 Open Access Article
Open Access ArticleMolecular characteristics of the refractory organic matter in the anaerobic and aerobic digestates of sewage sludge†
Xiaowei Li a,
Qingqing Meia,
Xiaofang Yana,
Bin Dong*bc,
Xiaohu Dai*bd,
Liangliang Yue,
Yibo Wangf,
Guoji Dinga,
Fang Yua and
John Zhoug
a,
Qingqing Meia,
Xiaofang Yana,
Bin Dong*bc,
Xiaohu Dai*bd,
Liangliang Yue,
Yibo Wangf,
Guoji Dinga,
Fang Yua and
John Zhoug
aSchool of Environmental and Chemical Engineering, Institute for the Conservation of Cultural Heritage, Shanghai University, Shanghai 200444, People’s Republic of China
bState Key Laboratory of Pollution Control and Resources Reuse, National Engineering Research Center for Urban Pollution Control, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, People’s Republic of China. E-mail: daixiaohu@tongji.edu.cn; lixiaowei419@shu.edu.cn
cSchool of Civil Engineering and Architecture, Ningbo Institute of Technology, Zhejiang University, Ningbo 315000, People's Republic of China
dShanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, People's Republic of China
eYanjin Senior High School, Shanghai 200122, P. R. China
fShanghai Key Lab of Bio-energy Crops, School of Life Sciences, Shanghai University, Shanghai 200444, People's Republic of China
gSchool of Civil and Environmental Engineering, University of Technology Sydney, 15 Broadway, Sydney, NSW 2007, Australia
First published on 25th September 2018
Abstract
The chemical characteristics of the refractory organic matter in anaerobic and aerobic digestates are hardly known although they are significant for further improving the degradation of organic matter during sludge digestion. Thus, in this study, various techniques are used to analyze the molecular properties of the total organic matter in raw sludge and mesophilic anaerobic and aerobic digestates (AnD and AoD, respectively). The results show that AnD has lower organic matter content, but the maturity and aromatization of its organic matter are lower than those of AoD. The FTIR and XPS spectra show that AoD has higher proportions of protein-like and aromatic groups and lower percentages of polysaccharide-like materials and ammonia nitrogen compared with AnD. The solid-phase fluorescence spectra indicate that AoD has a higher content of fluorescence organic matter, but its biodegradability and chemical accessibility are lower than those of AnD. Pyrolysis GC/MS analysis shows that the digestates are enriched with more lignin-like and aromatic groups and contain lower oxycompounds compared with raw sludge, especially AoD. These findings provide new insights into the molecular characteristics of the refractory organic matter in anaerobic and aerobic digestates and also provide a possible strategy to further enhance the degradation of organic matter in sewage sludge.
1. Introduction
Anaerobic and aerobic digestion are common processes for the stabilization of sewage sludge,1 which can ensure the stability of sewage sludge and decrease its corresponding volume. Unfortunately, anaerobic or aerobic digestion often results in a low degradation rate of organic matter (often less than 50%) for the sewage sludge treatment.2–5 This indicates that more than 50% of organic matter cannot be biologically degraded during anaerobic or aerobic digestion, thus causing limited sludge reduction. To date, the chemical characteristics of refractory organic matter are hardly known, and they need to be investigated because they can supply important information for further enhancing the degradation rate of organic matter during sludge digestion.Kumar6 suggested that organic matter in raw sewage sludge can be classified as four fractions: (I) a fraction that can be only degraded under aerobic conditions, (II) a fraction that can be only degraded under anaerobic conditions, (III) a fraction that can be degraded under both anaerobic and aerobic conditions, and (IV) a non-degradation fraction.7 However, information about the relative percentage and chemical composition of these organic fractions in sewage sludge is lacking. After anaerobic digestion, the residual organic matter in sewage sludge (anaerobic digestate, AnD) consists of organic fractions I and IV, and the organic fractions II and IV remain in sewage sludge after aerobic digestion (aerobic digestate, AoD). Therefore, it is feasible to resolve the characteristics of these organic fractions in sewage sludge by comparing the organic matter compositions in anaerobic and aerobic digestates.
Some studies have reported that the degradation of sludge organic matter during anaerobic and aerobic digestion is different due to different conditions1,8 such as different functional microbes.9 Shao et al.1 showed that the solubility of protein and the humification index of dissolved organic matter (DOM) in aerobic digestion are greater than that in anaerobic digestion for sewage sludge stabilization. Du and Li10 reported that anaerobic digestion results in faster hydrolysis and slower degradation, thus causing more DOM compared with aerobic digestion. Tomei et al.11 reported that dissolved polysaccharides and proteins considerably decrease under aerobic digestion but present clear enrichment under anaerobic digestion. Novak et al.12 showed that aerobic digestion leads to lower contents of dissolved protein and polysaccharides than anaerobic digestion. Li et al.13 observed that there is a different degradation order in sludge organic matter under anaerobic and aerobic conditions. However, these investigations were mainly based on DOM analysis and not total organic matter. It is well-known that the DOM content is often less than 5% of the total organic matter; thus, it is difficult to comprehensively reveal the characteristics of different organic fractions in sewage sludge.
FTIR spectroscopy is widely applied to characterize different functional groups of both fluorescent and non-fluorescent organic materials,14 whereas X-ray photoelectron spectroscopy (XPS) analysis is commonly used to determine the changes in chemical speciation of the main organic matter during sewage sludge treatment.9,15 Research has shown that solid-phase fluorescence excitation–emission matrix (SPF EEM) spectroscopy is a promising technique to investigate fluorescent organic matter such as humic acid and protein in solid samples;16–19 compared with the widely-used liquid-phase fluorescence spectroscopy on water-extractable organic matter, it is more rapid, comprehensive and accurate because no extraction step is required.16 In addition, pyrolysis coupled with gas chromatography (GC) and mass spectrometry (MS) is a fast, sensitive and reliable way to determine the molecular composition of complex organic mixtures.20,21 Thus, in this study, these techniques are applied to distinguish and quantify the chemical characteristics of the total organic matter in raw sludge, AnD and AoD at the molecular level to profoundly resolve the characteristics of different organic fractions in sewage sludge.
In addition, digestates contain many readily available nutrients such as nitrogen, phosphate and plant hormones,22 which make them suitable for the fertilization of crops.23 Muscolo et al.24 reported that compared with undigested wastes, digestates have a higher content of inorganic nitrogen (ammonium ion) and enhanced microbial stability and hygiene. However, depending on their chemical features, some digestates can have a negative impact on the environment or plant growth, whereas some may influence soil fertility and plant health positively.25 Thus, digestates cannot be definitively regarded as positive or negative for soil and agronomic utilization. Therefore, it is also indispensable to characterize each digestate from different digestion systems (anaerobic and aerobic) to determine the proper utilization of sludge digestates.
The objectives of this study are as follows: (1) to study the chemical characteristics of residual organic matter in solid samples of AnD and AoD using various techniques; (2) to analyze the molecular composition of different degradable organic fractions in sewage sludge; and (3) to compare the difference in potential values for land applications between AnD and AoD.
2. Materials and methods
2.1. Substrates and digestion processes
Dewatered sewage sludge samples as substrates were obtained from a wastewater treatment plant in Shenzhen City, China, and then stored at 4 °C. The sludge was obtained by collecting primary and excess sludge and dewatering with a high-molecular flocculant (polyacrylamide). The characteristics of the wastewater treatment plant are shown in Table S1 of the ESI.† The total solid (TS) and volatile solid (VS) contents of the sludge were 17.77 ± 0.10% and 63.43 ± 0.07%, respectively.The anaerobic digestion process was performed in a horizontal reactor with an effective volume of 12 L and operated in a batch mode for 20 days according to previous studies.26,27 Initially, 7.608 kg dewatered sewage sludge and 4.450 kg inoculated sludge were fed into the reactor. The inoculated sludge was obtained from the previous anaerobic reactor in our laboratory, and its characteristics are as follows: pH, 7.85 ± 0.18; total alkalinity, 17.8 ± 0.9 g CaCO3/L; TS, 13.07 ± 0.07%; VS/TS, 46.92 ± 0.41%; total Kjeldahl nitrogen (TKN), 8300 ± 121 mg L−1; and total ammonia nitrogen 3500 ± 43 mg L−1. The experiment was conducted in duplicate. The production of biogas in the anaerobic digestion reactor is shown in Fig. S1 in the ESI,† which indicates that the anaerobic reactor performance is normal and representative.
The aerobic digestion process was conducted in a reactor with an effective volume of 7.5 L and equipped with vertical-type stirrers with a rate of 20 rpm. Initially, 1.960 kg dewatered sewage sludge was added to the reactor and then, the total solid level was adjusted between 5% and 6% using deionized water according to previously reported methods.28,29 The aerobic reactor was run in batch mode at 35 ± 1 °C for 20 days and aerated continuously at a flow rate of 135 L h−1 through a microporous diffuser to maintain the dissolved oxygen at more than 2 mg L−1. Evaporation loss was compensated using distilled water every day.1,9 The experiment was conducted in duplicate.
2.2. Analysis of organic matter content
Raw sludge (RS) was sampled from the dewatered sewage sludge, whereas mesophilic anaerobic and aerobic digestates (AnD and AoD) were collected at the end of the experiment. The sludge samples were freeze-dried, sieved through a 0.149 mm sieve, and then applied for the following analysis. The VS content was estimated by heating the samples at 600 °C for 1 h in a muffle furnace. The ultimate analysis was conducted using a Vario EL III element analyzer (Elementar, Germany).Dissolved chemical oxygen demand (DCOD), protein, carbohydrate and total phosphorus (DTP) contents, and ammonia nitrogen (NH4–N) content were determined using the filtrate of the samples according to ref. 30. The filtrate was obtained according to ref. 13. The dissolved protein content was measured using the Lowry–Folin method,31 whereas the dissolved carbohydrate content was determined via the phenol-sulfuric method.32
2.3. Analysis of organic matter characteristics
FTIR spectra of the sludge samples were measured with pellets gained according to ref. 26 using a Nicolet iS50 FTIR spectrometer (Thermo Fisher Scientific Co., USA). The OMNIC 8.2.0.387 and Surfer 11.0 softwares were applied to analyze the FTIR spectral data. XPS spectra of the samples were obtained on an RBD-upgraded PHI-5000C ESCA photoelectron spectrometer (Perkin Elmer, USA) using Al Kα radiation (hv = 1486.6 eV) as the X-ray source for excitation according to Li et al.9 The RBD AugerScan 3.21 software was applied to analyze the XPS spectral data.SPF EEM spectra of the sludge samples were determined on an F-7000 fluorescence spectrometer (Hitachi High Technologies, Tokyo, Japan) using the solid module and without quartz glass according to ref. 18. Excitation spectra were measured in the range from 240 to 450 nm, whereas the corresponding emission range was from 290 to 550 nm at a scan speed of 1200 nm min−1. The variation and slit of both the excitation and emission spectra were 10 nm and 1 nm in the testing process, respectively. The Surfer 8.0 software was applied for the analysis of the EEM data.
The complexity index of the organic matter in the samples was further estimated using the EEM data according to ref. 33–36 with slight modifications. As shown in Fig. S2 of the ESI,† the obtained spectra were resolved into three zones (zones I–III).34 Zone I is related to protein-like materials, zone II is generally due to an inner filter phenomena, and zone III represents the lignocellulose-like and humic acid-like compounds.34 The complex index is defined as the ratio of the average fluorescence intensity of the complex fluorescence zones (II and III) to the average fluorescence intensity of the protein-like zones (I):
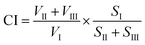 | (1) |
The sludge samples were used for pyrolysis GC/MS analysis according to ref. 20. Pyrolysis was conducted with a Py-2020iD pyrolyser (Frontier Laboratories, Japan) directly connected to a QP2010 GC/MS system (Shimadzu, Japan) equipped with a 30 m HP DB-5MS capillary column (0.25 mm diameter, 0.25 μm film thickness). The sample weight was about 1 mg, and the pyrolysis temperature was 500 °C. The GC-MS conditions were as follows: the oven temperature was 40 °C for 3 min and then increased to 300 °C at a rate of 15 °C min−1 and held for 10 min. Helium (He) was used as the carrier gas with a flow of 30 mL min−1 and split ratio of 20 before being introduced into the gas chromatograph. The pyrolysis products were appraised using the Wiley and NIST computer libraries according to the relative retention times and spectra reported in the literature. The relative percentage, Pi, of each pyrolysis product was estimated according to ref. 21 and 37.
2.4. Phytotoxicity test
The phytotoxicity test was conducted using plant seeds of wheat (Triticum aestivum) and radish (Raphanus sativus L.) according to ref. 9. Before the experiment, the seeds were soaked in deionized water for 6–10 h. The inhibition of seed germination was estimated with respect to that of the control according to ref. 38 and 39.3. Results and discussion
3.1. Organic matter contents
The organic matter contents of RS, AnD and AoD are shown in Table S2 in the ESI.† Compared with RS, AnD and AoD have lower VS contents. The VS removal rates of anaerobic and aerobic digestion are 47.14% and 42.07%, respectively, which are higher than the threshold value (40%) stipulated by the Chinese Discharge Standard (GB 18918-2002). The results imply that both anaerobic and aerobic digestions cause the biodegradation of sludge organic matter and exhibit acceptable performances for the stabilization of sewage sludge. However, it is found that about 52% and 58% of the organic matters in the sewage sludge are not degraded and still exist in the anaerobic and aerobic digestates, respectively. To date, information about the molecular characteristics of the refractory organic matters in AnD and AoD is limited.AnD and AoD have lower C, N and H contents and C/N and C/H ratios and slightly higher S content than RS. The C/N ratio is usually applied to evaluate the maturity and stabilization of the substrate,5 and the C/H ratio is used to represent the aromatization and condensation of organic matter such as humic substances.40 The results indicate that the anaerobic and aerobic digestion processes cause biodegradation of labile C- and N-containing (protein-like) and H-(carbohydrate-like) groups and an increase in the maturity and aromatization of organic matter in the sewage sludge.41 Compared with AnD, AoD has higher VS, C, N and H contents and slightly lower S content and C/N and C/H ratios, indicating that the content of residual organic matter in the anaerobic digestate is lower than that in the aerobic digestate, and its maturity and aromatization also seem to be lower than that of the latter.
Compared with the digestates of anaerobic digestion, the digestates from aerobic digestion have lower pH value as well as lower DCOD, NH4–N, dissolved protein, carbohydrate and DTP contents (Fig. 1), implying that AoD has less dissolved matter than AnD, which is in accordance with the findings of Du and Li.10 Meanwhile, AoD exhibits less inhibition on the germination rate and root length of the radish and wheat seeds than raw sludge, followed by AnD (Fig. S2 of the ESI†). This result indicates that compared with raw sludge, AoD has lower phytotoxicity, and AnD has higher phytotoxicity, which confirms the findings from the analysis of the total organic matter and DOM contents.
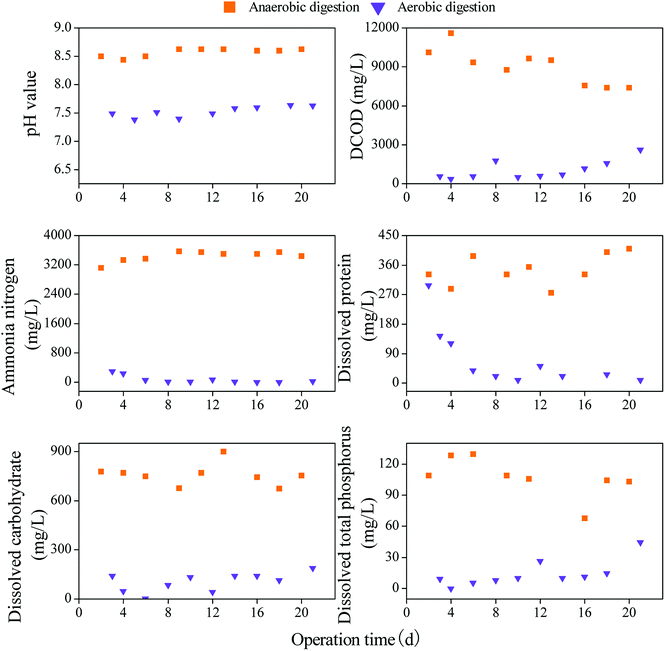 | ||
| Fig. 1 Comparison of the dissolved matter contents in the anaerobic and aerobic digestates of sewage sludge. | ||
The results indicate that AnD has less residual organic matters and higher DOMs than AoD, and the maturity and aromatization of the organic matter also appear to be lower than that of the latter; thus, AnD has higher phytotoxicity than AoD.
3.2. FTIR spectra
The 1800–900 cm−1 region in the FTIR spectra contains bands relative to amides, carboxylic acids, aliphatic groups and carbohydrates and thus is mainly analyzed and discussed,26,42 as shown in Fig. 2. The main absorption bands in this region and corresponding assignments are further analyzed and identified according to the literature13,26,41–43 (Table S3 in the ESI†). In general, the main differences in the FTIR spectra among RS, AnD and AoD are outlined as follows: (1) the relative intensity of the absorbance in the 1610–1700 cm−1 region is stronger for RS than for AoD, followed by AnD; (2) the peak in the 1530–1590 cm−1 region is weaker for AnD than for AoD, followed by RS; (3) the absorption peak in the 1370–1420 cm−1 region is more intense for AoD than for AnD, followed by RS; and (4) the peak in the 1000–1170 cm−1 region is stronger for AnD than for RS, followed by AoD. The results show that the anaerobic or aerobic digestates contain lower proportions of amide I and II groups (protein-like groups) and higher percentages of phenolic, COO− (carboxylic acids) and/or C–H (cellulose-like) groups compared with raw sewage sludge. This implies that the degradation rate of protein-like materials in the sewage sludge is higher than those of phenolic groups, carboxylic acids and/or cellulose-like groups during anaerobic or aerobic digestion, corresponding to the previous findings.5,44,45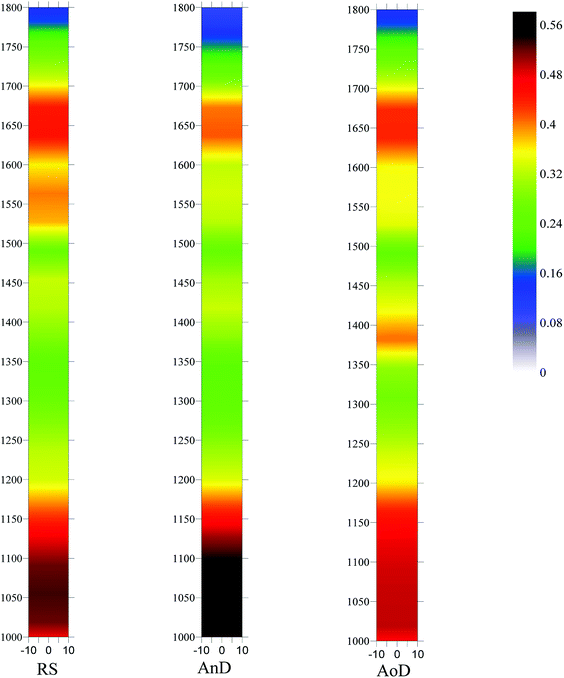 | ||
| Fig. 2 FTIR spectra of RS, AnD and AoD samples. RS, raw sludge; AnD, mesophilic anaerobic digestate; AoD, mesophilic aerobic digestate. | ||
Compared with AnD, AoD exhibited higher proportions of amide I and II groups (protein-like groups), phenolic groups, carboxylic acids and/or cellulose-like groups and lower proportion of polysaccharide-like substances. This result shows that the aerobic digestate has higher proportions of protein-like and aromatic groups and a lower percentage of polysaccharide-like materials compared with the anaerobic digestate. This indicates that the polysaccharide-like groups in the sewage sludge are more biodegraded via aerobic digestion than via anaerobic digestion, whereas the protein-like groups show the opposite trend.
3.3. XPS analysis
XPS analysis was applied to determine the chemical speciation of organic elements in the sludge samples. The representative peaks of the major elements, i.e., C, O and N are shown in Fig. 3. According to previous ref. 15, 46 and 47, each peak corresponds to different chemical bonds. The binding energy, assignments and quantitation of main elements (C, O and N) in the XPS spectra of RS, AnD and AoD are shown in Table 1. Both AnD and AoD have lower proportions of C–H, C–(O,N), C–OH/C–O–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds and higher percentages of C–C, C
N bonds and higher percentages of C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H bonds compared with RS. The results indicate that after anaerobic or aerobic digestion, the organic matter in the digestates is composed of lower proportions of protein-like (C
O and N–H bonds compared with RS. The results indicate that after anaerobic or aerobic digestion, the organic matter in the digestates is composed of lower proportions of protein-like (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N bonds) and carbohydrate-like (C–OH and C–H bonds) groups and higher percentages of aromatic materials (C–C bond), carboxylic acid (C
N and C–N bonds) and carbohydrate-like (C–OH and C–H bonds) groups and higher percentages of aromatic materials (C–C bond), carboxylic acid (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond) and ammonia (N–H). Furthermore, these results confirm the findings based on the FTIR spectra.
O bond) and ammonia (N–H). Furthermore, these results confirm the findings based on the FTIR spectra.
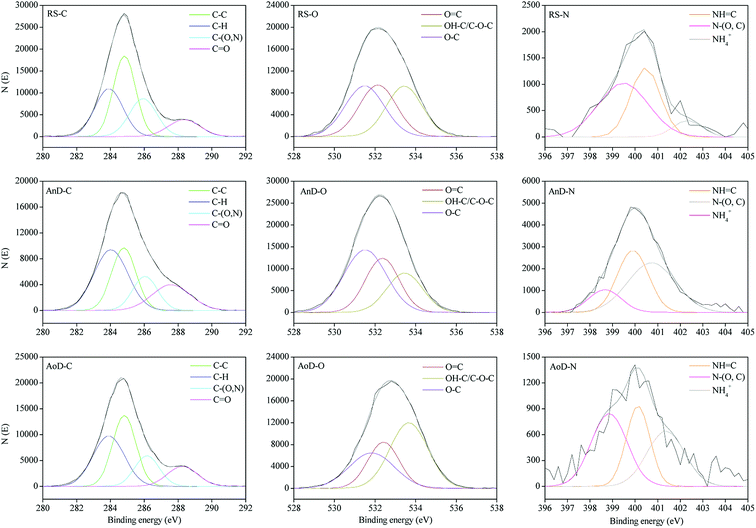 | ||
| Fig. 3 XPS spectra of RS, AnD and AoD samples. RS, raw sludge; AnD, mesophilic anaerobic digestate; AoD, mesophilic aerobic digestate. | ||
| Element | Peak (eV) | RS | AnD | AoD | Assignments |
|---|---|---|---|---|---|
| Atomic ratio (%) | |||||
| C 1s | |||||
| 1 | 283.95 ± 0.06 | 28.50 | 37.90 | 34.50 | C–C |
| 2 | 284.81 ± 0.02 | 38.10 | 28.20 | 36.00 | C–H |
| 3 | 286.05 ± 0.09 | 22.00 | 15.60 | 16.40 | C–(O,N) |
| 4 | 288.07 ± 0.34 | 11.40 | 18.20 | 13.10 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| O 1s | |||||
| 1 | 531.61 ± 0.16 | 34.70 | 45.00 | 28.70 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
| 2 | 532.31 ± 0.12 | 33.40 | 31.00 | 26.20 | OH–C/C–O–C |
| 3 | 533.52 ± 0.10 | 31.80 | 24.00 | 45.10 | C–O |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| N 1s | |||||
| 1 | 399.00 ± 0.35 | 51.50 | 14.60 | 39.50 | N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
| 2 | 400.16 ± 0.23 | 39.40 | 38.10 | 28.80 | N–(O,C) |
| 3 | 401.47 ± 0.66 | 9.10 | 47.30 | 31.70 | N–H |
Compared with AnD, AoD has higher percentages of C–H, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds and lower percentages of C
N bonds and lower percentages of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, OH–C/C–O–C, N-(O,C) and N–H bonds, implying that the refractory organic matter in AoD consists of higher percentages of protein-like and ester-like (such as phenolic) groups and lower proportions of polysaccharide-like materials and ammonia nitrogen. These results confirm the results from the FTIR spectra and the previous findings that there is considerable difference in the degradation characteristics of organic matter during anaerobic and aerobic digestion of sludge.1,11–13
O, OH–C/C–O–C, N-(O,C) and N–H bonds, implying that the refractory organic matter in AoD consists of higher percentages of protein-like and ester-like (such as phenolic) groups and lower proportions of polysaccharide-like materials and ammonia nitrogen. These results confirm the results from the FTIR spectra and the previous findings that there is considerable difference in the degradation characteristics of organic matter during anaerobic and aerobic digestion of sludge.1,11–13
3.4. SPF EEM spectra
SPF EEM spectroscopy was applied to determine the organic matter in the solid samples of RS, AnD and AoD, and the results are shown in Fig. 4. The samples are characterized by several fluorophores with their respective excitation/emission wavelength pairs and specific fluorescence intensity (Table 2). Three peaks (peak 1, 2 and 3) are observed for Ex/Em of 280/334 nm, 320/410 nm and 370/416–418 nm, respectively. According to Chen et al.,33 peak 1 corresponds to microbial by-products (i.e., tryptophan- and protein-like groups), and both peaks 2 and 3 are related to humic-like substances.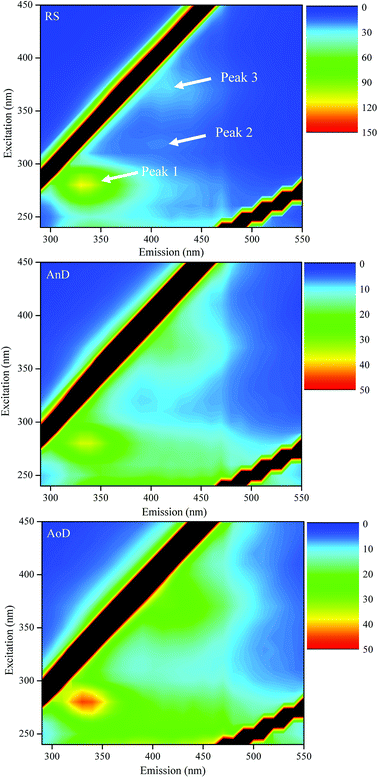 | ||
| Fig. 4 Solid-phase fluorescence EEM spectra of RS, AnD and AoD samples. RS, raw sludge; AnD, mesophilic anaerobic digestate; AoD, mesophilic aerobic digestate. | ||
The specific fluorescence intensity of peak 1 is higher for RS than for AoD and AnD, corresponding to the results of VS, C, N and H contents. The results show that the protein-like groups in the sewage sludge are biodegraded during anaerobic or aerobic digestion, which are consistent with the findings of the FTIR and XPS spectra. Li et al.26 reported that anaerobic digestion causes an increase in concentration of tyrosine-, protein- and humic-like materials by liquid-phase fluorescence EEM spectroscopy for samples with the same contents of dissolved organic carbon, which seems contrary to this result. They indicated that SPF EMM spectra might display different results as compared to liquid-phase spectra due to their different investigation methods. In general, anaerobic and aerobic digestions can cause decrease in the bulk content of fluorescence groups in the sludge, but they seem to lead to an enhancement in the percentage of fluorescence materials in DOM.
The complexity index of RS is lower than those of AnD and AoD, which indicates that organic matter of the digestates has a higher degree of complexity. Muller et al.34 and Maynaud et al.35 reported that the complexity index is mostly anti-correlated with biodegradability and sludge chemical accessibility. Thus, the results confirm the findings from the C/N ratio that the organic matter of the digestates has high biological stability. Additionally, compared with AnD, AoD has higher specific fluorescence intensities for peaks 1 and 3 and complexity index, indicating that AoD has a higher content of fluorescence organic matter, but its fluorescence organic matter has lower biodegradability and chemical accessibility than that in AnD, which is in accordance with the results of organic matter contents.
3.5. Pyrolysis GC/MS analysis
The pyrolysis GC/MS technique is commonly used to reveal the organic matter characteristics of soil and compost at the molecular level.21,48,49 Thus, in the present study, it is first applied to investigate the molecular characteristics of refractory organic matters in anaerobic and aerobic digestates. The total ion chromatograms derived from RS, AnD and AoD are shown in Fig. 5. The absolute intensities of ion currents from AnD and AoD are lower than that from RS, showing that the contents of pyrolytic products from the anaerobic and aerobic digestates are lower than that from raw sewage sludge. The possible reason is that anaerobic and aerobic digestates have lower organic matter contents (Table S2 of the ESI†) compared with raw sewage sludge due to biodegradation of organic matter during the digestion process. However, absolute intensities of ion current are higher in the AnD pyrogram than in the AoD pyrogram, which is not consistent with the results that VS, C, N and H contents of AnD are lower than that of AoD. The results based on C/N ratio and SPF EEM spectra reveal that the stability of the residual organic matter in AoD is higher than that in AnD. The possible reason is that a lower proportion of organic matter in AoD is cracked into pyrolysis products at the temperature used (500 °C) compared with that in AnD due to higher stabilization of the organic matter.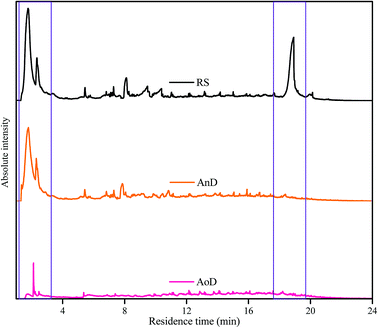 | ||
| Fig. 5 Analytical pyrograms (Py-GC/MS) of RS, AnD and AoD samples. RS, raw sludge; AnD, mesophilic anaerobic digestate; AoD, mesophilic aerobic digestate. | ||
The main difference in the pyrograms of RS, AnD and AoD is found at two elution times (1–4 min and 17–19 min). The ion current trace of RS in the range of 1–4 min is similar to that of AnD but different from that of AoD. González-Vila et al.20 reported that the low-molecular-weight products released at the elution time of 1–4 min are mainly polysaccharide- and protein-derived products. The result implies that RS and AnD have similar compositions of polysaccharides and proteins, which is different from that observed for AoD. Additionally, a distinct peak appears in the pyrogram of RS at the elution time of 17–19 min but disappears in those of AnD and AoD. The possible reason is that some lignin-like groups in the sewage sludge are decomposed during the anaerobic and aerobic digestions.20 Some specific microorganisms, such as certain strains of bacteria, have an enzyme pool that can degrade lignin and its derivatives.21
To further investigate the molecular composition of the pyrolysis products derived from RS, AnD and AoD, MS is used to analyze the chemical characteristics of the main peaks in the pyrograms, and the corresponding potential precursors are estimated according to ref. 20, 21 and 49–52, as outlined in Table 3. More than 50 different molecules are identified in the pyrograms of RS, AnD and AoD. The compounds are categorized into the following component classes according to their probable origins: aliphatic and aromatic groups, N-containing compounds, lignin, sulfocompounds and unspecified oxycompounds. Compared with AnD and AoD, RS has higher relative percentages of unspecified oxycompounds and fatty-acids products. Alvarez et al.53 found that oxygenated compounds originate primarily from the pyrolysis of undigested materials in sludge such as cellulose, lignin and fecal matter. Also, Wang et al.49 reported that chain fatty acids decrease gradually with increasing maturity of composting samples. Thus, the results confirm the above findings from the organic matter content analysis. In addition, AoD has higher relative percentages of N-containing compounds than AnD. Alvarez et al.53 suggested that the N-containing compounds are formed by the devolatilization of the protein fraction (proteins and nucleic acids) in the sludge derived from dead microorganisms. Thus, the result confirms the findings that the aerobic digestate contains a higher proportion of protein-like groups than the anaerobic digestate.
| Pyrolysis product | Molecular formula | Potential precursor | Relative percentage Pi (%) | ||
|---|---|---|---|---|---|
| RS | AnD | AoD | |||
| a ND, no data. | |||||
| 1-Propene, 2-methyl- | C4H8 | Aliphatic groups | ND | ND | 4.92 |
| 1-Heptene | C7H14 | Aliphatic groups | ND | 0.1 | ND |
| Heptane | C7H16 | Aliphatic groups | ND | 0.08 | ND |
| Hexane, 2,4-dimethyl- | C8H18 | Aliphatic groups | 0.26 | 0.41 | ND |
| 1-Nonene | C9H18 | Aliphatic groups | 0.47 | 0.23 | ND |
| 1-Decene | C10H20 | Aliphatic groups | 0.07 | 0.21 | ND |
| Decane | C10H22 | Aliphatic groups | 0.06 | ND | ND |
| 1-Undecene | C11H22 | Aliphatic groups | ND | 0.47 | 1.9 |
| Undecane | C11H24 | Aliphatic groups | ND | 0.4 | ND |
| 1-Dodecene | C12H24 | Aliphatic groups | ND | ND | 3.31 |
| Dodecane | C12H26 | Aliphatic groups | 0.18 | 0.77 | ND |
| 1-Tridecene | C13H26 | Aliphatic groups | 0.48 | 0.21 | ND |
| Tridecane | C13H28 | Aliphatic groups | 0.15 | ND | 3.28 |
| Undecane, 4,7-dimethyl- | C13H28 | Aliphatic groups | ND | 0.24 | ND |
| 3-Tetradecene, (Z)- | C14H28 | Aliphatic groups | 0.13 | 0.37 | 2.9 |
| Cetene | C16H32 | Aliphatic groups | ND | ND | 4.43 |
| 3-Hexadecene, (Z)- | C16H32 | Aliphatic groups | ND | 0.15 | ND |
| Hexadecane | C16H34 | Aliphatic groups | 0.15 | 0.37 | 0.8 |
| Pentadecane, 2,6,10-trimethyl- | C18H38 | Aliphatic groups | ND | 0.15 | ND |
| cis-2-Methyl-7-octadecene | C19H38 | Aliphatic groups | ND | 0.16 | ND |
| Hexadecane, 2,6,10,14-tetramethyl- | C20H42 | Aliphatic groups | ND | 0.22 | ND |
| Eicosane | C20H42 | Aliphatic groups | ND | 0.53 | ND |
| Toluene | C7H8 | Aromatic groups | 0.81 | 0.99 | 3.12 |
| Styrene | C8H8 | Aromatic groups | 0.68 | 1.19 | 1.61 |
| Ethylbenzene | C8H10 | Aromatic groups | 0.28 | ND | 1.24 |
| o-Xylene | C8H10 | Aromatic groups | ND | 0.82 | ND |
| Acetonitrile | C2H3N | N-containing compound | ND | ND | 5.06 |
| Benzyl nitrile | C8H7N | N-containing compound | ND | ND | 2.9 |
| Indolizine | C8H7N | N-containing compound | ND | ND | 6.97 |
| 1H-Indole, 2-methyl- | C9H9N | N-containing compound | ND | 0.23 | 4.61 |
| Pentadecanenitrile | C15H29N | N-containing compound | 0.3 | 0.09 | 1.9 |
| Oleanitrile | C18H33N | N-containing compound | ND | ND | 2.82 |
| Octadecanenitrile | C18H35N | N-containing compound | ND | ND | 1.63 |
| Butanamide | C4H9NO | N-containing compound | ND | 1.78 | ND |
| Hexadecanamide | C16H33NO | N-containing compound | 0.47 | ND | ND |
| 1-[(1-oxo-2-Propenyl)oxy]-2,5-pyrrolidinedione | C7H7NO4 | N-containing compound | 0.36 | ND | ND |
| p-Cresol | C7H8O | Lignin | ND | 1.26 | ND |
| Phenol, 2-methyl- | C7H8O | Lignin | 0.39 | 2.4 | 16.08 |
| 2,2-Dimethyl-1-oxa-spiro[2.3] hexane | C7H12O | Unspecified oxycompound | ND | 0.34 | ND |
| 1-Nonanol, 4,8-dimethyl- | C11H24O | Unspecified oxycompound | 0.06 | ND | ND |
| 2-Tridecanone | C13H26O | Unspecified oxycompound | ND | 0.08 | ND |
| 2-Heptadecanone | C17H34O | Unspecified oxycompound | ND | 0.05 | ND |
| Carbon dioxide | CO2 | Unspecified oxycompound | 57.66 | 78.9 | 27.07 |
| Acetic acid | C2H4O2 | Unspecified oxycompound | 4.58 | 5.02 | ND |
| Crotonic acid | C4H6O2 | Unspecified oxycompound | 2.88 | ND | ND |
| 2-Pentenoic acid | C5H8O2 | Unspecified oxycompound | 0.56 | ND | ND |
| trans-2-Pentenoic acid | C5H8O2 | Unspecified oxycompound | 2.56 | ND | ND |
| 2-Propenoic acid, 2-propenyl ester | C6H8O2 | Unspecified oxycompound | ND | 0.91 | ND |
| n-Hexadecanoic acid | C16H32O2 | Unspecified oxycompound | 25.64 | 0.7 | 3.45 |
| Oleic acid | C18H34O2 | Unspecified oxycompound | 0.82 | ND | ND |
| Sulfur dioxide | SO2 | Sulfocompound | ND | 0.17 | ND |
CO2 is a ubiquitous pyrolysis product and hence, it is of unspecified origin. A higher relative percentage of CO2 is observed for AnD than that for RS and AoD, which is possibly because anaerobic digestate often contains high contents of short-chain fatty acids54 and higher carbonate alkalinity.27 Hexadecanoic and oleic acids are common microbial constituents.49 AnD and AoD have lower percentages of hexadecanoic and oleic acids, which indicates that the microbial quantities in the anaerobic and aerobic digestates decrease with the decrease in readily biodegradable organic matters in the sewage sludge. Compared with the observations for RS, the relative proportion of phenolic compounds increases in the pyrograms of AnD and AoD, indicating that the biotic and abiotic oxidation of the lignin derivatives is relatively slower than the biodegradation rate of other sludge components during the digestion process. In addition, acetonitrile is mostly derived from condensed nitrogenous humic structures,55 and acetic fragments are principally derived from cellulose, lipids, and easily degradable C compounds.55 Acetonitrile is observed in the pyrogram of AoD, but acetic acid is absent, which imply that resistant N-compounds are present in the residual organic matter in AoD as a consequence of the degradation of the labile substrate, corresponding to the above results that AoD has high bio-stabilization and maturity.
In summary, the results show that the organic matters in the sludge digestates are composed of higher relative percentages of protein-like, lignin-like and aromatic groups but a lower relative percentage of oxycompounds compared with undigested sludge, especially for aerobic digestates.
3.6. The implication of this study
Kumar6 reported that there are four organic fractions in sewage sludge; however, the relative percentage and chemical composition of these fractions in sludge are hardly known. Novak et al.4 reported that combined anaerobic and aerobic digestion can increase the reduction of VS solids in sewage sludge by 12% compared with conventional mesophilic anaerobic digestion alone. They showed that fraction I is degradable only under aerobic conditions, which is 11.48–12.00% of the organic matter in sewage sludge. Then, the relative percentage of fraction III in organic matter of sewage sludge that is degradable under both anaerobic and aerobic conditions is 30.07–30.59%, according to the removal rate of organic matter in the anaerobic digestion of sewage sludge. Similarly, fractions II and IV account for 16.55–17.07% and 40.34–41.9% of organic matters in sewage sludge, respectively. These results indicate that fractions III and IV are the main organic matters in sewage sludge and thus, both anaerobic and aerobic digestions are feasible for the stabilization of sewage sludge.The FTIR and XPS spectra show that the polysaccharide-like groups in the sewage sludge are more biodegraded in aerobic digestion than in anaerobic digestion, whereas the protein-like groups show the opposite trend. The results imply that fraction I contains more polysaccharide-like materials, but fraction II has more protein-like and aromatic groups. This indicates that the pretreatment methods for improving the biodegradation of sludge organic matter should be different for anaerobic and aerobic digestions, especially enzymatic pretreatment. For example, glycohydrolase can be used to enhance the degradation of polysaccharide-like materials during sludge anaerobic digestion, whereas protease can be applied to improve the decomposition of protein-like groups in sludge aerobic digestion. However, the following observation needs to be further investigated: the polysaccharide-like materials in sludge are more easily degraded in aerobic digestion than in anaerobic digestion, but the protein-like groups show the opposite trend. Furthermore, the pyrolysis GC/MS analysis reveals that the organic matter in the aerobic or anaerobic digestate has higher relative percentages of protein-like, lignin-like and aromatic groups than that in raw sludge, implying that these organic matters belong to fraction IV. Therefore, it is significant to promote the biodegradation of fraction IV, which consists of protein-like, lignin-like and aromatic groups, for further enhancing the sludge degradation rate during the digestion process. In summary, these findings supply a new insight into the chemical composition of the different organic fractions in sewage sludge and lay a foundation for improving the sludge organic degradation rate.
Additionally, the results for total organic matter contents show that AoD has higher maturity and aromatization than AnD, implying that AoD has higher potential for land application, which is in accordance with the results of the phytotoxicity test. However, AnD has higher ammonia nitrogen than AoD (Fig. 1), indicating that AnD contains more available nutrients for plant growth such as nitrogen. Therefore, it is very important to balance the environmental safety and fertilizer efficiency for land utilization of digestates, which deserves further investigation.
4. Conclusion
The relative percentages of different organic fractions in sewage sludge and their degradation properties under anaerobic and aerobic digestion are estimated, implying that fractions III and IV are the main organic matters in the sewage sludge. More polysaccharide-like groups in sewage sludge are not biodegraded during anaerobic digestion; this trend is also observed for more protein-like and aromatic materials during aerobic digestion. Meanwhile, lignin-like and aromatic groups cannot be decomposed in both anaerobic and aerobic digestions. These results indicate that it is important to promote the biodegradation of these organic matters during sludge digestion using targeted pretreatment methods. Additionally, AnD has lower contents of organic matter but higher chemical accessibility, available nutrients (e.g., NH4–N), and phytotoxicity than AoD. Thus, it is very important to balance the environmental safety and fertilizer efficiency of sludge digestates for land utilization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was financially supported by the National Natural Scientific Foundation of China (51408423, 51578397, 51538008), Program of Shanghai Technology Research Leader Grant (17XD1420500), Key Program for International S&T Cooperation Projects of China (2016YFE0123500), Key Laboratory Open Project of Yangzi River Water Environment (YRWEF201604), Support Plan for Young Teachers by Shanghai Education Committee (ZZSD16027) and Cultural heritage protection Key Innovation Team in Shanghai High-level Local University.References
- L. Shao, T. Wang, T. Li, F. Lu and P. He, Bioresour. Technol., 2013, 140, 131–137 CrossRef CAS PubMed.
- S. Liu, N. Zhu and L. Y. Li, Bioresour. Technol., 2012, 104, 266–273 CrossRef CAS PubMed.
- G. Yang, P. Zhang, G. Zhang, Y. Wang and A. Yang, Bioresour. Technol., 2015, 192, 126–130 CrossRef CAS PubMed.
- J. T. Novak, S. Banjade and S. N. Murthy, Water Res., 2011, 45, 618–624 CrossRef CAS PubMed.
- X. Li, L. Chen, X. Dai, Q. Mei and G. Ding, J. Anal. Appl. Pyrolysis, 2017, 126, 288–297 CrossRef CAS.
- N. Kumar, Master Science thesis, Virginia Polytechnic Institute and State University, 2006.
- C. Rock, A. Alum and M. Abbaszadegan, Appl. Environ. Microbiol., 2010, 76, 8102–8109 CrossRef CAS PubMed.
- S. Banjade, Master degree thesis, Virginia Polytechnic Institute and State University, 2008.
- X. Li, Z. Li, X. Dai, B. Dong and Y. Tang, RSC Adv., 2016, 6, 76748–76758 RSC.
- H. Du and F. Li, Chemosphere, 2017, 168, 1022–1031 CrossRef CAS PubMed.
- M. C. Tomei, S. Rita and G. Mininni, New Biotechnol., 2011, 29, 17–22 CrossRef CAS PubMed.
- J. T. Novak, M. E. Sadler and S. N. Murthy, Water Res., 2003, 37, 3136–3144 CrossRef CAS PubMed.
- X. Li, X. Dai, L. Dai and Z. Liu, RSC Adv., 2015, 5, 82087–82096 RSC.
- X. Li, X. Dai, S. Yuan, N. Li, Z. Liu and J. Jin, Bioresour. Technol., 2015, 175, 245–253 CrossRef CAS PubMed.
- L. Hao, S. N. Liss and B. Q. Liao, Water Res., 2016, 89, 132–141 CrossRef CAS PubMed.
- R. Albrecht, E. Verrecchia and H. R. Pfeifer, Talanta, 2015, 134, 453–459 CrossRef CAS PubMed.
- F. Ammari, R. Bendoula, B. D. Jouanrimbaud, D. N. Rutledge and J. M. Roger, Talanta, 2014, 125, 146 CrossRef CAS PubMed.
- S. Mounier, G. Nicolodelli, R. Redon and D. M. Milori, Spectrochim. Acta, Part A, 2017, 177, 79–85 CrossRef CAS PubMed.
- M. Muller, D. M. Milori, S. Déléris, J. P. Steyer and Y. Dudal, Waste Manage., 2011, 31, 1916–1923 CrossRef CAS PubMed.
- F. J. González-Vila, J. A. González-Pérez, K. Akdi, M. D. Gómis, F. Pérez-Barrera and T. Verdejo, Bioresour. Technol., 2009, 100, 1304–1309 CrossRef PubMed.
- L. E. Fels, L. Lemee, A. Ambles and M. Hafidi, Int. Biodeterior. Biodegrad., 2014, 92, 26–35 CrossRef.
- X. Li, J. Guo, R. Dong, B. K. Ahring and W. Zhang, Sci. Total Environ., 2015, 544, 774–781 CrossRef PubMed.
- H. I. Owamah, S. O. Dahunsi, U. S. Oranusi and M. I. Alfa, Waste Manage., 2014, 34, 747–752 CrossRef CAS PubMed.
- A. Muscolo, G. Settineri, T. Papalia, E. Attinà, C. Basile and M. R. Panuccio, Sci. Total Environ., 2017, 586, 746–752 CrossRef CAS PubMed.
- M. R. Panuccio, E. Attinà, C. Basile, C. Mallamaci and A. Muscolo, Waste Biomass Valorization, 2016, 7, 267–280 CrossRef CAS.
- X. Li, X. Dai, J. Takahashi, N. Li, J. Jin, L. Dai and B. Dong, Bioresour. Technol., 2014, 159, 412–420 CrossRef CAS PubMed.
- N. Duan, B. Dong, B. WU and X. Dai, Bioresour. Technol., 2012, 104, 150–156 CrossRef CAS PubMed.
- S. Liu, N. Zhu and L. Y. Li, Bioresour. Technol., 2012, 104, 266–273 CrossRef CAS PubMed.
- S. Liu, N. Zhu and L. Y. Li, Chem. Eng. J., 2011, 174, 564–570 CrossRef CAS.
- SEPAC, Analytical and monitoring methods of water and wastewater, China environmental science press, Beijing, 2002 Search PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- D. Herbert, P. J. Philipps and R. E. Strange, Methods Enzymol., 1971, 5B, 265–277 Search PubMed.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Environ. Sci. Technol., 2003, 37, 5701–5710 CrossRef CAS PubMed.
- M. Muller, J. Jimenez, M. Antonini, Y. Dudal, E. Latrille, F. Vedrenne, J. P. Steyer and D. Patureau, Waste Manage., 2014, 34, 2572–2580 CrossRef CAS PubMed.
- G. Maynaud, C. Druilhe, M. Daumoin, J. Jimenez, D. Patureau, M. Torrijos, A. M. Pourcher and N. Wéry, Bioresour. Technol., 2017, 231, 65–74 CrossRef CAS PubMed.
- J. Jimenez, Q. Aemig, N. Doussiet, J.-P. Steyer, S. Houot and D. Patureau, Bioresour. Technol., 2015, 194, 344–353 CrossRef CAS PubMed.
- S. Amir, R. Abouelwafa, A. Meddich, S. Souabi, P. Winterton, G. Merlina, J. C. Revel, E. Pinelli and M. Hafidi, Int. Biodeterior. Biodegrad., 2010, 64, 614–621 CrossRef CAS.
- P. Oleszczuk, A. Malara, I. Jośko and A. Lesiuk, Water, Air, Soil Pollut., 2012, 223, 4937–4948 CrossRef CAS PubMed.
- G. K. Ofosubudu, J. N. Hogarh, J. N. Fobil, A. Quaye, S. K. A. Danso and D. Carboo, Resour., Conserv. Recycl., 2010, 54, 205–209 CrossRef.
- J. Polak, W. Sułkowski, M. Bartoszek and W. Papież, J. Mol. Struct., 2005, 744, 983–989 CrossRef.
- X. Li, M. Xing, J. Yang and Z. Huang, J. Hazard. Mater., 2011, 185, 740–748 CrossRef CAS PubMed.
- H. A. N. Abdulla, E. C. Minor, R. F. Dias and P. G. Hatcher, Geochim. Cosmochim. Acta, 2010, 74, 3815–3838 CrossRef CAS.
- E. Romero, C. Plaza, N. Senesi, R. Nogales and A. Polo, Geoderma, 2007, 139, 397–406 CrossRef CAS.
- M. J. Cuetos, X. Gomez, M. Otero and A. Moran, Biodegradation, 2010, 21, 543–556 CrossRef CAS PubMed.
- T. Abbasi, S. M. Tauseef and S. A. Abbasi, Renewable Sustainable Energy Rev., 2012, 16, 3228–3242 CrossRef CAS.
- H. Liu, G.-Q. Luo, H.-Y. Hu, Q. Zhang, J.-K. Yang and H. Yao, J. Hazard. Mater., 2012, 235, 298–306 CrossRef PubMed.
- B. Liao, H. Lin, S. Langevin, W. Gao and G. Leppard, Water Res., 2011, 45, 509–520 CrossRef CAS PubMed.
- S. Derenne and K. Quénéa, J. Anal. Appl. Pyrolysis, 2015, 111, 108–120 CrossRef CAS.
- K. Wang, C. He, S. You, W. Liu, W. Wang, R. Zhang, H. Qi and N. Ren, J. Hazard. Mater., 2015, 300, 745–753 CrossRef CAS PubMed.
- J. M. D. L. Rosa, J. A. González-Pérez, R. González-Vázquez, H. Knicker, E. López-Capel, D. A. C. Manning and F. J. González-Vila, Catena, 2008, 74, 296–303 CrossRef.
- M. F. Dignac, S. Houot and S. Derenne, J. Anal. Appl. Pyrolysis, 2006, 75, 128–139 CrossRef CAS.
- S. Xiong, J. Zhuo, B. Zhang and Q. Yao, J. Anal. Appl. Pyrolysis, 2013, 104, 632–639 CrossRef CAS.
- J. Alvarez, G. Lopez, M. Amutio, M. Artetxe, I. Barbarias, A. Arregi, J. Bilbao and M. Olazar, Fuel Process. Technol., 2016, 149, 169–175 CrossRef CAS.
- M. A. Bustamante, J. A. Alburquerque, A. P. Restrepo, C. de la Fuente, C. Paredes, R. Moral and M. P. Bernal, Biomass Bioenergy, 2012, 43, 26–35 CrossRef CAS.
- S. Marinari, G. Masciandaro, B. Ceccanti and S. Grego, Bioresour. Technol., 2007, 98, 2495–2502 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures associated with this article as mentioned in the main text. See DOI: 10.1039/c8ra05009k |
| This journal is © The Royal Society of Chemistry 2018 |
