Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oxygen vacancies of the TiO2 nano-based composite photocatalysts in visible light responsive photocatalysis
Buanya Beryl Adormaa†
,
Williams Kweku Darkwah†
 * and
Yanhui Ao
* and
Yanhui Ao
 *
*
Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Ministry of Education, Environmental Engineering Department, College of Environment, Hohai University, Nanjing, China. E-mail: andyao@hhu.edu.cn; wkdarkwah@hhu.edu.cn; williams.darkwah@stu.ucc.edu.gh; Tel: +8613770610843
First published on 4th October 2018
Abstract
The TiO2 nano-based composite photocatalyst is best known for application in solving the recent issues related to energy and environmental purification. Due to the low cost, nontoxicity, chemical stability and high efficiency of TiO2, it is unquestionably one of the most considered materials in environmental treatment. In this systematic review, we reveal the outstanding potential of oxygen vacancy in photocatalysis, and discuss the contemporary advancement in the photocatalytic activities, productivity, preparation methods and oxygen vacancy of the TiO2 nano-based composite photocatalyst for environmental treatment and energy as well as wastewater treatment. This exposé is anticipated to enlighten researchers and engineers on the specific management and assessment of the environment, which warrants prospective research into developing appropriate mechanisms for energy, wastewater treatment and environmental purification.
1. Introduction
Currently, research efforts have shifted toward semiconductor photocatalysts such as TiO2, ZnO, g-C3N4, etc., due to the ability of these metals to convert pollutants into CO2 and H2O for environmental applications such as biofuel production and wastewater treatment. For modest and cost-effective treatment, additional efficient photocatalysts are highly preferred to parallel the extensively used TiO2. The field of water photocatalysis has experienced tremendous growth, following the discovery of photocatalytic H2 production in the 1980s and the photocatalysis and hydrophilicity of TiO2 films by Honda and Fujishima in the 1990s, and other scientists in the early 70s.67–72 Further studies have revealed that industrial applications in this area have been wonderfully accomplished since the early 90s based on the outcomes from these basic research efforts.6,105–107Photocatalysis is the speeding up of oxidation and reduction reactions, brought about through the activation of a catalyst consisting of a semiconductor either alone or in combination with metal/organic/organometallic promoters, through light absorption and the subsequent charge and/or energy transfer, which can lead to the transformation of a pollutant. It must be noted that during the photocatalytic reaction, two actions must occur simultaneously in order for the successful production of reactive oxidizing species to occur. Typically, the first involves the oxidation of dissociatively adsorbed H2O by photogenerated holes, the second involves the reduction of an electron acceptor (typically dissolved oxygen) by photoexcited electrons; these reactions lead to the production of a hydroxyl and superoxide radical anion, respectively.68,69,97,103,104 In the area of photocatalysis, energy saving green tools are the ultimate, in accordance with photoinduced water cleavage to TiO2 electrodes, a phenomenon discovered by the pioneers in the field.1 It is believed that TiO2 photocatalysis is currently one of the best in the recent research in nanoscience and nanotechnology.
There have been many successful reports on the potential applications of TiO2 photocatalysts in water and environmental purification and management in the past few decades. In solving the recent issues related to energy and environmental purification, heterogeneous metal oxide semiconductor photocatalysts are best known. Due to the low-cost, nontoxicity, chemical stability and high efficiency of TiO2, it is unquestionably one of the most considered materials by researchers in the field of photocatalysis. However, the low quantum efficiency of TiO2 in photocatalytic mechanisms and the ineffective use of visible light for H2 harvesting, which mainly comes from its high recombination rate of photogenerated electron–hole pairs and wide band gap, have become problematic in prospective applications.9,73,74
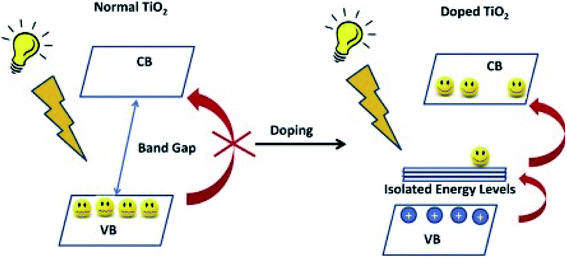
Scientists in the field have adopted various approaches for the improvement of the photocatalytic efficacy of TiO2. These strategies can be summarized as morphological modifications, such as increased surface area and porosity, or as chemical modifications, by the incorporation of additional components into the TiO2 structure. Three basic strategies have been adopted for the structural modification, namely, doping with metallic/non-metallic elements or co-doping of metallic and non-metallic elements,9,108–110 modification through the introduction of defects such as oxygen vacancies and Ti3+ in the band gap,111,112 and surface modification by treatment techniques.113–115 Currently, the attention is centered on oxygen vacancies, which occur naturally in oxides, due to the vital role they play in the physical characteristics of materials; oxygen vacancies may be positive or negative. Oxygen vacancies occur when the number of oxygens in a particular compound is less than what it is supposed to be to make a perfect crystal lattice. This results in materials such as ZnFe2O4, where ZnO and Fe2O3 are chosen as starting compounds.
This review is centered on the ability and effectiveness of the prospective applications of oxygen vacancies and photocatalysis using TiO2 composites for energy, wastewater and environmental treatment in order to design future implementations to solve environmental problems. This is attractive for ecological uses such as water purification, purified biofuel production and wastewater treatment. Researchers in this field are doing their best in one way or the other to use these composites to solve the environmental crises. This discussion tackles the use of oxygen vacancies of TiO2 nano-based composites in photocatalysis to solve environmental concerns such as water treatment and related issues.
2. Review
2.1. Photocatalysis
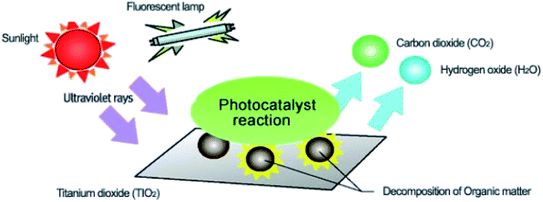
Environmental remediation technology such as aromatic oxygen vacancy compounds and NOx have been suggested by scientists in recent years for the treatment of urban pollution; thus leading to the use of photocatalytic self-cleaning and depolluting constituents. The photocatalytic properties of a thin layer of TiO2 entrenched in paints or concrete or placed at the surface of the particle influences the choice of these viable products. Photocatalysis is designed to harvest visible light (the major component of solar radiation that reaches the Earth's surface) using photocatalysts to drive chemical transformations (Fig. 1 and 3). The use of TiO2 photocatalysts as an evolving pollution control technology has been stated in numerous fields of science.9,22,31,37,38 It, therefore, seems that the actual influence and efficiency on the quality of water of these novel advancements have not been fully demonstrated to date but it has been revealed in a very restricted way. Photocatalysis embraces a class of reactions that use a catalyst activated by light and the decomposition of organic composites into water and carbon dioxide, leading to the fascinating properties of surfaces covered with a photocatalyst; these can shield against coating by fouling matter, are self-cleaning, antibacterial and viricidal.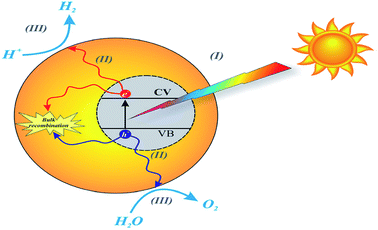 | ||
| Fig. 3 Schematic diagram of the basic mechanisms of the photocatalytic activity of water splitting. Reproduced with permission.64 Copyright 2015, Nanoscale. The Royal Society of Chemistry. | ||
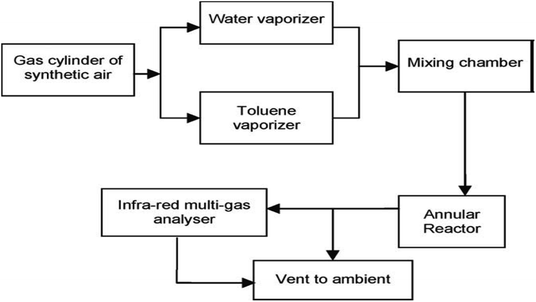
Heterogeneous photocatalysis in gas and liquid phase remediation has been intensively described by many researchers. Typically, the complete procedure is made up of five separate steps (Fig. 2): allocation of the reactants in the gas or liquid phase to the surface, adsorption of at least one of the reactants, reaction in the adsorbed phase, desorption of the product(s), and removal of the products from the interface region.
The third step is where the photocatalytic nature of certain metal oxides plays a role despite all the steps usually found in all heterogeneous processes. Semiconductor catalysts such as TiO2, ZnO, ZrO2, CeO2 etc., with photons carrying energy equal to or in excess of its band gap, create electron–hole pairs similar to photoinduced electron transfer and the absorption of light promotes one electron into the conduction band. The oxide may transfer its electron to any adsorbed electron acceptor (thereby promoting its reduction), while the hole (or the electron vacancy) may accept an electron from an adsorbed donor (promoting its oxidation).
TiO2 photocatalysis happens when the energy of the photons is enough to promote the electrons in the valence band to jump to the conduction band; this occurs in three steps:
(a) Photon absorption and electron–hole pair generation.
(b) Charge separation and migration to surface reaction sites or to recombination sites.
(c) Surface chemical reactions at active sites containing donor oxidation at valence-band holes and acceptor reduction at electron centers (Fig. 4).
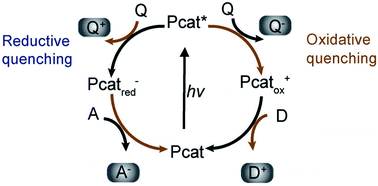 | ||
| Fig. 4 Photoredox catalysis by the photocatalyst. The oxidation steps are portrayed on the right; the reduction steps are shown on the left. Pcat: photocatalyst, Q: quencher, D: donor, A: acceptor. Reproduced with permission.62 Copyright 2009, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Numerous defects associated with photocatalytic principles have been identified by researchers. During photocatalysis, cation radicals17 can be produced by injecting charge from excited molecules into the conduction band of TiO2 (Fig. 4 and 5).
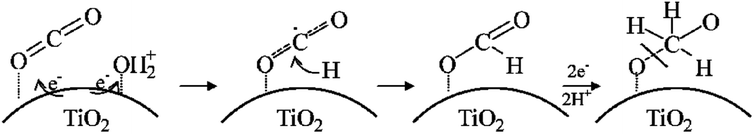 | ||
| Fig. 5 The mechanism of photoreduction of CO2 to the methoxyl radical on TiO2 in the presence of water during the photocatalytic reaction. Reproduced with permission.63 Copyright 2011, American Chemical Society. | ||
Irradiation is usually the starting process in TiO2 photocatalysis; thus, the excitation of electrons by photons at the ground state is the prerequisite. Periodically, the excitation stage and thus the photoexcitation of electrons at the ground state also occurs in most of the materials adsorbed on the surface of the semiconductors;97 e.g., the reaction occurring in dye-sensitized solar cells.42 There are different pathways that are mainly experienced by the charge carriers. Many of the individual materials such as TiO2 are mostly used for water splitting, oxidation/reduction, (Fig. 5) in both suspension and electrode systems.
Significant research attention is centered on metal oxides and silicates due to their photocatalytic activities and their wide range of applications in photocatalysis.90 Several semiconductors, such as TiO2,91 ZnO,81 Fe2O3,82 WO3,75 SrTiO3,76 NaTaO3,77 CdS,78 Ag3PO4,75 BiPO4,79 and g-C3N4,80 NiO,83 Cr2O3,84 Co3O4,85 Al2O3,89 are known photocatalysts, with their use for effective photocatalytic activities being dependent on their band gap86–88 (Fig. 6 and 7). The trapping experiments for holes and free radicals (ROS/RNS) are usually used to explain the photocatalytic schemes of photocatalysts such as TiO2. Also, the trapping experiments of holes, hydroxyl radicals (·OH), and superoxide radicals (·O2−) have been reported by many studies in photocatalysis as the main oxidative species that are found in photocatalytic processes.
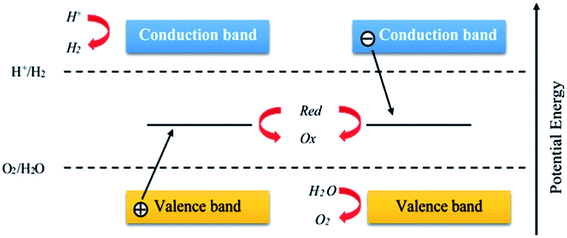 | ||
| Fig. 6 Schematic diagram showing the general water splitting ability of photocatalysts in the Z-scheme system. Reproduced with permission.64 Copyright 2015, Nanoscale. The Royal Society of Chemistry. | ||
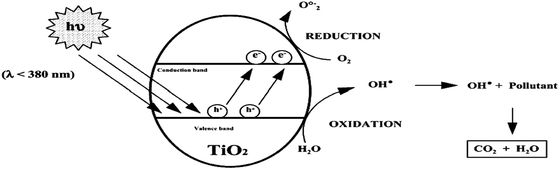 | ||
| Fig. 7 Schematic picture of the principles of the photocatalytic degradation of non-porous TiO2 particles. | ||
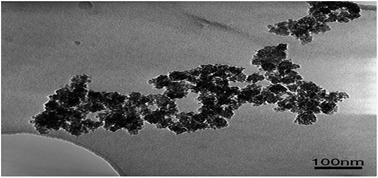 | ||
| Fig. 8 TEM image of the synthesized TiO2–SiO2 catalyst. Reproduced with permission.66 Copyright 2006, Chemical Engineering and Processing. Elsevier. | ||
In instituting whether transformation or water splitting (Fig. 5) is really a photocatalytic activity, with direct activation of reactants and intermediates through visible light absorption, it is essential to establish that the photocatalyst absorbs the photons rather than the absorbents.92,93
Photoexcited charge carriers (Fig. 7) drive the conversion of water (Fig. 5) and carbon dioxide into H2, CO, CH4, and CH3OH related oxygenates and hydrocarbons94–96 during photocatalytic production.
| Preparation methods | Comparison |
|---|---|
| (1) Hydrothermal | (1) Preparation of TiO2 powder using this method usually uses liquid solutions as solvents to harvest the precursors. This usually occurs under increased temperature, <250 °C,50 and high-pressure conditions |
| (2) Crystalline products are often formed (Fig. 8), considering the nucleation and crystal development. These products have different compositions, structures, and morphologies. TiO2 powders are obtained after critical washing and drying | |
| (3) NaOH or ethanol and water frequently serve as the solvents. TiCl4 and Ti(SO4)2 are also commonly used as precursors | |
| (4) The hydrothermal procedure is best used to improve crystallization on both the laboratory and commercial scales. The crystallization process51 is influenced by features such as reaction time, reaction temperature, the medium, and type of precursor | |
| (2) Water-in-oil microemulsion techniques | (1) In recent years, scientists in this field have shifted a lot of attention to monodisperse nanoparticle preparation using this technique49 due to the following factors: |
| (a) The water-in-oil microemulsion is thermodynamically stable52 | |
| (b) It is also the optically isotropic dispersion of surfactant stabilized microdroplets of water in an external oil phase.52 These extremely dispersed nanosized microdroplets are well-matched for particle synthesis. This is due to the ability to control the microenvironment, the site for chemical reactions | |
| 3. Coating methods | (1) Studies have revealed that active commercial TiO2 powder possesses less photocatalytic activity than the TiO2 film.53 Photocatalytic activity is often influenced by coating the TiO2.53 TiO2 doped with elements such as C and N for doped TiO2 synthesis is less expensive29 |
| (2) There are two main coating methods: | |
| (a) Directly sintering or dip-coating (sometimes called wash-coating) the catalyst powders54 | |
| (b) Formation of the TiO2 film on the support. This mostly uses the following preparative techniques: | |
| (i) Metal–organic CVD (MOCVD)19 | |
| (ii) Chemical vapor deposition (CVD) | |
| (iii) Sol–gel40,55 | |
| (iv) Spray coating |
The TiO2 nanomaterial obtained is mostly based on the conditions used in its preparation. These include the following:
(1) Gas-phase method.
(2) Liquid-phase method.
In contrast, the presence of the coordinate and disordered defects in diverse samples makes it barely possible to exactly compare the photocatalytic properties. Under ambient conditions at room temperature, distinct particles are uncommon.44 To simplify the study on the molecular scale, much research has been devoted to the hypothesis pertaining to well-defined particles. Consequently, there is a strong call for the understanding of the nature of photocatalytic reactions regarding the preparation of comparable standard specimens.
2.2. Oxygen vacancy
Oxygen vacancy occurs when the number of oxygens anticipated in a particular compound is less than what it is supposed to have to make it a perfect crystal lattice. Annealing in a reducing atmosphere is a prerequisite for the removal of oxygen from a compound made up of oxygen. Extra oxygenation requires annealing in an oxidizing atmosphere (O2). Simply put, this involves hole doping (the removal of two electrons as a result of adding one oxygen atom from the parent atom), and electron doping (addition of two electrons due to the removal of one oxygen atom from the parent atom). Magnetic and transport properties are mostly determined by the final material's structure, which is also dependent on its total charge/spin state. This idea is expected to be true in nanomaterials or bulk materials; however, anomalous properties are seen in nanomaterials, due to the ability of these materials to exist in new or active electronic states or environments (Fig. 9).10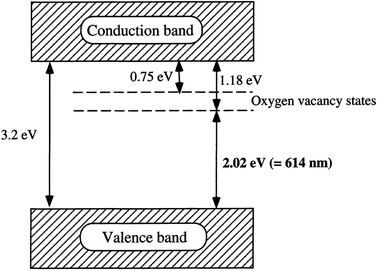 | ||
| Fig. 9 A proposed band structure model for the anatase TiO2 with oxygen vacancies. Reproduced with permission.65 Copyright 2000, Journal of Molecular Catalysis A: Chemical. Elsevier. | ||
Considering spinel type ferrites, the exchange of cations in the tetrahedral and octahedral sites in these magnetic oxides are well known to induce vacancies or lattice defects. There is, therefore, the inversion of normal spinel structures due to the occupation of most divalent transitional metal ions. At this point, the superexchange mechanism is produced due to the relative positions of both octahedral and tetrahedral sites of the atoms. Interatomic distances, anions, cations and bond angles are extensively close. The hopping mechanism that has a give-and-take of an e− to a half-filled shell is often determined by the oxygen atom in the structure. Antiferromagnetic coupling to the unpaired spin of oxygen, as described by the Pauli exclusion and Hund's rule, or contribution to the net magnetism occurs due to an outstanding exchange with the closest neighbor transition metal. Negative and positive oxygen vacancies are two main types of oxygen vacancy. The concentration is centered on the oxygen vacancy, one of the natural spots in oxides, due to the vital role it plays in the physical characteristics of materials.
| Doped TiO2xNx photocatalyst | TiO2 photocatalyst |
|---|---|
| (a) Several nitrogen-doped TiO2 samples (Fig. 10)7 were found to photodegrade gaseous formaldehyde,8 acetaldehyde,8,9 acetone,10 2-propanol,11,12 toluene,8,13 and methylene blue | (a) Pure TiO2 photocatalyst is usually less effective for photo depletion of methylene blue39 |
| (b) TiO2xNx (films and powders) has better photoactivity than TiO2 under visible light irradiation29 due to higher surface acidity (Fig. 10)29,30 | (b) TiO2 has less photocatalytic activity under visible light irradiation |
| (c) The active wavelength of TiO2xNx, of less than 500 nm covers the main peak of the solar irradiation energy beyond the Earth's atmosphere (around 460 nm)9 | (c) A similar active wavelength of 500 nm for TiO2 does not cover the main peak of the solar irradiation energy beyond Earth's atmosphere9 |
| (d) Introduction of ZrO2 into TiO2xNx exhibited higher porosity, higher specific surface area, and an enhanced thermal stability14 | (d) This feature was absent in TiO2 |
| (e) Decreases the deactivation of the photocatalysts28 | (e) Deactivation of the surface occurs very quickly |
| Metals | Properties | References |
|---|---|---|
| (1) Transition metal ions such as V, Cr, Mn, Fe, Co, Ni, or Cu | (1) Extend light absorption into the visible region | 27 and 41 |
| (2) There is a considerable reduction in the photocatalytic activity in the UV region | ||
| (2) Presence of metals, such as Li+, Zn2+, Cd2+, Ce3+, Co3+, Cr3+, Fe3+, Al3+, Mn2+ and Pt | (1) Considerably change the photocatalytic activity of TiO2 | 39 and 41 |
| (2) Sol–gel systems can be used to prepare the Mn+/TiO2 layers for phenol degradation | ||
| (3) The presence of Co3+, Cr3+, Ce3+, Mn2+, Al3+ and Fe3+ ion (5 mol% Mn+: Ti4+) | (1) Has an opposing influence on the photocatalytic activity of the TiO2 photocatalyst | 41 |
| (2) There is a decline in the photocatalytic activity of TiO2 under UV irradiation | ||
| (3) These metal ions act as recombination sites for the photogenerated charge carriers |
The Wendt group's experiment5 on adsorption and desorption, where they used oxygen and water as probe molecules that were observed in STM images to bridge oxygen vacancies, and oxygen atoms on surface Ti atoms, reported the production of pure and reduced TiO2 surfaces. Their work further addressed the criteria to determine the cleanliness by STM imaging.5 Yang and colleagues in their research in 2010 on Chinese housing energy and environment introduced a novel technique for the characterization of oxygen vacancy associates in mostly hydrogen modified TiO2 photocatalyst using positron annihilation lifetime spectroscopy (PALS). They concluded that small neutral Ti3± oxygen vacancies (large quantity), vacancy clusters (appreciably large in size), and voids of vacancies (just a few of them) were actually in hydrogenated TiO2. In the research by Fujishima and coworkers,2 there was a remarkable improvement in the photocatalytic activity of 2% WO3–TiO2 catalysts when an appropriate oxygen vacancy was employed by using Fe3+ as an electron acceptor under UV irradiation in 12 hours.
Researchers have found out that the controlled combustion of Ti metal in a natural gas flame can also be used to synthesize chemically modified n-type TiO2 by using carbon as a doping agent.8,15 Inhibition of the charge recombination and trapping of the photoexcited electrons using these doped impurities (Table 3) also helps to increase the photocatalytic activity.33–36 There are three methods for preparing the visible light responsive photocatalyst, namely, doping TiO2 with transition metal ions, doping nitrogen into TiO2 and utilizing sensitizing dyes.
Semiconductors such as sensitizing dye (higher band-gap), change the electron-transfer processes during the photocatalytic reaction as described by Vinodgopal and Kamat in the principle of photosensitization of a semiconductor.16 Dye cation radicals17 can be produced by injecting charge from the excited dye molecule into the conduction band of TiO2.
(1) The maximum entropy method (MEM).
(2) X-ray diffraction (XRD).
Most research efforts have also proposed a method for the analysis of oxygen vacancies using conventional XRD and MEM techniques (Table 4).
| Analysis tool | Comparison |
|---|---|
| (1) X-ray diffraction (XRD) | (1) X-ray diffraction (XRD) is a simple and useful tool for the analysis of oxygen vacancy because it reveals the crystal structure and the electron density distribution of periodic arrays of atoms |
| (2) Analysis of X-ray diffraction data using Rietveld refinement has been attempted for the quantitative analysis of oxygen vacancies in terms of oxygen site occupancy | |
| (3) X-ray diffraction requires the use of neutrons or synchrotron X-rays | |
| (2) Maximum entropy technique | (1) The maximum entropy method (MEM) is also a suitable tool for the analysis of oxygen because it uses the more precise Rietveld refinement that resolves summation-terminated errors and affords a better structural model |
| (2) The maximum entropy technique presents insignificant modeling errors via the least-biased electronic reconstruction of X-ray diffraction patterns in real space |
The efficiency of a particular photocatalyst depends strongly on the competence of electron–hole pair separation and the adsorption ability of gaseous oxygen vacancy compounds. Coupling TiO2 catalysts18-20 with other semiconductor oxides or depositing metals or doping TiO2 photocatalysts with some other metal ions help to improve its photocatalytic activities. Studies have18 established that the photoreactivity for both oxidation and reduction significantly improved when they doped with Fe3+, Mo5+, Ru3+, Os3+, Re5+, V4+, and Rh3+ at 0.1–0.5%, but Co3+ and Al3+ doping decreased the photoreactivity.22 When the active sites on the reaction surface21 of a gas–solid photocatalyst is reduced, the activity also decreases.21 It can be concluded that the activity of catalysts depends on their lifetime, which is potentially essential to the economic process.21 Studies on toluene,23,24 ethanol25 and trichloroethylene, dimethyl sulfide, and trichloropropene22 have found out that deactivation in these compounds is really uncommon. Sauer and Ollis26 in their work stated that particle materials block pores on the photocatalysts' surfaces, hence, change the surfaces of these catalysts.27 The chemical or physical adsorption of organic substrates on the TiO2 matrix is improved when of lanthanide ions, for instance, La3+, Eu3+, Pr3+, Nd3+, and Sm3+ amalgamated into the matrix19.
(a) Between the electrons and the conduction band in the presence of delocalized free electrons.
(b) Between the electrons and the oxygen vacancies in the form of oxygen vacancy complexes and the ionized shallow-donor impurities.
Considering the theoretical viewpoint, the highest occupied molecular orbital and the lowest unoccupied molecular orbital often shifts due to the influence exerted by the defect states.59 It was realized from experiment59 that an occupied defect state of 0.7 eV below the bottom of the conduction band was decreased by the oxygen vacancy.7 Research on the characterization analysis of anatase, rutile, and brookite showed that anatase and brookite were made up of oxygen vacancies, which remarkably improved the photocatalytic activity. In conclusion, it is presumed that the difference in the absorptions on the surfaces of the active sites and the reactants (CO2, H2O, CO2−, and CO) were due to oxygen vacancies.
Lattice distortion makes use of the original energy provided by an election or a photon. The swiftly vanishing approach for the charge carriers7 has been reported by many scientists as annihilation at the recombination center. Mitsuhara and coworkers' studies43 on semiconductors led to a proposal of three recombination mechanisms (Table 5).
| Recombination mechanism | Properties | Limitations | References |
|---|---|---|---|
| (1) Band-to-band recombination | (1) It occurs between the excited electron and the hole lying in the empty valence band | (1) The production of available electrons and holes limits this reaction | 61 |
| (2) It is second order to the concentration of the charge Carrier | |||
| (2) Trap-assisted recombination | (1) This mechanism transpires with the help of the “trap” state | (1) Shockley–Read–Hall model (SRH model) confirmed that the concentration of charge carriers hinders this reaction or mechanism | |
| (2) It happens between the excited electrons and holes in the valence band | |||
| (3) Auger recombination | (1) This usually happens during the recombination process of the excited electron and hole | 20 | |
| (2) Releasing the energy to improve the energy of another electron or hole |
(i) To alter the band energy structure of the pristine TiO2 as the defect states.
(ii) To trap charge carriers in the migration pathways as the electron pool or recombination center.
(iii) To power the adsorption of reactants (e.g., H2O, O2, CO2, and organic pollutants) as the active sites.61
3. Conclusion
This systematic review sums up the novel developments in the photocatalytic applications of TiO2-based composite photocatalysts with oxygen vacancies in the areas of energy, wastewater treatment and environmental purification. Nonporous TiO2 has revealed its greatness as one of the best candidates in designing and engineering advanced composite photocatalysts. There is little doubt that the considerable progress in TiO2 nano-based composites will continue in the near future. More studies are necessary in order to make full use of the excellent properties resulting from the oxygen vacancy of the nonporous TiO2 photocatalysts.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was financially supported by grants from National Science Funds for Creative Research Groups of China (No. 51421006), Natural Science Foundation of China (51679063), the Key Program of National Natural Science Foundation of China (No. 41430751), the National Key Plan for Research and Development of China (2016YFC0502203), Fundamental Research Funds (No. 2016B43814), and PAPD. Buanya Beryl Adormaa and Williams Kweku Darkwah were the recipients of scholarship from the Nanjing Government and China Scholarship Council (CSC) respectively for the duration of this work.References
- A. Fujishima and K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- A. Fujishima, et al., Combination effect of activated carbon with TiO2 for the photodegradation of binary pollutants at typical indoor air level, Sci. 80, 2006, 4, 2–4 Search PubMed.
- Y. Wang, et al., Coiled-coil networking shapes cell molecular machinery, Mol. Biol. Cell, 2012, 23, 3911–3922 CrossRef CAS PubMed.
- J. H. Park, et al., Analysis of oxygen vacancy in Co-doped ZnO using the electron density distribution obtained using MEM, Nanoscale Res. Lett., 2015, 10, 186 CrossRef PubMed.
- D. J. Wendt, et al., Neutral Endopeptidase-Resistant C-Type Natriuretic Peptide Variant Represents a New Therapeutic Approach for Treatment of Fibroblast Growth Factor Receptor 3-Related Dwarfism, J. Pharmacol. Exp. Ther., 2015, 353, 132–149 CrossRef CAS PubMed.
- X. Yang, Y. Jiang, M. Yang and M. Shan, Energy and environment in Chinese rural housing: Current status and future perspective, Front. Energy Power Eng. China, 2010, 4, 35–46 CrossRef.
- Y. Liu and J. Brenan, Partitioning of platinum-group elements (PGE) and chalcogens (Se, Te, As, Sb, Bi) between monosulfide-solid solution (MSS), intermediate solid solution (ISS) and sulfide liquid at controlled fO2-fS2 conditions, Geochim. Cosmochim. Acta, 2015, 159, 139–161 CrossRef CAS.
- Y. Irokawa, et al., Photodegradation of toluene over TiO2–xNx under visible light irradiation, Phys. Chem. Chem. Phys., 2006, 8, 1116 RSC.
- R. Asahi, T. Mikawa, T. Ohwaki, K. Aoki and Y. Taga, Visible Light Photocatalysis in Nitrogen-Doped Titanium Oxides, Sci. 80, 2001, 293, 269–271 CrossRef CAS PubMed.
- T. Ihara, M. Miyoshi, Y. Iriyama, O. Matsumoto and S. Sugihara, Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping, Appl. Catal., B, 2003, 42, 403–409 CrossRef CAS.
- H. Irie, Y. Watanabe and K. Hashimoto, Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-x Nx Powders, J. Phys. Chem. B, 2003, 107, 5483–5486 CrossRef CAS.
- S. Miyauchi, et al., Adaptation to left-right reversed vision rapidly activates ipsilateral visual cortex in humans, J. Physiol., 2004, 98, 207–219 CrossRef PubMed.
- S. Wu, L. J. Mickley, D. J. Jacob, D. Rind and D. G. Streets, Effects of 2000-2050 changes in climate and emissions on global tropospheric ozone and the policy-relevant background surface ozone in the United States, J. Geophys. Res.: Atmos., 2008, 113, D18312 CrossRef.
- P. Wang, The economics of foreign exchange and global finance, Econ. Foreign Exch. Glob. Financ., 2005, DOI:10.1007/3-540-28524-5.
- R. Li, et al., First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from raphidiopsis curvata ( cyanobacteria ) 1 A strain of Raphidiopsis ( Cyanobacteria ) isolated from a fish pond in Wuhan, P. R. China was examined for its taxonomy and pr, J. Phycol., 2001, 1126, 1121–1126 CrossRef.
- K. Vinodgopal and P. V. Kamat, Enhanced Rates of Photocatalytic Degradation of an Azo Dye Using SnO2/TiO2 Coupled Semiconductor Thin Films, Environ. Sci. Technol., 1995, 29, 841–845 CrossRef CAS PubMed.
- C. Nasr, et al., Environmental photochemistry on semiconductor surfaces. Visible light induced degradation of a textile diazo dye, naphthol blue black, on TiO2 nanoparticles, J. Phys. Chem., 1996, 100, 8436–8442 CrossRef CAS.
- W. Choi, A. Termin and M. R. Hoffmann, The role of metal ion dopants in quantum-sized TiO\n 2: Correlation between photoreactivity and charge carrier recombination dynamics, J. Phys. Chem., 1994, 98, 13669–13679 CrossRef.
- L. J. yi, M. W. hong, L. P. xiang and Z. J. cai, Detection of intermediates in the TiO2-assisted photodegradation of Rhodamine B under visible light irradiation, J. Environ. Sci., 2007, 19, 892–896 CrossRef.
- B. Zhang, X. Pan, C. H. Cannon, G. P. Cobb and T. A. Anderson, Conservation and divergence of plant microRNA genes, Plant J., 2006, 46, 243–259 CrossRef CAS PubMed.
- M. L. Sauer and D. F. Ollis, Photocatalyzed oxidation of ethanol and acetaldehyde in humidified air, J. Catal., 1996, 158, 570–582 CrossRef CAS.
- D. F. Ollis, Photocatalytic purification and remediation of contaminated air and water, Comptes Rendus de l Académie des Sciences - Series IIC - Chemistry, 2000, 3, 405–411 CrossRef CAS.
- M. A. Blanco, A. M. Pendás, E. Francisco, J. M. Recio and R. Franco, Thermodynamical properties of solids from microscopic theory: applications to MgF2 and Al2O3, J. Mol. Struct.: THEOCHEM, 1996, 368, 245–255 CrossRef CAS.
- O. D'Hennezel, P. Pichat and D. F. Ollis, Benzene and toluene gas-phase photocatalytic degradation over H2O and HCL pretreated TiO2: By-products and mechanisms, J. Photochem. Photobiol., A, 1998, 118, 197–204 CrossRef.
- E. Piera, J. Ayllón, X. Doménech and J. Peral, TiO 2 deactivation during gas-phase photocatalytic oxidation of ethanol, Catal. Today, 2002, 76, 259–270 CrossRef CAS.
- M. L. Sauer and D. F. Ollis, Catalyst Deactivation in Gas – Solid Photocatalysis, J. Catal., 1996, 217, 215–217 CrossRef.
- J. Zhao and X. Yang, Photocatalytic oxidation for indoor air purification: A literature review, Building and Environment, 2003, 38, 645–654 CrossRef.
- C. Belver, M. J. López-Muñoz, J. M. Coronado and J. Soria, Palladium enhanced resistance to deactivation of titanium dioxide during the photocatalytic oxidation of toluene vapors, Appl. Catal., B, 2003, 46, 497–509 CrossRef CAS.
- X. Fu, L. a. Clark, Q. Yang and M. a. Anderson, Enhanced Photocatalytic Performance of Titania-Based Binary Metal Oxides: TiO 2/SiO 2 and TiO 2/ZrO 2, Environ. Sci. Technol., 1996, 30, 647–653 CrossRef CAS.
- R. Méndez-Román and N. Cardona-Martínez, Relationship between the formation of surface species and catalyst deactivation during the gas-phase photocatalytic oxidation of toluene, Catal. Today, 1998, 40, 353–365 CrossRef.
- M. Chang and H. C. Chang, Development of a screening method for biogenic amine producing Bacillus spp., Int. J. Food Microbiol., 2012, 153, 269–274 CrossRef CAS PubMed.
- H. Kisch and W. Macyk, Visible-light photocatalysis by modified titania, ChemPhysChem, 2002, 3, 399–400 CrossRef CAS PubMed.
- X. Chen, M. C. Schmidt, W. F. Goins and J. C. Glorioso, Two herpes simplex virus type 1 latency-active promoters differ in their contributions to latency-associated transcript expression during lytic and latent infections, J. Virol., 1995, 69, 7899–7908 CAS.
- K. Nagaveni, M. S. Hegde, N. Ravishankar, G. N. Subbanna and G. Madras, Synthesis and Structure of Nanocrystalline TiO 2 with Lower Band Gap Showing High Photocatalytic Activity, Langmuir, 2004, 20, 2900–2907 CrossRef CAS PubMed.
- K. Nagaveni, G. Sivalingam, M. S. Hegde and G. Madras, Solar photocatalytic degradation of dyes: High activity of combustion synthesized nano TiO2, Appl. Catal., B, 2004, 48, 83–93 CrossRef CAS.
- L. T. Wong, K. W. Mui and P. S. Hui, A statistical model for characterizing common air pollutants in air-conditioned offices, Atmos. Environ., 2006, 40, 4246–4257 CrossRef CAS.
- D. T. Ho, A. J. Bardwell, S. Grewal, C. Iverson and L. Bardwell, Interacting JNK-docking sites in MKK7 promote binding and activation of JNK mitogen-activated protein kinases, J. Biol. Chem., 2006, 281, 13169–13179 CrossRef CAS PubMed.
- F. P. An, et al., Observation of electron-antineutrino disappearance at Daya Bay, Phys. Rev. Lett., 2012, 108, 1–7 Search PubMed.
- E. C. Yang, K. M. Kim, J. Runess and S. M. Boo, Morphology and molecular phylogeny of Psilothallia dentata (Ceramiaceae, Rhodophyta), Algae, 2004, 19(4), 283–292 CrossRef.
- W. T. Tseng, Z. Dörnyei and N. Schmitt, A new approach to assessing strategic learning: The case of self-regulation in vocabulary acquisition, Appl. Linguist., 2006, 27, 78–102 CrossRef.
- P. V. Kamat and D. Meisel, Nanoparticles in advanced oxidation processes, Curr. Opin. Colloid Interface Sci., 2002, 7, 282–287 CrossRef CAS.
- T. Mitsuhara, et al., A Case of a Primary Radiation-Induced Malignant Peripheral Nerve Sheath Tumor in the Cauda Equina in a Patient with Neurofibromatosis, Journal of Spine, 2013, 2, 2–4 Search PubMed.
- C. Di Valentin, L. Ferrighi and G. Fazio, Theoretical Studies of Oxygen Reactivity of Free-Standing and Supported Boron-Doped Graphene, ChemSusChem, 2016, 9, 1061–1077 CrossRef CAS PubMed.
- C. Di Valentin, et al., Adsorption of Water on Reconstructed Rutile TiO 2 (011)-(2×1): TiO Double Bonds and Surface Reactivity, J. Am. Chem. Soc., 2005, 127, 9895–9903 CrossRef CAS PubMed.
- H. Yoneyama and T. Torimoto, Titanium dioxide/adsorbent hybrid photocatalysts for photodestruction of organic substances of dilute concentrations, Catal. Today, 2000, 58, 133–140 CrossRef CAS.
- C. H. Ao and S. C. Lee, Combination effect of activated carbon with TiO2 for the photodegradation of binary pollutants at typical indoor air level, J. Photochem. Photobiol., A, 2004, 161, 131–140 CrossRef CAS.
- H. Yu, Y. Ye, S. Zhao and G. Wang, A backprojection-filtration algorithm for nonstandard spiral cone-beam CT with an n-PI-window, Phys. Med. Biol., 2005, 50, 2099–2111 CrossRef PubMed.
- L. Zeatoun and D. Feke, Characterization of TiO2 smoke prepared using gas-phase hydrolysis of TiCl4, Part. Part. Syst. Charact., 2006, 22, 276–281 CrossRef CAS.
- P. Monnoyer, A. Fonseca and J. B. Nagy, Preparation of colloidal AgBr particles from microemulsions, Colloids Surf., A, 1995, 100, 233–243 CrossRef CAS.
- S. Guo, et al., Pulp and fiber characterization of wheat straw and eucaluptus pulps - A comparison, BioResources, 2009, 4, 1006–1016 CAS.
- J. Sun, L. Qiao, S. Sun and G. Wang, Photocatalytic degradation of Orange G on nitrogen-doped TiO2catalysts under visible light and sunlight irradiation, J. Hazard. Mater., 2008, 155, 312–319 CrossRef CAS PubMed.
- Y. Qi, S. Bai and G. M. Heisler, Changes in ultraviolet-B and visible optical properties and absorbing pigment concentrations in pecan leaves during a growing season, Agric. For. Meteorol., 2003, 120, 229–240 CrossRef.
- C. C. Huang, Z. Yang, K. H. Lee and H. T. Chang, Synthesis of highly fluorescent gold nanoparticles for sensing mercury(II), Angew. Chem., Int. Ed., 2007, 46, 6824–6828 CrossRef CAS PubMed.
- T. N. Obee and R. T. Brown, TiO2 Photocatalysis for Indoor Air Applications: Effects of Humidity and Trace Contaminant Levels on the Oxidation Rates of Formaldehyde, Toluene, and 1,3-Butadiene, Environ. Sci. Technol., 1995, 29, 1223–1231 CrossRef CAS PubMed.
- J. Yu, H. Elderfield and B. Hönisch, B/Ca in planktonic foraminifera as a proxy for surface seawater pH, Paleoceanography, 2007, 22, PA2202 CrossRef.
- X. Feng, et al., Significantly Enhanced Visible Light Photocatalytic Efficiency of Phosphorus doped TiO2 with surface oxygen vacancies for Ciprofloxacin Degradation: Synergistic Effect and Intermediates Analysis, J. Hazard. Mater., 2018 DOI:10.1016/j.jhazmat.2018.03.013.
- Y. Lv, Y. Zhu and Y. Zhu, Enhanced Photocatalytic Performance for the BiPO 4–x Nanorod Induced by Surface Oxygen Vacancy, J. Phys. Chem. C, 2013, 117, 18520–18528 CrossRef CAS.
- L. Zou, Y. Luo, M. Hooper and E. Hu, Removal of VOCs by photocatalysis process using adsorption enhanced TiO2-SiO2catalyst, Chem. Eng. Process., 2006, 45, 959–964 CrossRef CAS.
- B. J. Morgan and G. W. Watson, Intrinsic n-type Defect Formation in TiO_2: A Comparison of Rutile and Anatase from GGA+\textitU Calculations, J. Phys. Chem. C, 2010, 114, 2321–2328 CrossRef CAS.
- H. Kisch, et al., Pseudotumor cerebri, Neurol. Clin., 2004, 22, 99–131 CrossRef.
- J. Of, et al., Oxygen Vacancy Structure Associated Photocatalytic Water Oxidation of BiOCl, J. Phys. Chem. C, 2013, 117, 3601 Search PubMed.
- K. Zeitler, Angew. Chem., Int. Ed., 2009, 48, 9785 CrossRef CAS PubMed.
- N. M. Dimitrijevic, B. K. Vijayan, O. G. Poluektov, T. Rajh, K. A. Gray, H. He and P. Zapol, J. Am. Chem. Soc., 2011, 133, 3964 CrossRef CAS PubMed.
- M. R. Gholipour, C.-T. Dinh, F. Béland and T.-O. Do, Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting, Nanoscale, 2015, 7, 8187–8208 RSC.
- I. Nakamura, N. Negishi, S. Kutsuna, T. Ihara, S. Sugihara and K. Takeuchi, Role of oxygen vacancy in the plasma-treated TiO photocatalyst with visible light activity for NO removal, J. Mol. Catal. A: Chem., 2000, 161, 205–212 CrossRef CAS.
- L. Zou, Y. Luo, M. Hooper and E. Hu, Removal of VOCs by photocatalysis process using .adsorption enhanced TiO2–SiO2 catalyst, Chem. Eng. Process., 2006, 45, 959–964 CrossRef CAS.
- Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han and C. Li, Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations, Chem. Rev., 2014, 114, 9987–10043 CrossRef CAS PubMed.
- U. I. Gaya and A. H. Abdullah, Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems, J. Photochem. Photobiol., C, 2008, 9, 1–12 CrossRef CAS.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Understanding TiO2 Photocatalysis: Mechanisms and Materials, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors, Angew. Chem., Int. Ed., 2013, 52, 7372–7408 CrossRef CAS PubMed.
- R. Daghrir, P. Drogui and D. Robert, Modified TiO2 for Environmental Photocatalytic Applications: A Review, Ind. Eng. Chem. Res., 2013, 52, 3581–3599 CrossRef CAS.
- K. Nakata and A. Fujishima, TiO2 photocatalysis: Design and applications, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS.
- S. G. Kumar and L. G. Devi, Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics, J. Phys. Chem. A, 2011, 115, 13211–13241 CrossRef CAS PubMed.
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z.-X. Guo and J. Tang, Visible-light driven heterojunction photocatalysts for water splitting—A critical review, Energy Environ. Sci., 2015, 8, 731–759 RSC.
- W. Wang, B. Cheng, J. Yu, G. Liu and W. Fan, Visible-Light Photocatalytic Activity and Deactivation Mechanism of Ag3PO4 Spherical Particles, Chem.–Asian J., 2012, 7, 1902–1908 CrossRef CAS PubMed.
- H. Kato and A. Kudo, Visible-Light-Response and Photocatalytic Activities of TiO2 and SrTiO3 Photocatalysts Codoped with Antimony and Chromium, J. Phys. Chem. B, 2002, 106, 5029–5034 CrossRef CAS.
- H. Kato and A. Kudo, Water Splitting into H2 and O2 on Alkali Tantalate Photocatalysts ATaO3 (A = Li, Na, and K), J. Phys. Chem. B, 2001, 105, 4285–4292 CrossRef CAS.
- D. Jing and L. Guo, A Novel Method for the Preparation of a Highly Stable and Active CdS Photocatalyst with a Special Surface Nanostructure, J. Phys. Chem. B, 2006, 110, 11139–11145 CrossRef CAS PubMed.
- C. Pan and Y. Zhu, New Type of BiPO4 Oxy-Acid Salt Photocatalyst with High Photocatalytic Activity on Degradation of Dye, Environ. Sci. Technol., 2010, 44, 5570–5574 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, A metal-free polymeric photocatalyst for hydrogen production from water under visible light, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- Y. Lan, X. Li, G. Li and Y. Luo, Sol-gel method to prepare graphene/Fe2O3 aerogel and its catalytic application for the thermal decomposition of ammonium perchlorate, J. Nanopart. Res., 2015, 17, 395 CrossRef.
- M. Krumm, C. L. Pueyo and S. Polarz, Monolithic zinc oxide aerogels from organometallic sol-gel precursors, Chem. Mater., 2010, 22, 5129–5136 CrossRef CAS.
- J. G. Seo, M. H. Youn, Y. Bang and I. K. Song, Effect of Ni/Al atomic ratio of mesoporous Ni-Al2O3 aerogel catalysts on their catalytic activity for hydrogen production by steam reforming of liquefied natural gas (LNG), Int. J. Hydrogen Energy, 2010, 35, 12174–12181 CrossRef CAS.
- A. E. Gash, T. M. Tillotson, J. H. Satcher, Jr., L. W. Hrubesh and R. L. Simpson, New sol-gel synthetic route to transition and main-group metal oxide aerogels using inorganic salt precursors, J. Non-Cryst. Solids, 2001, 285, 22–28 CrossRef CAS.
- T. Zeng, X. Zhang, S. Wang, H. Niu and Y. Cai, Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants, Environ. Sci. Technol., 2015, 49, 2350–2357 CrossRef CAS PubMed.
- Z. Novak, P. Kotnik and Ž. Knez, Preparation of WO3 aerogel catalysts using supercritical CO2 drying, J. Non-Cryst. Solids, 2004, 350, 308–313 CrossRef CAS.
- A. Cabañas, E. Enciso, M. C. Carbajo, M. J. Torralvo, C. Pando and J. A. R. Renuncio, Synthesis of SiO2-aerogel inverse opals in supercritical carbon dioxide, Chem. Mater., 2005, 17, 6137–6145 CrossRef.
- R. Sui, A. Rizkalla and P. A. Charpentier, Experimental study on the morphology and porosity of TiO2 aerogels synthesized in supercritical carbon dioxide, Microporous Mesoporous Mater., 2011, 142, 688–695 CrossRef CAS.
- M. B. Chowdhury, R. Sui, R. A. Lucky and P. A. Charpentier, One-pot procedure to synthesize high surface area alumina nanofibers using supercritical carbon dioxide, Langmuir, 2009, 26, 2707–2713 CrossRef PubMed.
- G. R. Patzke, Y. Zhou, R. Kontic and F. Conrad, Oxide nanomaterials: Synthetic developments, mechanistic studies, and technological innovations, Angew. Chem., Int. Ed., 2011, 50, 826–859 CrossRef CAS PubMed.
- E. Alonso, I. Montequi and M. Cocero, Effect of synthesis conditions on photocatalytic activity of TiO2 powders synthesized in supercritical CO2, J. Supercrit. Fluids, 2009, 49, 233–238 CrossRef CAS.
- M. Rochkind, S. Pasternak and Y. Paz, Using Dyes for Evaluating Photocatalytic Properties: A Critical Review. Molecules, Molecules, 2015, 20, 88–110 CrossRef CAS PubMed.
- N. Barbero and D. Vione, Why Dyes Should Not Be Used to Test the Photocatalytic Activity of Semiconductor Oxides, Environ. Sci. Technol., 2016, 50, 2130–2131 CrossRef CAS PubMed.
- S. Styring, Artificial photosynthesis for solar fuels, Faraday Discuss., 2012, 155, 357–376 RSC.
- A. Listorti, J. Durrant and J. Barber, Solar to fuel, Nat. Mater., 2009, 8, 929–930 CrossRef CAS PubMed.
- D. Chen, X. Zhang and A. F. Lee, Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals, J. Mater. Chem. A, 2015, 3, 14487–14516 RSC.
- Y. Nosaka and A. Y. Nosaka, Generation and Detection of Reactive Oxygen Species in Photocatalysis, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS PubMed.
- D. Hollmann, M. Karnahl, S. Tschierlei, K. Kailasam, M. Schneider, J. Radnik, K. Grabow, U. Bentrup, H. Junge and M. Beller, et al., Structure-Activity Relationships in Bulk Polymeric and Sol-Gel-Derived Carbon Nitrides during Photocatalytic Hydrogen Production, Chem. Mater., 2014, 26, 1727–1733 CrossRef CAS.
- T. Li, L. Zhao, Y. He, J. Cai, M. Luo and J. Lin, Synthesis of g-C3N4/SmVO4 composite photocatalyst with improved visible light photocatalytic activities in RhB degradation, Appl. Catal., B, 2013, 129, 255–263 CrossRef CAS.
- J. B. Priebe, J. Radnik, A. J. J. Lennox, M.-M. Pohl, M. Karnahl, D. Hollmann, K. Grabow, U. Bentrup, H. Junge and M. Beller, et al., Solar Hydrogen Production by Plasmonic Au-TiO2 Catalysts: Impact of Synthesis Protocol and TiO2 Phase on Charge Transfer Efficiency and H2 Evolution Rates, ACS Catal., 2015, 5, 2137–2148 CrossRef CAS.
- K. Miyashita, S.-I. Kuroda, S. Tajima, K. Takehira, S. Tobita and H. Kubota, Photoluminescence study of electron-hole recombination dynamics in the vacuum-deposited SiO2/TiO2 multilayer film with photo-catalytic activity, Chem. Phys. Lett., 2003, 369, 225–231 CrossRef CAS.
- A. Nashim, S. Martha and K. M. Parida, Heterojunction conception of n-La2Ti2O7/p-CuO in the limelight of photocatalytic formation of hydrogen under visible light, RSC Adv., 2014, 4, 14633–14643 RSC.
- E. Karamian and S. Sharifnia, On the general mechanism of photocatalytic reduction of CO2, J. CO2 Util., 2016, 16, 194–203 CrossRef CAS.
- J. L. White, M. F. Baruch, J. E. Pander, Y. Hu, I. C. Fortmeyer, J. E. Park, T. Zhang, K. Liao, J. Gu and Y. Yan, et al., Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes, Chem. Rev., 2015, 115, 12888–12935 CrossRef CAS PubMed.
- S. A. Lee, K. H. Choo, C. H. Lee, H. I. Lee, T. Hyeon, W. Choi and H. H. Kwon, Use of ultrafiltration membranes for the separation of TiO2 photocatalysts in drinking water treatment, Ind. Eng. Chem. Res., 2001, 40, 1712–1719 CrossRef CAS.
- R. Molinari, L. Palmisano, E. Drioli and M. Schiavello, Studies on various reactor configurations for coupling photocatalysis and membrane processes in water purification, J. Membr. Sci., 2002, 206, 399–415 CrossRef CAS.
- W. Xi and S. Geissen, Separation of titanium dioxide from photocatalytically treated water by cross-flow microfiltration, Water Res., 2001, 35, 1256–1262 CrossRef CAS PubMed.
- D. B. Hamal, et al., A multifunctional biocide/sporocide and photocatalyst based on titanium dioxide (TiO2) codoped with silver, carbon, and sulfur, Langmuir, 2010, 26, 2805–2810 CrossRef CAS PubMed.
- B. Peng, et al., General synthesis and optical properties of monodisperse multifunctional metal-ion-doped TiO2 hollow particles, J. Phys. Chem. C, 2009, 113, 20240–20245 CrossRef CAS.
- M. E. Kurtoglu, T. Longenbach, K. Sohlberg and Y. Gogotsi, Strong Coupling of Cr and N in Cr -N-doped TiO2 and Its Effect on Photocatalytic Activity, J. Phys. Chem. C, 2011, 115, 17392–17399 CrossRef CAS.
- F. Amano, M. Nakata, A. Yamamoto and T. Tanaka, Effect of Ti3+Ions and Conduction Band Electrons on Photocatalytic and Photoelectrochemical Activity of Rutile Titania for Water Oxidation, J. Phys. Chem. C, 2016, 120, 6467–6474 CrossRef CAS.
- C. S. Chen, et al., Effect of Ti3+ on TiO2-supported Cu catalysts used for CO oxidation, Langmuir, 2012, 28, 9996–10006 CrossRef CAS PubMed.
- H. Liu, et al., The enhancement of TiO2 photocatalytic activity by hydrogen thermal treatment, Chemosphere, 2003, 50, 39–46 CrossRef CAS PubMed.
- I. Nakamura, S. Sugihara and K. Takeuchi, Mechanism for NO Photooxidation over the Oxygen-Deficient TiO2 Powder under Visible Light Irradiation, Chem. Lett., 2000, 29, 1276–1277 CrossRef.
- Z. K. Zhang, M. L. Bai, D. Z. Guo, S. M. Hou and G. M. Zhang, Plasma-electrolysis synthesis of TiO2 nano/microspheres with optical absorption extended into the infra-red region, Chem. Commun., 2011, 47, 8439–8441 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |