Open Access Article
Open Access ArticleCyclodextrin modified niosomes to encapsulate hydrophilic compounds
Noelia D. Machado,
O. Fernando Silva ,
Rita H. de Rossi and
Mariana A. Fernández
,
Rita H. de Rossi and
Mariana A. Fernández *
*
Instituto de Investigaciones en Físico-Química de Córdoba (INFIQC-CONICET), Departamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Ciudad Universitaria, X5000HUA, Córdoba, Argentina. E-mail: marfer@fcq.unc.edu.ar
First published on 23rd August 2018
Abstract
Niosomes were prepared from equimolar mixtures of two non-ionic surfactants, Span 80 and Tween 80. The capability of the vesicular systems was studied through the encapsulation of two azo dyes as molecular probes of different hydrophobicity (methyl orange (MO) and methyl yellow (MY)). To improve the efficiency of the niosomes to encapsulate the dyes, we employed an additional modification of the vesicular system, adding β-cyclodextrin (β-CD) or a modified amphiphilic β-CD (Mod-β-CD) to the niosomes. Neither the inclusion of dyes nor the incorporation of β-CD to the niosomes produces considerable modifications in size and morphology of the vesicles. However, in the presence of Mod-β-CD the niosomes became smaller, probably due to the anchoring of the cyclodextrin at the surface of vesicles through the hydrophobic chain, altering the curvature of the outer monolayer and reducing the surface charge of the interphase. The entrapment efficiency (EE) for MY was higher than that for MO in niosomes without cyclodextrin, however, the content of MO in the presence of β-CD increased considerably. Besides, the release of this dye under the same conditions was faster and reached 70% in 24 hours whereas in the absence of the macrocycle, the release was 15%, in the same time. UV-visible spectrophotometry and induced circular dichroism analysis allowed it to be established that MO is complexed with cyclodextrins inside vesicles, whereas MY interacts mainly with the niosome bilayer instead of with CD. Besides, the cavity of cyclodextrins is probably located in the interphase and preferably in the polar region of niosomes.
Introduction
In recent years, the concept of active agent controlled release systems, from which encapsulated compounds are delivered at the right time and place and in the desired concentration, has become one of the challenges in developing new active compound delivery devices for different applications in the pharmaceutical and food industries. For this reason, different strategies have been proposed, based on the use of delivery devices such as microspheres, microemulsions, nanoparticles, microcapsules, implantable pumps, and vesicular systems among others.1–7There are several advantages of using active compound delivery systems, for example, to protect drugs from a hostile environment (enzymes, pH, heat, air, light, moisture) or to improve poor aqueous solubility of the active agents that limits bioavailability and efficiency in the action site.8,9 Among the promising drug targeting vehicles are vesicles. They carry active ingredients that increase bioavailability and produce the desired effect over an extended period of time.10–12 These systems consist of lamellar structures formed by amphiphilic molecules surrounded by an aqueous compartment. They are useful for the release of both hydrophilic and hydrophobic compounds, which are encapsulated in their internal hydrophilic compartment or in the outer lipid layer, respectively.
Vesicular systems can be of different types depending on the main components that are used in their preparation.13,14 Liposomes are the best known and they have been widely investigated as models to study the cell membrane, and as carriers for protection and/or delivery of bioactive agents. They are formed by natural or synthetic phospholipids.15 The high costs of their formulation and low chemical stability are their main disadvantages that promote the seeking of new vesicular systems with improved properties.13
Niosomes are microscopic vesicles formed by non-ionic surfactants which form closed bilayer structures due to their amphiphilic nature.16,17 The lipophilic groups are located within the membrane while the hydrophilic groups are exposed to the aqueous medium18,19 as in liposomes. Non-ionic surfactants are preferred in comparison with ionic ones due to their ability to increase the solubility of highly water insoluble compounds,20 great compatibility with biological systems (lower toxicity, less irritation effect and non-immunogenicity),21 improved chemical stability against oxidation, and feasibility of surface modification. In addition, they are generally derived from renewable sources, allowing their sustainable production,22 and also are biodegradable. Niosomes allow to entrap both hydrophilic and lipophilic compounds. A lipophilic one, could interact with alkyl chains of non-ionic surfactants in the domain of the bilayers, whereas an hydrophilic compound could interact either with non-ionic surfactant polar head groups (adsorbing in the bilayers surfaces) or be located in the aqueous inner of niosomes.23,24 Due to this characteristics, niosomes present a wide range of potentials applications in cosmetics,25 pharmaceutics,26–28 food,29 and agrochemical industries.
Incorporation of some macrocycles like cyclodextrins to vesicular systems has been used as additional strategy to improve the loaded amount, stability and bioavailability of very hydrophobic compounds in liposomes.30–32 Cyclodextrins (CDs) have been employed due to their ability to form inclusion complexes with a wide range of compounds,33 however the strategies to load cyclodextrin–drug complexes in niosomes was barely used due to the need to previously form the inclusion complex before the incorporation in vesicles.34,35
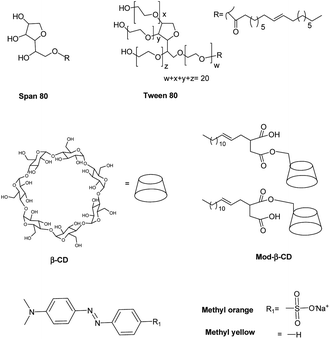
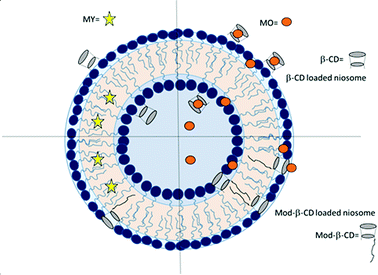
In this work we prepared niosomes from equimolar mixtures of two non-ionic surfactants, Span 80 (sorbitan monooleate) and Tween 80 (polyoxyethylen (20) sorbitan monooleate), whose chemical structures are shown in Scheme 1. To evaluate the transport properties of the systems we studied the encapsulation of two azo dyes as molecular probes of different hydrophobicity (methyl orange (MO) and methyl yellow (MY)), Scheme 1. MO has a high water solubility at 20 °C, 5000 mg L−1,36 thus MO is a model of hydrophilic active agent. In the other way, the aqueous solubility of MY at 20 °C is 13.6 mg L−1,37 so this compound is a model of an hydrophobic active molecule.
With the aim to improve the efficiency of the niosomes as vectors of active agents, we made a modification of the vesicular system, adding β-cyclodextrin (β-CD) to the vesicles. Additionally, we included an amphiphilic modified β-CD in the niosomes (Mod-β-CD, Scheme 1) with the idea that this compound probably could be located in a different place than the native CD, due to the presence of the aliphatic chain. Thus, the interaction of the active compounds with the CDs loaded niosomes could be different. Mod-β-CD has been synthetized and studied in our laboratory38,39 and it was demonstrated that it can form aggregates in aqueous and organic media, and in both systems, the cavity is available to interact with external guests.40–42
It is worth to mention that all the molecules used in this work for the synthesis of the carriers are fatty acids and sugar derivated. They are biodegradable, food grade, and their production is sustainable. The results obtained from the studies of these systems (niosomes-macrocycles) are important for further applications of these systems as delivery agents.
Experimental
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz y 170 W) for 30 min at 60 °C, and then were extruded 15 times to produce 100 nm diameter niosomes, using an Avanti Mini Extruder (Avanti Polar Lipids, Inc). The final surfactant concentration in the niosomes was 0.01 M.
000 Hz y 170 W) for 30 min at 60 °C, and then were extruded 15 times to produce 100 nm diameter niosomes, using an Avanti Mini Extruder (Avanti Polar Lipids, Inc). The final surfactant concentration in the niosomes was 0.01 M.Size and morphology determinations
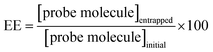 | (1) |
Release studies were performed using Franz diffusion cells. The experiments were carried out at 37 °C under stirring, and with a dialysis benzoylated membrane (Sigma Aldrich cut off 2 kDa MW) separating both compartments. The donor compartment was filled with molecular probe loaded niosomes and the receptor compartment was filled with water.
At predetermined time intervals and up to 24 h, receptor solution were sampled for analysis and replaced with the same volume of fresh solution. The receptor compartment volume was constant during all the experiment. This procedure was needed to ensure sink and quantitative conditions43 to determine small amounts of permeated molecular probe. The dye content in samples was measured by UV-visible spectrophotometry using 4 cm path length quartz cells. The permeation of molecular probe aqueous solution was also investigated in the same way, and used as control.
Induced circular dichroism (ICD) experiments were carried out using a JASCO J-810 instrument. The path length used in all spectroscopic experiments was 1 cm unless otherwise mentioned.
Results and discussion
Niosomes were prepared from a mixture of non-ionic surfactants, whose chemical structures are shown in Scheme 1. A practical, adimensional and empirical parameter that allows predicting vesicles formation is called hydrophilic–lipophilic balance (HLB), which is a measure of the relative contribution of the hydrophilic and lipophilic regions of the surfactant molecules. It was reported in literature that compounds or blends with values of HLB > 12 are water soluble and tend to form micelles, although in some cases, with the adding of some additives as cholesterol, can form vesicles. On the other way, compounds with HLB < 6 are soluble in organic solvents and have the tendency to form water in oil (W/O) emulsions. Instead, compounds with an intermediate value of HLB in the range of 7–11, can form bilayers and in consequence vesicles.13,19,21,44,45In this regard, an aqueous solution of T80 (HLB = 15) tends to form micelles, whereas a solution of S80 (HLB = 4) tends to form W/O emulsions as were experimentally corroborated. Each of surfactants can not form vesicles, however, an equimolar mixture of T80-S80 form vesicles as the parameter HLB = 10 predicted. The total concentration of surfactants was 0.01 M. In the case of niosomes prepared with added CD, the final concentration of β-CD was 1.5 mM and of Mod-β-CD was 1.7 mM. It is worth to notice that it is not possible to dissolve this amount of Mod-β-CD in pure water.
Size and morphology
Table 1 shows the mean diameter and PDI (polydispersity index) obtained by DLS for empty and loaded niosomes in water. In general, mean diameters obtained for the different niosomal formulations (with and without β-CD) were not remarkable different and had a mean diameter of ∼110 nm. In addition, mean diameter does not change considerably due the entrapped molecular probe. In regards to niosomes loaded with Mod-β-CD, the size of the vesicles tends to decrease by the presence of the amphiphilic cyclodextrin, and this result suggests that this CD interacts with the vesicular aggregates. It has been demonstrated in literature that the number of carbohydrate residues in the oligosaccharide chain of the polar headgroup plays a crucial rol to determine the characteristics of the self-assembled structure such as the radius of curvature.46 In this way, it has been reported that the loading of synthetic polymer-lipids47 or hydroxypropyl-β-cyclodextrin48 (HP-β-CD) to liposomes leads to decrease the size of the vesicles. The authors explained that the use of polymer-lipids molecules leads to an asymmetric distribution of synthetic lipids between the outer and inner monolayers as a consequence of a significant bending of the outer layer. In case of using HP-β-CD, the cyclodextrins act shelling the surface charge of the interphase.48 However, the mechanism of particle size reduction by cyclodextrins is unclear and in literature to date are reflected the difficulties to demonstrate, in a direct way, structural evidence that show the location of CDs in surfactants assemblies.49 With regards to the mentioned above, we explained our results considering that the modified cyclodextrin could anchor at the surface of niosomes penetrating in the layer through the hydrophobic chain50 and due the insertion in the outer layer and the presence of cyclodextrin moiety, the area of polar headgroup at the interphase increase and alters the radius of curvature, and thus a decreasing in the size of niosomes was observed. Table 1 also shows that PDI values obtained for all niosomes were equal or lower than 0.5, which indicate that the vesicles are monodisperse.25,29,51,52| Without CDb | With β-CDa | With Mod-β-CDb | |
|---|---|---|---|
| a The errors of diameters correspond to the standard deviation of three measurements.b The values in brackets are the averaged PDI values (n = 3). | |||
| MY | 110 ± 10 (0.5) | 106.9 ± 0.7 (0.24) | 72 ± 1 (0.25) |
| MO | 110 ± 10 (0.5) | 107 ± 2 (0.15) | 71 ± 2 (0.27) |
| Empty | 130 ± 20 (0.40) | 114.2 ± 0.8 (0.18) | 83 ± 8 (0.4) |
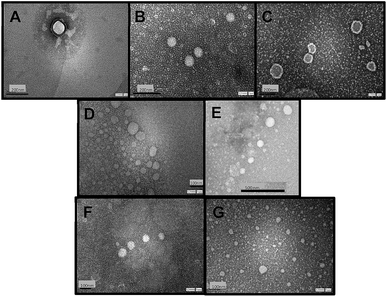
Fig. 1 shows TEM images of empty niosomes (Fig. 1 A), loaded niosomes with molecular probes (Fig. 1 B and C), and niosomes with the incorporation of β-CD (Fig. 1 D) or Mod-β-CD (Fig. 1 E). Fig. 1F and G present β-CD loaded niosomes with the incorporation of MO and MY respectively.
The presence of molecular probes (MY or MO) do not alter the morphology of niosomes, neither empty or cyclodextrin loaded niosomes (Fig. 1). It follows that loading process and addition of the macrocycle do not modify niosomes morphology.24,25 Besides, the size of the vesicles determined from TEM measurements are in good agreement with those determined by DLS measurement.
All niosomal formulations were stable for at least 2 months storaged at 4 °C. The stability of niosomes was assessed by measuring their vesicle size and morphology several times during two months.
Spectrophotometric characterization of the systems
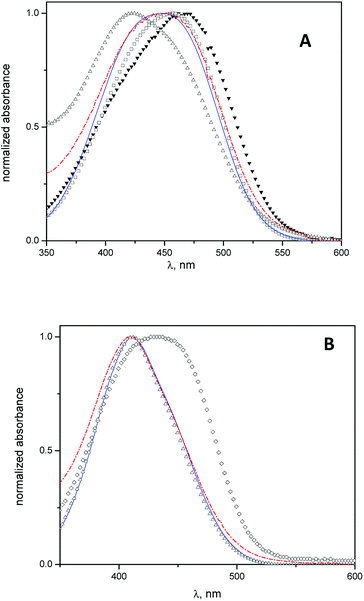
Spectrophotometric studies were carried out using MO and MY as molecular probes with the aim to determine the location of the cyclodextrins into the vesicles.In Fig. 2 A, the absorption spectra of MO in water, β-CD aqueous solution, niosomes, and niosomes with β-CD or Mod-β-CD are shown. The wavelength of MO maximum absorption shifts from 467 nm in water to lower wavelength (λ = 425 nm) in niosomes media. This hypsochromic shift indicates that in niosomes, MO is sensing a less polar environment41 and suggest that it resides in the polar region of interphase but we cannot discard the presence of MO in the entrapped aqueous.
In presence of CDs, the results show that the MO absorption band shifts to lower wavelengths, i.e., λmax = 457 nm in β-CD aqueous solution and λmax = 445 nm in niosomes with β-CD (or Mod-β-CD), in comparison with that obtained in water. These features were attributed to the interaction between MO and CDs by the formation of an inclusion complex.41
On the other hand, the electronic band of the complex MO:CDs in niosomes is quite different, in position and in shape, when they were compared with the spectrum of the complex MO:β-CD in aqueous solution. We interpret our results considering that in niosomes systems, the inclusion process occurs preferably in lower polarity regions such as the interphases. We described previously that a maximum absorption wavelength of 445 nm corresponded to an absorption band of the inclusion complex MO:HP-β-CD in a media with low polarity (reverse micelles of 1,4-bis(2-ethylhexyl)-sulfosuccinate, AOT). In that case we explained the results considering that the complex was formed in the confined water pool inside the reverse micelles.41
Equivalent spectrophotometric analysis was performed for the interaction of MY with niosomes, β-CD niosomes and Mod-β-CD niosomes (Fig. 2B). It is shown that the maximum absorption wavelengths of MY are 432 nm in β-CD aqueous solution, and 411 nm in niosomes (empty or with CDs). As can be seen, there is no differences between the maximum of MY in the last three systems. These results indicate that MY interacts mainly with the niosomes bilayer, instead of with CD.
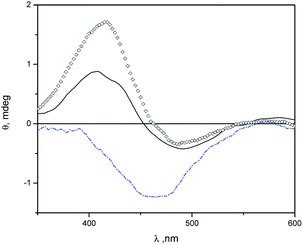
In Fig. 3 we show induced circular dichroism (ICD) spectra of MO in niosomes loaded with β-CD and niosomes loaded with Mod-β-CD. The presence of signals indicate that MO is interacting with the chiral cavity of the CDs in the niosomes. It has been fully demonstrated that when an achiral chromophore is in a chiral environment like that produced by cyclodextrin, ICD signals at wavelengths close to the maximum of absorption of the achiral compound are observed.53
The ICD spectra of MO in aqueous solution of β-CD and in β-CD-niosomes have a splitted signal (a positive and a negative band) whereas in the system Mod-β-CD-niosomes the ICD signal of MO is negative (Fig. 3). These results indicate that the complex MO:Mod-β-CD in niosomes is different from that formed between MO:β-CD (with or without niosomes).
An empirical rule for the analysis of the inclusion complexes of cyclodextrins with chromophoric molecules states that the sign of the induced circular dichroism depends on the orientation of the transition dipole moment in the cyclodextrin's cavity.53–55 Briefly, an ICD positive signal is observed when the electric transition dipole moment of a chromophore included into the cavity is parallel to the principal axis of the cyclodextrin, and the ICD signal is negative if the electric transition dipole moment is perpendicular to the principal axis of the host. The opposite was observed with the electric transition dipole moment of a chromophore that was not included into the cavity of cyclodextrins, but it is close enough to detect the chiral effect of the macrocycle. We proposed that in the niosomes containing β-CD, MO can be included in a deeper form into the cavity, meanwhile in Mod-β-CD niosomes, due to the CD is anchored onto the surface of niosomes, the chromophore can be partially located into the cavity. The presence of a small negative ICD signal, besides the positive one when MO is in presence of β-CD, could be indicating that the chromophore can take different orientations in this macrocycle, probably because the cavity is more available. Additionally, no ICD signals were observed with MY:cyclodextrin niosomes. Probably due to the low water solubility and lower association constant of MY with β-CD, the dye is located in the hydrophobic region of vesicles. We explained these results considering the affinities of MO for the interphase and the cavities of cyclodextrins. The association constant (Kas) for MO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD (1
β-CD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) inclusion complex is 2.97 × 103 M−1.56 In the same conditions MY is very insoluble in water even in the presence of cyclodextrin. In literature, there is a value reported for the apparent formation constant of MY:β-CD complex at pH 1.1 of (49 ± 1) × 101 M−1.57 In those experimental conditions the protonated azo compound is more soluble than at pH 7, however the reported value of Kas allow us estimate that the concentration of complex MY
1) inclusion complex is 2.97 × 103 M−1.56 In the same conditions MY is very insoluble in water even in the presence of cyclodextrin. In literature, there is a value reported for the apparent formation constant of MY:β-CD complex at pH 1.1 of (49 ± 1) × 101 M−1.57 In those experimental conditions the protonated azo compound is more soluble than at pH 7, however the reported value of Kas allow us estimate that the concentration of complex MY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD (1
β-CD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is considerably lower than the value for the complex MO
1) is considerably lower than the value for the complex MO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD (1
β-CD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Considering the log
1). Considering the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for MY58 and MO,59 that are 4.60 and 2.06 respectively, it is reasonable that MY shows stronger affinity for the hydrophobic region of bilayers than MO.
P values for MY58 and MO,59 that are 4.60 and 2.06 respectively, it is reasonable that MY shows stronger affinity for the hydrophobic region of bilayers than MO.
Considering the difference in cyclodextrins' hydrophilicity, we suggest that β-CD is probably located in the polar region of the interphase, in the outer and in the inner monolayers. The fact that β-CD can be incorporated into the polar region may be attributed to the interaction of the OH groups with the polar headgroup of the surfactants. It is known that the OH groups on the exterior of cyclodextrins can form hydrogen bonds with water, or in the same way, interact with the headgroup of surfactants.42,60 We also know from the absorption spectra of MO that this compound is at the interphase, located close to a chiral environment, and this is another evidence of the presence of CD in this region of the vesicle. In the other way, the location of Mod-β-CD in T80-S80 niosomes was explained considering that the presence of the long hydrocarbon tail in its structure helps the incorporation into the interphase since Mod-β-CD is less soluble in water. According with the results of DLS and TEM, Mod-β-CD is distributed mostly in the outer monolayers and thus conduct to a reduction in the size of vesicles.
The ICD experiments showed that the cavities of Mod-β-CD are available to interact with MO and the negative signal indicate that the MO cannot be located in a deeper form, and we suggest that is due to the presence of the tails of surfactants that also competes for the cavities. Scheme 2 summarizes the above discussion.
Entrapment efficiency (EE) and in vitro release studies
According to eqn (1), EE was determined for niosomal formulations with and without CDs. The concentrations of the initial amount and the finally entrapped concentration of molecular probes into niosomes were determined with the corresponding calibration plot. Table 2 shows the obtained values.| Niosomes formulation | MY | MO |
|---|---|---|
| a The errors correspond to the standard deviation of three measurements. | ||
| T80-S80 | 54 ± 8 | 45 ± 8 |
| Mod-β-CD-T80-S80 | 48 ± 5 | 40 ± 5 |
| β-CD-T80-S80 | 60 ± 10 | 57 ± 9 |
It was observed that EE obtained for MY was higher than that obtained for MO in niosomes without CDs, probably due to the MY poor water solubility, therefore it has more affinity for the lipophilic bilayer of the niosome.
EE for MY was not modified by the presence of CDs, meanwhile, the value of EE for MO was slightly increased in niosomes containing β-CD, however the values of EE in Mod-β-CD were not different from those obtained in T80-S80 niosomes (initial concentrations of the dyes were keeping constant).
We explained these results considering the affinities of MO for the interphase and the cavities of cyclodextrins. The increase in EE for MO due to niosomes with β-CD can be attributed to the presence of a complex between MO:β-CD since the association constant for the inclusion complex MO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD (1
β-CD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is considerable, about 3000 M−1 (ref. 56) as was mentioned. In the same conditions MY is very insoluble in water even in the presence of cyclodextrin, so the amount of complex formed with the last compound is considerably lower than with MO. There are some reports of improvements of EE when the drug is incorporated in liposomes as drug/CD inclusion complexes in a similar way to the results here obtained. For instance, the entrapment of curcumin, a broad-spectrum anticancer drug, increased from 30% in CD-free liposomes to 50% when hydroxypropyl-β-CD (HP-β-CD) was added to the system.61 In another study, indomethacin (a nonsteroid anti-inflammatory drug) was included in liposomes of soybean phosphatidylcholine as a complex with β-CD or HP-β-CD, obtaining improvements in the loaded amount of indomethacin of approximately 1.5 times in both cases, in comparison with the experiments without CD.62
1) is considerable, about 3000 M−1 (ref. 56) as was mentioned. In the same conditions MY is very insoluble in water even in the presence of cyclodextrin, so the amount of complex formed with the last compound is considerably lower than with MO. There are some reports of improvements of EE when the drug is incorporated in liposomes as drug/CD inclusion complexes in a similar way to the results here obtained. For instance, the entrapment of curcumin, a broad-spectrum anticancer drug, increased from 30% in CD-free liposomes to 50% when hydroxypropyl-β-CD (HP-β-CD) was added to the system.61 In another study, indomethacin (a nonsteroid anti-inflammatory drug) was included in liposomes of soybean phosphatidylcholine as a complex with β-CD or HP-β-CD, obtaining improvements in the loaded amount of indomethacin of approximately 1.5 times in both cases, in comparison with the experiments without CD.62
In the other hand, Mod-β-CD is located among the tails of surfactants and the hydrocarbon chains are included into the cavity of CD decreasing the complex MO:Mod-β-CD. It worth to mention that not all the cavities are occupied by the tails of surfactants and many of them are able to interact with external guests as we observed from ICD measurement. The higher affinity of MO by β-CD could increase the encapsulated amount of this molecule that is included in the niosome.
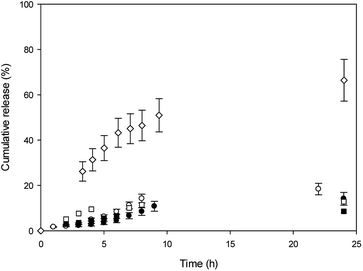
In vitro release experiments were carried out in water at 37 °C, and release profiles are shown in Fig. 4.
With the aim to evaluate CDs effect in the niosomes, the release profiles were shown for comparison. It was observed that MO cumulative release from β-CD niosomes is higher than MO released from T80-S80 niosomes during elapsed time measurements. After 24 h, 70% of MO was released from the niosomes with β-CD, whereas almost only 20% of MO was released from niosomes without the macrocycle. The initial concentration of this dye was the same in both niosome systems. We explained our results considering that in absence of β-CD, the azo dye is released from the aqueous core and the polar region of interphase. In the presence of β-CD, a part of the MO is included into the cavity, and in that way could be more available to the aqueous outside because the location of the macrocycle.
In the other hand, we mentioned that the lower affinity of MO for the cavities of Mod-β-CD in T80-S80 niosomes can be responsible of the lack of assistance of Mod-β-CD during the release experiments and only a release from the niosome was observed.
MY release from T80-S80 niosomes was slower than MO release and we explain these results considering that MY have a high affinity for niosomes due to its lower aqueous solubility, and the presence of Mod-β-CD do not alter the release profile as was expected considering the absence of interaction between MY and Mod-β-CD.
Conclusions
Spectrophotometric studies between molecular probes and cyclodextrin loaded niosomes enabled to infer about the location of the macrocyclic cavity and the dyes in the vesicular system.The niosomes in presence of β-CD allowed to improve entrapped hydrophilic molecular probe amount and in turn, to produce a faster dye release. Even though β-CD presence does not modify considerably niosomes size and shape, positively modifies entrapment ability and release, particularly when the compound is hydrophilic and with certain affinity by cyclodextrin. The incorporation of Mod-β-CD, instead, did not produce entrapment and release differences.
The results presented here shows that cyclodextrin may form part of niosomes. The cyclodextrin modified niosomes have promising properties for entrapment and delivery of different molecules that may be interesting active compounds, fundamentally as food and pharmaceutical additives, due to the adequate nature of the components of the vesicles. We will continue exploring this interesting area.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported in part by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP 112-201101-00441 y PIP 112-2015-01-00242), Agencia Nacional de Ciencia y Tecnología (FONCyT, PICT 2014-2516), Ministerio de Ciencia y Tecnología de la Provincia de Córdoba (MINCyT-Cba, PID 2009-2011) and Universidad Nacional de Córdoba, Argentina (SECyT 30720150100659CB). OFS, RHR and MAF hold researcher positions at CONICET. NDM thanks CONICET for the fellowship granted.Notes and references
- C. Y. Wong, H. Al-Salami and C. R. Dass, Int. J. Pharm., 2018, 537, 223 CrossRef PubMed.
- M. Bai, J. He, L. Kang, J. Nie and R. Yin, Int. J. Biol. Macromol., 2018, 113, 889 CrossRef PubMed.
- C. M. Asensio, A. J. Paredes, M. P. Martin, D. A. Allemandi, V. Nepote and N. R. Grosso, J. Food Sci., 2017, 82, 2864 CrossRef PubMed.
- M. D. Chatzidaki, K. Papadimitriou, V. Alexandraki, F. Balkiza, M. Georgalaki, V. Papadimitriou, E. Tsakalidou and A. Xenakis, Food Chem., 2018, 255, 97 CrossRef PubMed.
- S. Gopalakrishnan and A. Chenthilnathan, Res. J. Pharm., Biol. Chem. Sci., 2012, 3, 1090 Search PubMed.
- N. Akhtar, Curr. Drug Delivery, 2014, 11, 87 CrossRef.
- M. Imran, M. R. Shah, F. Ullah, S. Ullah, A. M. A. Elhissi, W. Nawaz, F. Ahmad, A. Sadiq and I. Ali, Drug Delivery, 2016, 23, 3653 CrossRef PubMed.
- E. Soo, S. Thakur, Z. Qu, S. Jambhrunkar, H. S. Parekh and A. Popat, J. Colloid Interface Sci., 2016, 462, 368 CrossRef PubMed.
- Y. Liu, D. Liu, L. Zhu, Q. Gan and X. Le, Food Res. Int., 2015, 74, 97 CrossRef PubMed.
- T. Uchino, F. Lefeber, G. Gooris and J. Bouwstra, Eur. J. Pharm. Biopharm., 2014, 86, 156 CrossRef PubMed.
- L. Tavano and R. Muzzalupo, Colloids Surf., B, 2016, 147, 161 CrossRef PubMed.
- D. J. McClements, Adv. Colloid Interface Sci., 2015, 219, 27 CrossRef PubMed.
- C. Marianecci, L. Di Marzio, F. Rinaldi, C. Celia, D. Paolino, F. Alhaique, S. Esposito and M. Carafa, Adv. Colloid Interface Sci., 2014, 205, 187 CrossRef PubMed.
- C. Marianecci, S. Petralito, F. Rinaldi, P. N. Hanieh and M. Carafa, J. Drug Delivery Sci. Technol., 2016, 32, 256 CrossRef.
- P. Freixeiro, E. Diéguez-Casal, L. Costoya, B. Seijo, C. M. Ferreirós, M. T. Criado and S. Sánchez, Int. J. Pharm., 2013, 443, 1 CrossRef PubMed.
- I. F. Uchegbu and A. T. Florence, Adv. Colloid Interface Sci., 1995, 58, 1 CrossRef.
- I. F. Uchegbu and S. P. Vyas, Int. J. Pharm., 1998, 172, 33 CrossRef.
- C. Marianecci, L. Di Marzio, E. Del Favero, L. Cantù, P. Brocca, V. Rondelli, F. Rinaldi, L. Dini, A. Serra, P. Decuzzi, C. Celia, D. Paolino, M. Fresta and M. Carafa, Langmuir, 2016, 32, 1241 CrossRef PubMed.
- N. B. Mahale, P. D. Thakkar, R. G. Mali, D. R. Walunj and S. R. Chaudhari, Adv. Colloid Interface Sci., 2012, 183–184, 46 CrossRef PubMed.
- D. Pando, G. Gutiérrez, J. Coca and C. Pazos, J. Food Eng., 2013, 117, 227 CrossRef.
- S. Moghassemi and A. Hadjizadeh, J. Controlled Release, 2014, 185, 22 CrossRef PubMed.
- M. Mashal, N. Attia, G. Puras, G. Martínez-Navarrete, E. Fernández and J. L. Pedraz, J. Controlled Release, 2017, 254, 55 CrossRef PubMed.
- V. Sharma, S. Anandhakumar and M. Sasidharan, Mater. Sci. Eng., C, 2015, 56, 393 CrossRef PubMed.
- S. K. Mehta and N. Jindal, Colloids Surf., B, 2013, 101, 434 CrossRef PubMed.
- L. Tavano, R. Muzzalupo, N. Picci and B. De Cindio, Colloids Surf., B, 2014, 114, 144 CrossRef PubMed.
- L. Tavano, R. Aiello, G. Ioele, N. Picci and R. Muzzalupo, Colloids Surf., B, 2014, 118, 7 CrossRef PubMed.
- D. Pando, M. Matos, G. Gutiérrez and C. Pazos, Colloids Surf., B, 2015, 128, 398 CrossRef PubMed.
- A. H. Alomrani, M. H. Al-Agamy and M. M. Badran, J. Drug Delivery Sci. Technol., 2015, 28, 37 CrossRef.
- D. Pando, M. Beltrán, I. Gerone, M. Matos and C. Pazos, Food Chem., 2015, 170, 281 CrossRef PubMed.
- L. Zhang, Q. Zhang, X. Wang, W. Zhang, C. Lin, F. Chen, X. Yang and W. Pan, Int. J. Pharm., 2015, 492, 40 CrossRef PubMed.
- C. Aloisio, S. G. Antimisiaris and M. R. Longhi, J. Mol. Liq., 2017, 229, 106 CrossRef.
- R. Gharib, H. Greige-Gerges, S. Fourmentin, C. Charcosset and L. Auezova, Carbohydr. Polym., 2015, 129, 175 CrossRef PubMed.
- E. M. M. del Valle, Process Biochem., 2004, 39, 1033 CrossRef.
- O. Elsie, S. B. Tiwari, N. Udupa, K. Ravindra and D. Uma, Indian J. Pharmacol., 1999, 31, 279 Search PubMed.
- I. P. Sheena, U. V. Singh, A. Shirinivas K and N. Udupa, J. Pharm. Pharmacol., 1997, 3, 383 Search PubMed.
- The Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals, ed. S. Budavari, Merck and Co., Inc., Whitehouse Station, NJ, 12th edn, 1996, p. 1041 Search PubMed.
- H. Jaber, W. Mabey, A. Liu, T. Chou, H. Johnson, T. Mill, R. T. Podoll and J. S. Winterle, Data Acquisition for Environmental Transport and Fate Screening for Compounds of Interest to the Office of Emergency and Remedial Response, EPA/600/6-84/011, U.S. Environmental Protection Agency, Washington, D.C., 1984, p. 43 Search PubMed.
- O. F. Silva, M. A. Fernández, S. L. Pennie, R. R. Gil and R. H. de Rossi, Langmuir, 2008, 24, 3718 CrossRef PubMed.
- O. F. Silva, R. H. de Rossi, N. M Correa, J. J. Silber and R. D. Falcone, RSC Adv., 2018, 8, 12535 RSC.
- P. S. Sales, R. H. de Rossi and M. A. Fernández, Chemosphere, 2011, 84, 1700 CrossRef PubMed.
- O. F. Silva, J. J. Silber, R. H. de Rossi, N. M. Correa and M. A. Fernández, J. Phys. Chem. B, 2007, 111, 10703 CrossRef PubMed.
- O. F. Silva, N. M. Correa, J. J. Silber, R. H. de Rossi and M. A. Fernández, Langmuir, 2014, 30, 3354 CrossRef PubMed.
- S.-F. Ng, J. J. Rouse, F. D. Sanderson, V. Meidan and G. M. Eccleston, AAPS PharmSciTech, 2010, 11, 1432 CrossRef PubMed.
- M. Salim, H. Minamikawa, A. Sugimura and R. Hashim, Med. Chem. Commun., 2014, 5, 1602 RSC.
- T. Yoshioka, B. Sternberg and A. T. Florence, Int. J. Pharm., 1994, 105, 1 CrossRef.
- B. Maggio, Biochim. Biophys. Acta, Biomembr., 1985, 815, 245 CrossRef.
- M. Rovira-Bru, D. H. Thompson and I. Szleifer, Biophys. J., 2002, 83, 2419 CrossRef PubMed.
- A. H. Alomrani, G. A. Shazly, A. A. A. F. Amara and M. M. Badran, Colloids Surf., B, 2014, 121, 74 CrossRef PubMed.
- M. Tsianou and A. I. Fajalia, Langmuir, 2014, 30, 13754 CrossRef PubMed.
- J. J. Pinzón Barrantes, B. Maggio, R. H. De Rossi and R. V. Vico, J. Phys. Chem. B, 2017, 121, 4482 CrossRef PubMed.
- D. D. Verma, S. Verma, G. Blume and A. Fahr, Int. J. Pharm., 2003, 258, 141 CrossRef PubMed.
- L. Tavano, L. Gentile, C. Oliviero Rossi and R. Muzzalupo, Colloids Surf., B, 2013, 110, 281 CrossRef PubMed.
- S. Allenmark, Chirality, 2003, 15, 409 CrossRef PubMed.
- K. Harata and H. Uedaira, Bull. Chem. Soc. Jpn., 1975, 48, 375 CrossRef.
- K. Harata, Bioorg. Chem., 1981, 10, 255 CrossRef.
- J. Carrazana, B. Reija, P. R. Cabrer, W. Al-Soufi, M. Novo and J. Vázquez Tato, Supramol. Chem., 2004, 16, 549 CrossRef.
- K. M. Tawarah and H. M. Abu-Shamleh, J. Inclusion Phenom. Mol. Recognit. Chem., 1991, 11, 29 CrossRef.
- https://pubchem.ncbi.nlm.nih.gov.
- Calculated using Chem Draw Ultra 8, Cambridge Soft. Corp., 1985–2003 Search PubMed.
- C. Zhou, X. Cheng, Q. Zhao, Y. Yan, J. Wang and J. Huang, Langmuir, 2013, 29, 13175 CrossRef PubMed.
- S. S Dhule, P. Penfornis, T. Frazier, R. Walker, J. Feldman, G. Tan, J. He, A. Alb, V. John and R. Pochampally, Nanomedicine, 2012, 8, 440 CrossRef PubMed.
- H. Chen, J. Gao, F. Wang and W. Liang, Drug Delivery, 2007, 14, 201 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |