMS (ESI) calcd for C22H34O5N [M + H]+ 392.2431, found 392.2434.
1); [α]25D +29.8 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.37 (s, 1H), 5.30 (s, 1H), 4.97 (s, 1H), 4.93 (s, 1H), 4.55 (t, J = 7.8 Hz, 1H), 4.05 (t, J = 9.6 Hz, 1H), 3.72 (s, 3H), 3.30 (t, J = 7.3 Hz, 1H), 2.91–2.84 (m, 2H), 2.84–2.77 (m, 1H), 2.72 (dd, J = 11.9, 4.6 Hz, 1H), 2.50 (td, J = 13.3, 4.6 Hz, 1H), 2.44–2.26 (m, 3H), 2.26–2.11 (m, 2H), 2.06–1.98 (m, 1H), 1.79–1.67 (m, 2H), 1.43–1.28 (m, 2H), 1.53–1.43 (m, 2H), 0.94–0.88 (m, 6H); 13C NMR (100 MHz, CDCl3) δC 177.4, 176.1, 153.4, 148.8, 113.7, 111.1, 84.1, 73.6, 60.7, 51.8, 49.7, 48.0, 46.1, 46.0, 43.7, 42.6, 38.9, 35.9, 32.5, 25.0, 22.8, 22.3; LCMS (ESI): m/z 414.27 (M + Na)+; HRMS (ESI) calcd for C22H34O5N [M + H]+ 392.2431, found 392.2434.
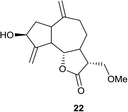
(3R,3aS,6aR,8S,9aR,9bS)-8-Hydroxy-3-(methoxymethyl)-6,9-dimethylenedecahydroazuleno[4,5-b]furan-2(3H)-one (22)
Yellow viscous liquid (87%); Rf 0.40 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]26D +62.4 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.38 (m, 1H), 5.30 (m, 1H), 4.97 (m, 1H), 4.93 (m, 1H), 4.55 (t, J = 7.3 Hz, 1H), 4.05 (t, J = 8.7 Hz, 1H), 3.73–3.68 (m, 1H), 3.63 (dd, J = 9.6, 1.8 Hz, 1H), 3.37 (s, 3H), 2.93–2.80 (m, 2H), 2.51 (td, J = 12.8, 4.6 Hz, 1H), 2.42–2.29 (m, 3H), 2.24–2.16 (m, 1H), 2.09–2.01 (m, 1H), 1.80–1.71 (m, 1H), 1.42–1.28 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 176.1, 153.4, 148.9, 113.7, 110.9, 84.1, 73.6, 68.9, 59.4, 49.5, 48.1, 45.1, 43.6, 38.9, 36.2, 32.6; LCMS (ESI): m/z 300.99 (M + Na)+; HRMS (ESI) calcd for C16H22O4Na [M + Na]+ 301.1410, found 301.1398.
1); [α]26D +62.4 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δH 5.38 (m, 1H), 5.30 (m, 1H), 4.97 (m, 1H), 4.93 (m, 1H), 4.55 (t, J = 7.3 Hz, 1H), 4.05 (t, J = 8.7 Hz, 1H), 3.73–3.68 (m, 1H), 3.63 (dd, J = 9.6, 1.8 Hz, 1H), 3.37 (s, 3H), 2.93–2.80 (m, 2H), 2.51 (td, J = 12.8, 4.6 Hz, 1H), 2.42–2.29 (m, 3H), 2.24–2.16 (m, 1H), 2.09–2.01 (m, 1H), 1.80–1.71 (m, 1H), 1.42–1.28 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 176.1, 153.4, 148.9, 113.7, 110.9, 84.1, 73.6, 68.9, 59.4, 49.5, 48.1, 45.1, 43.6, 38.9, 36.2, 32.6; LCMS (ESI): m/z 300.99 (M + Na)+; HRMS (ESI) calcd for C16H22O4Na [M + Na]+ 301.1410, found 301.1398.
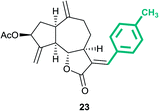
(3aS,6aR,8S,9aR,9bS)-3-((E)-4-Methylbenzylidene)-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (23)
Brown viscous liquid (48%); Rf 0.45 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (500 MHz, CDCl3) δH 7.63 (d, J = 2.7 Hz, 1H), 7.30 (d, J = 7.9 Hz, 2H), 7.24 (d, J = 7.9 Hz, 2H), 5.61 (t, J = 7.2 Hz, 1H), 5.56 (s, 1H), 5.33 (s, 1H), 5.01 (s, 1H), 4.94 (s, 1H), 4.21 (dd, J = 9.9, 8.1 Hz, 1H), 3.41 (td, J = 7.4, 3.8 Hz, 1H), 3.03 (q, J = 8.2 Hz, 1H), 2.95–2.83 (m, 1H), 2.49–2.44 (m, 1H), 2.42 (s, 3H), 2.37–2.29 (m, 2H), 2.21–2.15 (m, 1H), 2.14 (s, 3H), 1.89–1.81 (m, 1H); 13C NMR (125 MHz, CDCl3) δC 171.8, 170.9, 148.0, 147.5, 139.9, 138.1, 131.0, 129.7, 129.3, 128.4, 114.7, 113.8, 83.7, 74.7, 50.6, 45.2, 43.5, 36.7, 33.3, 28.4, 21.5, 21.3; HRMS (ESI) calcd for C24H26O4Na [M + Na]+ 401.1723, found 401.1720.
4); 1H NMR (500 MHz, CDCl3) δH 7.63 (d, J = 2.7 Hz, 1H), 7.30 (d, J = 7.9 Hz, 2H), 7.24 (d, J = 7.9 Hz, 2H), 5.61 (t, J = 7.2 Hz, 1H), 5.56 (s, 1H), 5.33 (s, 1H), 5.01 (s, 1H), 4.94 (s, 1H), 4.21 (dd, J = 9.9, 8.1 Hz, 1H), 3.41 (td, J = 7.4, 3.8 Hz, 1H), 3.03 (q, J = 8.2 Hz, 1H), 2.95–2.83 (m, 1H), 2.49–2.44 (m, 1H), 2.42 (s, 3H), 2.37–2.29 (m, 2H), 2.21–2.15 (m, 1H), 2.14 (s, 3H), 1.89–1.81 (m, 1H); 13C NMR (125 MHz, CDCl3) δC 171.8, 170.9, 148.0, 147.5, 139.9, 138.1, 131.0, 129.7, 129.3, 128.4, 114.7, 113.8, 83.7, 74.7, 50.6, 45.2, 43.5, 36.7, 33.3, 28.4, 21.5, 21.3; HRMS (ESI) calcd for C24H26O4Na [M + Na]+ 401.1723, found 401.1720.
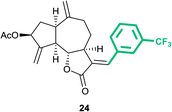
(3aS,6aR,8S,9aR,9bS)-6,9-Dimethylene-2-oxo-3-((E)-3-(trifluoro-methyl)benzylidene)dodecahydroazuleno[4,5-b]furan-8-yl acetate (24)
Brown viscous liquid (53%); Rf 0.42 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (400 MHz, CDCl3) δH 7.67–7.61 (m, 3H), 7.57–7.54 (m, 2H), 5.63–5.56 (m, 1H), 5.56–5.53 (m, 1H), 5.35–5.31 (m, 1H), 5.01 (s, 1H), 4.92 (s, 1H), 4.23 (dd, J = 10.4, 7.7 Hz, 1H), 3.42 (td, J = 7.5, 3.8 Hz, 1H), 3.03 (q, J = 8.3 Hz, 1H), 2.91–2.82 (m, 1H), 2.43 (dt, J = 14.2, 8.0 Hz, 1H), 2.34–2.21 (m, 3H), 2.19–2.13 (m, 1H), 2.11 (s, 3H), 1.87–1.78 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 170.9, 170.8, 147.9, 146.9, 135.9, 134.7, 132.8, 131.7, 129.2, 128.6, 125.9, 125.9, 122.4, 115.1, 114.2, 83.6, 74.6, 50.6, 45.3, 43.3, 36.7, 32.3, 28.1, 21.3; HRMS (ESI) calcd for C24H23O4F3Na [M + Na]+ 455.1441, found 455.1436.
4); 1H NMR (400 MHz, CDCl3) δH 7.67–7.61 (m, 3H), 7.57–7.54 (m, 2H), 5.63–5.56 (m, 1H), 5.56–5.53 (m, 1H), 5.35–5.31 (m, 1H), 5.01 (s, 1H), 4.92 (s, 1H), 4.23 (dd, J = 10.4, 7.7 Hz, 1H), 3.42 (td, J = 7.5, 3.8 Hz, 1H), 3.03 (q, J = 8.3 Hz, 1H), 2.91–2.82 (m, 1H), 2.43 (dt, J = 14.2, 8.0 Hz, 1H), 2.34–2.21 (m, 3H), 2.19–2.13 (m, 1H), 2.11 (s, 3H), 1.87–1.78 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 170.9, 170.8, 147.9, 146.9, 135.9, 134.7, 132.8, 131.7, 129.2, 128.6, 125.9, 125.9, 122.4, 115.1, 114.2, 83.6, 74.6, 50.6, 45.3, 43.3, 36.7, 32.3, 28.1, 21.3; HRMS (ESI) calcd for C24H23O4F3Na [M + Na]+ 455.1441, found 455.1436.
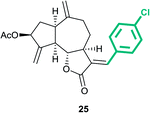
(3aS,6aR,8S,9aR,9bS)-3-((E)-4-Chlorobenzylidene)-6,9-dimethylene -2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (25)
Brown viscous liquid (64%); Rf 0.42 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (400 MHz, CDCl3) δH 7.60–7.54 (m, 1H), 7.39 (d, J = 8.1 Hz, 2H), 7.31 (d, J = 8.1 Hz, 2H), 5.59 (t, J = 7.1 Hz, 1H), 5.53 (s, 1H), 5.32 (s, 1H), 4.99 (s, 1H), 4.92 (s, 1H), 4.24–4.17 (m, 1H), 3.39–3.30 (m, 1H), 3.01 (q, J = 8.1 Hz, 1H), 2.85 (t, J = 9.3 Hz, 1H), 2.48–2.38 (m, 1H), 2.36–2.26 (m, 2H), 2.20–2.14 (m, 1H), 2.11 (s, 3H), 1.87–1.77 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 171.3, 170.8, 147.9, 147.2, 136.5, 135.4, 132.4, 130.8, 130.3, 128.9, 115.0, 114.1, 83.5, 74.6, 50.6, 45.2, 43.4, 36.7, 32.9, 28.3, 21.3; HRMS (ESI) calcd for C23H23O4ClNa [M + Na]+ 421.1177, found 421.1171.
4); 1H NMR (400 MHz, CDCl3) δH 7.60–7.54 (m, 1H), 7.39 (d, J = 8.1 Hz, 2H), 7.31 (d, J = 8.1 Hz, 2H), 5.59 (t, J = 7.1 Hz, 1H), 5.53 (s, 1H), 5.32 (s, 1H), 4.99 (s, 1H), 4.92 (s, 1H), 4.24–4.17 (m, 1H), 3.39–3.30 (m, 1H), 3.01 (q, J = 8.1 Hz, 1H), 2.85 (t, J = 9.3 Hz, 1H), 2.48–2.38 (m, 1H), 2.36–2.26 (m, 2H), 2.20–2.14 (m, 1H), 2.11 (s, 3H), 1.87–1.77 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 171.3, 170.8, 147.9, 147.2, 136.5, 135.4, 132.4, 130.8, 130.3, 128.9, 115.0, 114.1, 83.5, 74.6, 50.6, 45.2, 43.4, 36.7, 32.9, 28.3, 21.3; HRMS (ESI) calcd for C23H23O4ClNa [M + Na]+ 421.1177, found 421.1171.
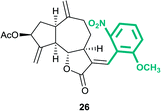
(3aS,6aR,8S,9aR,9bS)-3-((E)-2-Methoxy-6-nitrobenzylidene)-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (26)
Brown viscous liquid (42%); Rf 0.37 (EtOAc–petroleum ether, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7); 1H NMR (400 MHz, CDCl3) δH 7.67 (d, J = 7.8 Hz, 1H), 7.42 (t, J = 8.3 Hz, 1H), 7.15 (d, J = 8.1 Hz, 1H), 6.86 (brs, 1H), 5.56 (t, J = 7.3 Hz, 1H), 5.45 (s, 1H), 5.27 (s, 1H), 4.98 (s, 2H), 4.10 (t, J = 9.5 Hz, 1H), 3.83 (s, 3H), 3.11–3.01 (m, 1H), 2.97 (q, J = 8.1 Hz, 1H), 2.90–2.82 (m, 1H), 2.57–2.50 (m, 1H), 2.48–2.36 (m, 2H), 2.28–2.19 (m, 1H), 2.11 (s, 3H), 1.86–1.78 (m, 1H), 1.64–1.53 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 170.9, 170.9, 148.6, 148.0, 147.8, 132.4, 129.3, 122.7, 119.7, 116.4, 115.4, 114.3, 113.7, 83.1, 74.7, 56.5, 50.3, 46.4, 44.7, 36.5, 34.3, 30.6, 21.3; HRMS (ESI) calcd for C24H25O7NNa [M + Na]+ 462.1523, found 462.1521.
7); 1H NMR (400 MHz, CDCl3) δH 7.67 (d, J = 7.8 Hz, 1H), 7.42 (t, J = 8.3 Hz, 1H), 7.15 (d, J = 8.1 Hz, 1H), 6.86 (brs, 1H), 5.56 (t, J = 7.3 Hz, 1H), 5.45 (s, 1H), 5.27 (s, 1H), 4.98 (s, 2H), 4.10 (t, J = 9.5 Hz, 1H), 3.83 (s, 3H), 3.11–3.01 (m, 1H), 2.97 (q, J = 8.1 Hz, 1H), 2.90–2.82 (m, 1H), 2.57–2.50 (m, 1H), 2.48–2.36 (m, 2H), 2.28–2.19 (m, 1H), 2.11 (s, 3H), 1.86–1.78 (m, 1H), 1.64–1.53 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 170.9, 170.9, 148.6, 148.0, 147.8, 132.4, 129.3, 122.7, 119.7, 116.4, 115.4, 114.3, 113.7, 83.1, 74.7, 56.5, 50.3, 46.4, 44.7, 36.5, 34.3, 30.6, 21.3; HRMS (ESI) calcd for C24H25O7NNa [M + Na]+ 462.1523, found 462.1521.
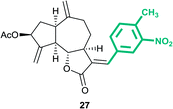
(3aS,6aR,8S,9aR,9bS)-3-((E)-4-Methyl-3-nitrobenzylidene)-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (27)
Light brown semi solid (54%); Rf 0.56 (EtOAc–petroleum ether, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7); 1H NMR (400 MHz, CDCl3) δH 8.00 (s, 1H), 7.57 (d, J = 3.4 Hz, 1H), 7.53–7.48 (m, 1H), 7.41 (d, J = 7.8 Hz, 1H), 5.63–5.55 (m, 1H), 5.55–5.51 (m, 1H), 5.32 (t, J = 1.7 Hz, 1H), 5.02 (s, 1H), 4.96 (s, 1H), 4.23 (dd, J = 10.4, 7.7 Hz, 1H), 3.47–3.39 (m, 1H), 3.10–2.94 (m, 1H), 2.93–2.80 (m, 1H), 2.65 (s, 3H), 2.49–2.38 (m, 1H), 2.38–2.26 (m, 2H), 2.26–2.15 (m, 1H), 2.11 (s, 3H), 1.87–1.78 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 170.8, 170.8, 149.1, 147.9, 146.9, 134.7, 133.9, 133.2, 132.6, 131.9, 126.6, 125.1, 115.3, 114.1, 83.6, 74.6, 50.6, 45.2, 43.3, 36.6, 32.5, 28.2, 21.2, 20.5; HRMS (ESI) calcd for C24H25O6NNa [M + Na]+ 446.1574, found 446.1569.
7); 1H NMR (400 MHz, CDCl3) δH 8.00 (s, 1H), 7.57 (d, J = 3.4 Hz, 1H), 7.53–7.48 (m, 1H), 7.41 (d, J = 7.8 Hz, 1H), 5.63–5.55 (m, 1H), 5.55–5.51 (m, 1H), 5.32 (t, J = 1.7 Hz, 1H), 5.02 (s, 1H), 4.96 (s, 1H), 4.23 (dd, J = 10.4, 7.7 Hz, 1H), 3.47–3.39 (m, 1H), 3.10–2.94 (m, 1H), 2.93–2.80 (m, 1H), 2.65 (s, 3H), 2.49–2.38 (m, 1H), 2.38–2.26 (m, 2H), 2.26–2.15 (m, 1H), 2.11 (s, 3H), 1.87–1.78 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 170.8, 170.8, 149.1, 147.9, 146.9, 134.7, 133.9, 133.2, 132.6, 131.9, 126.6, 125.1, 115.3, 114.1, 83.6, 74.6, 50.6, 45.2, 43.3, 36.6, 32.5, 28.2, 21.2, 20.5; HRMS (ESI) calcd for C24H25O6NNa [M + Na]+ 446.1574, found 446.1569.
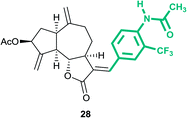
(3aS,6aR,8S,9aR,9bS)-3-((E)-4-Acetamido-3-(trifluoromethyl) benzylidene)-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b] furan-8-yl acetate (28)
Brown viscous liquid (68%); Rf 0.15 (EtOAc–petroleum ether, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7); 1H NMR (500 MHz, CDCl3) δH 8.37 (d, J = 7.9 Hz, 1H), 7.62 (s, 1H), 7.59–7.53 (m, 3H), 5.62–5.56 (m, 1H), 5.54 (s, 1H), 5.34–5.31 (m, 1H), 5.02 (s, 1H), 4.94 (s, 1H), 4.23 (dd, J = 10.4, 7.6 Hz, 1H), 3.40 (dt, J = 7.6, 3.7 Hz, 1H), 3.03 (q, J = 8.2 Hz, 1H), 2.90–2.82 (m, 1H), 2.43 (td, J = 14.0, 8.1 Hz, 1H), 2.35–2.29 (m, 2H), 2.26 (s, 3H), 2.24–2.15 (m, 2H), 2.12 (s, 3H), 1.87–1.79 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 171.1, 170.8, 168.5, 147.9, 147.0, 136.0, 135.4, 133.9, 130.7, 127.4, 123.8, 115.2, 114.1, 83.6, 74.6, 50.6, 45.3, 43.2, 36.7, 32.4, 28.1, 24.9, 21.3; HRMS (ESI) calcd for C26H26O5NF3Na [M + Na]+ 512.1655, found 512.1650.
7); 1H NMR (500 MHz, CDCl3) δH 8.37 (d, J = 7.9 Hz, 1H), 7.62 (s, 1H), 7.59–7.53 (m, 3H), 5.62–5.56 (m, 1H), 5.54 (s, 1H), 5.34–5.31 (m, 1H), 5.02 (s, 1H), 4.94 (s, 1H), 4.23 (dd, J = 10.4, 7.6 Hz, 1H), 3.40 (dt, J = 7.6, 3.7 Hz, 1H), 3.03 (q, J = 8.2 Hz, 1H), 2.90–2.82 (m, 1H), 2.43 (td, J = 14.0, 8.1 Hz, 1H), 2.35–2.29 (m, 2H), 2.26 (s, 3H), 2.24–2.15 (m, 2H), 2.12 (s, 3H), 1.87–1.79 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 171.1, 170.8, 168.5, 147.9, 147.0, 136.0, 135.4, 133.9, 130.7, 127.4, 123.8, 115.2, 114.1, 83.6, 74.6, 50.6, 45.3, 43.2, 36.7, 32.4, 28.1, 24.9, 21.3; HRMS (ESI) calcd for C26H26O5NF3Na [M + Na]+ 512.1655, found 512.1650.
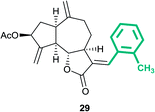
(3aS,6aR,8S,9aR,9bS)-3-((E)-2-Methylbenzylidene)-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (29)
Brown viscous liquid (56%); Rf 0.45 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (500 MHz, CDCl3) δH 7.73 (d, J = 3.4 Hz, 1H), 7.27–7.16 (m, 4H), 5.62–5.55 (m, 1H), 5.53 (t, J = 2.0 Hz, 1H), 5.32 (t, J = 1.8 Hz, 1H), 4.94 (s, 1H), 4.88–4.82 (m, 1H), 4.17 (dd, J = 10.4, 8.2 Hz, 1H), 3.33–3.23 (m, 1H), 2.97 (q, J = 8.0 Hz, 1H), 2.89–2.81 (m, 1H), 2.44–2.38 (m, 1H), 2.31 (s, 3H), 2.27–2.15 (m, 2H), 2.11 (s, 3H), 2.06–2.00 (m, 2H), 1.84–1.76 (m, 1H); 13C NMR (125 MHz, CDCl3) δC 171.4, 170.9, 148.0, 147.4, 137.5, 136.9, 133.4, 130.6, 130.3, 129.3, 127.9, 125.7, 114.6, 114.1, 83.4, 74.7, 50.7, 45.1, 43.9, 36.6, 33.0, 28.7, 21.3, 19.9; HRMS (ESI) calcd for C24H26O4Na [M + Na]+ 401.1723, found 401.1718.
4); 1H NMR (500 MHz, CDCl3) δH 7.73 (d, J = 3.4 Hz, 1H), 7.27–7.16 (m, 4H), 5.62–5.55 (m, 1H), 5.53 (t, J = 2.0 Hz, 1H), 5.32 (t, J = 1.8 Hz, 1H), 4.94 (s, 1H), 4.88–4.82 (m, 1H), 4.17 (dd, J = 10.4, 8.2 Hz, 1H), 3.33–3.23 (m, 1H), 2.97 (q, J = 8.0 Hz, 1H), 2.89–2.81 (m, 1H), 2.44–2.38 (m, 1H), 2.31 (s, 3H), 2.27–2.15 (m, 2H), 2.11 (s, 3H), 2.06–2.00 (m, 2H), 1.84–1.76 (m, 1H); 13C NMR (125 MHz, CDCl3) δC 171.4, 170.9, 148.0, 147.4, 137.5, 136.9, 133.4, 130.6, 130.3, 129.3, 127.9, 125.7, 114.6, 114.1, 83.4, 74.7, 50.7, 45.1, 43.9, 36.6, 33.0, 28.7, 21.3, 19.9; HRMS (ESI) calcd for C24H26O4Na [M + Na]+ 401.1723, found 401.1718.
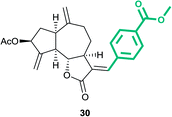
Methyl-4-((E)-((3aS,6aR,8S,9aR,9bS)-8-acetoxy-6,9-dimethylene-2-oxodecahydroazuleno[4,5-b]furan-3(2H)-ylidene)methyl)benzoate (30)
Brown viscous liquid (67%); Rf 0.24 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (400 MHz, CDCl3) δH 8.07–8.02 (m, 1H), 7.58–7.52 (m, 1H), 7.48–7.43 (m, 1H), 7.36–7.23 (m, 2H), 5.60–5.51 (m, 2H), 5.45 (d, J = 11.2 Hz, 1H), 4.95–4.89 (m, 2H), 4.17 (dd, J = 10.3, 8.3 Hz, 1H), 3.90 (s, 3H), 3.24–3.17 (m, 1H), 2.98–2.90 (m, 1H), 2.86–2.78 (m, 1H), 2.59–2.51 (m, 1H), 2.47–2.32 (m, 2H), 2.21–2.12 (m, 1H), 2.10 (s, 3H), 2.01–1.93 (m, 1H), 1.79–1.72 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 171.0, 170.8, 166.6, 147.9, 147.3, 138.3, 136.4, 132.2, 130.9, 129.3, 128.9, 128.8, 128.4, 114.5, 114.3, 83.0, 74.7, 52.4, 50.8, 45.2, 43.7, 36.6, 32.6, 29.0, 21.3; HRMS (ESI) calcd for C25H26O6Na [M + Na]+ 445.1622, found 445.1616.
4); 1H NMR (400 MHz, CDCl3) δH 8.07–8.02 (m, 1H), 7.58–7.52 (m, 1H), 7.48–7.43 (m, 1H), 7.36–7.23 (m, 2H), 5.60–5.51 (m, 2H), 5.45 (d, J = 11.2 Hz, 1H), 4.95–4.89 (m, 2H), 4.17 (dd, J = 10.3, 8.3 Hz, 1H), 3.90 (s, 3H), 3.24–3.17 (m, 1H), 2.98–2.90 (m, 1H), 2.86–2.78 (m, 1H), 2.59–2.51 (m, 1H), 2.47–2.32 (m, 2H), 2.21–2.12 (m, 1H), 2.10 (s, 3H), 2.01–1.93 (m, 1H), 1.79–1.72 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 171.0, 170.8, 166.6, 147.9, 147.3, 138.3, 136.4, 132.2, 130.9, 129.3, 128.9, 128.8, 128.4, 114.5, 114.3, 83.0, 74.7, 52.4, 50.8, 45.2, 43.7, 36.6, 32.6, 29.0, 21.3; HRMS (ESI) calcd for C25H26O6Na [M + Na]+ 445.1622, found 445.1616.
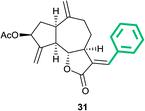
(3aS,6aR,8S,9aR,9bS)-3-((E)-Benzylidene)-6,9-dimethylene-2-oxo dodecahydroazuleno[4,5-b]furan-8-yl acetate (31)
Light brown viscous liquid (55%); Rf 0.57 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (400 MHz, CDCl3) δH 7.41–7.36 (m, 6H), 5.63–5.56 (m, 1H), 5.54 (t, J = 2.0 Hz, 1H), 5.32 (t, J = 2.0 Hz, 1H), 4.98 (s, 1H), 4.92 (s, 1H), 4.20 (dd, J = 10.3, 7.8 Hz, 1H), 3.43–3.35 (m, 1H), 3.01 (q, J = 8.1 Hz, 1H), 2.92–2.80 (m, 1H), 2.48–2.36 (m, 2H), 2.33–2.27 (m, 1H), 2.19–2.13 (m, 1H), 2.11 (s, 3H), 1.96–1.89 (m, 1H), 1.87–1.78 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 171.6, 170.9, 148.0, 147.4, 138.0, 133.9, 129.6, 129.4, 128.6, 128.4, 114.8, 113.9, 83.6, 74.7, 50.6, 45.2, 43.5, 36.7, 33.1, 28.4, 21.3; HRMS (ESI) calcd for C23H24O4Na [M + Na]+ 387.1567, found 387.1563.
4); 1H NMR (400 MHz, CDCl3) δH 7.41–7.36 (m, 6H), 5.63–5.56 (m, 1H), 5.54 (t, J = 2.0 Hz, 1H), 5.32 (t, J = 2.0 Hz, 1H), 4.98 (s, 1H), 4.92 (s, 1H), 4.20 (dd, J = 10.3, 7.8 Hz, 1H), 3.43–3.35 (m, 1H), 3.01 (q, J = 8.1 Hz, 1H), 2.92–2.80 (m, 1H), 2.48–2.36 (m, 2H), 2.33–2.27 (m, 1H), 2.19–2.13 (m, 1H), 2.11 (s, 3H), 1.96–1.89 (m, 1H), 1.87–1.78 (m, 1H); 13C NMR (100 MHz, CDCl3) δC 171.6, 170.9, 148.0, 147.4, 138.0, 133.9, 129.6, 129.4, 128.6, 128.4, 114.8, 113.9, 83.6, 74.7, 50.6, 45.2, 43.5, 36.7, 33.1, 28.4, 21.3; HRMS (ESI) calcd for C23H24O4Na [M + Na]+ 387.1567, found 387.1563.
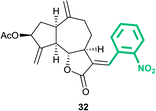
(3aS,6aR,8S,9aR,9bS)-6,9-Dimethylene-3-((E)-2-nitrobenzylidene)-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (32)
Brown viscous liquid (47%); Rf 0.20 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (400 MHz, CDCl3) δH 8.23–8.13 (m, 1H), 7.87 (d, J = 3.7 Hz, 1H), 7.73–7.65 (m, 1H), 7.63–7.55 (m, 1H), 7.42 (d, J = 7.3 Hz, 1H), 5.59–5.51 (m, 2H), 5.36–5.29 (m, 1H), 4.93 (s, 1H), 4.81 (s, 1H), 4.20 (dd, J = 10.3, 8.3 Hz, 1H), 3.24–3.12 (m, 1H), 2.94 (q, J = 8.4 Hz, 1H), 2.87–2.77 (m, 1H), 2.43–2.34 (m, 1H), 2.23–2.14 (m, 1H), 2.11 (s, 3H), 2.01–1.95 (m, 1H), 1.83–1.73 (m, 1H), 1.66–1.53 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 170.9, 170.3, 147.7, 147.5, 146.9, 134.2, 133.6, 132.5, 130.8, 130.1, 129.9, 125.1, 114.9, 114.6, 82.9, 74.6, 50.7, 45.2, 43.7, 36.6, 32.2, 29.0, 21.3; HRMS (ESI) calcd for C23H23O6NNa [M + Na]+ 432.1418, found 432.1413.
4); 1H NMR (400 MHz, CDCl3) δH 8.23–8.13 (m, 1H), 7.87 (d, J = 3.7 Hz, 1H), 7.73–7.65 (m, 1H), 7.63–7.55 (m, 1H), 7.42 (d, J = 7.3 Hz, 1H), 5.59–5.51 (m, 2H), 5.36–5.29 (m, 1H), 4.93 (s, 1H), 4.81 (s, 1H), 4.20 (dd, J = 10.3, 8.3 Hz, 1H), 3.24–3.12 (m, 1H), 2.94 (q, J = 8.4 Hz, 1H), 2.87–2.77 (m, 1H), 2.43–2.34 (m, 1H), 2.23–2.14 (m, 1H), 2.11 (s, 3H), 2.01–1.95 (m, 1H), 1.83–1.73 (m, 1H), 1.66–1.53 (m, 2H); 13C NMR (100 MHz, CDCl3) δC 170.9, 170.3, 147.7, 147.5, 146.9, 134.2, 133.6, 132.5, 130.8, 130.1, 129.9, 125.1, 114.9, 114.6, 82.9, 74.6, 50.7, 45.2, 43.7, 36.6, 32.2, 29.0, 21.3; HRMS (ESI) calcd for C23H23O6NNa [M + Na]+ 432.1418, found 432.1413.
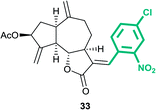
(3aS,6aR,8S,9aR,9bS)-3-((E)-4-Chloro-2-nitrobenzylidene)-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (33)
Brown viscous liquid (52%); Rf 0.26 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (500 MHz, CDCl3) δH 8.18 (d, J = 2.1 Hz, 1H), 7.78 (d, J = 3.7 Hz, 1H), 7.66 (dd, J = 8.2, 1.8 Hz, 1H), 7.37 (d, J = 8.2 Hz, 1H), 5.60–5.55 (m, 1H), 5.52 (t, J = 1.8 Hz, 1H), 5.33 (t, J = 1.8 Hz, 1H), 4.96–4.93 (m, 1H), 4.84 (s, 1H), 4.21 (dd, J = 10.4, 8.2 Hz, 1H), 3.19–3.12 (m, 1H), 2.98–2.92 (m, 1H), 2.84–2.77 (m, 1H), 2.42–2.35 (m, 1H), 2.23–2.17 (m, 1H), 2.11 (s, 3H), 2.04–1.98 (m, 1H), 1.91–1.82 (m, 1H), 1.82–1.76 (m, 1H), 1.67–1.60 (m, 1H); 13C NMR (125 MHz, CDCl3) δC 170.8, 170.0, 147.9, 147.6, 146.7, 135.8, 133.7, 133.3, 132.8, 131.2, 129.1, 125.4, 115.1, 114.7, 82.8, 74.6, 50.8, 45.2, 43.7, 36.6, 32.0, 29.1, 21.3; HRMS (ESI) calcd for C23H22O6NClNa [M + Na]+ 466.1028, found 466.1027.
4); 1H NMR (500 MHz, CDCl3) δH 8.18 (d, J = 2.1 Hz, 1H), 7.78 (d, J = 3.7 Hz, 1H), 7.66 (dd, J = 8.2, 1.8 Hz, 1H), 7.37 (d, J = 8.2 Hz, 1H), 5.60–5.55 (m, 1H), 5.52 (t, J = 1.8 Hz, 1H), 5.33 (t, J = 1.8 Hz, 1H), 4.96–4.93 (m, 1H), 4.84 (s, 1H), 4.21 (dd, J = 10.4, 8.2 Hz, 1H), 3.19–3.12 (m, 1H), 2.98–2.92 (m, 1H), 2.84–2.77 (m, 1H), 2.42–2.35 (m, 1H), 2.23–2.17 (m, 1H), 2.11 (s, 3H), 2.04–1.98 (m, 1H), 1.91–1.82 (m, 1H), 1.82–1.76 (m, 1H), 1.67–1.60 (m, 1H); 13C NMR (125 MHz, CDCl3) δC 170.8, 170.0, 147.9, 147.6, 146.7, 135.8, 133.7, 133.3, 132.8, 131.2, 129.1, 125.4, 115.1, 114.7, 82.8, 74.6, 50.8, 45.2, 43.7, 36.6, 32.0, 29.1, 21.3; HRMS (ESI) calcd for C23H22O6NClNa [M + Na]+ 466.1028, found 466.1027.
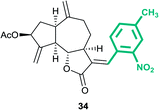
(3aS,6aR,8S,9aR,9bS)-3-((E)-4-Methyl-2-nitrobenzylidene)-6,9-dimethylene-2-oxododecahydroazuleno[4,5-b]furan-8-yl acetate (34)
Brown viscous liquid (67%); Rf 0.26 (EtOAc–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); 1H NMR (400 MHz, CDCl3) δH 8.02–7.94 (m, 1H), 7.82 (d, J = 3.7 Hz, 1H), 7.48 (d, J = 7.3 Hz, 1H), 7.30 (d, J = 7.8 Hz, 1H), 5.62–5.50 (m, 2H), 5.36–5.27 (m, 1H), 5.03–4.90 (m, 2H), 4.82 (s, 1H), 4.25–4.10 (m, 1H), 3.21–3.14 (m, 1H), 2.99–2.90 (m, 1H), 2.88–2.75 (m, 1H), 2.51 (s, 3H), 2.42–2.36 (m, 1H), 2.22–2.16 (m, 1H), 2.11 (s, 3H), 2.03–1.95 (m, 1H), 1.87–1.63 (m, 3H); 13C NMR (100 MHz, CDCl3) δC 170.9, 170.4, 147.8, 147.4, 147.0, 140.8, 134.3, 132.0, 129.9, 127.8, 125.4, 124.9, 114.8, 114.5, 82.9, 74.6, 50.7, 45.2, 43.8, 36.6, 32.3, 29.0, 21.3, 21.2; HRMS (ESI) calcd for C24H25O6NNa [M + Na]+ 446.1574, found 446.1571.
4); 1H NMR (400 MHz, CDCl3) δH 8.02–7.94 (m, 1H), 7.82 (d, J = 3.7 Hz, 1H), 7.48 (d, J = 7.3 Hz, 1H), 7.30 (d, J = 7.8 Hz, 1H), 5.62–5.50 (m, 2H), 5.36–5.27 (m, 1H), 5.03–4.90 (m, 2H), 4.82 (s, 1H), 4.25–4.10 (m, 1H), 3.21–3.14 (m, 1H), 2.99–2.90 (m, 1H), 2.88–2.75 (m, 1H), 2.51 (s, 3H), 2.42–2.36 (m, 1H), 2.22–2.16 (m, 1H), 2.11 (s, 3H), 2.03–1.95 (m, 1H), 1.87–1.63 (m, 3H); 13C NMR (100 MHz, CDCl3) δC 170.9, 170.4, 147.8, 147.4, 147.0, 140.8, 134.3, 132.0, 129.9, 127.8, 125.4, 124.9, 114.8, 114.5, 82.9, 74.6, 50.7, 45.2, 43.8, 36.6, 32.3, 29.0, 21.3, 21.2; HRMS (ESI) calcd for C24H25O6NNa [M + Na]+ 446.1574, found 446.1571.
Biology
Conflicts of interest
The authors declare no competing interests.Acknowledgements
Generous financial support received from Science and Engineering Research Board (SERB), Department of Science & Technology, Government of India (SR/S1/OC-02/2011) is gratefully acknowledged. Authors are grateful to Dr K. Kumaravel, Department of Marine Biotechnology, Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai-608502, Tamil Nadu for the collection of the plant material from Kolli Hills, Tamilnadu. Authors thank Prof. Dr N. Parthasarathy, Department of Ecology and Environmental Sciences, Pondicherry University, India for the taxonomic identification of the plant material. Authors are also grateful to Prof. Dr G. N. Krishna Kumari, Department of Medicinal Chemistry, Sri Ramachandra College of Biomedical Sciences, Technology and Research, Porur, Chennai for an authentic sample of zaluzanin D. Authors wish to thank Dr Rajesh G. Gonnade, Principle Scientist, Centre for Material Characterization, CSIR-NCL, Pune for his kind help with X-ray crystallography of one of our samples. E.K.A. and N.A.G. are grateful to the University Grants Commission (UGC), New Delhi for the award of their Senior Research Fellowships.Notes and references
- (a) G. M. Cragg, D. J. Newman and K. M. Snader, J. Nat. Prod., 1997, 60, 52–60 CrossRef CAS PubMed; (b) D. J. Newman, G. M. Cragg and K. M. Snader, J. Nat. Prod., 2003, 66, 1022–1037 CrossRef CAS PubMed; (c) M. Gordaliza, Clin. Transl. Oncol., 2007, 9, 767–776 CrossRef CAS PubMed; (d) D. J. Newman and G. M. Cragg, J. Nat. Prod., 2007, 70, 461–477 CrossRef CAS PubMed; (e) A. L. Harvey, Drug Discovery Today, 2008, 13, 894–901 CrossRef CAS PubMed; (f) D. J. Newman and G. M. Cragg, J. Nat. Prod., 2012, 75, 311–335 CrossRef CAS PubMed; (g) E. C. Barnes, R. Kumar and R. A. Davis, Nat. Prod. Rep., 2016, 33, 372–381 RSC.
- (a) G. W. Wang, J. J. Qin, X. R. Cheng, Y. H. Shen, L. Shan, H. Z. Jin and W. D. Zhang, Expert Opin. Invest. Drugs, 2014, 23, 317–345 CrossRef CAS PubMed; (b) M. H. R. Amorim, R. M. Gil da Costa, C. Lopes and M. M. Bastos, Crit. Rev. Toxicol., 2013, 43, 559–579 CrossRef CAS PubMed; (c) A. Ghantous, H. Gali-Muhtasib, H. Vuorela, N. A. Saliba and N. Darwiche, Drug Discovery Today, 2010, 15, 668–678 CrossRef CAS PubMed; (d) M. Tanasova and S. J. Sturla, Chem. Rev., 2012, 112, 3578–3610 CrossRef CAS PubMed; (e) I. Merfort, Curr. Drug Targets, 2011, 12, 1560–1573 CrossRef CAS PubMed; (f) A. Janecka, A. Wyrębska, K. Gach, J. Fichna and T. Janecki, Drug Discovery Today, 2012, 17, 561–572 CrossRef CAS PubMed; (g) E. Rodriguez, G. H. N. Towers and J. C. Mitchell, Phytochemistry, 1976, 15, 1573–1580 CrossRef CAS; (h) A. K. Picman and G. H. N. Towers, Biochem. Syst. Ecol., 1983, 11, 321–327 CrossRef CAS; (i) A. K. Picman, Biochem. Syst. Ecol., 1986, 14, 255–281 CrossRef CAS; (j) R. R. Kitson, A. Millemaggi and R. J. Taylor, Angew. Chem., Int. Ed., 2009, 48, 9426–9451 CrossRef CAS PubMed; (k) A. K. Bhattacharya and R. P. Sharma, Heterocycles, 1999, 51, 1681–1745 CrossRef CAS.
- https://en.wikipedia.org/wiki/Vernonia.
- (a) A. Bordignon, M. Frédérich, A. Ledoux, P. E. Campos, P. Clerc, T. Hermann, J. Quetin-Leclercq and E. Cieckiewicz, Nat. Prod. Res., 2017, 1–4 Search PubMed; (b) C. Zdero, F. Bohlmann and G. M. Mungai, Phytochemistry, 1990, 29, 3668–3669 CrossRef CAS; (c) A. M. Marzouk and O. B. Abd Elhalim, Nat. Prod. Res., 2016, 30, 741–749 CrossRef CAS PubMed; (d) C. N. S. Antonio, B. d. S. Elnatan and O. dos S. F. Raquel, J. Med. Plants Res., 2015, 9, 838–850 CrossRef; (e) U. J. Youn, E. J. Park, T. P. Kondratyuk, C. J. Simmons, R. P. Borris, P. Tanamatayarat, S. Wongwiwatthananukit, O. Toyama, T. Songsak and J. M. Pezzuto, Bioorg. Med. Chem. Lett., 2012, 22, 5559–5562 CrossRef CAS PubMed; (f) N. Kimani, J. Matasyoh, M. Kaiser, R. Brun and T. Schmidt, Molecules, 2017, 22, 597 CrossRef PubMed; (g) A. Chea, S. Hout, C. Long, L. Marcourt, R. Faure, N. Azas and R. Elias, Chem. Pharm. Bull., 2006, 54, 1437–1439 CrossRef CAS PubMed; (h) J. C. Chukwujekwu, C. A. Lategan, P. J. Smith, F. R. Van Heerden, J. Van Staden and S. Afr, J. Bot., 2009, 75, 176–179 CAS; (i) U. J. Youn, G. Miklossy, X. Chai, S. Wongwiwatthananukit, O. Toyama, T. Songsak, J. Turkson and L. C. Chang, Fitoterapia, 2014, 93, 194–200 CrossRef CAS PubMed; (j) A. Sinisi, E. Millán, S. M. Abay, A. Habluetzel, G. Appendino, E. Muñoz and O. Taglialatela-Scafati, J. Nat. Prod., 2015, 78, 1618–1623 CrossRef CAS PubMed; (k) R. B. Williams, A. Norris, C. Slebodnick, J. Merola, J. S. Miller, R. Andriantsiferana, V. E. Rasamison and D. G. I. Kingston, J. Nat. Prod., 2005, 68, 1371–1374 CrossRef CAS PubMed; (l) N. J. Toyang, H. K. Wabo, E. N. Ateh, H. Davis, P. Tane, L. B. Sondengam, J. Bryant and R. Verpoorte, J. Ethnopharmacol., 2013, 146, 552–556 CrossRef CAS PubMed; (m) N. J. Toyang, H. K. Wabo, E. N. Ateh, H. Davis, P. Tane, L. B. Sondengam, J. Bryant and R. Verpoorte, J. Ethnopharmacol., 2013, 146, 552–556 CrossRef CAS PubMed; (n) O. Kos, V. Castro, R. Murillo, L. Poveda and I. Merfort, Phytochemistry, 2006, 67, 62–69 CrossRef CAS PubMed; (o) W. G. Padolina, H. Yoshioka, N. Nakatani, T. J. Mabry, S. A. Monti, R. E. Davis, P. J. Cox, O. A. Sim, W. H. Watson and I. B. Wu, Tetrahedron, 1974, 30, 1161–1170 CrossRef CAS; (p) C. A. Catalán, D. I. De Iglesias, J. Kavka, V. E. Sosa and W. Herz, Phytochemistry, 1988, 27, 197–202 CrossRef; (q) A. Bardón, N. I. Kamiya, C. A. D. P. De Léon, C. A. Catalán, J. G. Díaz and W. Herz, Phytochemistry, 1992, 31, 609–613 CrossRef; (r) L. Zhang, Y. L. Shao, L. Hua, Y. Li, S. H. Hussain, M. Arfan and K. Gao, Phytochem. Lett., 2014, 7, 14–18 CrossRef CAS; (s) F. Bohlmann, C. Zdero, R. M. King and H. Robinson, Phytochemistry, 1982, 21, 695–699 CrossRef CAS; (t) C. Kraft, K. Jenett-Siems, K. Siems, J. Jakupovic, S. Mavi, U. Bienzle and E. Eich, Phytother. Res., 2003, 17, 123–128 CrossRef CAS PubMed; (u) I. Ganjian, I. Kubo and P. Fludzinski, Phytochemistry, 1983, 22, 2525–2526 CrossRef CAS; (v) B. Mompon and R. Toubian, Tetrahedron, 1976, 32, 2545–2548 CrossRef CAS; (w) G. Roos, H. Prawat, C. U. Walter, I. Klaiber, B. Vogler, J. H. Guse and W. Kraus, Planta Med., 1998, 64, 673–674 CrossRef CAS PubMed; (x) T. Ito, S. Aimaiti, N. N. Win, T. Kodama and H. Morita, Bioorg. Med. Chem. Lett., 2016, 26, 3608–3611 CrossRef CAS PubMed.
- (a) J. Jakupovic, S. Banerjee, V. Castro, F. Bohlmann, A. Schuster, J. D. Msonthi and S. Keeley, Phytochemistry, 1986, 25, 1359–1364 CrossRef CAS; (b) A. B. Aliyu, N. A. Koorbanally, B. Moodley, P. Singh and H. Y. Chenia, Phytochemistry, 2016, 126, 23–33 CrossRef CAS PubMed; (c) A. B. Aliyu, B. Moodley, H. Chenia and N. A. Koorbanally, Phytochemistry, 2015, 111, 163–168 CrossRef CAS PubMed; (d) N. Kimani, J. Matasyoh, M. Kaiser, R. Brun and T. Schmidt, Molecules, 2018, 23, 248 CrossRef PubMed; (e) L. Jakupovic, D. A. Gage, F. Bohlmann and T. J. Mabry, Phytochemistry, 1986, 25, 1179–1183 CrossRef; (f) H. Buskuhl, F. L. de Oliveira, L. Z. Blind, R. A. de Freitas, A. Barison, F. R. Campos, Y. E. Corilo, M. N. Eberlin, G. F. Caramori and M. W. Biavatti, Phytochemistry, 2010, 71, 1539–1544 CrossRef CAS PubMed; (g) S. M. Kupchan, R. J. Hemingway, A. Karim and D. Werner, J. Org. Chem., 1969, 34, 3908–3911 CrossRef CAS PubMed; (h) J. Jakupovic, C. Zdero, R. Boeker, U. Warning, F. Bohlmann and S. B. Jones, Eur. J. Org. Chem., 1987, 111–123 CAS; (i) T. J. Mabry, Z. Abdel-Baset, W. G. Padolina and S. B. Jones Jr, Biochem. Syst. Ecol., 1975, 2, 185–192 CrossRef CAS; (j) A. Koziol, L. Mroczko, M. Niewiadomska and S. Lochyński, Polish Journal of Natural Sciences, 2017, 32, 495–511 Search PubMed; (k) A. Bardón, C. A. Catalán, A. B. Gutiérrez and W. Herz, Phytochemistry, 1990, 29, 313–315 CrossRef; (l) F. Tsichritzis, K. Siems, J. Jakupovic, F. Bohlmann and G. M. Mungai, Phytochemistry, 1991, 30, 3808–3809 CrossRef CAS; (m) S. M. Kupchan, R. J. Hemingway, D. Werner, A. Karim, A. T. McPhail and G. A. Sim, J. Am. Chem. Soc., 1968, 90, 3596–3597 CrossRef CAS PubMed; (n) M. Jisaka, H. Ohigashi, K. Takegawa, M. A. Huffman and K. Koshimizu, Biosci., Biotechnol., Biochem., 1993, 57, 833–834 CrossRef CAS PubMed; (o) M. Jisaka, M. Kawanaka, H. Sugiyama, K. Takegawa, M. A. Huffman, H. Ohigashi and K. Koshimizu, Biosci., Biotechnol., Biochem., 1992, 56, 845–846 CrossRef CAS.
- R. R. A. Kitson, A. Millemaggi and R. J. K. Taylor, Angew. Chem., Int. Ed., 2009, 48, 9426–9451 CrossRef CAS PubMed.
- (a) N. J. Lawrence, A. T. McGown, J. Nduka, J. A. Hadfield and R. G. Pritchard, Bioorg. Med. Chem. Lett., 2001, 11, 429–431 CrossRef CAS PubMed; (b) S. K. Srivastava, A. Abraham, B. Bhat, M. Jaggi, A. T. Singh, V. K. Sanna, G. Singh, S. K. Agarwal, R. Mukherjee and A. C. Burman, Bioorg. Med. Chem. Lett., 2006, 16, 4195–4199 CrossRef CAS PubMed; (c) S. Neelakantan, S. Nasim, M. L. Guzman, C. T. Jordan and P. A. Crooks, Bioorg. Med. Chem. Lett., 2009, 19, 4346–4349 CrossRef CAS PubMed; (d) S. Nasim and P. A. Crooks, Bioorg. Med. Chem. Lett., 2008, 18, 3870–3873 CrossRef CAS PubMed; (e) D. R. Hwang, Y. S. Wu, C. W. Chang, T. W. Lien, W. C. Chen, U. K. Tan, J. T. A. Hsu and H. P. Hsieh, Bioorg. Med. Chem., 2006, 14, 83–91 CrossRef CAS PubMed; (f) S. G. Klochkov, S. V. Afanas’eva, A. N. Pushin, G. K. Gerasimova, N. K. Vlasenkova and Y. N. Bulychev, Chem. Nat. Compd., 2009, 46, 817–823 CrossRef; (g) K. H. Lee, H. Furukawa and E. S. Huang, J. Med. Chem., 1972, 15, 609–611 CrossRef CAS PubMed; (h) E. Hejchman, R. D. Haugwitz and M. Cushman, J. Med. Chem., 1995, 38, 3407–3410 CrossRef CAS PubMed; (i) N. R. Unde, S. V. Hiremath, G. H. Kulkarni and G. R. Kelkar, Tetrahedron Lett., 1968, 9, 4861–4862 CrossRef; (j) J. R. Woods, H. Mo, A. A. Bieberich, T. Alavanjac and D. A. Colby, J. Med. Chem., 2011, 54, 7934–7941 CrossRef CAS PubMed.
- J. R. Woods, H. Mo, A. A. Bieberich, T. Alavanjac and D. A. Colby, Med. Chem. Commun., 2013, 4, 27–33 RSC and references cited therein.
- (a) A. K. Bhattacharya, D. C. Jain, R. P. Sharma, R. Roy and A. T. McPhail, Tetrahedron, 1997, 53, 14975–14990 CrossRef CAS; (b) A. K. Bhattacharya, M. Pal, D. C. Jain, B. S. Joshi, R. Roy, U. Rychlewska and R. P. Sharma, Tetrahedron, 2003, 59, 2871–2876 CrossRef CAS; (c) A. K. Bhattacharya and K. C. Rana, Indian J. Chem., 2013, 52B, 901–903 CAS; (d) A. K. Bhattacharya, H. R. Chand, J. John and M. V. Deshpande, Eur. J. Med. Chem., 2015, 94, 1–7 CrossRef CAS PubMed; (e) T. R. Valkute, M. Arkile, D. Sarkar and A. K. Bhattacharya, Planta Medica International Open, 2016, 3, e55–e59 CrossRef.
- (a) G. N. Krishna Kumari, S. Masilamani, M. R. Ganesh and S. Aravind, Phytochemistry, 2003, 62, 1101–1104 CrossRef; (b) G. N. Krishna Kumari, S. Masilamani, M. R. Ganesh, S. Aravind and S. R. Sridhar, Fitoterapia, 2003, 74, 479–482 CrossRef CAS PubMed.
- Crystallographic data (excluding structure factors): CCDC-1525170 contains the supplementary crystallographic data for this paper.
- H. Matsuda, T. Kageura, Y. Inoue, T. Morikawa and M. Yoshikawa, Tetrahedron, 2000, 56, 7763–7777 CrossRef CAS.
- (a) C. Han, F. J. Barrios, M. V. Riofski and D. A. Colby, J. Org. Chem., 2009, 74, 7176–7179 CrossRef CAS PubMed; (b) P. R. R. Vadaparthi, C. P. Kumar, K. Kumar, A. Venkanna, V. L. Nayak, S. Ramakrishna and K. S. Babu, Med. Chem. Res., 2015, 24, 2871–2878 CrossRef CAS; (c) E. E. Shul’ts, A. V. Belovodskii, M. M. Shakirov and G. A. Tolstikov, Russ. Chem. Bull., 2012, 61, 1975–1985 CrossRef; (d) A. S. Kihkentayeva, E. E. Shults, Y. V. Gatilov, S. S. Patrushev, S. Karim, G. A. Atazhanova and S. M. Adekenov, Chem. Heterocycl. Compd., 2016, 52, 788–796 CrossRef CAS; (e) Y. H. Ding, H. X. Fan, J. Long, Q. Zhang and Y. Chen, Bioorg. Med. Chem. Lett., 2013, 23, 6087–6092 CrossRef CAS PubMed.
- I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed.
- M. Herrmann, H. M. Lorenz, R. Voll, M. Grünke, W. Woith and J. R. Kalden, Nucleic Acids Res., 1994, 22, 5506–5507 CrossRef CAS PubMed.
- E. A. Slee, M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green and S. J. Martin, J. Cell Biol., 1999, 144, 281–292 CrossRef CAS PubMed.
- S. Fuldaand and K. M. Debatin, Oncogene, 2006, 25, 4798–4811 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available:Additional information includes spectral copies. CCDC 1525170. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra06238b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |