Open Access Article
Open Access ArticleIdentification of the dominant hydrogeochemical processes and characterization of potential contaminants in groundwater in Qingyuan, China, by multivariate statistical analysis†
Zuobing Liang ,
Jianyao Chen*,
Tao Jiang,
Kun Li,
Lei Gao,
Zhuowei Wang,
Shaoheng Li and
Zhenglan Xie
,
Jianyao Chen*,
Tao Jiang,
Kun Li,
Lei Gao,
Zhuowei Wang,
Shaoheng Li and
Zhenglan Xie
Guangdong Provincial Key Laboratory of Urbanization and Geo-simulation, School of Geography and Planning, Sun Yat-Sen University, Guangzhou, PR China. E-mail: chenjyao@mail.sysu.edu.cn; Tel: +86 20 84115930
First published on 26th September 2018
Abstract
In karst areas, groundwater is an important water source for drinking and irrigation purposes; however, karst aquifers are vulnerable and recovery from damage is difficult. We collected surface water (pond and river water) and groundwater (hand-pump well, dug well, and borehole water) samples in Qingyuan city, China, to determine the major chemicals in the water with the primary goals of evaluating the geochemical composition, identifying the geochemical processes governing the water chemistry, and identifying the probable sources of potential contaminants in shallow and deep groundwater in the study area. The results revealed marked differences in water chemistry between shallow and deep groundwater. The groundwater composition was largely controlled by rock–water interactions, particularly the dissolution of evaporite minerals (e.g., calcite, gypsum, and anhydrite), and ion exchange processes were important drivers of the chemical compositions of groundwater in the study area. Moreover, in shallow and deep groundwater, Mg2+ and SO42− concentrations were increased due to the long residence time of deep groundwater, while K+ and Na+ concentrations were decreased due to anthropogenic input. Finally, factor analysis of the major and trace elements differentiated between anthropogenic and geogenic sources of potential contaminants in karst aquifers.
1. Introduction
Over the last few decades, rapid growth of urban population has resulted in dramatic increases in water use and wastewater production in many developing countries worldwide.1 Because groundwater is a major water resource, anthropogenic degradation of groundwater quality has attracted significant interest. In particular, groundwater from karst aquifers is an important drinking water resource in karst regions, but is particularly vulnerable to anthropogenic contamination due to the porous nature of karst terrain.2,3 Groundwater hydrochemistry and quality evaluations are used to clarify mineralization processes, and are also useful for exploring water–rock interactions and analyzing area-specific environmental issues.4Groundwater studies largely focus on the main hydrochemical processes that affect groundwater quality and hydrochemical profiles.5,6 Hydrochemical analyses can help define chemical reactions produced by water–rock interactions, and water–rock interactions or anthropogenic sources of pollution can also be interpreted in the context of related reactions and processes.7,8
Qingyuan city is located in a typical karst area in Guangdong Province, China, characterized by high spatial hydrogeological heterogeneities. This karst environment is vulnerable and recovery of function of karst aquifers is difficult once damaged. Issues related to pollution-intense industry relocation driven by globalization, at both a national and regional scale, are becoming increasingly relevant under current policy-making and environmental regulations. Guangdong Province is considered a pollution haven for overseas industries, and Qingyuan, located in northern Guangdong Province, is becoming a pollution haven for the Pearl River Delta.9 Qingyuan is the largest prefecture-level city in Guangdong Province, and anthropogenic sources of pollution therein are mainly derived from extensive urbanization, farming and agriculture practices, and industrial activities. Evaluating the groundwater quality and impact of human activities in this large karst area is a necessary and pressing task. Therefore, the objectives of this study were to (1) examine seasonal variations in water chemistry, (2) determine the geochemical processes and factors controlling the chemical compositions of karst water, (3) elucidate the chemical evolution of surface water and groundwater, and (4) identify probable sources of potential contaminants.
2. Study area
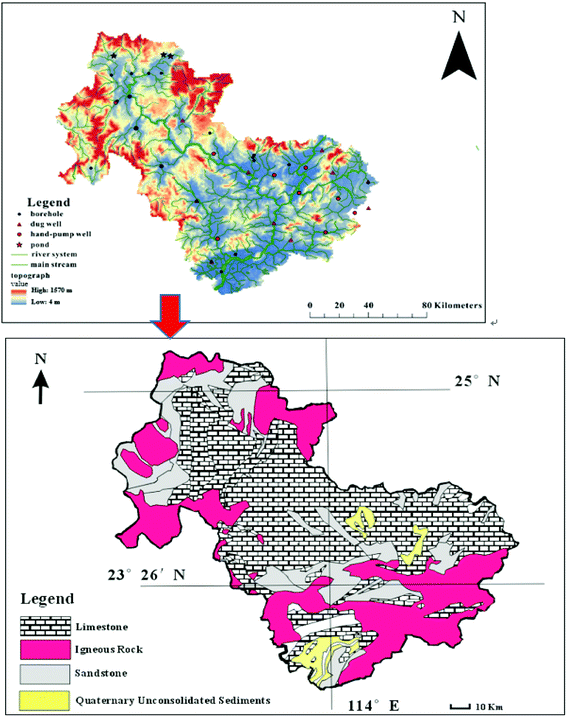
The study area was in Qingyuan (23°26′–25°11′N, 111°55′–113°55′E), northern Guangdong Province, China, and covered about 1.9 × 104 km2 (Fig. 1). There are three morphologically distinct formations in the study area: plains, hills, and mountains. The maximum elevation, in the mountainous region, is nearly 1570 m and the minimum elevation, in the plains, is approximately 4 m. The area has a subtropical monsoon climate with an annual mean temperature of 20.6 °C and annual precipitation of 1556 mm. Several rivers run through the plain, including Lianjiang River, Pajiang River, Beijiang River, and their tributaries.Two types of rocks are exposed in the study area: limestone formed from the Late Paleozoic to Mesozoic (Triassic) and granite formed during the Yanshanian. Carboniferous carbonate rocks are widely exposed and typical synclinal–tectonic karst basins associated with peak-forest plains and peak-cluster depressions are widely distributed. Fissures and fractures have extensively developed in carbonate rocks, promoting karstification of the basin, and the high precipitation ensures abundant groundwater resources.
2.1 Sample collection and pretreatment in the field
The water samples were collected from three ponds, 13 hand-pump wells, 11 dug wells, 19 boreholes, and nine other locations along various rivers. The sampling locations are shown in Fig. 1. To investigate seasonal variability, samples were collected at the three ponds and 13 of the boreholes on select days during both the dry season (January 2017) and rainy season (September 2017).Before sample collection, each sample bottle was washed with nitric acid to remove cations and then rinsed with distilled water. In addition, the sample bottles were rinsed thrice with the same water before sample collection.
2.2 Analytical methods
Environmental parameters (pH, electrical conductivity (EC), and temperature) were stabilized and measured with a multi-parameter measurement device (DR/800; HACH, Loveland, CO, USA) during pumping. Water samples were filtered through 0.22 μm pore-size membrane filters (Millipore, Burlington, MA, USA) to remove suspended particles and microorganisms, after which major cation samples were acidified to pH < 2 with HCl immediately after sampling. All major ions (Cl−, NO3−, PO43−, and SO42−) except HCO3− were analyzed using a DX-600 ion chromatograph (Dionex, Sunnyvale, CA, USA) in the laboratory of the School of Environmental Science and Engineering, Sun Yat-sen University (Guangzhou, China), with a detection limit of 0.1 ppm. HCO3− was titrated immediately in the field using a portable testing kit (Merck KGaA Co., Darmstadt, Germany), with an accuracy of 0.05 mmol L−1. The major cations were detected with inductively coupled plasma optical emission spectrometry (Optima 8300; Perkin-Elmer, Waltham, MA, USA) with a detection limit of 0.05 ppm for Ca2+, Mg2+, K+, and Na+, and 0.01 ppm for Ba, Sr, Fe, Cr, Mn, Co, Ni, Cu, Zn, As, Se, Mo, Cd, and Pb at the laboratory of the Instrumental Analysis & Research Center, Sun Yat-sen University. The analytical precision of the major ions was within 1%. The ion balance errors of most water samples were <10%, and some were >10 (Appendix A). The ion balance error higher than 10% in some samples may result from the existence of unanalyzed anions and cations such as organic acids in water.10,11 Still, there should be further research on the factors affecting the higher error of ion balance.3. Results and discussion
3.1 Field parameters
Table 1 presents the major physiochemical and chemical properties of the water samples, which were analyzed on select days in both the dry (January 2017) and rainy (September 2017) seasons. The average temperature varied seasonally in pond water and was 17.3 ± 2.35 °C and 20.1 ± 7.65 °C in the dry and rainy seasons, respectively. Meanwhile, the average temperature of the river samples in the rainy season was 29.5 ± 2.25 °C. During the dry season, the average temperatures of the hand-pump and dug well water were 20.1 ± 2.66 °C and 22.8 ± 1.93 °C, respectively. The average temperatures of deep groundwater (i.e., boreholes) in the dry and rainy seasons were 19.9 ± 2.53 °C and 25.5 ± 1.92 °C, respectively, indicative of seasonal variation according to surface air temperature in the karst area.| Sample | EC (μs cm−1) | pH | DO (mg L−1) | Temp. (°C) | HCO3− | Cl− | NO3− | SO42− | K+ | Na+ | Ca2+ | Mg2+ | TDS (mg L−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| meq L−1 | |||||||||||||||
| Ponds | Rainy season (n = 3) | Min | 12.2 | 6.94 | 3.22 | 23.8 | 0.50 | 0.03 | 0.01 | 0.22 | 0.01 | 0.02 | 0.05 | 0.01 | 34.2 |
| Mean | 281 | 7.51 | 3.61 | 26.6 | 2.53 | 0.35 | 0.36 | 0.35 | 0.13 | 0.42 | 2.25 | 0.46 | 245 | ||
| Max | 572 | 8.23 | 1.84 | 31 | 4.00 | 0.92 | 1.04 | 0.38 | 0.35 | 0.76 | 4.74 | 0.21 | 437 | ||
| SD | 280 | 3.63 | 1.99 | 7.65 | 1.82 | 0.49 | 0.59 | 0.12 | 0.19 | 0.38 | 2.35 | 0.62 | 202 | ||
| Dry season (n = 3) | Min | 12.1 | 5.46 | 3.62 | 14.6 | 0.10 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.07 | 0.05 | 9.28 | |
| Mean | 444 | 6.60 | 4.97 | 17.3 | 3.57 | 0.36 | 0.10 | 0.25 | 0.13 | 0.22 | 2.61 | 0.64 | 313 | ||
| Max | 721 | 7.13 | 7.44 | 18.4 | 5.84 | 0.99 | 0.25 | 0.40 | 0.35 | 0.43 | 4.55 | 1.62 | 470 | ||
| SD | 379 | 0.99 | 2.14 | 2.35 | 3.05 | 0.55 | 0.13 | 0.21 | 0.19 | 0.21 | 2.30 | 0.85 | 263 | ||
| Rivers | Rainy season (n = 9) | Min | 35.7 | 6.70 | 2.50 | 26.8 | 0.00 | 0.04 | 0.04 | 0.10 | 0.01 | 0.03 | 0.98 | 0.21 | 104 |
| Mean | 213 | 7.75 | 5.65 | 29.5 | 1.15 | 0.12 | 0.14 | 0.48 | 0.05 | 0.13 | 3.30 | 0.56 | 208 | ||
| Max | 339 | 8.12 | 7.13 | 31.5 | 3.00 | 0.27 | 0.54 | 1.21 | 0.10 | 0.33 | 6.35 | 1.18 | 344 | ||
| SD | 111 | 0.62 | 1.87 | 2.25 | 1.25 | 0.07 | 0.15 | 0.35 | 0.03 | 0.09 | 1.80 | 0.35 | 86.6 | ||
| Hand pump wells | Dry season (n = 13) | Min | 167 | 5.81 | 3.24 | 13.5 | 0.59 | 0.12 | 0.00 | 0.14 | 0.01 | 0.02 | 0.67 | 0.10 | 105 |
| Mean | 448 | 6.82 | 4.61 | 20.1 | 2.95 | 0.45 | 0.09 | 0.62 | 0.17 | 0.23 | 3.32 | 0.44 | 310 | ||
| Max | 675 | 7.43 | 6.16 | 22.3 | 5.20 | 1.05 | 0.22 | 1.21 | 0.48 | 0.49 | 5.30 | 1.21 | 503 | ||
| SD | 144 | 0.46 | 0.90 | 2.66 | 1.21 | 0.30 | 0.07 | 0.34 | 0.14 | 0.17 | 1.32 | 0.31 | 109 | ||
| Dug wells | Dry season (n = 11) | Min | 72.9 | 5.70 | 3.11 | 16.2 | 0.44 | 0.05 | 0.00 | 0.08 | 0.00 | 0.00 | 0.39 | 0.07 | 46 |
| Mean | 362 | 6.65 | 4.98 | 20.1 | 2.16 | 0.55 | 0.10 | 0.46 | 0.20 | 0.30 | 2.31 | 0.40 | 240 | ||
| Max | 942 | 7.56 | 7.48 | 22.8 | 5.35 | 1.82 | 0.30 | 1.21 | 0.87 | 0.91 | 6.05 | 1.37 | 620 | ||
| SD | 254 | 0.60 | 2.31 | 1.93 | 1.78 | 0.58 | 0.10 | 0.39 | 0.26 | 0.31 | 1.68 | 0.41 | 173 | ||
| Boreholes | Rainy season (n = 19) | Min | 32 | 6.08 | 1.13 | 22.6 | 1.10 | 0.03 | 0.02 | 0.01 | 0.02 | 0.03 | 0.30 | 0.08 | 131 |
| Mean | 377 | 7.33 | 2.22 | 25.5 | 3.61 | 0.17 | 0.08 | 2.51 | 0.10 | 0.47 | 2.98 | 0.96 | 432 | ||
| Max | 1735 | 8.41 | 6.51 | 30.0 | 7.20 | 0.48 | 0.28 | 42.0 | 0.60 | 3.51 | 17.0 | 5.68 | 2653 | ||
| SD | 353 | 0.56 | 1.27 | 1.92 | 1.66 | 0.14 | 0.08 | 9.56 | 0.14 | 0.83 | 3.61 | 1.28 | 550 | ||
| Dry season (n = 13) | Min | 201 | 6.40 | 1.71 | 14.2 | 1.08 | 0.02 | 0.00 | 0.01 | 0.01 | 0.01 | 0.75 | 0.11 | 134 | |
| Mean | 473 | 7.40 | 2.81 | 19.9 | 3.24 | 0.37 | 0.02 | 1.98 | 0.09 | 0.21 | 2.52 | 1.25 | 381 | ||
| Max | 1494 | 8.49 | 3.80 | 22.4 | 10.61 | 1.69 | 0.21 | 15.78 | 0.26 | 0.90 | 8.80 | 6.03 | 1301 | ||
| SD | 375 | 0.60 | 0.65 | 2.15 | 2.53 | 0.45 | 0.06 | 4.55 | 0.07 | 0.27 | 2.30 | 1.53 | 334 | ||
The average EC values varied substantially among water samples but which did not show a variation characteristic in the vertical spatial distribution. However, the average EC values for ponds and boreholes were lower in the dry season than the rainy season. Meanwhile, the pH of pond water was 5.46–7.13 in the dry season and 6.94–8.23 in the rainy season. The minimum value of pond water was sampled in a small pond underlaid by sandy stone that provided drinking water for nearby residents and was mainly recharged by rain water. According to Huang et al.,12 the pH values in a coastal mega-city in South China was 4.56 over a 5 years period. The short water–rock reaction time in the small pond and low EC values of rain water may have been responsible for the lower pH and EC values.
The dissolved oxygen (DO) concentration of the surface (i.e., pond and river) waters varied from 1.84 mg L−1 to 7.44 mg L−1, whereas those of shallow groundwater (i.e., hand-pump well and dug well water) ranged widely from 3.11 mg L−1 to 7.48 mg L−1 (average: 5.00 mg L−1). Meanwhile, the DO concentration of deep groundwater was 1.71–3.80 mg L−1 (average: 2.81 mg L−1) in the dry season and 1.13–6.51 mg L−1 (average: 2.22 mg L−1) in the rainy season.
3.2 Water chemistry
Piper trilinear diagrams summarize the differences in hydrochemical composition among water sources.13 Fig. 2 presents the piper trilinear equivalence diagrams of the samples. Overall, the hydrochemistry was dominated by alkaline earth metal ions, although weak acids, Ca2+, and bicarbonate also made substantial contributions to the water chemistry composition. Overall, according to the analysis of major ionic composition, Ca–HCO3 and Ca–SO4 were the two main waters types, although three of the pond samples had Ca–HCO3-type water. The majority of hand-pump and dug well samples had Ca–HCO3-type water, probably as a consequence of calcite dissolution. Most borehole and river samples were Ca–HCO3 type, accompanied by Ca–SO4 in several boreholes and river samples. Ca2+ and HCO3− ions result not only from dissolution of carbonate rocks, but potentially also from the hydrolysis of sandstone cement or, to a limited degree, feldspar dissolution in basement rocks.14,15 | ||
| Fig. 2 Piper diagram for hydro-chemical types of the analyzed water samples ((a): dry season, (b): rainy season). | ||
Most river samples and some hand-pump and dug well samples tended to be slightly enriched in SO42− and Cl−. The Cl− concentrations in the dug well water reached 1.82 meq L−1, and the maximum SO42− concentrations in the river, hand-pump well, and dug well samples were 1.21 meq L−1, respectively (Table 1). Na+ and K+ concentrations in shallow groundwater also tended to be enriched compared to the deep groundwater samples. In addition to natural sources, anthropogenic activities (e.g., deicing, fertilizer, and wastewater) produce K+ and Na+ ions that can enter the groundwater,14,16 and almost all shallow groundwater samples from both the residential and karst areas were more vulnerable to contamination. By contrast, the deep groundwater tended to be more enriched in Mg2+ than the shallow groundwater. According to the incongruent dissolution of dolomite, the increase in Mg2+ corresponded to the increased residence time in limestone.17 Since the deep groundwater was in the karst area, the enrichment in Mg2+ may have resulted from the long residence time of groundwater.
3.3 Chemical evolution of groundwater
The soluble ions in natural waters mainly originated from rock and soil weathering and anthropogenic inputs, and partly from atmospheric inputs. The influence of rock weathering, evaporation, and precipitation on water chemistry was evaluated with Gibbs' diagrams. Fig. 3a and b show Gibbs' plots of total dissolved solids according to the ion ratios Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) of groundwater in the two seasons. Most data points were distributed in areas of end members of the dominant rocks, confirming that the groundwater chemistry in the study area was dominated by the interaction between water and aquifer materials. In addition, one pond sample in the study area was dominated by atmospheric precipitation. | ||
| Fig. 3 Ratios of Na+ to (Na+ + Ca2+) as a function of TDS for all samples (a: dry season, b: rainy season). | ||
The chemical properties of the groundwater in the study area were mainly controlled by natural geochemical processes, although anthropogenic activities also influenced the chemical properties of groundwater, especially in the shallow part of the aquifer. In aquifer systems, the chemical evolution of groundwater results from interactions among various processes, which are difficult to identify based on the solute balance.14 However, the origins of ions and processes that control their concentrations can be determined by plotting bivariate diagrams.18 Therefore, we created several plots of the relationships among major elements to identify the origin and processes contributing to groundwater mineralization.
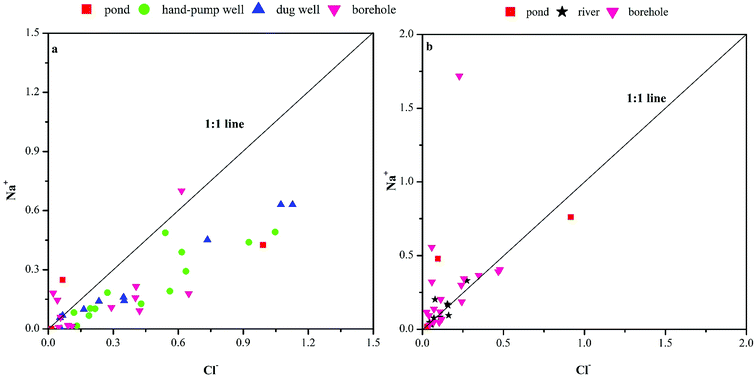
The Na+/Cl− ratio is considered to be one of the most important indicators of the origin of groundwater, where a ratio of 1 represents halite dissolution.19 The plot of Na+ versus Cl− revealed a trend from excess Cl− (Cl− > Na+) toward the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio line for hand-pump well, dug well, and borehole water samples in the dry season (Fig. 4a). Meanwhile, most points for river water were situated along the halite dissolution line, although several high Na+/Cl− ratios were observed in borehole water samples in the rainy season (Fig. 4b). The excess Na+ over Cl− ions may have resulted from three processes:20 (1) cation exchange reactions; (2) weathering of feldspars in sandstone samples, and (3) reaction of silicate minerals derived mainly from sandstone weathering. Conversely, the depletion of Na+ concentrations relative to Cl− concentrations in the upper aquifer may have resulted from the reaction of silicate minerals, or a second type of ionic exchange in which Na+ was removed from the complex and replaced by Ca2+. Hence, during the dry season, the abnormal samples in the borehole waters above the 1
1 stoichiometric ratio line for hand-pump well, dug well, and borehole water samples in the dry season (Fig. 4a). Meanwhile, most points for river water were situated along the halite dissolution line, although several high Na+/Cl− ratios were observed in borehole water samples in the rainy season (Fig. 4b). The excess Na+ over Cl− ions may have resulted from three processes:20 (1) cation exchange reactions; (2) weathering of feldspars in sandstone samples, and (3) reaction of silicate minerals derived mainly from sandstone weathering. Conversely, the depletion of Na+ concentrations relative to Cl− concentrations in the upper aquifer may have resulted from the reaction of silicate minerals, or a second type of ionic exchange in which Na+ was removed from the complex and replaced by Ca2+. Hence, during the dry season, the abnormal samples in the borehole waters above the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line indicated that halite dissolution was not the only source of Na+. Samples with Na+/Cl− ratios lower than 1 accounted for 92% and 64% of shallow and deep groundwater samples, respectively, further indicative of additional Na+ sources. However, in the rainy season, the excess of Na+ relative to Cl− concentrations in most borehole samples might have reflected cation exchange reactions or mineral dissolution in deep groundwater. Overall, precipitation appeared to greatly influence the deep groundwater chemistry in the karst area.
1 line indicated that halite dissolution was not the only source of Na+. Samples with Na+/Cl− ratios lower than 1 accounted for 92% and 64% of shallow and deep groundwater samples, respectively, further indicative of additional Na+ sources. However, in the rainy season, the excess of Na+ relative to Cl− concentrations in most borehole samples might have reflected cation exchange reactions or mineral dissolution in deep groundwater. Overall, precipitation appeared to greatly influence the deep groundwater chemistry in the karst area.
In most samples, HCO3− was the dominant anion, accounting for approximately 60–80% of the total dissolved anions. The HCO3− concentrations were 0.10–4.76 meq L−1 for pond water, 0.44–5.35 meq L−1 for shallow groundwater and 1.08–10.61 meq L−1 for deep groundwater during the dry season (Table 1). During the rainy season, the HCO3− concentrations were 0.00–3.00 meq L−1 for river water and 1.10–7.20 meq L−1 for deep groundwater. In the plot of HCO3− versus Ca2+, most hand-pump well water points were above the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio line, dug well water points were clustered around the 1
1 stoichiometric ratio line, dug well water points were clustered around the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio line, and borehole water points were below the 1
1 stoichiometric ratio line, and borehole water points were below the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric line (Fig. 5a and b). Meanwhile, in the Ca2+ + Mg2+ versus HCO3− plot (Fig. 6a and b), the dug well, river, and hand-pump well waters mostly had (Ca2+ + Mg2+)/HCO3− ratios above 1
1 stoichiometric line (Fig. 5a and b). Meanwhile, in the Ca2+ + Mg2+ versus HCO3− plot (Fig. 6a and b), the dug well, river, and hand-pump well waters mostly had (Ca2+ + Mg2+)/HCO3− ratios above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating that additional sources besides carbonated weathering accounted for the excess Mg2+ and Ca2+ relative to HCO3−.
1, indicating that additional sources besides carbonated weathering accounted for the excess Mg2+ and Ca2+ relative to HCO3−.
Related studies reported that excess Ca2+ + Mg2+ with respect to HCO3− in karst aquifers may result from calcite dissolution, and the majority of Ca2+, Mg2+, and HCO3− ions from shallow groundwater may have largely originated from carbonate dissolution. Meanwhile, the plot of Ca2+ + Mg2+ versus HCO3− during the dry season (Fig. 6a) showed that most borehole water points were clustered along the dolomite line, indicating that the Mg2+, Ca2+, and HCO3− in this aquifer shared the same origin; however, an excess of HCO3− over Ca2+ + Mg2+ was observed during the rainy season (Fig. 6b). Thus, rock type was not the only factor influencing the Ca2+, Mg2+ and HCO3− ions in the borehole waters among different seasons.
In the studied aquifer system, carbonate dissolution is the dominant process contributing to groundwater solutes (Ca2+, Mg2+, and HCO3−). The mean Mg2+/Ca2+ ratios of the hand-pump well, dug well, and borehole water samples were 0.15, 0.17, and 0.55, respectively. The Mg2+/Ca2+ ratio sometimes approached a value of 0.33, indicating the influence of calcite dissolution.14 According to Appendix B, the lithology in deep groundwater (borehole) is mainly controlled by limestone, but the lithology in shallow groundwater (hand-pump well and dug well) all belongs to unconsolidated sediments. Therefore, lithology may not the main factor affecting Mg2+/Ca2+ ratio in groundwater. As we can see from Fig. 7a and b, Mg2+/Ca2+ ratios in deep groundwater were higher than shallow groundwater in general, well depth in hand-pump well and dug well are all below 8 m, and all above 60 m in borehole (Appendix B). It main conclude that higher Mg2+/Ca2+ ratio in deep groundwater was likely resulted from the longer residence time of groundwater. During the rainy season, the karst aquifer was mainly recharged by precipitation, resulting in a short groundwater residence time based on the heterogeneity of karst aquifers and depleted Mg2+ compared with the deep groundwater in the dry season. Hence, the groundwater residence time within carbonate aquifers may explain the difference in Mg2+ proportions among different aquifers.
The plots of (Ca2+ + Mg2+) versus (HCO3− + SO42−) (Fig. 8a and b) and Ca2+ versus SO42− (Fig. 8c and d) were used to identify the sources of Ca2+, Mg2+, and SO42−. Most groundwater samples, except the hand-pump well water samples, showed a trend toward excess Ca2+ (Ca2+ > SO42−) above the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line in the Ca2+ versus SO42− plot, and a strong, significant linear relationship following a 1
1 line in the Ca2+ versus SO42− plot, and a strong, significant linear relationship following a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in the (Ca2+ + Mg2+) versus (HCO3− + SO42−) plot (Fig. 8a and b). This indicated that Ca2+, Mg2+, SO42+, and HCO3− were derived from the dissolution of calcite, dolomite, and gypsum.21 In addition, the Ca2+ versus SO42− plots of the river and borehole water samples showed a trend toward excess Ca2+ (Ca2+ > SO42−) above the 2
1 ratio in the (Ca2+ + Mg2+) versus (HCO3− + SO42−) plot (Fig. 8a and b). This indicated that Ca2+, Mg2+, SO42+, and HCO3− were derived from the dissolution of calcite, dolomite, and gypsum.21 In addition, the Ca2+ versus SO42− plots of the river and borehole water samples showed a trend toward excess Ca2+ (Ca2+ > SO42−) above the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line during the rainy season. Meanwhile, good correlations between Ca2+ and SO42− were observed among the hand-pump well, dug well, and borehole water samples (Fig. 8a), suggesting that the increased SO42− concentrations were driven by gypsum and anhydrite dissolution.22
1 line during the rainy season. Meanwhile, good correlations between Ca2+ and SO42− were observed among the hand-pump well, dug well, and borehole water samples (Fig. 8a), suggesting that the increased SO42− concentrations were driven by gypsum and anhydrite dissolution.22
The studied aquifer system is composed of silicate minerals; therefore, the dissolution resulting from silicate weathering may have contributed to the groundwater chemistry. The bivariate mixing diagrams of Na+-normalized Mg2+ versus Na+-normalized Ca2+ (Fig. 9a and b) could be used to evaluate the influence of silicate weathering.14 The observed waters were marked by carbonate dissolution, but one of the pond water samples was close to the global average silicate domain, indicating that carbonate dissolution was prominent, although silicate weathering also influenced the groundwater solutes (Fig. 9a and b).
Ca2+ and Mg2+ can be produced from silicate and carbonate weathering.23 Calcite and anorthite produce similar molar concentrations of Ca2+ and HCO3−, but no SiO2. Meanwhile, the incongruent dissolution of feldspars, micas, and pyroxenes, as well as anthropogenic activities (e.g., deicing, fertilizer, and wastewater) produce K+ and Na+, which enter the groundwater. Moreover, HCO3− in groundwater can originate from carbonate dissolution, silicate weathering, oxidation of organic matter or biogenic CO2 dissolution.
In this study, most water of the samples were depleted in Na+ relative to Cl−. The excess of Cl− relative to Na+ was suggestive of silicate mineral reactions24 or other forms of ionic exchange.25 Because pronounced silicate weathering was not seen in the studies waters, a bivariate graph of (Ca2+ + Mg2+)–(SO42− + HCO3−) versus (Na+ + K+–Cl−) (Fig. 9a and b) was plotted to clarify the ionic exchange.20,26 In the graph (Fig. 10a), a slope of approximately −1 would indicate active cation exchange in the aquifer. The dug well and dry season borehole water samples in the observed area had slopes of −0.53 and 0.04 (Fig. 10a), respectively, indicating that cation exchange had no influence on their water chemistry. Meanwhile, the river and rainy season borehole water samples had slopes of −15.8 and −4.1, respectively, indicating that cation exchange had a slight influence on the water chemistry. Finally, hand-pump well water had a slope of −0.94 (approximately −1), indicating that cation exchange had a substantial influence on the chemistry of hand-pump well water during the dry season.
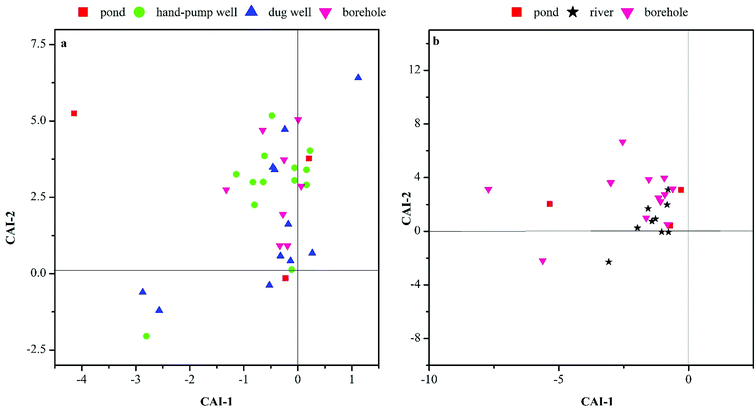
We also examined two indices of base exchange, namely the chloro-alkaline indices (CAI-1 and CAI-2),27 to evaluate ion exchange between Na+ and K+ in groundwater with Ca2+ and Mg2+ in the aquifer material.
| CAI-1 = Cl− − [(Na+ + K+)/Cl−] | (1) |
| CAI-2 = Cl− − [(Na+ + K+)/SO42−] + HCO3− + CO3− + NO3− | (2) |
The CAI-1 values were negative in most of the water samples (Fig. 11a and b), especially in the pond and borehole water samples during the dry season and all samples during the rainy season. By contrast, the CAI-2 values were positive in the pond and borehole water samples and most of the river, hand-pump well, and dug well water samples. This indicated that Mg2+ and Ca2+ in groundwater samples are exchanged with Na+ and K+ in the aquifer material, and that silicate dissolution or cation exchange may have caused the depletion of some cations in the groundwater samples.
Ion exchange processes are closely related to groundwater residence time and hydrological conditions.28 Therefore, the ion exchange in deep water samples was closely related to the residence time according to the aquifer depth. Meanwhile, the shallow groundwater samples were located in a densely populated area. As a consequence of anthropogenic influences, the shallow groundwater samples had higher N, K+, and Cl− contents. Generally, groundwater residence time and anthropogenic influences are the main causes of ion exchange in groundwater chemistry.
3.4 Probable sources of potential contaminants
The water samples obtained in the study area were analyzed for heavy metals such as Cr, Mn, Ni, Cu, Zn, As, Cd, and Pb, and the results are shown in Table 2. Mn and Zn were found in relatively high concentrations. The Mn concentrations in ponds, shallow groundwater, and deep groundwater were 0.00–31.4, 0.00–1,871, and 4.94–521 μg L−1, respectively. The Zn concentrations in the surface, shallow, and deep water were 1.32–7.76, 0.00–470, and 0.00–477 μg L−1, respectively. Among the 40 tested water samples, none had heavy metal concentrations exceeding the World Health Organization guidelines29 or Standards for Drinking Water Quality in China (GB5749-2006).| μg L−1 | Pond water (n = 3) | Shallow groundwater (n = 24) | Deep groundwater (n = 13) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | SD | Range | Mean | SD | Range | Mean | SD | |
| a Shallow groundwater: hand pump well + dug well. | |||||||||
| Cr | 0.02–0.07 | 0.05 | 0.02 | 0.00–0.53 | 0.08 | 0.13 | 0.00–0.51 | 0.07 | 0.14 |
| Mn | 0.00–31.4 | 10.60 | 18.0 | 0.00–1871 | 88.9 | 380 | 4.94–521 | 124 | 161 |
| Ni | 0.16–0.59 | 0.32 | 0.24 | 0.00–1.33 | 0.40 | 0.33 | 0.11–4.64 | 0.32 | 1.25 |
| Cu | 0.20–0.35 | 0.28 | 0.07 | 0.00–1.40 | 0.34 | 0.28 | 0.00–2.65 | 0.24 | 0.72 |
| Zn | 1.32–7.76 | 3.58 | 3.63 | 0.00–470 | 32.2 | 98.7 | 0.00–477 | 34.5 | 132 |
| As | 0.00–7.57 | 3.29 | 3.88 | 0.00–1.44 | 0.35 | 0.37 | 0.00–0.65 | 1.97 | 0.22 |
| Cd | 0.00 | 0.00 | 0.00 | 0.00–0.49 | 0.03 | 0.11 | 0.00–0.81 | 0.03 | 0.22 |
| Pb | 0.00–0.04 | 0.01 | 0.02 | 0.00–26.0 | 0.03 | 0.06 | 0.00–0.80 | 0.03 | 0.22 |
We performed a principal component analysis (PCA) based on an eigenanalysis of the correlation matrix to identify associations among variables, and indicate the participation of the individual chemical parameters in several influence factors, which is a common phenomenon in hydrochemistry.30 Only parameters with correlation coefficients exceeding 0.50 were considered. Table 3 presents the correlation matrix of eight heavy metals (Cu and Ni, r = 0.70**; Cr and Zn; r = 0.55**; As and Zn, r = 0.51**; Cd and Cr, r = 0.60**; Cd and Zn, r = 0.94**; Pb and Cr, r = 0.56**; Pb and Zn, r = 0.71**; Pb and Cd, r = 0.86**). One interpretation of these observations is that, in groundwater, these trace elements had similar hydrochemical characteristics. Trace elements such as Cr and As typically occur as soluble oxyanions in oxidizing waters. Mn is highly soluble in low-pH (reducing) waters. Meanwhile, the redox-sensitive element As is relatively more soluble in oxidized groundwater, where it exists as the oxyanions AsO42− and H2AsO4−. However, in reducing waters, As is readily incorporated in insoluble minerals.
| Cr | Mn | Ni | Cu | Zn | As | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|
| a Correlation is significant at the 0.05 level.b Correlation is significant at the 0.01 level. | ||||||||
| Cr | 1.00 | −0.16 | 0.04 | 0.09 | 0.55b | 0.29 | 0.60b | 0.56b |
| Mn | 1.00 | 0.15 | −0.08 | −0.09 | −0.25 | −0.07 | −0.11 | |
| Ni | 1.00 | 0.70b | 0.10 | 0.10 | 0.14 | 0.27 | ||
| Cu | 1.00 | 0.09 | 0.21 | 0.12 | 0.36a | |||
| Zn | 1.00 | 0.51b | 0.94b | 0.71b | ||||
| As | 1.00 | 0.37a | 0.25 | |||||
| Cd | 1.00 | 0.86b | ||||||
| Pb | 1.00 | |||||||
Multivariate statistical methods have been applied widely to investigate environmental phenomena31 and reduce the dimensionality of data, to allow extraction of information for water quality assessments and surface water management.32 Among these methods, PCA is often used to simplify large, complex datasets and identify correlated variables, to reduce the number of variables explaining the total variance of the data.33 In this study, PCA was applied to a dataset containing almost 814 values (22 parameters at 37 sampling sites) to compare the compositional patterns among the examined water systems and identify interrelated factors.
Seven principal components (PCs) had eigenvalues >1, accounting for almost 81.0% of the total variance in the dataset (Table 4). PCs 1–3 explained 19.3%, 17.1%, and 14.4% of the total variance, respectively, accounting for the majority of the variance in the original dataset. PCs 4–7 were less important, and each of these four components explained similar amounts of variance (6.10–10.6%).
| Variable | Component | ||||||
|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | |
| a Significant factor loadings are bold faced. | |||||||
| EC | 0.78 | −0.01 | 0.29 | −0.12 | −0.04 | 0.47 | 0.12 |
| pH | 0.28 | 0.13 | −0.24 | 0.31 | −0.41 | 0.21 | 0.07 |
| SO42− | 0.95 | −0.07 | 0.09 | −0.06 | 0.07 | 0.01 | −0.01 |
| NO3− | −0.09 | −0.06 | 0.71 | −0.21 | 0.05 | 0.40 | 0.13 |
| Cl− | 0.14 | −0.04 | 0.87 | −0.08 | 0.04 | 0.09 | 0.02 |
| PO43− | 0.06 | 0.11 | −0.05 | −0.00 | −0.17 | 0.90 | −0.01 |
| K+ | −0.10 | −0.10 | 0.79 | 0.05 | −0.10 | −0.05 | 0.00 |
| Na+ | 0.34 | −0.04 | 0.85 | 0.04 | 0.078 | −0.09 | 0.04 |
| Cr | −0.06 | 0.68 | −0.20 | 0.02 | −0.06 | 0.15 | 0.55 |
| Mn | 0.20 | −0.07 | −0.06 | 0.06 | 0.95 | −0.11 | −0.06 |
| Fe | 0.95 | 0.08 | −0.04 | 0.03 | 0.01 | −0.14 | −0.05 |
| Co | −0.06 | 0.01 | −0.00 | 0.02 | 0.98 | −0.08 | −0.00 |
| Ni | −0.03 | 0.02 | −0.08 | 0.90 | 0.17 | 0.09 | 0.01 |
| Cu | −0.12 | 0.12 | 0.25 | 0.84 | −0.07 | −0.04 | −0.03 |
| Zn | 0.00 | 0.91 | −0.02 | 0.01 | 0.00 | 0.23 | −0.06 |
| As | −0.11 | 0.37 | 0.25 | 0.11 | −0.11 | 0.63 | 0.01 |
| Se | −0.06 | −0.02 | 0.18 | −0.11 | −0.08 | −0.00 | 0.90 |
| Sr2+ | 0.96 | −0.06 | −0.10 | 0.00 | 0.01 | −0.05 | −0.05 |
| Mo | 0.05 | 0.21 | −0.23 | 0.72 | −0.09 | −0.04 | −0.17 |
| Cd | 0.02 | 0.96 | −0.11 | 0.07 | −0.01 | 0.11 | −0.05 |
| Ba2+ | −0.14 | 0.37 | 0.41 | 0.11 | −0.07 | 0.07 | −0.39 |
| Pb | −0.01 | 0.86 | −0.03 | 0.32 | −0.07 | −0.07 | −0.01 |
| % Total variance | 19.3 | 17.1 | 14.4 | 10.6 | 9.80 | 8.20 | 6.10 |
| % Cumulative variance | 19.3 | 36.4 | 50.8 | 61.3 | 70.1 | 75.6 | 81.0 |
PC 1 was significantly correlated with SO42−, Fe, and Sr2+ concentrations, as well as EC. In the water samples, SO42− was mainly derived from the dissolution of gypsum and anhydrite. Meanwhile, the source of Sr might be the dissolution of carbonate rocks or Sr-bearing clay materials.34 Finally, highly positive loadings for Fe2+ may indicate similar geochemical behaviors of these parameters. Therefore, these elements could originate from the dissolution of Fe-oxides and oxidation of sulfide minerals.
PC 2 was correlated with Cr, Zn, Cd, and Pb ion concentrations. Studies have reported that groundwater contamination by heavy metals (e.g., Cd, Cr, Zn, Pb, and Cu) due to agricultural activities is increasing. Cu and Cd are largely related to agronomic practices.35,36 For example, Cd mainly originates from pesticide application, while fungicides contain large quantities of Cu sulfate.37 In addition, Zn and Pb originate from industrial and mining activities.38 Hence, the Cr, Zn, Cd, and Pb ions in the water samples in this study were probably derived from a combination of agricultural, mining, and industrial activities.
PC 3 was correlated with NO3−, Cl−, K+, and Na+ concentrations. As mentioned by Busico et al.,39 NO3−, Cl−, K+, and Na+ can be produced via agricultural fertilizer use in rural and suburban areas, as well as from livestock manure and sewer leakages.40 Therefore, NO3−, Cl−, K+, and Na+ were likely the result of agricultural fertilizer inputs, livestock manure, or sewage.
PCs 4–7 explained about 30.2% of the total variance; however, more detailed information is still needed to explore the sources of the ions of these components.
4. Conclusions
We used descriptive statistics, Piper trilinear diagrams, major ion ratios, and PCA to identify the predominant hydrochemical processes and characterize potential contaminants of groundwater in Qingyuan, China. Several conclusions were obtained from the results, as summarized below.(1) Groundwater in the karst aquifer in Qingyuan is dominated by two main water types, Ca–HCO3 and Ca–SO4, controlled mainly by the dissolution of evaporite minerals (e.g., calcite, gypsum, and/or anhydrite) and ion exchange processes.
(2) Na+ and K+ concentrations were decreased, while Mg2+ was increased in shallow and deep groundwater samples. Overall, shallow groundwater was more vulnerable to pollution than deep groundwater. In addition, the Mg2+/Ca2+ ratio was increased in deep groundwater samples because of its relatively long residence time.
(3) Potential contaminants in the karst groundwater were identified by PCA, including NO3−, Cl−, K+, and Na+ from agricultural fertilizers, livestock manure, and sewer leakages, as well as Cr, Zn, Cd, and Pb ions, likely from a combination of agricultural, mining, and industrial activities.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was Supported by the National Natural Science Foundation of China (41701585, 41771027 and 41611140112), the Fundamental Research Fund for the Central Universities of China (17lgpy40), and the Natural Science Foundation of Guangdong, China (2017A030310309), Provincial Special Fund for Economic Development (Marine Economic Development) (GDME2018E004), and the Scientific and Technological Innovation Project of the Water Sciences Department of Guangdong Province (2018-2021) and 1616F G@GPS INQUA.References
- X. D. Li, C. Q. Liu, M. Harue, S. L. Li and X. L. Liu, The use of environmental isotopic (C, Sr, S) and hydrochemical tracers to characterize anthropogenic effects on karst groundwater quality: a case study of the Shuicheng Basin, SW China, Appl. Geochem., 2010, 25(12), 1924–1936 CrossRef CAS.
- N. Goldscheider and N. Goldscheider, Karst groundwater vulnerability mapping: application of a new method in the Swabian Alb, Germany, Hydrogeol. J., 2005, 13(4), 555–564 CrossRef CAS.
- D. K. S. Y. Klaas, M. A. Imteaz, A. Arulrajah, D. K. S. Y. Klaas, M. A. Imteaz and A. Arulrajah, Development of groundwater vulnerability zones in a data-scarce eogenetic karst area using Head-Guided Zonation and particle-tracking simulation methods, Water Res., 2017, 122, 17–26 CrossRef CAS PubMed.
- Q. Yang, L. Wang, H. Ma, K. Yu and J. D. MartãN, Hydrochemical characterization and pollution sources identification of groundwater in Salawusu aquifer system of Ordos Basin, China, Environ. Pollut., 2016, 216, 340–349 CrossRef CAS PubMed.
- M. Karroum, M. Elgettafi, A. Elmandour, C. Wilske, M. Himi and A. Casas, Geochemical processes controlling groundwater quality under semi arid environment: a case study in central Morocco, Sci. Total Environ., 2017, 609, 1140 CrossRef CAS PubMed.
- J. Xiao, Z. Jin and J. Wang, Geochemistry of trace elements and water quality assessment of natural water within the Tarim River Basin in the extreme arid region, NW China, J. Geochem. Explor., 2014, 136(1), 118–126 CrossRef CAS.
- S. Armengol, M. Manzano, S. A. Bea and S. Martínez, Identifying and quantifying geochemical and mixing processes in the Matanza-Riachuelo Aquifer System, Argentina, Sci. Total Environ., 2017, 599-600, 1417 CrossRef CAS PubMed.
- P. W. Swarzenski, C. D. Reich, R. M. Spechler, J. L. Kindinger and W. S. Moore, Using multiple geochemical tracers to characterize the hydrogeology of the submarine spring off Crescent Beach, Florida, Chem. Geol., 2001, 179(1), 187–202 CrossRef CAS.
- H. C. LI Yan, Characteristics and mechanism of manufacturing industry shift in the Pearl River Delta during 1998-2009, Progress in Geography, 2013, 32(5), 777–787 Search PubMed.
- H. Ji and Y. Jiang, Carbon flux and C, S isotopic characteristics of river waters from a karstic and a granitic terrain in the Yangtze River system, J Asian Earth Sci., 2012, 57(3), 38–53 CrossRef.
- P. Négrel, C. J. Allègre, B. Dupré and E. Lewin, Erosion sources determined by inversion of major and trace element ratios and strontium isotopic ratios in river water: The Congo Basin case, Earth Planet. Sci. Lett., 1993, 120(1–2), 59–76 CrossRef.
- X. F. Huang, X. A. Li, L. Y. He, N. Feng, M. Hu, Y. W. Niu and L. W. Zeng, 5-Year study of rainwater chemistry in a coastal mega-city in South China, Atmos. Res., 2010, 97(1), 185–193 CrossRef CAS.
- C. Soulsby, M. Chen, R. C. Ferrier, R. C. Helliwell, A. Jenkins and R. Harriman, Hydrogeochemistry of shallow groundwater in an upland Scottish catchment, Hydrol. Processes, 2015, 12(7), 1111–1127 CrossRef.
- Ž. Brkić, M. Briški and T. Marković, Use of hydrochemistry and isotopes for improving the knowledge of groundwater flow in a semiconfined aquifer system of the Eastern Slavonia (Croatia), Catena, 2016, 142, 153–165 CrossRef.
- H. Jiráková, F. Huneau, Z. Hrkal, H. Celle-Jeanton and P. L. Coustumer, Carbon isotopes to constrain the origin and circulation pattern of groundwater in the north-western part of the Bohemian Cretaceous Basin (Czech Republic), Appl. Geochem., 2010, 25(8), 1265–1279 CrossRef.
- S. Jakóbczyk-Karpierz, S. Sitek, R. Jakobsen and A. Kowalczyk, Geochemical and isotopic study to determine sources and processes affecting nitrate and sulphate in groundwater influenced by intensive human activity - carbonate aquifer Gliwice (Southern Poland), Appl. Geochem., 2016, 76, 168–181 CrossRef.
- H. Celle-Jeanton, C. Emblanch, J. Mudry and A. Charmoille, Contribution of time tracers (Mg2+, TOC, δ13CTDIC, NO3−) to understand the role of the unsaturated zone: A case study—Karst aquifers in the Doubs valley, eastern France, Geophys. Res. Lett., 2016, 30(6), 7027 Search PubMed.
- K. A. R. Kpegli, A. Alassane, R. Trabelsi, K. Zouari, M. Boukari, D. Mama, F. L. Dovonon, Y. V. Yoxi and L. E. Toro-Espitia, Geochemical processes in Kandi Basin, Benin, West Africa: A combined hydrochemistry and stable isotopes approach, Quat. Int., 2015, 369, 99–109 CrossRef.
- M. Meybeck, Global chemical weathering of surficial rocks estimated from river dissolved loads, Am. J. Sci., 1987, 287(5), 401–428 CrossRef CAS.
- I. Farid, R. Trabelsi, K. Zouari and R. Beji, Geochemical and isotopic study of surface and groundwaters in Ain Bou Mourra basin, central Tunisia, Quat. Int., 2013, 303(4), 210–227 CrossRef.
- J. He, Y. An and F. Zhang, Geochemical characteristics and fluoride distribution in the groundwater of the Zhangye Basin in Northwestern China, J. Geochem. Explor., 2013, 135(6), 22–30 CrossRef CAS.
- Q. Yang, L. Wang, H. Ma, K. Yu and J. D. Martín, Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the Southeastern part of Ordos basin, China, Environ. Pollut., 2016, 218, 879–888 CrossRef CAS PubMed.
- F. Marcantonio, Principles and Applications of Geochemistry, Trans., Am. Geophys. Union, 2013, 79(30), 356 CrossRef.
- W. Edmunds, A. Guendouz, A. Mamou, A. Moulla, P. Shand and K. Zouari, Groundwater evolution in the Continental Intercalaire aquifer of southern Algeria and Tunisia: trace element and isotopic indicators, Appl. Geochem., 2003, 18(6), 805–822 CrossRef CAS.
- J. Ma, Z. Ding, W. M. Edmunds, J. B. Gates and T. Huang, Limits to recharge of groundwater from Tibetan plateau to the Gobi desert, implications for water management in the mountain front, J. Hydrol., 2009, 364(1), 128–141 CrossRef.
- A. B. Moussa, H. Mzali, K. Zouari and H. Hezzi, Hydrochemical and isotopic assessment of groundwater quality in the Quaternary shallow aquifer, Tazoghrane region, north-eastern Tunisia, Quat. Int., 2014, 338(4), 51–58 CrossRef.
- H. Schoeller, Hydrodynamique dans le karst, Chron. Hydrogeol., 1967, 10, 7–21 Search PubMed.
- H. Guo and Y. Wang, Hydrogeochemical processes in shallow quaternary aquifers from the northern part of the Datong Basin, China, Appl. Geochem., 2004, 19(1), 19–27 CrossRef CAS.
- F. Edition, Guidelines for drinking-water quality, WHO chronicle, 2011, vol. 38, pp. 104–108 Search PubMed.
- K. Chen, J. J. Jiao and J. Huang, Multivariate statistical evaluation of trace elements in groundwater in a coastal area in Shenzhen, China, Environ. Pollut., 2007, 147(3), 771–780 CrossRef CAS PubMed.
- K. Anazawa and H. Ohmori, The hydrochemistry of surface waters in andesitic volcanic area, Norikura volcano, central Japan, Chemosphere, 2005, 59(5), 605–615 CrossRef CAS PubMed.
- V. Simeonov, J. Stratis, C. Samara, G. Zachariadis, D. Voutsa, A. Anthemidis, M. Sofoniou and T. Kouimtzis, Assessment of the surface water quality in Northern Greece, Water Res., 2003, 37(17), 4119–4124 CrossRef CAS PubMed.
- R. P. Hooper, Diagnostic tools for mixing models of stream water chemistry, Water Resour. Res., 2003, 39(3), 249–256 CrossRef.
- M. Zieliński, J. Dopieralska, Z. Belka, A. Walczak, M. Siepak, M. Jakubowicz, M. Zieliński, J. Dopieralska and Z. Belka, et al., The strontium isotope budget of the Warta River (Poland): between silicate and carbonate weathering, and anthropogenic pressure, Appl. Geochem., 2017, 81, 1–11 CrossRef.
- J. Nouri, A. H. Mahvi, G. R. Jahed and A. A. Babaei, Regional distribution pattern of groundwater heavy metals resulting from agricultural activities, Environ. Geol., 2008, 55(6), 1337–1343 CrossRef CAS.
- P. Wongsasuluk, S. Chotpantarat, W. Siriwong and M. Robson, Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand, Environ. Geochem. Health, 2014, 36(1), 169–182 CrossRef CAS PubMed.
- L. Gao, Z. Wang, J. Shan, J. Chen, C. Tang and M. Yi, Aquatic environmental changes and anthropogenic activities reflected by the sedimentary records of the Shima River, Southern China, Environ. Pollut., 2017, 224, 70–81 CrossRef CAS PubMed.
- A. Bartkowiak, J. Lemanowicz and B. Breza-Boruta, Evaluation of the content of Zn, Cu, Ni and Pb as well as the enzymatic activity of forest soils exposed to the effect of road traffic pollution, Environ. Sci. Pollut. Res., 2017, 24(30), 23893–23902 CrossRef CAS PubMed.
- G. Busico, E. Cuoco, N. Kazakis, N. Colombani, M. Mastrocicco, D. Tedesco and K. Voudouris, Multivariate statistical analysis to characterize/discriminate between anthropogenic and geogenic trace elements occurrence in the Campania Plain, Southern Italy, Environ. Pollut., 2018, 234, 260–269 CrossRef CAS PubMed.
- E. Cuoco, N. Colombani, Ograve, T. H. Darrah, M. Mastrocicco, D. Tedesco, E. Cuoco, N. Colombani and T. H. Darrah, et al., Geolithological and anthropogenic controls on the hydrochemistry of the Volturno river (Southern Italy), Hydrol. Processes, 2017, 31, 627–638 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06051g |
| This journal is © The Royal Society of Chemistry 2018 |