DOI:
10.1039/C8RA05338C
(Paper)
RSC Adv., 2018,
8, 28997-29007
Novel total syntheses of oxoaporphine alkaloids enabled by mild Cu-catalyzed tandem oxidation/aromatization of 1-Bn-DHIQs†
Received
21st June 2018
, Accepted 6th August 2018
First published on 14th August 2018
Abstract
Novel total syntheses of oxoaporphine alkaloids such as liriodenine, dicentrinone, cassameridine, lysicamine, oxoglaucine and O-methylmoschatoline were developed. The key step of these total syntheses is Cu-catalyzed conversion of 1-benzyl-3,4-dihydro-isoquinolines (1-Bn-DHIQs) to 1-benzoyl-isoquinolines (1-Bz-IQs) via tandem oxidation/aromatization. This novel Cu-catalyzed conversion has been studied in detail, and was successfully used for constructing the 1-Bz-IQ core.
Introduction
Oxoaporphines, which have a characteristic carbonyl junction between aromatic B and D rings (see Fig. 1), are a sub-class of aporphinoid alkaloids.1 Oxoaporphine alkaloids are widespread in the kingdom of plants.1,2 For example, liriodenine 1a,3 dicentrinone 1b,4 cassameridine 1c,5 lysicamine 1d,6 oxoglaucine 1e7 and O-methylmoschatoline 1f8 (also referred to as liridine9 and homomoschatoline10) have been isolated from various botanic natural resources.3–10 Since oxoaporphine alkaloids 1a–f have shown a broad spectrum of interesting biological activities,11 so the total syntheses of them have aroused much interest from chemists.12 However, efficient, benign and practical total syntheses of these alkaloids remained highly desirable. Therefore, we herein report novel total syntheses of oxoaporphine alkaloids 1a–f via a key Cu-catalyzed conversion of 1-benzyl-3,4-dihydro-isoquinolines (1-Bn-DHIQs) to 1-benzoyl-isoquinolines (1-Bz-IQs).
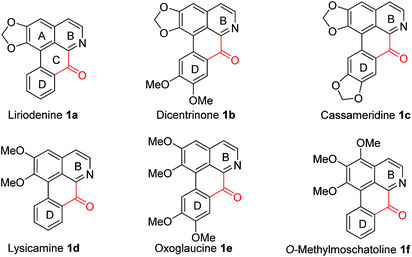 |
| | Fig. 1 The targeted oxoaporphine alkaloids 1a–f. | |
A general retrosynthetic analysis of the above several oxoaporphine alkaloids 1a–f is depicted in Scheme 1. As can be seen from the Scheme 1, C-ring could be constructed via Pschorr cyclization of anilines A, which could be obtained from the reduction of nitro groups of compounds B. The 1-Bz-IQ core of compounds B could be constructed via Cu-catalyzed tandem oxidation/aromatization of 1-Bn-DHIQs C. B-ring of compounds C could be constructed via Bischler–Napieralski cyclization of amides D, which could be derived from amines E and acyl chlorides F. Amines E could be prepared from aryl aldehydes G.
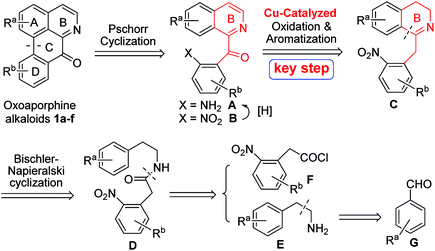 |
| | Scheme 1 A novel general retrosynthetic analysis of oxoaporphine alkaloids 1a–f. | |
Results and discussion
According to the above general retrosynthetic analysis, novel total syntheses of the six targeted oxoaporphine alkaloids 1a–f are depicted in Scheme 2. As can be seen from the Scheme 2, EDA (ethylenediamine)-catalyzed condensation of aryl aldehydes with nitromethane13 first produced nitroalkenes 2a–c in high yields. Next, when compounds 2a–c were treated with 6.0 equiv. of LiAlH4 in tetrahydrofuran at reflux, simultaneous reduction of both nitro group and double bond occurred in one-pot to furnish 2-aryl ethanamines 3a–c, which then immediately exposed to 1.1 equiv. of 2-arylacetyl chlorides and 3.0 equiv. of K2CO3 at 0 °C in a mixed solvent of dichloromethane and water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford amides 4a–f. Subsequently, treatment of compounds 4a–f with 3.0 equiv. of POCl3 in anhydrous acetonitrile at reflux gave 1-Bn-DHIQs 5a–f via Bischler–Napieralski cyclization.14 It was observed that compounds 5a–f and compounds 5′a–f were interchangeable at room temperature, we failed to separate them by chromatography due to rapid tautomerization between imines (5a–f) and enamines (5′a–f), so they were used as such for the next step without separation; when the tautomeric mixture of 5a–f and 5′a–f were treated with 0.1 equiv. (10 mol%) of anhydrous CuBr2 and 1.0 equiv. of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in dimethyl sulfoxide (DMSO) at 35 °C under an open air (O2) atmosphere, Cu-catalyzed tandem benzylic oxidation and aromatization occurred smoothly in one-pot to afford 1-Bz-IQs 6a–f in good yields over two steps (from 4a–f). Nitro (NO2) groups of compounds 6a–f could be rapidly reduced by acid-activated iron;15 when compounds 6a–f were treated with 10 equiv. of iron powder and 30 equiv. of HOAc at 70 °C under N2 atmosphere in aqueous ethanol (EtOH/H2O = 9
1) to afford amides 4a–f. Subsequently, treatment of compounds 4a–f with 3.0 equiv. of POCl3 in anhydrous acetonitrile at reflux gave 1-Bn-DHIQs 5a–f via Bischler–Napieralski cyclization.14 It was observed that compounds 5a–f and compounds 5′a–f were interchangeable at room temperature, we failed to separate them by chromatography due to rapid tautomerization between imines (5a–f) and enamines (5′a–f), so they were used as such for the next step without separation; when the tautomeric mixture of 5a–f and 5′a–f were treated with 0.1 equiv. (10 mol%) of anhydrous CuBr2 and 1.0 equiv. of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in dimethyl sulfoxide (DMSO) at 35 °C under an open air (O2) atmosphere, Cu-catalyzed tandem benzylic oxidation and aromatization occurred smoothly in one-pot to afford 1-Bz-IQs 6a–f in good yields over two steps (from 4a–f). Nitro (NO2) groups of compounds 6a–f could be rapidly reduced by acid-activated iron;15 when compounds 6a–f were treated with 10 equiv. of iron powder and 30 equiv. of HOAc at 70 °C under N2 atmosphere in aqueous ethanol (EtOH/H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), reduction of nitro groups took place smoothly to produce anilines 7a–f in high yields. Finally, compounds 7a–f were first treated with 1.0 equiv. of NaNO2 at 0–8 °C to produce diazonium intermediates, which were then immediately treated with 10 equiv. of copper powder at 60 °C in an aqueous H2SO4 (10% w/w) solution, Pschorr cyclization16 happened rapidly to afford oxoaporphines 1a–f in good yields. Thus, the targeted oxoaporphine alkaloids liriodenine 1a, dicentrinone 1b, cassameridine 1c, lysicamine 1d, oxoglaucine 1e and O-methyl-moschatoline 1f were synthesized from aryl aldehydes via 7 steps in 39%, 26%, 29%, 38%, 31% and 41% overall yields, respectively.
1), reduction of nitro groups took place smoothly to produce anilines 7a–f in high yields. Finally, compounds 7a–f were first treated with 1.0 equiv. of NaNO2 at 0–8 °C to produce diazonium intermediates, which were then immediately treated with 10 equiv. of copper powder at 60 °C in an aqueous H2SO4 (10% w/w) solution, Pschorr cyclization16 happened rapidly to afford oxoaporphines 1a–f in good yields. Thus, the targeted oxoaporphine alkaloids liriodenine 1a, dicentrinone 1b, cassameridine 1c, lysicamine 1d, oxoglaucine 1e and O-methyl-moschatoline 1f were synthesized from aryl aldehydes via 7 steps in 39%, 26%, 29%, 38%, 31% and 41% overall yields, respectively.
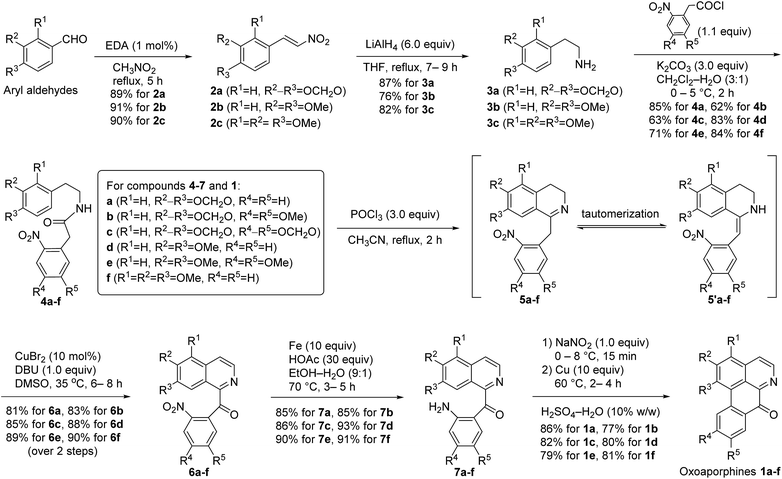 |
| | Scheme 2 Total syntheses of the targeted oxoaporphine alkaloids 1a–f starting from aryl aldehydes. | |
In the above-described total syntheses of oxoaporphine alkaloids 1a–f, the key step is Cu-catalyzed conversion of 1-Bn-THIQs 5a–f to 1-Bz-IQs 6a–f. This key conversion can be achieved by some known methods.17–21 However, these known methods often suffered from drawbacks such as use of poisonous and hazardous strong oxidants including SeO2,17 Pb(OAc)4,18 and CAN,19 inconvenient use of the photoactivated singlet O2,20 or need of high reaction temperature21 (120 °C). Therefore, development of an efficient and benign method for this particular conversion might be very helpful for total syntheses of oxoaporphine alkaloids.
Copper is a cheap transition metal with low toxicity; and air (O2) is an eco-friendly clean oxidant. Hence, an increasing amount of copper-catalyzed aerobic oxidations of various compounds have been recently developed.22 For the above conversion of 1-Bn-THIQs 5a–f to 1-Bz-IQs 6a–f, CuBr2 was used as the catalyst, and air (O2) was used as the clean oxidant. So this Cu-catalyzed method might be much more benign, eco-friendly and practical than the previous known methods.17–21 Advantages such as mildness (at 35 °C), high efficiency and eco-friendliness prompted us to investigate the details of this potential green chemical method.
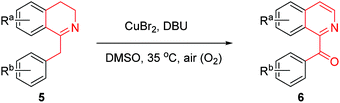
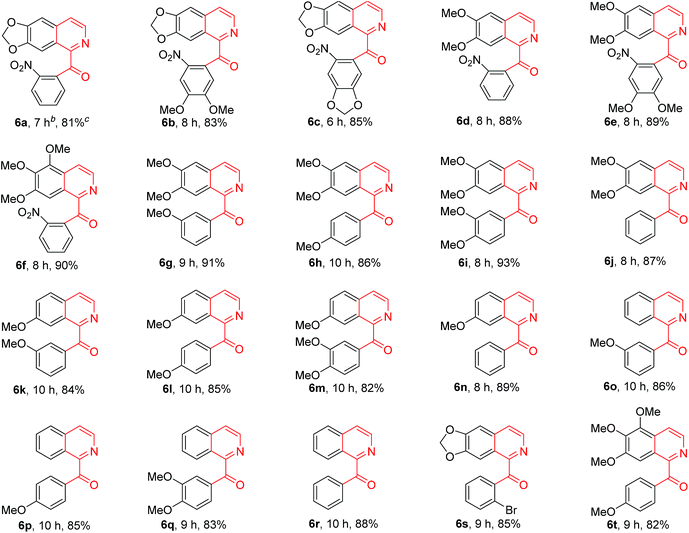
In order to know the scope and limitation of the method, more variously substituted 1-Bn-DHIQs 5 were prepared via Bischler–Napieralski cyclization, and a total of twenty substrates 5a–t have been tested for the CuBr2-catalyzed aerobic oxidation under the standard reaction conditions (see footnote of Table 1); the results are summarized in Table 1 (20 examples). As can be seen from the Table 1, the scope of the reaction is wide, it could be applicable to all the tested substrates, and afforded the desired 1-Bz-IQs 6a–t in good to high yields. We have found that 1-benzyl-3,4-dihydro-β-carbolines could undergo aerobic oxidation in DMSO to afford 1-benzoyl-β-carbolines without a copper catalyst,23 but herein we found that a copper catalyst is necessary for this one-pot aerobic oxidation, it was sluggish in the absence of copper catalyst. Several copper salts have been tested as the catalyst, CuBr2 obviously worked better than CuCl2, Cu(OAc)2, CuSO4, Cu2(OH)2CO3, CuO and CuI. Moreover, a base is also necessary for the reaction, DBU worked obviously better than Et3N, diisopropylethylamine, pyridine, N,N-dimethylaminopyridine (DMAP), 1,5-diazabicyclo[4,3,0]non-5-ene (DBN), Na2CO3 and K2CO3; DMSO as the solvent is better than DMF, CH3CN, THF, EtOH, Me2CO, CH2Cl2 and 1,4-dioxane.
Table 1 CuBr2-catalyzed conversion of variously substituted 1-Bn-DHIQs 5 to 1-Bz-IQs 6a
|
| Standard reaction conditions: 1-Bn-DHIQs 5 (2 mmol), CuBr2 (0.2 mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (2 mmol), DMSO (6 mL), 35 °C (inner temperature), air (O2). Reaction time. Isolated yields. |
 |
A possible mechanism for the CuBr2-catalyzed conversion of 1-Bn-DHIQs 5 to 1-Bz-IQs 6 was proposed in Scheme 3. 1-Bn-DHIQs 5 would first react with CuBr2 and a base (B = DBU, etc.) to form enamine-CuBr complexes 5′-CuBr, which would then undergo Jenkins-like aerobic oxidation24 to produce peroxide-CuBr complexes I-A. The intermediate Cu-complexes I-A would be unstable enough to decompose immediately to furnish 1-Bz-DHIQs 8. Compounds 8 would finally undergo DBU-promoted oxidation by O2 to produce 1-Bz-IQs 6 according to Kumar's reports.25 The intermediate compounds 8 could be detected by TLC during the reaction, and could also be isolated if the reaction was stopped at a middle point. For example, when CuBr2-catalyzed conversion of compound 5a to compound 6a was stopped at 3 h, the intermediate compound 8a was isolated in 38% yield.
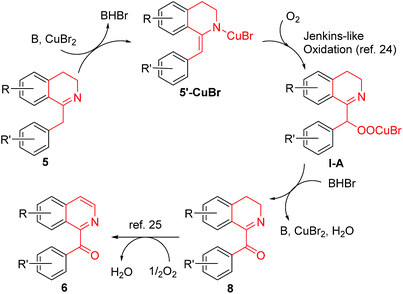 |
| | Scheme 3 A possible mechanism for the CuBr2-catalyzed conversion of 1-Bn-DHIQs 5 to 1-Bz-IQs 6. | |
Conclusions
In conclusion, novel total syntheses of oxoaporphine alkaloids including liriodenine 1a, dicentrinone 1b, cassameridine 1c, lysicamine 1d, oxoglaucine 1e and O-methyl-moschatoline 1e were achieved via a benign and ecofriendly Cu-catalyzed general approach. They were synthesized from the cheap and commercially available aryl aldehydes via seven steps in 39%, 26%, 29%, 38%, 31% and 41% overall yields, respectively.
In addition, a novel practical Cu-catalyzed conversion of 1-Bn-DHIQs 5 to 1-Bz-IQs 6, which was used as the key step in the above total syntheses, has also been investigated in detail; this Cu-catalyzed method has some advantages such as mildness, eco-friendliness, wide scope, ease of experimental procedure and high yields. It would provide a new general basic approach towards the total syntheses of oxoaporphine alkaloids and their derivatives.
Experimental
General
1H NMR and 13C NMR spectra were acquired on a Bruker AM 400 instrument, chemical shifts are given on the δ scale as parts per million (ppm) with tetramethylsilane (TMS) as the internal standard. IR spectra were recorded on a Nicolet Magna IR-550 instrument. Mass spectra were performed with a HP1100 LC-MS spectrometer. Melting points were measured on a Mei-TEMP II melting point apparatus. Column chromatography was performed on silica gel (Qingdao Chemical Factory). All reagents and solvents were analytically pure, and were used as such as received from the chemical suppliers.
Preparation of nitroalkenes 2a–c
An aryl aldehyde (∼40 mmol) was dissolved in nitromethane (30 mL), and then ethylenediamine (0.025 g, 0.416 mmol) was added. The resulting solution was then heated and stirred at reflux for 5 h. After the reaction was complete (checked by TLC, eluent: CH2Cl2/hexane = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the solution was concentrated under vacuum to give crude product as yellow solid, which was triturated in aqueous methanol (MeOH/H2O = 9
2), the solution was concentrated under vacuum to give crude product as yellow solid, which was triturated in aqueous methanol (MeOH/H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and was then filtered by suction. Compounds 2a–c were thus obtained in 89%, 90% and 91% yields, respectively. Characterization data of compounds 2a–c are as follows:
1), and was then filtered by suction. Compounds 2a–c were thus obtained in 89%, 90% and 91% yields, respectively. Characterization data of compounds 2a–c are as follows:
(E)-5-(2-Nitrovinyl)benzo[d][1,3]dioxole (2a). Yellow crystals, mp 158–159 °C. 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 13.5 Hz, 1H), 7.48 (d, J = 13.5 Hz, 1H), 7.09 (d, J = 7.8 Hz, 1H), 7.00 (s, 1H), 6.88 (d, J = 7.8 Hz, 1H), 6.07 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 151.39, 148.77, 139.13, 135.37, 126.69, 124.18, 109.08, 106.98, 102.10.
(E)-1,2-Dimethoxy-4-(2-nitrovinyl)benzene (2b). Pale yellow crystals, mp 141–142 °C. 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 13.6 Hz, 1H), 7.56 (d, J = 13.6 Hz, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.02 (s, 1H), 6.92 (d, J = 8.2 Hz, 1H), 3.95 (s, 3H), 3.93 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 152.78, 149.51, 139.39, 135.13, 124.71, 122.78, 111.30, 110.18, 56.10, 56.02.
(E)-1,2,3-Trimethoxy-4-(2-nitrovinyl)benzene (2c). Pale yellow crystals, mp 192–193 °C. 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 13.6 Hz, 1H), 7.77 (d, J = 13.6 Hz, 1H), 7.21 (d, J = 8.8 Hz, 1H), 6.73 (d, J = 8.8 Hz, 1H), 4.00 (s, 3H), 3.93 (s, 3H), 3.88 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 157.36, 154.27, 142.39, 136.52, 135.37, 126.65, 117.02, 107.68, 61.19, 60.94, 56.20.
Preparation of 2-aryl ethanamines 3a–c
A solution of compound 2 (∼18 mmol) in THF (40 mL) was dropwise added into a stirred suspension of LiAlH4 (4.102 g, 108.1 mmol) in THF (100 mL) at 0 °C over 20 min. After the addition was finished, the mixture was then heated and stirred at reflux for 7–9 h. The mixture was cooled to 0 °C by an ice-bath. While the mixture was vigorously stirred, water (5 mL) was dropwise added into the reaction mixture over 30 min, and an aqueous solution of NaOH (28 mL, 15% w/w) was then slowly added into the mixture at 0 °C. The ice-bath was removed, and the mixture was further stirred at room temperature for 3 h. The mixture was filtered through a thin layer of celite, and the filter cake was washed twice with THF (2 × 30 mL). The filtrates were combined and dried over anhydrous MgSO4. The solution was concentrated under vacuum to give crude product as yellow oil, which was purified by formation of hydrochloride ammonium salt. Pure 2-aryl ethanamines 3a–c were obtained after neutralization in 87%, 76% and 82% yields, respectively. Characterization data of compounds 3a–c are as follows:
2-(Benzo[d][1,3]dioxol-5-yl)ethanamine (3a). 1H NMR (400 MHz, CDCl3) δ 6.74 (d, J = 7.8 Hz, 1H), 6.69 (s, 1H), 6.64 (d, J = 7.8 Hz, 1H), 5.92 (s, 2H), 2.91 (t, J = 6.9 Hz, 2H), 2.67 (t, J = 6.9 Hz, 2H), 1.56 (br. s, 3H, NH2); 13C NMR (100 MHz, CDCl3) δ 147.57, 145.80, 133.46, 121.55, 109.02, 108.09, 100.71, 43.49, 39.49.
2-(3,4-Dimethoxyphenyl)ethanamine (3b). 1H NMR (400 MHz, CDCl3) δ 6.79 (d, J = 7.9 Hz, 1H), 6.76 (d, J = 7.9 Hz, 1H), 6.73 (s, 1H), 3.87 (s, 3H), 3.85 (s, 3H), 2.93 (t, J = 6.9 Hz, 2H), 2.68 (t, J = 6.9 Hz, 2H), 1.43 (br. s, 2H, NH2); 13C NMR (100 MHz, CDCl3) δ 148.81, 147.34, 132.35, 120.65, 111.96, 111.22, 55.83, 55.73, 43.58, 39.51.
2-(2,3,4-Trimethoxyphenyl)ethanamine (3c). 1H NMR (400 MHz, CDCl3) δ 6.84 (d, J = 8.5 Hz, 1H), 6.62 (d, J = 8.5 Hz, 1H), 3.88 (s, 3H), 3.87 (s, 3H), 3.84 (s, 3H), 2.91 (t, J = 7.0 Hz, 2H), 2.71 (t, J = 7.0 Hz, 2H), 2.32 (br. s, 2H, NH2); 13C NMR (100 MHz, CDCl3) δ 152.31, 152.08, 142.29, 125.46, 124.35, 107.16, 60.92, 60.69, 55.97, 42.81, 33.84.
Preparation of amides 4a–f
2-Aryl acetic acid (∼11 mmol) was dissolved in CH2Cl2 (20 mL), and SOCl2 (1.790 g, 15.05 mmol) was added. The resulting solution was then heated and stirred at reflux for 4 h. The reaction solution was concentrated under vacuum to dryness, oily residue was then dissolved in dry CH2Cl2 (10 mL), the solution was immediately used bellow. 2-Aryl ethanamine 3 (10 mmol) was dissolved in 40 mL CH2Cl2, and an aqueous solution of K2CO3 (4.150 g, 30.03 mmol) in water (30 mL) was added. The biphasic mixture was cooled by an ice-bath, and was stirred at 0 to 5 °C. The above freshly prepared solution of 2-aryl acetyl chloride was added dropwise into the reaction mixture over 2 min. After the addition was finished, stirring was continued at 0 to 5 °C for 2 h. When the reaction was complete (checked by TLC, eluent: EtOAc/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the reaction mixture was transferred into a separatory funnel. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (20 mL). The organic extracts were combined, dried over anhydrous MgSO4, and then concentrated under vacuum to give crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/hexane = 1
3), the reaction mixture was transferred into a separatory funnel. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (20 mL). The organic extracts were combined, dried over anhydrous MgSO4, and then concentrated under vacuum to give crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Amides 4a–f were thus obtained in 85%, 62%, 63%, 83%, 71% and 84% yields, respectively. Characterization data of compounds 4a–f are as follows:
4). Amides 4a–f were thus obtained in 85%, 62%, 63%, 83%, 71% and 84% yields, respectively. Characterization data of compounds 4a–f are as follows:
N-(2-(Benzo[d][1,3]dioxol-5-yl)ethyl)-2-(2-nitrophenyl)aceta-mide (4a). White crystals, mp 125–126 °C. 1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.2 Hz, 1H), 7.60 (dd, J1 = 8.0 Hz, J2 = 7.8 Hz, 1H), 7.48–7.42 (m, 2H), 6.69 (d, J = 7.9 Hz, 1H), 6.60 (s, 1H), 6.55 (d, J = 7.9 Hz, 1H), 5.92 (s, 2H), 5.84 (br. s, 1H, CONH), 3.79 (s, 2H), 3.49–3.42 (m, 2H), 2.69 (t, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 168.93, 148.80, 147.72, 146.11, 133.57, 133.33, 132.44, 130.41, 128.37, 125.12, 121.61, 109.03, 108.30, 100.87, 41.06, 40.89, 35.24; IR (KBr film) 3280, 3070, 2921, 1640, 1552, 1521, 1492, 1350, 1247, 1192, 1034, 923, 809, 790, 715 cm−1.
N-(2-(Benzo[d][1,3]dioxol-5-yl)ethyl)-2-(4,5-dimethoxy-2-nitrophenyl)acetamide (4b). White crystals, mp 206–207 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.97 (t, J = 5.8 Hz, 1H, CONH), 7.64 (s, 1H), 7.05 (s, 1H), 6.81 (d, J = 7.9 Hz, 1H), 6.79 (s, 1H), 6.65 (d, J = 7.9 Hz, 1H), 5.96 (s, 2H), 3.87 (s, 3H), 3.85 (s, 3H), 3.82 (s, 2H), 3.25–3.17 (m, 2H), 2.61 (t, J = 7.2 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 168.62, 152.57, 147.16, 147.10, 145.42, 140.92, 133.25, 126.01, 121.43, 115.36, 108.98, 108.02, 107.94, 100.58, 56.10, 55.90, 40.78, 40.00, 34.84; IR (KBr film) 3270, 3092, 2933, 1641, 1580, 1525, 1502, 1420, 1330, 1274, 1239, 1186, 1063, 1034, 923, 875, 804, 748 cm−1.
N-(2-(Benzo[d][1,3]dioxol-5-yl)ethyl)-2-(6-nitrobenzo[d][1,3]dioxol-5-yl)acetamide (4c). White crystals, mp 208–209 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.02 (t, J = 5.7 Hz, 1H, CONH), 7.63 (s, 1H), 7.01 (s, 1H), 6.82 (d, J = 7.9 Hz, 1H), 6.79 (s, 1H), 6.65 (d, J = 7.9 Hz, 1H), 6.22 (s, 2H), 5.96 (s, 2H), 3.76 (s, 2H), 3.26–3.18 (m, 2H), 2.61 (t, J = 7.2 Hz, 2H);13C NMR (100 MHz, DMSO-d6) δ 168.40, 151.26, 147.11, 146.53, 145.43, 142.63, 133.20, 128.10, 121.45, 111.87, 108.99, 108.03, 105.08, 103.20, 100.58, 40.61, 39.95, 34.82; IR (KBr film) 3280, 3064, 2920, 1637, 1548, 1524, 1504, 1480, 1442, 1378, 1328, 1249, 1192, 1032, 924, 877, 816, 722 cm−1.
N-(3,4-Dimethoxyphenethyl)-2-(2-nitrophenyl)acetamide (4d). White crystals, mp 129–130 °C. 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 8.2 Hz, 1H), 7.58 (dd, J1 = 8.0 Hz, J2 = 8.2 Hz, 1H), 7.46–7.40 (m, 2H), 6.75 (d, J = 8.1 Hz, 1H), 6.69 (s, 1H), 6.67 (d, J = 8.1 Hz, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 3.78 (s, 2H), 3.52–3.45 (m, 2H), 2.73 (t, J = 7.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 168.88, 148.98, 148.76, 147.62, 133.58, 133.37, 131.20, 130.38, 128.39, 125.12, 120.60, 111.90, 111.32, 55.92, 55.84, 40.94, 40.85, 35.10; IR (KBr film) 3424, 3065, 2944, 2839, 1651, 1602, 1479, 1280, 1256, 1163, 1125, 1028, 935, 823, 718 cm−1; HRMS (ESI) m/z calcd for C18H21N2O5 [M + H]+: 345.1450, found: 345.1451.
N-(3,4-Dimethoxyphenethyl)-2-(4,5-dimethoxy-2-nitrophenyl)acetamide (4e). White crystals, mp 131–132 °C. 1H NMR (400 MHz, CDCl3) δ 7.60 (s, 1H), 6.83 (s, 1H), 6.74 (d, J = 8.1 Hz, 1H), 6.67 (s, 1H), 6.65 (d, J = 8.1 Hz, 1H), 5.96 (br. s, 1H, CONH), 3.96 (s, 3H), 3.94 (s, 3H), 3.85 (s, 3H), 3.84 (s, 3H), 3.77 (s, 2H), 3.53–3.44 (m, 2H), 2.73 (t, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 168.90, 153.35, 148.96, 148.07, 147.59, 140.80, 131.14, 125.48, 120.56, 114.44, 111.88, 111.23, 108.15, 56.57, 56.35, 55.87, 55.82, 41.39, 40.90, 35.09; IR (KBr film) 3467, 3072, 2945, 2832, 1653, 1600, 1475, 1282, 1251, 1168, 1125, 1018, 925, 827, 724 cm−1; HRMS (ESI) m/z calcd for C20H25N2O7 [M + H]+: 405.1662, found: 405.1665.
N-(2,3,4-Trimethoxyphenethyl)-2-(2-nitrophenyl)acetamide (4f). White crystals, mp 106–107 °C. 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 7.8 Hz, 1H), 7.57 (d, J = 7.2 Hz, 1H), 7.48–7.41 (m, 2H), 6.78 (d, J = 8.2 Hz, 1H), 6.58 (d, J = 8.2 Hz, 1H), 6.21 (br. s, 1H, CONH), 3.85 (s, 3H), 3.845 (s, 3H), 3.843 (s, 3H), 3.80 (s, 2H), 3.47–3.41 (m, 2H), 2.73 (t, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 168.94, 152.58, 151.80, 148.89, 142.19, 133.49, 133.42, 130.53, 128.28, 125.06, 124.74, 124.51, 107.43, 60.98, 60.74, 56.00, 40.95, 40.94, 29.63; IR (KBr, film) 3420, 3293, 2958, 2939, 1644, 1548, 1524, 1495, 1467, 1417, 1349, 1240, 1101, 933, 792, 716 cm−1; HRMS (ESI) m/z calcd for C19H23N2O6 [M + H]+: 375.1556, found: 375.1537.
Preparation of 1-benzyl-3,4-dihydroisoquinolines 5
A corresponding amide 4 (∼5 mmol) was dissolved in anhydrous acetonitrile (40 mL), and phosphorus oxychloride (2.350 g, 15.33 mmol) was slowly added into the mixture. The resulting solution was then heated and stirred at reflux for 2 h. After the reaction was complete (checked by TLC, eluent: CH2Cl2/hexane = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the solution was concentrated under vacuum to dryness, the residue was dissolved in CH2Cl2 (50 mL). An aqueous solution of K2CO3 (20 mL, 15% w/w) was added. After the mixture was vigorously stirred for 5 min, two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (20 mL). The organic extracts were combined, dried over anhydrous MgSO4, and then concentrated under vacuum to give crude solid product as a tautomeric mixture of 1-benzyl-3,4-dihydroisoquinoline 5 and enamine 5′, which was used as such for the next step.
1), the solution was concentrated under vacuum to dryness, the residue was dissolved in CH2Cl2 (50 mL). An aqueous solution of K2CO3 (20 mL, 15% w/w) was added. After the mixture was vigorously stirred for 5 min, two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (20 mL). The organic extracts were combined, dried over anhydrous MgSO4, and then concentrated under vacuum to give crude solid product as a tautomeric mixture of 1-benzyl-3,4-dihydroisoquinoline 5 and enamine 5′, which was used as such for the next step.
General procedure for the CuBr2-catalyzed conversion of 1-benzyl-3,4-dihydroisoquinolines 5 to 1-benzoyl-isoquinolines 6
The above tautomeric mixture of 1-benzyl-3,4-dihydroisoquinoline 5 and enamine 5′ were dissolved in DMSO (10 mL), DBU (0.755 g, 4.959 mmol) and CuBr2 (0.113 g, 0.506 mmol) were added. The resulting solution was then stirred at 35 °C for 6–10 h (see Table 1) under an atmosphere of air. After the reaction was complete (checked by TLC, eluent: EtOAc/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), a dilute ammonia aqueous solution (5% w/w, 50 mL) and CH2Cl2 (40 mL) were added into the mixture. Two phases were separated, and the aqueous phase was twice extracted again with CH2Cl2 (2 × 30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. Removal of solvent by vacuum distillation gave crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1
4), a dilute ammonia aqueous solution (5% w/w, 50 mL) and CH2Cl2 (40 mL) were added into the mixture. Two phases were separated, and the aqueous phase was twice extracted again with CH2Cl2 (2 × 30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. Removal of solvent by vacuum distillation gave crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to afford pure 1-benzoyl-isoquinoline 6 as crystals in a yield (over two steps) as indicated in Table 1. Characterization data of 1-benzoyl-isoquinolines 6a–t are as follows:
10) to afford pure 1-benzoyl-isoquinoline 6 as crystals in a yield (over two steps) as indicated in Table 1. Characterization data of 1-benzoyl-isoquinolines 6a–t are as follows:
([1,3]Dioxolo[4,5-g]isoquinolin-5-yl)(2-nitrophenyl)metha-none (6a). Pale yellow crystals, mp 244–245 °C. 1H NMR (400 MHz, CDCl3-TFA) δ 8.42 (s, 1H), 8.30 (d, J = 8.2 Hz, 1H), 8.19 (d, J = 6.4 Hz, 1H), 8.09 (d, J = 6.4 Hz, 1H), 7.98 (dd, J1 = 7.5 Hz, J1 = 7.8 Hz, 1H), 7.92–7.84 (m, 2H), 7.40 (s, 1H), 6.40 (s, 2H); 13C NMR (100 MHz, CDCl3-TFA) δ 186.14, 156.93, 154.17, 146.24, 142.08, 141.68, 135.87, 134.04, 131.80, 131.08, 130.20, 126.47, 125.39, 124.52, 104.36, 103.78, 103.16; IR (KBr, film) 3077, 2917, 1684, 1576, 1518, 1460, 1348, 1275, 1214, 1036, 1017, 946, 867, 788, 706 cm−1; HRMS (ESI) m/z calcd for C17H11N2O5 [M + H]+: 323.0668, found: 323.0665.
(4,5-Dimethoxy-2-nitro-phenyl)([1,3]dioxolo[4,5-g]isoquino-lin-5-yl)methanone (6b). Pale yellow crystals, mp 258–259 °C. 1H NMR (400 MHz, CDCl3-TFA) δ 8.37 (s, 1H), 8.16 (d, J = 6.3 Hz, 1H), 8.06 (d, J = 6.3 Hz, 1H), 7.70 (s, 1H), 7.38 (s, 1H), 7.27 (s, 1H), 6.40 (s, 2H), 4.07 (s, 3H), 4.05 (s, 3H); 13C NMR (100 MHz, CDCl3-TFA) δ 187.14, 155.78, 154.82, 153.24, 152.19, 144.56, 140.54, 139.96, 131.12, 126.75, 125.71, 124.55, 112.93, 107.34, 103.84, 103.81, 103.35, 56.88, 56.73; IR (KBr, film) 3096, 2916, 1682, 1579, 1520, 1500, 1463, 1397, 1326, 1282, 1261, 1228, 1210, 1077, 1034, 942, 863, 778, 758 cm−1; HRMS (ESI) m/z calcd for C19H15N2O7 [M + H]+: 383.0879, found: 383.0881.
([1,3]Dioxolo[4,5-g]isoquinolin-5-yl)(6-nitrobenzo[d][1,3] dioxol-5-yl)methanone (6c). Pale yellow crystals, mp 229–230 °C. 1H NMR (400 MHz, CDCl3-TFA) δ 8.38 (s, 1H), 8.21 (d, J = 6.3 Hz, 1H), 8.07 (d, J = 6.3 Hz, 1H), 7.64 (s, 1H), 7.38 (s, 1H), 7.16 (s, 1H), 6.39 (s, 2H), 6.30 (s, 2H); 13C NMR (100 MHz, CDCl3-TFA) δ 185.29, 156.70, 154.12, 153.93, 151.96, 142.54, 141.76, 141.62, 130.04, 128.24, 126.18, 124.54, 109.97, 105.43, 104.87, 104.22, 103.62, 103.61; IR (KBr, film) 3065, 2917, 1677, 1609, 1577, 1519, 1500, 1464, 1424, 1398, 1326, 1273, 1237, 1203, 1031, 988, 920, 871, 764 cm−1; HRMS (ESI) m/z calcd for C18H11N2O7 [M + H]+: 367.0566, found: 367.0565.
(6,7-Dimethoxyisoquinolin-1-yl)(2-nitrophenyl)methanone (6d). Pale yellow crystals, mp 195–196 °C. 1H NMR (400 MHz, CDCl3) δ 8.75 (s, 1H), 8.24 (d, J = 5.6 Hz, 1H), 8.15 (d, J = 5.6 Hz, 1H), 7.80 (dd, J1 = 8.0 Hz, J2 = 7.7 Hz, 1H), 7.73–7.58 (m, 3H), 7.10 (s, 1H), 4.14 (s, 3H), 4.05 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 195.63, 153.08, 152.47, 148.35, 147.59, 140.15, 137.94, 134.54, 134.02, 130.29, 129.66, 123.67, 123.66, 123.55, 104.74, 104.53, 56.26, 56.07; IR (KBr film) 2945, 2835, 1650, 1600, 1477, 1329, 1282, 1255, 1168, 1125, 1020, 915, 809 cm−1; HRMS (ESI) m/z calcd for C18H15N2O5 [M + H]+: 339.0981, found: 339.0985.
(6,7-Dimethoxyisoquinolin-1-yl)(4,5-dimethoxy-2-nitrophenyl)methanone (6e). Pale yellow crystals, mp 212–213 °C. 1H NMR (400 MHz, CDCl3) δ 8.65 (s, 1H), 8.24 (d, J = 5.4 Hz, 1H), 7.65 (s, 1H), 7.61 (d, J = 5.4 Hz, 1H), 7.12 (s, 1H), 7.09 (s, 1H), 4.13 (s, 3H), 4.04 (s, 3H), 4.02 (s, 3H), 4.01 (S, 3H); 13C NMR (100 MHz, CDCl3) δ 195.33, 153.69, 153.06, 152.30, 149.68, 149.06, 140.57, 140.01, 134.44, 132.05, 123.54, 111.05, 106.46, 104.72, 104.71, 104.61, 56.64, 56.57, 56.22, 56.07; IR (KBr film) 2981, 2845, 1652, 1603, 1473, 1328, 1282, 1255, 1166, 1123, 1021, 934, 865, 812 cm−1; HRMS (ESI) m/z calcd for C20H19N2O7 [M + H]+: 399.1192, found: 399.1190.
(2-Nitrophenyl)(5,6,7-trimethoxyisoquinolin-1-yl)methanone (6f). Pale yellow crystals, mp 113–114 °C. 1H NMR (400 MHz, CDCl3) δ 8.56 (s, 1H), 8.26 (d, J = 5.2 Hz, 1H), 8.15 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 5.2 Hz, 1H), 7.80 (dd, J1 = 8.0 Hz, J2 = 7.7 Hz, 1H), 7.70 (d, J = 7.5 Hz, 1H), 7.65 (dd, J1 = 7.7 Hz, J2 = 7.5 Hz, 1H), 4.12 (s, 3H), 4.05 (s, 3H), 4.04 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 195.45, 156.02, 148.63, 147.48, 146.51, 144.13, 139.55, 137.92, 134.07, 130.33, 129.84, 129.69, 124.45, 123.58, 119.13, 100.86, 61.70, 61.23, 56.26; IR (KBr, film) 2938, 1687, 1612, 1577, 1528, 1476, 1353, 1274, 1243, 1124, 1048, 941, 855, 758, 637 cm−1; HRMS (ESI) m/z calcd for C19H17N2O6 [M + H]+: 369.1087, found: 369.1085.
(6,7-Dimethoxyisoquinolin-1-yl)(3-methoxyphenyl)methanone (6g). White crystals, mp 168–169 °C. 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 5.5 Hz, 1H), 7.66 (d, J = 5.5 Hz, 1H), 7.60 (s, 1H), 7.56 (s, 1H), 7.44 (d, J = 7.6 Hz, 1H), 7.37 (dd, J = 7.9 Hz, J = 7.6 Hz, 1H), 7.17 (d, J = 7.9 Hz, 1H), 7.14 (s, 1H), 4.05 (s, 3H), 3.96 (s, 3H), 3.85 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 195.18, 159.62, 153.22, 153.02, 151.21, 140.05, 138.40, 134.05, 129.37, 123.99, 122.95, 121.64, 120.13, 114.55, 104.88, 103.95, 56.14, 56.08, 55.47. IR (KBr film) 2937, 2834, 1660, 1591, 1485, 1439, 1310, 1270, 1259, 1148, 1036, 746, 634 cm−1; HRMS (ESI) m/z calcd for C19H18NO4 [M + H]+: 324.1236, found: 324.1219.
(6,7-Dimethoxyisoquinolin-1-yl)(4-methoxyphenyl)methanone (6h). White crystals, mp 157–158 °C (lit. 21 150–152 °C). 1H NMR (400 MHz, CDCl3) δ 8.46 (d, J = 5.5 Hz, 1H),7.96 (d, J = 8.9 Hz, 2H), 7.65 (d, J = 5.5 Hz), 7.57 (s, 1H), 7.15 (s, 1H), 6.96 (d, J = 8.9 Hz, 2H), 4.06 (s, 3H), 3.96 (s, 3H), 3.88 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 193.92, 163.95, 153.69, 153.15, 151.00, 140.02, 133.98, 133.28 (2C), 129.81, 122.80, 121.29, 113.71 (2C), 104.86, 104.06, 56.12, 56.07, 55.55; IR (KBr film) 2961, 2940, 2836, 1650, 1607, 1511, 1442, 1321, 1264, 1156, 1054, 916, 862, 836, 613 cm−1.
(6,7-Dimethoxyisoquinolin-1-yl)(3,4-dimethoxyphenyl)metha-none (6i). White crystals, mp 209–210 °C. 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 5.5 Hz, 1H), 7.72 (s, 1H), 7.69 (d, J = 5.5 Hz, 1H), 7.55 (s, 1H), 7.42 (d, J = 8.4 Hz, 1H), 7.15 (s, 1H), 6.87 (d, J = 8.4 Hz, 1H), 4.06 (s, 3H), 3.97 (s, 3H), 3,96 (s, 3H), 3.95 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 193.94, 153.79, 153.68, 153.15, 150.98, 149.00, 140.01, 133.93, 129.89, 126.91, 122.80, 121.26, 111.88, 109.94, 104.85, 104.01, 56.11, 56.09, 56.04, 55.99; IR (KBr film) 2970, 2934, 1657, 1505, 1460, 1270, 1227, 1140, 1025, 860, 749 cm−1; HRMS (ESI) m/z calcd for C20H20NO5 [M + H]+: 354.1341, found: 354.1343.
(6,7-Dimethoxyisoquinolin-1-yl)(phenyl)methanone (6j). White crystals, mp 130–131 °C (lit. 21 131–132 °C). 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 5.5 Hz, 1H), 7.96 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 5.5 Hz, 1H), 7.65 (s, 1H), 7.63 (t, J = 7.8 Hz, 1H), 7.50 (dd, J1 = 7.8 Hz, J2 = 8.0 Hz, 2H), 7.15 (s, 1H), 4.06 (s, 3H), 3.97 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 195.39, 153.20, 152.96, 151.22, 140.07, 137.11, 134.07, 133.42, 130.87 (2C), 128.38 (2C), 123.03, 121.66, 104.88, 103.99, 56.13, 56.08; IR (KBr film) 2971, 2841, 1657, 1510, 1456, 1260, 1232, 1154, 1052, 864, 709, 649 cm−1.
(7-Methoxyisoquinolin-1-yl)(3-methoxyphenyl)methanone (6k). White crystals, mp 106–107 °C. 1H NMR (400 MHz, CDCl3) δ 8.50 (d, J = 5.5 Hz, 1H), 7.82 (d, J = 8.5 Hz, 1H), 7.75 (d, J = 5.5 Hz, 1H), 7.58 (s, 1H), 7.57 (s, 1H), 7.45–7.34 (m, 3H), 7.16 (d, J = 8.2 Hz, 1H), 3.89 (s, 3H), 3.86 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 194.98, 159.68, 159.33, 154.03, 139.35, 138.32, 132.64, 129.41, 128.66, 127.93, 124.31, 124.00, 122.73, 120.26, 114.48, 103.22, 55.53, 55.50; IR (KBr film) 3448, 2923, 1660, 1622, 1593, 1452, 1315, 1283, 1263, 1235, 1038, 869, 848, 646 cm−1; HRMS (ESI) m/z calcd for C18H16NO3 [M + H]+: 294.1130, found: 294.1136.
(7-Methoxyisoquinolin-1-yl)(4-methoxyphenyl)methanone (6l). White crystals, mp 101–103 °C. 1H NMR (400 MHz, CDCl3) δ 8.49 (d, J = 5.5 Hz, 1H), 7.96 (d, J = 8.9 Hz, 2H), 7.81 (d, J = 9.0 Hz, 1H), 7.73 (d, J = 5.5 Hz, 1H), 7.54 (s, 1H), 7.38 (d, J = 9.0 Hz, 1H), 6.96 (d, J = 8.9 Hz, 2H), 3.88 (s, 3H), 3.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 193.74, 164.02, 159.15, 154.69, 139.33, 133.27 (2C), 132.58, 129.76, 128.60, 127.82, 124.19, 122.35, 113.76 (2C), 103.35, 55.56, 55.51. IR (KBr film) 2926, 2837, 1652, 1622, 1601, 1506, 1427, 1251, 1215, 1185, 1166, 1044, 843, 613 cm−1. HRMS (ESI) m/z calcd for C18H16NO3 [M + H]+: 294.1130, found: 294.1127.
(3,4-Dimethoxyphenyl)(7-methoxyisoquinolin-1-yl)methanone (6m). White crystals, mp 150–152 °C. 1H NMR (400 MHz, CDCl3) δ 8.49 (d, J = 5.4 Hz, 1H), 7.82 (d, J = 9.0 Hz, 1H), 7.74 (d, J = 5.4 Hz, 1H), 7.72 (s, 1H), 7.52 (s, 1H), 7.44–7.38 (m, 2H), 6.87 (d, J = 8.5 Hz, 1H), 3.97 (s, 3H), 3.95 (s, 3H), 3.88 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 193.76, 159.17, 154.68, 153.92, 149.11, 139.27, 132.57, 129.87, 128.60, 127.86, 126.92, 124.23, 122.35, 111.89, 109.99, 103.37, 56.14, 56.04, 55.52; IR (KBr film) 2998, 2931, 1653, 1597, 1585, 1516, 1265, 1232, 1143, 1027, 847, 762, 641 cm−1. HRMS (ESI) m/z calcd for C19H18NO4 [M + H]+: 324.1236, found: 324.1233.
(7-Methoxyisoquinolin-1-yl)(phenyl)methanone (6n). White crystals, mp 120–122 °C. 1H NMR (400 MHz, CDCl3) δ 8.50 (d, J = 5.4 Hz, 1H), 7.96 (d, J = 8.4 Hz, 2H), 7.82 (d, J = 9.0 Hz, 1H), 7.75 (d, J = 5.4 Hz, 1H), 7.62 (t, J = 8.0 Hz, 1H), 7.61 (s, 1H), 7.49 (dd, J1 = 8.0 Hz, J2 = 8.4 Hz, 2H), 7.40 (d, J = 9.0 Hz, 1H), 3.89 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 195.13, 159.37, 153.95, 139.32, 137.04, 133.50, 132.68, 130.85 (2C), 128.65 (2C), 128.42, 128.00, 124.31, 122.75, 103.29, 55.53; IR (KBr film) 2978, 2923, 1661, 1624, 1502, 1455, 1317, 1247, 1216, 1168, 851, 715, 646 cm−1. HRMS (ESI) m/z calcd for C17H14NO2 [M + H]+: 264.1025, found: 264.1018.
(Isoquinolin-1-yl)(3-methoxyphenyl)methanone (6o). White crystals, mp 62–63 °C. 1H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 5.6 Hz, 1H), 8.19 (d, J = 8.5 Hz, 1H), 7.93 (d, J = 8.4 Hz, 1H), 7.82 (d, J = 5.6 Hz, 1H), 7.75 (dd, J1 = 8.2 Hz, J2 = 8.4 Hz, 1H), 7.63 (dd, J1 = 8.2 Hz, J2 = 8.5 Hz, 1H), 7.58 (s, 1H), 7.41 (d, J = 7.6 Hz, 1H), 7.35 (dd, J1 = 7.6 Hz, J2 = 8.0 Hz, 1H), 7.16 (d, J = 8.0 Hz, 1H), 3.86 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 194.61, 159.73, 156.49, 141.16, 137.93, 136.67, 130.78, 129.50, 128.36, 127.14, 126.35, 126.12, 123.99, 122.63, 120.44, 114.33, 55.50; IR (KBr film) 3005, 2937, 1665, 1594, 1463, 1281, 1266, 1147, 1039, 834, 769, 637 cm−1; HRMS (ESI) m/z calcd for C17H14NO2 [M + H]+: 264.1025, found: 264.1030.
(Isoquinolin-1-yl)(4-methoxyphenyl)methanone (6p). White crystals, mp 67–69 °C. 1H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 5.7 Hz, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.95 (d, J = 8.8 Hz, 2H), 7.94 (d, J = 8.0 Hz, 1H), 7.81 (d, J = 5.7 Hz, 1H), 7.75 (dd, J1 = 8.3 Hz, J2 = 8.5 Hz, 1H), 7.61 (dd, J1 = 8.0 Hz, J2 = 8.3 Hz, 1H), 6.95 (d, J = 8.8 Hz, 2H), 3.88 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 193.40, 164.16, 157.08, 141.16, 136.67, 133.19 (2C), 130.72, 129.52, 128.19, 127.08, 126.32, 126.30, 122.30, 113.83 (2C), 55.58; IR (KBr film) 3008, 2936, 1651, 1600, 1576, 1412, 1249, 1154, 1022, 828, 745 cm−1.
(3,4-Dimethoxyphenyl)(isoquinolin-1-yl)methanone (6q). White crystals, mp 145–146 °C. 1H NMR (400 MHz, CDCl3) δ 8.60 (d, J = 5.7 Hz, 1H), 8.16 (d, J = 8.5 Hz, 1H), 7.93 (d, J = 8.1 Hz, 1H), 7.81 (d, J = 5.7 Hz, 1H), 7.76 (dd, J1 = 8.3 Hz, J2 = 8.5 Hz, 1H), 7.74 (s, 1H), 7.62 (dd, J1 = 8.1 Hz, J2 = 8.3 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H), 6.84 (d, J = 8.4 Hz, 1H), 3.97 (s, 3H), 3.94 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 193.45, 157.03, 154.04, 149.18, 141.12, 136.66, 130.76, 129.62, 128.20, 127.08, 127.02, 126.36, 126.29, 122.31, 111.52, 109.97, 56.17, 56.08; IR (KBr film) 3002, 2938, 1660, 1582, 1510, 1410, 1266, 1150, 1134, 1019, 825, 752 cm−1; HRMS (ESI) m/z calcd for C18H16NO3 [M + H]+: 294.1130, found: 294.1131.
(Isoquinolin-1-yl)(phenyl)methanone (6r). White crystals, mp 74–75 °C. 1H NMR (400 MHz, CDCl3) δ 8.61 (d, J = 5.6 Hz, 1H), 8.22 (d, J = 8.5 Hz, 1H), 8.00–7.91 (m, 3H), 7.81 (d, J = 5.6 Hz, 1H), 7.74 (dd, J1 = 8.2 Hz, J2 = 8.5 Hz, 1H), 7.64–7.58 (m, 2H), 7.48 (dd, J1 = 7.8 Hz, J2 = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 194.81, 156.44, 141.18, 136.71, 136.63, 133.73, 130.78 (2C), 130.74, 128.51, 128.37 (2C), 127.15, 126.42, 126.16, 122.65; IR (KBr film) 2978, 2922, 1663, 1596, 1449, 1402, 1335, 1317, 1279, 1252, 1153, 924, 709, 678, 639 cm−1.
(2-Bromophenyl)([1,3]dioxolo[4,5-g]isoquinolin-5-yl)metha-none (6s). White crystals, mp 180–181 °C. 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 5.4 Hz, 1H), 8.20 (s, 1H), 7.65–7.57 (m, 3H), 7.46 (dd, J1 = 7.7 Hz, J2 = 7.9 Hz, 1H), 7.36 (dd, J1 = 7.9 Hz, J2 = 8.1 Hz, 1H), 7.15 (s, 1H), 6.15 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 197.22, 151.43, 151.04, 150.38, 141.64, 140.61, 135.97, 133.08, 131.88, 130.72, 127.35, 124.68, 123.63, 120.46, 102.78, 102.53, 102.00; IR (KBr film) 2975, 2908, 1679, 1576, 1497, 1460, 1317, 1261, 1208, 1037, 951, 867, 723, 631 cm−1; HRMS (ESI) m/z calcd for C17H11NO3Br [M + H]+: 355.9922, found: 355.9917.
(4-Methoxyphenyl)(5,6,7-trimethoxyisoquinolin-1-yl)methan-one (6t). White crystals, mp 154–156 °C. 1H NMR (400 MHz, CDCl3) δ 8.48 (d, J = 5.5 Hz, 1H), 8.01 (d, J = 5.5 Hz, 1H), 7.94 (d, J = 8.3 Hz, 2H), 7.36 (s, 1H), 6.96 (d, J = 8.3 Hz, 2H), 4.08 (s, 3H), 4.03 (s, 3H), 3.93 (s, 3H), 3.88 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 193.72, 164.04, 154.58, 154.01, 146.71, 144.30, 139.44, 133.26 (2C), 129.73, 129.21, 123.77, 116.58, 113.75 (2C), 100.36, 61.64, 61.23, 56.10, 55.55; IR (KBr film) 2944, 2839, 1651, 1602, 1479, 1327, 1280, 1256, 1163, 1125, 1028, 935, 823, 702 cm−1. HRMS (ESI) m/z calcd for C20H20NO5 [M + H]+: 354.1341, found: 354.1336.
Preparation of anilines 7a–f
Nitro compound 6 (∼2 mmol) was dissolved in a mixed solvent of EtOH (18 mL) and H2O (2 mL). Iron powder (1.130 g, 20.23 mmol) and acetic acid (3.610 g, 60.12 mmol) were added into the solution. The mixture was then heated and stirred under N2 at 70 °C for 3–5 h. After the reaction was complete (checked by TLC, eluent: EtOAc/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the mixture was cooled down to room temperature and then filtered. The cake was washed twice with EtOH (2 × 10 mL). The filtrate was concentrated under vacuum to give a residue, which was dissolved in CH2Cl2 (30 mL). An aqueous solution of Na3PO4 (20 mL, 10% w/w) was added, the mixture was vigorously stirred for 5 min. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. Solvent was removed under vacuum to give crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/hexane = 1
2), the mixture was cooled down to room temperature and then filtered. The cake was washed twice with EtOH (2 × 10 mL). The filtrate was concentrated under vacuum to give a residue, which was dissolved in CH2Cl2 (30 mL). An aqueous solution of Na3PO4 (20 mL, 10% w/w) was added, the mixture was vigorously stirred for 5 min. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. Solvent was removed under vacuum to give crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Anilines 7a–f were thus obtained in 85%, 85%, 86%, 93%, 90% and 91% yields, respectively. Characterization data of compounds 7a–f are as follows:
2). Anilines 7a–f were thus obtained in 85%, 85%, 86%, 93%, 90% and 91% yields, respectively. Characterization data of compounds 7a–f are as follows:
(2-Aminophenyl)([1,3]dioxolo[4,5-g]isoquinolin-5-yl)metha-none (7a). Pale yellow crystals, mp 246–247 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.37 (d, J = 5.6 Hz, 1H), 7.78 (d, J = 5.6 Hz, 1H), 7.56 (br. s, 2H, NH2), 7.47 (s, 1H), 7.29 (dd, J1 = 8.2 Hz, J2 = 8.5 Hz, 1H), 7.03 (s, 1H), 6.92–6.87 (m, 2H), 6.37 (dd, J1 = 8.0 Hz, J2 = 8.3 Hz, 1H), 6.22 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 196.49, 156.03, 152.65, 150.85, 148.81, 140.43, 135.06, 134.73, 134.12, 122.04, 120.89, 116.90, 115.83, 114.16, 102.77, 102.21, 100.59; IR (KBr film) 3421, 3124, 3078, 2918, 1684, 1576, 1518, 1460, 1348, 1314, 1275, 1260, 1214, 1036, 946, 867, 788, 706, 636 cm−1; HRMS (ESI) m/z calcd for C17H13N2O3 [M + H]+: 293.0926, found: 293.0919.
(2-Amino-4,5-dimethoxy-phenyl)([1,3]dioxolo[4,5-g]isoqui-nolin-5-yl)methanone (7b). Pale yellow crystals, mp 182–183 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.37 (d, J = 5.6 Hz, 1H), 7.74 (d, J = 5.6 Hz, 1H), 7.56 (br. s, 2H, NH2), 7.41 (s, 1H), 7.05 (s, 1H), 6.48 (s, 1H), 6.40 (s, 1H), 6.19 (s, 2H), 3.80 (s, 3H), 3.43 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 193.52, 156.42, 156.34, 150.85, 150.76, 148.67, 140.36, 138.72, 134.74, 122.05, 120.74, 115.72, 108.04, 102.75, 102.17, 100.79, 98.61, 56.07, 55.41; IR (KBr film) 3415, 3310, 2916, 1645, 1585, 1545, 1507, 1465, 1403, 1264, 1221, 1204, 1150, 1110, 1037, 948, 873, 854, 734, 675 cm−1; HRMS (ESI) m/z calcd for C19H17N2O5 [M + H]+: 353.1137, found: 353.1142.
(6-Aminobenzo[d][1,3]dioxol-5-yl)([1,3]dioxolo[4,5-g]iso-quinolin-5-yl)methanone (7c). Pale yellow crystals, mp 240–241 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.37 (d, J = 5.6 Hz, 1H), 7.84 (br. s, 2H, NH2), 7.75 (d, J = 5.6 Hz, 1H), 7.44 (s, 1H), 7.02 (s, 1H), 6.46 (s, 1H), 6.21 (s, 2H), 6.19 (s, 1H), 5.91 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 193.22, 156.33, 153.63, 152.51, 150.77, 148.66, 140.41, 137.27, 134.70, 121.90, 120.70, 109.29, 108.08, 102.75, 102.17, 101.29, 100.70, 95.69; IR (KBr film) 3393, 3286, 2890, 1684, 1648, 1565, 1500, 1462, 1421, 1392, 1270, 1230, 1207, 1091, 1035, 944, 855, 839, 778, 669 cm−1; HRMS (ESI) m/z calcd for C18H13N2O5 [M + H]+: 337.0824, found: 337.0826.
(2-Aminophenyl)(6,7-dimethoxyisoquinolin-1-yl)methanone (7d). Pale yellow crystals, mp 170–171 °C. 1H NMR (400 MHz, CDCl3) δ 8.42 (d, J = 5.6 Hz, 1H), 7.57 (d, J = 5.6 Hz, 1H), 7.25 (s, 1H), 7.25–7.18 (m, 2H), 7.10 (s, 1H), 6.72 (d, J = 8.2 Hz, 1H), 6.60 (br. s, 2H, NH2), 6.46 (dd, J1 = 8.0 Hz, J2 = 8.2 Hz, 1H), 3.99 (s, 3H), 3.88 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 197.64, 155.57, 153.14, 152.11, 150.72, 140.34, 135.20, 134.79, 133.77, 122.06, 120.45, 117.46, 117.06, 115.57, 104.95, 103.94, 56.10, 56.04; IR (KBr film) 3412, 3302, 2967, 2844, 1672, 1645, 1477, 1282, 1212, 1127, 1090, 938, 854, 735, 674 cm−1; HRMS (ESI) m/z calcd for C18H17N2O3 [M + H]+: 309.1239, found: 309.1236.
(2-Amino-4,5-dimethoxyphenyl)(6,7-dimethoxyisoquinolin-1-yl)methanone (7e). Pale yellow crystals, mp 156–157 °C. 1H NMR (400 MHz, CDCl3) δ 8.44 (d, J = 5.5 Hz, 1H), 7.59 (d, J = 5.5 Hz, 1H), 7.33 (s, 1H), 7.13 (s, 1H), 6.68 (s, 1H), 6.56 (br. s, 2H, NH2), 6.20 (s, 1H), 4.04 (s, 3H), 3.93 (s, 3H), 3.89 (s, 3H), 3.50 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 195.00, 156.42, 155.80, 153.14, 150.65, 149.96, 140.32, 140.01, 133.86, 122.09, 120.34, 115.97, 109.84, 104.88, 104.21, 98.82, 56.38, 56.12, 56.10, 55.93; IR (KBr film) 3421, 3312, 2982, 2834, 1658, 1601, 1470, 1285, 1252, 1203, 1143, 1027, 937, 828, 689 cm−1; HRMS (ESI) m/z calcd for C20H21N2O5 [M + H]+: 369.1450, found: 369.1449.
(2-Aminophenyl)(5,6,7-trimethoxyisoquinolin-1-yl)methanone (7f). Pale yellow crystals, mp 105–106 °C. 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 5.7 Hz, 1H), 7.94 (d, J = 5.7 Hz, 1H), 7.27 (dd, J1 = 8.1 Hz, J2 = 7.8 Hz, 1H), 7.20 (d, J = 8.1 Hz, 1H), 7.10 (s, 1H), 6.73 (d, J = 8.2 Hz, 1H), 6.55 (br. s, 2H, NH2), 6.50 (dd, J1 = 8.2 Hz, J2 = 7.8 Hz, 1H), 4.07 (s, 3H), 4.02 (s, 3H), 3.89 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 197.48, 155.80, 154.30, 152.05, 146.79, 144.28, 139.82, 135.27, 134.87, 129.00, 123.14, 117.48, 117.04, 115.73, 115.69, 100.23, 61.62, 61.23, 56.10; IR (KBr, film) 3457, 3310, 2958, 2922, 2851, 1668, 1619, 1584, 1547, 1475, 1262, 1203, 1157, 1122, 1049, 940, 749, 657 cm−1; HRMS (ESI): calcd for C19H19N2O4 [M + H]+: 339.1345, found: 339.1337.
Preparation of oxoaporphines 1a–f
Aniline 7 (∼1 mmol) was dissolved in an aqueous solution (5 mL, 10% w/w) of sulfuric acid. The solution was cooled to 0–8 °C with an ice bath. A freshly prepared aqueous solution (1 mL, 1 M) of sodium nitrite was dropwise added over 5 min. Stirring was continued at 0–8 °C for 15 min to give a clear pale orange aqueous solution of diazonium salt, which was immediately used below. Copper powder (0.640 g, 10.07 mmol) was added into an aqueous solution (5 mL, 10% w/w) of sulfuric acid. The suspension was warmed and stirred at 60 °C. The above aqueous solution of diazonium salt was dropwise added over 10 min. The mixture was further stirred at 60 °C for 2–4 h. After the reaction was complete (checked by TLC, eluent: EtOAc/hexane = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the mixture was cooled to room temperature. CH2Cl2 (30 mL) and concentrated ammonia (10 mL, 25% w/w) were added, and the mixture was then vigorously stirred for 5 min. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. The organic solution was concentrated under vacuum to give crude product, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1
1), the mixture was cooled to room temperature. CH2Cl2 (30 mL) and concentrated ammonia (10 mL, 25% w/w) were added, and the mixture was then vigorously stirred for 5 min. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. The organic solution was concentrated under vacuum to give crude product, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Compounds 1a–f were thus obtained in 86%, 77%, 82%, 80%, 79% and 81% yields, respectively. Characterization data of oxoaporphines 1a–f are as follows:
4). Compounds 1a–f were thus obtained in 86%, 77%, 82%, 80%, 79% and 81% yields, respectively. Characterization data of oxoaporphines 1a–f are as follows:
Liriodenine (1a). Pale yellow crystals, mp 283–284 °C (lit.3d mp 282 °C). 1H NMR (400 MHz, CDCl3-TFA) δ 8.78 (d, J = 5.9 Hz, 1H), 8.66 (d, J = 8.1 Hz, 1H), 8.43 (d, J = 8.0 Hz, 1H), 8.36 (d, J = 5.9 Hz, 1H), 7.93 (dd, J1 = 7.8 Hz, J2 = 8.1 Hz, 1H), 7.67 (dd, J1 = 7.8 Hz, J2 = 8.0 Hz, 1H), 7.48 (s, 1H), 6.63 (s, 2H); 13C NMR (100 MHz, CDCl3-TFA) δ 176.51, 158.50, 152.03, 143.26, 137.16, 134.99, 133.94, 132.15, 130.37, 129.11, 128.27, 128.08, 126.79, 122.36, 108.14, 105.20, 103.66; IR (KBr film) 3034, 2920, 1660, 1597, 1575, 1469, 1486, 1421, 1309, 1260, 1229, 1206, 1125, 1041, 1014, 958, 869, 778, 689, 568 cm−1; HRMS (ESI) m/z calcd for C17H10NO3 [M + H]+: 276.0661, found: 276.0665.
Dicentrinone (1b). Pale yellow crystals, mp 292–293 °C (lit.4b mp 293–295 °C). 1H NMR (400 MHz, CDCl3-TFA) δ 8.74 (d, J = 5.6 Hz, 1H), 8.33 (d, J = 5.6 Hz, 1H), 8.05 (s, 1H), 7.82 (s, 1H), 7.43 (s, 1H), 6.63 (s, 2H), 4.10 (s, 3H), 4.04 (s, 3H); 13C NMR (100 MHz, CDCl3-TFA) δ 174.96, 158.63, 157.13, 152.03, 150.56, 143.27, 135.07, 133.57, 129.04, 126.63, 122.59, 121.78, 109.71, 109.64, 108.06, 105.40, 103.58, 56.46, 56.16; IR (KBr film) 3002, 2918, 2839, 1640, 1593, 1577, 1513, 1476, 1453, 1425, 1368, 1344, 1307, 1276, 1253, 1217, 1136, 1056, 1001, 966, 855, 777, 676, 636, 577 cm−1; HRMS (ESI) m/z calcd for C19H14NO5 [M + H]+: 336.0872, found: 336.0870.
Cassameridine (1c). Pale yellow crystals, mp 301–302 °C (lit.5a mp 300 °C). 1H NMR (400 MHz, CDCl3-TFA) δ 8.72 (d, J = 6.2 Hz, 1H), 8.35 (d, J = 6.2 Hz, 1H), 8.11 (s, 1H), 7.76 (s, 1H), 7.45 (s, 1H), 6.61 (s, 2H), 6.23 (s, 2H); 13C NMR (100 MHz, CDCl3-TFA) δ 174.76, 158.64, 156.46, 152.30, 150.01, 143.18, 134.79, 133.71, 130.79, 126.58, 124.25, 121.60, 108.03, 107.52, 107.12, 105.34, 103.55, 103.54; IR (KBr film) 3029, 2917, 2848, 1648, 1611, 1572, 1503, 1482, 1467, 1444, 1376, 1294, 1268, 1240, 1123, 1101, 1079, 1040, 965, 899, 818, 604; HRMS (ESI) m/z calcd for C18H10NO5 [M + H]+: 320.0559, found: 320.0560.
Lysicamine (1d). Pale yellow crystals, mp 206–207 °C (lit.26 mp 207–208 °C). 1H NMR (400 MHz, CDCl3) δ 9.17 (d, J = 8.2 Hz, 1H), 8.90 (d, J = 5.3 Hz, 1H), 8.59 (d, J = 7.8 Hz, 1H), 7.80 (d, J = 5.3 Hz, 1H), 7.78 (dd, J1 = 7.8 Hz, J2 = 8.0 Hz, 1H), 7.57 (dd, J1 = 8.0 Hz, J2 = 8.2 Hz, 1H), 7.22 (s, 1H), 4.10 (s, 3H), 4.02 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 182.71, 156.85, 152.08, 152.05, 145.39, 145.05, 135.50, 134.34, 132.11, 128.90, 128.80, 128.45, 123.58, 122.11, 119.84, 106.48, 60.67, 56.23; IR (KBr film) 2968, 2920, 2837, 1662, 1610, 1552, 1512, 1465, 1415, 1375, 1260, 1235, 1110, 1079, 1041, 962, 887, 792, 605; HRMS (ESI) m/z calcd for C18H14NO3 [M + H]+: 292.0974, found: 292.0970.
Oxoglaucine (1e). Pale yellow crystals, mp 225–226 °C (lit.27 mp 227–229 °C). 1H NMR (400 MHz, CDCl3) δ 8.82 (d, J = 5.2 Hz, 1H), 8.72 (s, 1H), 7.95 (s, 1H), 7.69 (d, J = 5.2 Hz, 1H), 7.11 (s, 1H), 4.06 (s, 3H), 4.04 (s, 3H), 4.03 (s, 3H), 3.99 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 181.28, 156.60, 153.65, 150.91, 149.39, 145.38, 144.81, 135.25, 129.05, 126.76, 123.30, 121.53, 119.73, 110.13, 109.67, 105.98, 60.57, 56.18, 56.17, 55.98; IR (KBr film) 2979, 2924, 2841, 1662, 1600, 1554, 1501, 1463, 1403, 1378, 1292, 1266, 1246, 1217, 1100, 1081, 1037, 975, 890, 827, 739, 624; HRMS (ESI) m/z calcd for C20H18NO5 [M + H]+: 352.1185, found: 352.1187.
O-Methylmoschatoline (1f). Pale yellow crystals, mp 184–185 °C (lit.10b mp 186–188 °C). 1H NMR (400 MHz, CDCl3) δ 9.00 (d, J = 8.2 Hz, 1H), 8.86 (d, J = 5.2 Hz, 1H), 8.46 (d, J = 7.7 Hz, 1H), 8.11 (d, J = 5.2 Hz, 1H), 7.64 (dd, J1 = 8.2 Hz, J2 = 7.9 Hz, 1H), 7.42 (dd, J1 = 7.7 Hz, J2 = 7.9 Hz, 1H), 4.11 (s, 3H), 4.03 (s, 3H), 4.00 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 182.59, 156.48, 148.45, 147.25, 145.43, 144.52, 134.51, 134.34, 131.38, 131.01, 128.85, 128.08, 127.64, 122.77, 119.12, 115.56, 61.81, 61.46, 60.99; IR (KBr, film) 2956, 2923, 2853, 1660, 1579, 1466, 1393, 1311, 1261, 1205, 1092, 970, 800, 723, 612 cm−1; HRMS (ESI): calcd for C19H16NO4 [M + H]+: 322.1079, found: 322.1072.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 20972048, 20172015) for the financial support of this work.
Notes and references
-
(a) M. Shamma and H. Guinaudeau, Nat. Prod. Rep., 1986, 3, 345 RSC;
(b) M. Shamma and H. Guinaudeau, Nat. Prod. Rep., 1984, 1, 201 RSC.
-
(a) D. L. Custodio and V. F. da Vegia Junior, RSC Adv., 2014, 4, 21864 RSC;
(b) N. K. N. A. Zawawi, N. Ahmat, R. Ahmad, F. M. Jaafar and N. A. Ghani, Biochem. Syst. Ecol., 2012, 43, 7 CrossRef;
(c) Z.-Y. Liang, X.-S. Yang, W. Ye and X.-J. Hao, Chin. J. Chem., 2005, 23, 895 CrossRef;
(d) C. Xu, Y. Zhang and R. X. Tan, Fitoterapia, 2004, 75, 239 CrossRef PubMed;
(e) L. Killer, F. G. Vogt, A. J. Freyer, M. D. Menachery and C. M. Adelman, J. Nat. Prod., 2003, 66, 115 CrossRef PubMed;
(f) T.-J. Hsich, F.-R. Chang, Y.-C. Chia, C.-Y. Chen, H.-C. Liu, H.-F. Chiu and Y.-C. Wu, J. Nat. Prod., 2001, 64, 1157 CrossRef;
(g) W.-L. Lo, F.-R. Chang and Y.-C. Wu, J. Chin. Chem. Soc., 2000, 47, 1251 CrossRef;
(h) N. Xie, Z. H. Cheng and X. G. Yu, Chin. Chem. Lett., 1999, 10, 675 Search PubMed;
(i) F.-R. Chang, Y.-C. Chao, C.-M. Teng and Y.-C. Wu, J. Nat. Prod., 1998, 61, 863 CrossRef PubMed;
(j) M. D. Menachery, H. M. Mandell, S. A. DeSaw, N. A. DeAntonio, A. J. Freyer and L. B. Killmer, J. Nat. Prod., 1997, 60, 1328 CrossRef;
(k) J.-J. Chen, I.-L. Tsai and I.-S. Chen, J. Nat. Prod., 1996, 59, 156 CrossRef;
(l) G.-G. Harrigan, A. A. L. Gunatilaka, D. G. I. Kingston, G. W. Chan and R. K. Johnson, J. Nat. Prod., 1994, 57, 68 CrossRef PubMed.
-
(a) M. Castro-Moreno, C. L. Tinoco-Ojanguren, M. R. Cruz-Ortega and A. R. Gonzalez-Esquinca, J. Plant Res., 2013, 126, 529 CrossRef PubMed;
(b) I. de la Cruz Chacon and A. R. Gonzalez-Esquinca, Nat. Prod. Res., 2012, 26, 42 CrossRef PubMed;
(c) T. Kametani, H. Terasawa, M. Ihara and J. Iriarte, Phytochemistry, 1975, 14, 1884 CrossRef;
(d) M. A. Buchanan and E. E. Dickey, J. Org. Chem., 1960, 25, 1389 CrossRef.
-
(a) W. N. N. Wan Othman, Y. Sivasothy, S. Y. Liew, J. Mohamad, M. A. Nafiah, K. Ahmad, M. Litaudon and K. Awang, Phytochem. Lett., 2017, 21, 230 CrossRef;
(b) K.-S. Chen, Y.-C. Wu, C.-M. Teng, F.-N. Ko and T.-S. Wu, J. Nat. Prod., 1997, 60, 645 CrossRef PubMed;
(c) E. M. K. Wijeratne, Y. Hatanaka, T. Kikuchi, Y. Tezuka and A. A. L. Gunatilaka, Phytochemistry, 1996, 42, 1703 CrossRef;
(d) C.-C. Chen, C.-F. Lin, Y.-L. Huang, F.-N. Ko and C. M. Teng, J. Nat. Prod., 1995, 58, 1423 CrossRef.
-
(a) M. P. Cava, K. V. Rao, B. Douglas and J. A. Weisbach, J. Org. Chem., 1968, 33, 2443 CrossRef;
(b) Y.-C. Wu, Y.-C. Chao, F.-R. Chang and Y.-Y. Chen, Phytochemistry, 1997, 46, 181 CrossRef;
(c) D. Y. Si, S. X. Zhao and J. Z. Deng, J. Nat. Prod., 1992, 55, 828 CrossRef.
-
(a) H. Omar, N. M. Hashim, A. Zajmi, N. Nordin, S. I. Abdelwahab, A. H. S. Azizan, A. H. A. Hadi and H. M. Ali, Molecules, 2013, 18, 8994 CrossRef PubMed;
(b) E. V. Costa, M. L. B. Pinheiro, A. Barison, F. R. Campos, M. J. Salvador, B. H. L. N. S. Maia, E. C. Cabral and M. N. Eberlin, J. Nat. Prod., 2010, 73, 1180 CrossRef PubMed;
(c) M. D. Menachery and D. L. Edgren, J. Nat. Prod., 1988, 51, 1283 CrossRef.
-
(a) F.-R. Chang, J.-L. Wei, C.-M. Teng and Y.-C. Wu, J. Nat. Prod., 1998, 61, 1457 CrossRef PubMed;
(b) Y.-Y. Chen, F.-R. Chang and Y.-C. Wu, J. Nat. Prod., 1996, 59, 904 CrossRef;
(c) E. Martinez, A. M. P. de Diaz and P. Joseph-Nathan, Planta Med., 1988, 54, 361 CrossRef PubMed.
-
(a) B. Liu, L. Jian, G. Chen, X. Song, C. Han and J. Wang, Chem. Nat. Compd., 2014, 49, 1172 CrossRef;
(b) D. S. Swaffar, C. J. Holley, R. W. Fitch, K. R. Elkin, C. Zhang, J. P. Sturgill and M. D. Menachery, Planta Med., 2012, 78, 230 CrossRef PubMed;
(c) J. Sichaem, T. Ruksilp, W. Worawalai, P. Siripong, S. Khumkratok and S. Tip-pyang, Fitoterapia, 2011, 82, 422 CrossRef PubMed;
(d) S. Fujita, M. Ninomiya, M. Efdi, K. Ohguchi, Y. Nozawa and M. Koketsu, Nat. Prod. Res., 2010, 24, 1630 CrossRef PubMed.
- K. K. Tan, T. J. Khoo, M. Rajagopal and C. Wiart, Nat. Prod. Res., 2015, 29, 2346 CrossRef PubMed.
-
(a) R. M. Brash and A. T. Sneden, J. Nat. Prod., 1983, 46, 437 CrossRef;
(b) M. P. Cava, K. T. Buck, I. Noguchi, M. Srinivasan, M. G. Rao and A. I. DaRocha, Tetrahedron, 1975, 31, 1667 CrossRef.
-
(a) Z.-H. Li, J. Gao, P.-H. Hu and J.-P. Xiong, Oncol. Lett., 2017, 14, 1979 CrossRef PubMed;
(b) N. L. Andreazza, C. C. de Lourenco, A. J. Hernandez-Tasco, M. L. B. Pinheiro, M. E. A. Stefanello, E. V. Costa and M. J. Salvador, J. Photochem. Photobiol., B, 2016, 160, 154 CrossRef PubMed;
(c) M. Arita, S. Philipov and A. S. Galabov, Microbiol. Immunol., 2015, 59, 338 CrossRef PubMed;
(d) Y.-B. Wei, Y.-X. Li, H. Song and X.-J. Feng, J. Enzyme Inhib. Med. Chem., 2014, 29, 722 CrossRef PubMed;
(e) C.-C. Wu, C.-L. Wu, S.-L. Huang and H.-T. Chang, Wood Sci. Technol., 2012, 46, 737 CrossRef;
(f) E. V. Costa, M. L. B. Pinheiro, A. D. L. de Souza, A. Barison, F. R. Campos, R. H. Valdez, T. Ueda-Nakamura, B. P. D. Filho and C. V. Nakamura, Molecules, 2011, 16, 9714 CrossRef PubMed;
(g) E. V. Costa, M. L. B. Pinheiro, C. M. Xavier, J. R. A. Silva, A. C. F. Amaral, A. D. L. Souza, A. Barison, F. R. Campos, A. G. Ferreira, G. M. C. Machado and L. L. P. Leon, J. Nat. Prod., 2006, 69, 292 CrossRef PubMed;
(h) R. Saleem, M. Ahmed, S. I. Ahmed, M. Azeem, R. A. Khan, N. Rasool, H. Saleem, F. Noor and S. Faizi, Phytother. Res., 2005, 19, 881 CrossRef PubMed;
(i) T.-J. Hsieh, T.-Z. Liu, C.-L. Chern, D.-A. Tsao, F.-J. Lu, Y.-H. Syu, P.-Y. Hsieh, H.-S. Hu, T.-T. Chang and C.-H. Chen, Food Chem. Toxicol., 2005, 43, 1117 CrossRef PubMed;
(j) S. H. Woo, M. C. Reynolds, N. J. Sun, J. M. Cassady and R. M. Snapka, Biochem. Pharmacol., 1997, 54, 467 CrossRef PubMed.
-
(a) A. F. C. Rossini, A. C. A. Muraca, G. A. Casagrande and C. Raminelli, J. Org. Chem., 2015, 80, 10033 CrossRef PubMed;
(b) B. Melzer and F. Bracher, Org. Biomol. Chem., 2015, 13, 7664 RSC;
(c) T.-H. Chuang, C.-F. Li, H.-Z. Lee and Y.-C. Wen, J. Org. Chem., 2013, 78, 4974 CrossRef PubMed;
(d) O. V. Sigh, V.-J. Huang, C.-H. Chen and S.-S. Lee, Tetrahedron Lett., 2007, 48, 8166 CrossRef;
(e) K. Orito, S. Uchiito, Y. Satoh, T. Tatsuzawa, R. Harade and M. Tokuda, Org. Lett., 2000, 2, 307 CrossRef PubMed;
(f) N. Atanes, L. Castedo, E. Guitian, C. Saa, J. M. Saa and R. Suau, J. Org. Chem., 1991, 56, 2984 CrossRef;
(g) L. Castedo, C. Saa, J. M. Saa and R. Suau, J. Org. Chem., 1982, 47, 513 CrossRef;
(h) P. Wiriyachitra and M. P. Cava, J. Org. Chem., 1977, 42, 2274 CrossRef PubMed;
(i) M. P. Cava, I. Noguchi and K. T. Buck, J. Org. Chem., 1973, 38, 2394 CrossRef.
- J. Yang, J. Dong, X. Lu, Q. Zhang, W. Ding and X. Shi, Chin. J. Chem., 2012, 30, 2827 CrossRef.
-
(a) A. Bischler and B. Napieralski, Ber. Dtsch. Chem. Ges., 1893, 26, 1903 CrossRef;
(b) W. M. Whaley and T. R. Govindachari, Preparation of 3,4-dihydro-isoquinolines and related compounds by Bischler–Napieralski reaction, in Organic Reactions, ed. R. Adams, John Wiley and Sons, New York, 1951, vol. VI, p. 74 Search PubMed.
-
(a) A. Agrawal and P. G. Tratnyek, Environ. Sci. Technol., 1996, 30, 153 CrossRef;
(b) M. d. F. Pereira and V. Thiery, Org. Lett., 2012, 14, 4754 CrossRef PubMed.
- P. H. Leake, Chem. Rev., 1956, 56, 27 CrossRef.
-
(a) Y.-F. Zhu, K. Wilcoxen, T. Gross, P. Connors, N. Strack, R. Gross, C. Q. Huang, J. R. McCarthy, Q. Xie, N. Ling and C. Chen, Bioorg. Med. Chem. Lett., 2003, 13, 1927 CrossRef PubMed;
(b) Y. Qian, M. Ahmad, S. Chen, P. Gillespie, N. Le, F. Mennona, S. Mischke, S.-S. So, H. Wang, C. Burghardt, S. Tannu, K. Conde-Knape, J. Kochan and D. Bolin, Bioorg. Med. Chem. Lett., 2011, 21, 6264 CrossRef PubMed.
- G. R. Lenz and C. Costanza, J. Org. Chem., 1988, 53, 1176 CrossRef.
- R.-Y. Kuo, F.-R. Chang, C.-C. Wu, R. Patnam, W.-Y. Wang, Y.-C. Du and Y.-C. Wu, Bioorg. Med. Chem. Lett., 2003, 13, 2789 CrossRef PubMed.
- N. H. Martin and C. W. Jefford, Helv. Chim. Acta, 1982, 65, 762 CrossRef.
- H. Gan, Y. Lu, Y. Huang, L. Ni, J. Xu, H. Yao and X. Wu, Tetrahedron Lett., 2011, 52, 1320 CrossRef.
-
(a) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef PubMed;
(b) S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef PubMed;
(c) S. D. McCann and S. S. Stahl, Acc. Chem. Res., 2015, 48, 1756 CrossRef PubMed;
(d) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC.
- T. H. Trieu, J. Dong, Q. Zhang, B. Zheng, T.-Z. Meng, X. Lu and X.-X. Shi, Eur. J. Org. Chem., 2013, 3271 CrossRef.
- M. D. Garcia, A. J. Wilson, D. P. G. Emmerson and P. R. Jenkins, Chem. Commun., 2006, 2586 RSC.
-
(a) R. Shankar, S. S. More, M. V. Madhubabu, N. Vembu and V. K. S. Kumar, Synlett, 2012, 23, 1013 CrossRef;
(b) M. V. Madhubabu, R. Shankar, R. Akula, U. K. S. Kumar and M. V. B. Rao, Pharma Chem., 2014, 6, 50 Search PubMed.
- J. W. Skiles, J. M. Saa and M. P. Cava, Can. J. Chem., 1979, 57, 1642 CrossRef.
- J. Cohen, W. Von Langenthal and W. I. Taylor, J. Org. Chem., 1961, 26, 4143 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05338c |
|
| This journal is © The Royal Society of Chemistry 2018 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford amides 4a–f. Subsequently, treatment of compounds 4a–f with 3.0 equiv. of POCl3 in anhydrous acetonitrile at reflux gave 1-Bn-DHIQs 5a–f via Bischler–Napieralski cyclization.14 It was observed that compounds 5a–f and compounds 5′a–f were interchangeable at room temperature, we failed to separate them by chromatography due to rapid tautomerization between imines (5a–f) and enamines (5′a–f), so they were used as such for the next step without separation; when the tautomeric mixture of 5a–f and 5′a–f were treated with 0.1 equiv. (10 mol%) of anhydrous CuBr2 and 1.0 equiv. of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in dimethyl sulfoxide (DMSO) at 35 °C under an open air (O2) atmosphere, Cu-catalyzed tandem benzylic oxidation and aromatization occurred smoothly in one-pot to afford 1-Bz-IQs 6a–f in good yields over two steps (from 4a–f). Nitro (NO2) groups of compounds 6a–f could be rapidly reduced by acid-activated iron;15 when compounds 6a–f were treated with 10 equiv. of iron powder and 30 equiv. of HOAc at 70 °C under N2 atmosphere in aqueous ethanol (EtOH/H2O = 9
1) to afford amides 4a–f. Subsequently, treatment of compounds 4a–f with 3.0 equiv. of POCl3 in anhydrous acetonitrile at reflux gave 1-Bn-DHIQs 5a–f via Bischler–Napieralski cyclization.14 It was observed that compounds 5a–f and compounds 5′a–f were interchangeable at room temperature, we failed to separate them by chromatography due to rapid tautomerization between imines (5a–f) and enamines (5′a–f), so they were used as such for the next step without separation; when the tautomeric mixture of 5a–f and 5′a–f were treated with 0.1 equiv. (10 mol%) of anhydrous CuBr2 and 1.0 equiv. of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in dimethyl sulfoxide (DMSO) at 35 °C under an open air (O2) atmosphere, Cu-catalyzed tandem benzylic oxidation and aromatization occurred smoothly in one-pot to afford 1-Bz-IQs 6a–f in good yields over two steps (from 4a–f). Nitro (NO2) groups of compounds 6a–f could be rapidly reduced by acid-activated iron;15 when compounds 6a–f were treated with 10 equiv. of iron powder and 30 equiv. of HOAc at 70 °C under N2 atmosphere in aqueous ethanol (EtOH/H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), reduction of nitro groups took place smoothly to produce anilines 7a–f in high yields. Finally, compounds 7a–f were first treated with 1.0 equiv. of NaNO2 at 0–8 °C to produce diazonium intermediates, which were then immediately treated with 10 equiv. of copper powder at 60 °C in an aqueous H2SO4 (10% w/w) solution, Pschorr cyclization16 happened rapidly to afford oxoaporphines 1a–f in good yields. Thus, the targeted oxoaporphine alkaloids liriodenine 1a, dicentrinone 1b, cassameridine 1c, lysicamine 1d, oxoglaucine 1e and O-methyl-moschatoline 1f were synthesized from aryl aldehydes via 7 steps in 39%, 26%, 29%, 38%, 31% and 41% overall yields, respectively.
1), reduction of nitro groups took place smoothly to produce anilines 7a–f in high yields. Finally, compounds 7a–f were first treated with 1.0 equiv. of NaNO2 at 0–8 °C to produce diazonium intermediates, which were then immediately treated with 10 equiv. of copper powder at 60 °C in an aqueous H2SO4 (10% w/w) solution, Pschorr cyclization16 happened rapidly to afford oxoaporphines 1a–f in good yields. Thus, the targeted oxoaporphine alkaloids liriodenine 1a, dicentrinone 1b, cassameridine 1c, lysicamine 1d, oxoglaucine 1e and O-methyl-moschatoline 1f were synthesized from aryl aldehydes via 7 steps in 39%, 26%, 29%, 38%, 31% and 41% overall yields, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the solution was concentrated under vacuum to give crude product as yellow solid, which was triturated in aqueous methanol (MeOH/H2O = 9
2), the solution was concentrated under vacuum to give crude product as yellow solid, which was triturated in aqueous methanol (MeOH/H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and was then filtered by suction. Compounds 2a–c were thus obtained in 89%, 90% and 91% yields, respectively. Characterization data of compounds 2a–c are as follows:
1), and was then filtered by suction. Compounds 2a–c were thus obtained in 89%, 90% and 91% yields, respectively. Characterization data of compounds 2a–c are as follows:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the reaction mixture was transferred into a separatory funnel. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (20 mL). The organic extracts were combined, dried over anhydrous MgSO4, and then concentrated under vacuum to give crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/hexane = 1
3), the reaction mixture was transferred into a separatory funnel. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (20 mL). The organic extracts were combined, dried over anhydrous MgSO4, and then concentrated under vacuum to give crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Amides 4a–f were thus obtained in 85%, 62%, 63%, 83%, 71% and 84% yields, respectively. Characterization data of compounds 4a–f are as follows:
4). Amides 4a–f were thus obtained in 85%, 62%, 63%, 83%, 71% and 84% yields, respectively. Characterization data of compounds 4a–f are as follows:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the solution was concentrated under vacuum to dryness, the residue was dissolved in CH2Cl2 (50 mL). An aqueous solution of K2CO3 (20 mL, 15% w/w) was added. After the mixture was vigorously stirred for 5 min, two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (20 mL). The organic extracts were combined, dried over anhydrous MgSO4, and then concentrated under vacuum to give crude solid product as a tautomeric mixture of 1-benzyl-3,4-dihydroisoquinoline 5 and enamine 5′, which was used as such for the next step.
1), the solution was concentrated under vacuum to dryness, the residue was dissolved in CH2Cl2 (50 mL). An aqueous solution of K2CO3 (20 mL, 15% w/w) was added. After the mixture was vigorously stirred for 5 min, two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (20 mL). The organic extracts were combined, dried over anhydrous MgSO4, and then concentrated under vacuum to give crude solid product as a tautomeric mixture of 1-benzyl-3,4-dihydroisoquinoline 5 and enamine 5′, which was used as such for the next step.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), a dilute ammonia aqueous solution (5% w/w, 50 mL) and CH2Cl2 (40 mL) were added into the mixture. Two phases were separated, and the aqueous phase was twice extracted again with CH2Cl2 (2 × 30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. Removal of solvent by vacuum distillation gave crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1
4), a dilute ammonia aqueous solution (5% w/w, 50 mL) and CH2Cl2 (40 mL) were added into the mixture. Two phases were separated, and the aqueous phase was twice extracted again with CH2Cl2 (2 × 30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. Removal of solvent by vacuum distillation gave crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to afford pure 1-benzoyl-isoquinoline 6 as crystals in a yield (over two steps) as indicated in Table 1. Characterization data of 1-benzoyl-isoquinolines 6a–t are as follows:
10) to afford pure 1-benzoyl-isoquinoline 6 as crystals in a yield (over two steps) as indicated in Table 1. Characterization data of 1-benzoyl-isoquinolines 6a–t are as follows:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the mixture was cooled down to room temperature and then filtered. The cake was washed twice with EtOH (2 × 10 mL). The filtrate was concentrated under vacuum to give a residue, which was dissolved in CH2Cl2 (30 mL). An aqueous solution of Na3PO4 (20 mL, 10% w/w) was added, the mixture was vigorously stirred for 5 min. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. Solvent was removed under vacuum to give crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/hexane = 1
2), the mixture was cooled down to room temperature and then filtered. The cake was washed twice with EtOH (2 × 10 mL). The filtrate was concentrated under vacuum to give a residue, which was dissolved in CH2Cl2 (30 mL). An aqueous solution of Na3PO4 (20 mL, 10% w/w) was added, the mixture was vigorously stirred for 5 min. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. Solvent was removed under vacuum to give crude product as pale yellow solid, which was purified by flash chromatography (eluent: EtOAc/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Anilines 7a–f were thus obtained in 85%, 85%, 86%, 93%, 90% and 91% yields, respectively. Characterization data of compounds 7a–f are as follows:
2). Anilines 7a–f were thus obtained in 85%, 85%, 86%, 93%, 90% and 91% yields, respectively. Characterization data of compounds 7a–f are as follows:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the mixture was cooled to room temperature. CH2Cl2 (30 mL) and concentrated ammonia (10 mL, 25% w/w) were added, and the mixture was then vigorously stirred for 5 min. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. The organic solution was concentrated under vacuum to give crude product, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1
1), the mixture was cooled to room temperature. CH2Cl2 (30 mL) and concentrated ammonia (10 mL, 25% w/w) were added, and the mixture was then vigorously stirred for 5 min. Two phases were separated, and the aqueous phase was extracted again with CH2Cl2 (30 mL). The organic extracts were combined, and dried over anhydrous MgSO4. The organic solution was concentrated under vacuum to give crude product, which was purified by flash chromatography (eluent: EtOAc/CH2Cl2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Compounds 1a–f were thus obtained in 86%, 77%, 82%, 80%, 79% and 81% yields, respectively. Characterization data of oxoaporphines 1a–f are as follows:
4). Compounds 1a–f were thus obtained in 86%, 77%, 82%, 80%, 79% and 81% yields, respectively. Characterization data of oxoaporphines 1a–f are as follows:






