Open Access Article
Open Access ArticleRetracted Article: Down-regulated LncR-MALAT1 suppressed cell proliferation and migration by inactivating autophagy in bladder cancer
Jiude Qi†
a,
Yanfeng Chu†b,
Guangyan Zhangc,
Hongjun Lid,
Dongdong Yange and
Qi Wang *f
*f
aDepartment of Oncology, People's Hospital of Laiwu, Shandong 271100, China
bDepartment of Laboratory, Yantaishan Hospital, Shandong 264000, China
cDepartment of Laboratory, People's Hospital of Zhangqiu District, Shandong 250200, China
dDepartment of EGG Laboratory, Traditional Chinese Medicine Hospital of Zhangqiu Dirtrict, Shandong 250200, China
eDepartment of Nursing, People's Hospital of Zhangqiu District, Shandong 250000, China
fDepartment of Urology, Qingdao Municipal Hospital, No. 5 Donghai Middle Road, Shinan District, Qingdao, Shandong 266001, China. E-mail: wangqisdqd@163.com; Tel: +86-0523-82789159
First published on 4th September 2018
Abstract
Long non-coding RNA-metastasis-associated lung adenocarcinoma transcript (LncR-MALAT) is highly expressed in a variety of tumors, which can affect the progression of tumor cells. LncR-MALAT1 was reported to affect the proliferation of pancreatic cancer and glioma cells by regulating autophagy, but how LncR-MALAT1 affects the proliferation and invasion of various cancer cells by regulating autophagy in bladder cancer has not been reported. Therefore, in this study, we aimed to investigate the effect of LncR-MALAT1 on cell proliferation, apoptosis, invasion and autophagy of bladder cancer and the possible mechanism in vitro. The results showed that LncR-MALAT1 was highly expressed in bladder cancer tissues and cells. The silence of LncR-MALAT1 inhibited the proliferation and invasion and promoted apoptosis in bladder cancer cells. In addition, MALAT1 shRNA down-regulated the expression of Beclin1 and the LC3 II/I ratio, enhanced the expression of p62 and played a significant role in autophagy inhibition. By further investigating the relevant regulatory mechanisms, we found that MALATI shRNA reduced the phosphorylation of AMPK and increased the phosphorylation level of mTOR, thereby inhibiting the activation of the AMPK/mTOR pathway. It is noteworthy that the AMPK/mTOR pathway activator, metformin, partially reversed the effect of MALAT1 shRNA on the inhibition of autophagy in bladder cancer cells. At the same time, the proliferation and invasion ability of HT-1376 cells inhibited by MALAT1 shRNA were also enhanced. The results showed that down-regulation of LncR-MALAT1 could inhibit the proliferation and invasion of bladder cancer cells by attenuating autophagy via the regulation of the AMPK/mTOR pathway.
Introduction
Bladder cancer is the ninth most common tumor in the world.1 In recent years, the incidence of bladder cancer has increased year by year. There were about 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bladder cancer cases in 2012 and the mortality rate was nearly 60%.2 Bladder cancer can be divided into urothelial cell carcinoma, squamous cell carcinoma and adenocarcinoma, among which urothelial cell carcinoma is the most common one.3 At present, the treatment for bladder cancer mainly depends on surgery, but the 5 year survival rate is less than 50% because of the high recurrence rate and metastasis risk.4,5 Therefore, getting a better understanding of the pathogenesis of bladder cancer and finding a new therapeutic target have become urgent tasks.
000 bladder cancer cases in 2012 and the mortality rate was nearly 60%.2 Bladder cancer can be divided into urothelial cell carcinoma, squamous cell carcinoma and adenocarcinoma, among which urothelial cell carcinoma is the most common one.3 At present, the treatment for bladder cancer mainly depends on surgery, but the 5 year survival rate is less than 50% because of the high recurrence rate and metastasis risk.4,5 Therefore, getting a better understanding of the pathogenesis of bladder cancer and finding a new therapeutic target have become urgent tasks.
Human genome sequencing results showed that only 2% of the human genome was the protein coding gene, and more than 80% of the DNA is transcribed as RNA without protein coding. Such RNA is called noncoding RNA.6 Recent researches have shown that lncRNAs play an important role in gene transcription, shear, cell cycle regulation and immune response.7,8 LncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a long-chain non-coding RNA found in 2003, which is located on chromosome 11q13.1.9 Investigations have shown that LncR-MALAT1 was highly expressed in a variety of tumor tissues, such as lung cancer, breast cancer, liver cancer, pancreatic cancer and bladder cancer.10–13 Previous reports demonstrated that knockdown of MALAT1 inhibited the proliferation of human osteosarcoma cell and suppressed the expression of proliferating cell nuclear antigen (PCNA).14 Others also reported that the abnormal expression of MALAT1 in bladder cancer was closely related to cancer development and prognosis.15
Autophagy is a highly conserved metabolic pathway in eukaryotic cells which removes harmful substances in the cell, provides nutrients and energy for cells, thus maintaining cell metabolic balance.16 Investigations have shown that autophagy is closely related to a variety of diseases, such as aging, neurodegenerative diseases and cancer.17–19 Among them, autophagy plays a dual role as tumor suppressor and tumor promoter in the process of cancer development. It affects cell mobility by influencing the phenotype of cells and also provides nutritional support for the proliferation of tumor cells.19,20 It has also been reported that LncR-MALAT1 can influence the proliferation, mobility and multidrug resistance of pancreatic cancer cells by regulating autophagy.21 However, how LncR-MALAT1 affects the proliferation and invasion of various cancer cells by regulating autophagy in bladder cancer has not been reported.
The aim of this investigation was to investigate the related mechanism of LncR-MALAT1 in regulating the proliferation and invasion in bladder cancer cells. We found that MALAT1 showed a high expression in bladder cancer. MALAT1 shRNA inhibited the proliferation and invasion of bladder cancer cells, promoted cell apoptosis, inhibited autophagy and inactivated AMPK/mTOR pathway. The activation of AMPK/mTOR pathway reversed the effect of MALAT1 shRNA on cell proliferation, apoptosis, invasion and autophagy. Taken together, our study demonstrated that down-regulation of LncRNA MALAT1 inhibited the proliferation and invasion of bladder cancer cells by inhibiting autophagy via the AMPK/mTOR pathway.
Materials and methods
Samples collection
The specimens were obtained from 30 patients who were underwent surgical treatment for bladder cancer between March 2016 and March 2017 at the Qingdao Municipal Hospital. All patients had no chemotherapy and radiotherapy before surgery. All of 30 patients, 23 were male and 7 were female, aged 58.23 ± 9.41 years (range 30–76). Tissue samples were immediately snap-frozen in liquid nitrogen after cutting then stored in −80 °C until RNA extraction. In this experiment, the patients signed the informed consent and got the approval from the ethics committee of the Qingdao Municipal Hospital (KYLL-2017(ks)-1243). All experiments were performed in compliance with the relevant laws or guidelines of People's Hospital of LaiwuCell lines culture
The bladder cancer cell lines RT4, HT-1376, RT112, 253J, T24 and normal urinary tract epithelial cells SV-HUC-1 were derived from the Chinese Academy of Sciences cell bank. SV-HUC-1 cells were cultured with 10% fetal bovine serum F-12K medium (21127030, Gibco, Rockville, MD). Other cells were cultured in RPMI 1640 medium (11875119, Gibco, Rockville, MD) containing 10% fetal bovine serum. All cells were cultured in a humidified incubator at 37 °C with an atmosphere of 5% CO2.Quantitative real-time polymerase chain reaction
Total RNA was extracted from cells and tissues according to the manufacturer protocols of TRIzol kit (12183555, Invitrogen, Carlsbad, USA) and was quantified by NanoDrop2000 (Thermo Fisher Scientific, Waltham, USA). Then cDNA was synthesized using reverse transcription kits (AM1314, Thermo Fisher Scientific, Waltham, USA) following the manufacturer protocols. Quantitative analysis of cDNA was performed by using a fluorescence quantitative PCR kit (F416XL, Thermo Fisher Scientific, Waltham, USA) following the manufacturer protocols. The amplification primers used were as follows: MALAT1 sense 5′-CTCACTAAA GGCACCGAAGG-3′, MALAT1 anti-sense 5′-GGCAGAGAAGTT GCTTGTGG-3′. The expression level of MALAT1 was detected with GAPDH as the internal reference. GAPDH sense 5′-GAAGGTGAAGGTCGGAGTCA-3′, GAPDH anti-sense 5′-TGGACTCCACGACGTACTCA-3′. Relative expression was calculated by 2−ΔΔCt method.Cell transfection
Cell transfection was transfected according to the DharmaFECT kit (13778075, Life Technologies, Grand Island, NY) transfection instructions. The MALAT1 used in shRNA was purchased from Shanghai Aibos Biological Technology Co., Ltd. The sequence was as follows: 5′-GACCTTGAAATCCATCACG-3′, with a shRNA vector without the intended sequence as a negative control. Cells were cultured in 6-well plates at 60% confluency. Next day, 100 nM of MALAT1 shRNA or negative control (MALAT1 scramble) and DharmaFECT Transfection Reagent (Life Technologies, Grand Island, NY) were diluted in Opti-MEM Reduced Serum Medium (Gibco), respectively, co-incubated for 20 min at room temperature and added into each well. After transfection of MALAT1 shRNA or MALAT1 scramble for 48 h, the cells were collected for subsequent experiments.Cell proliferation test
The cell proliferation was examined in accordance with the CCK-8 kit (C0038, Dojindo; Kumamoto, Japan) instructions after the HT-1376 cells and 253J cells were transfected with MALAT1 shRNA. Cells were incubated in 10% CCK-8 solution diluted with normal medium for 4 h at 37 °C. At last the absorbance at 450 nm was detected with a spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA). The proliferation folds were calculated by formula the absorbance post transfection/the absorbance before transfection.Transwell invasion experiment
Cell invasion was measured by using a 24-well plate containing transwell chamber (8 μm pore size, Corning). The cells were grown in a pre-coated matrigel chamber, and a complete culture medium containing 10% fetal bovine serum was cultured in the lower chamber. The cells on the surface of the membrane were wiped off using a sterile cotton swab after 24 hours of incubation. The cells that hit the lower layers of the cells were stained with hematoxylin and the ten fields were randomly selected for statistical purposes.Flow cytometric analysis
Cells in each group were harvested at 48 h post-transfection. For the apoptosis analysis, cells were collected (1 × 106) and resuspend in 200 μl binding buffer (1×). Cell density should be 5 × 105/ml, then were stained using the Annexin V-fluorescein isothiocyanate (FITC) and PI (BMS500FI-100, Annexin V-FITC Apoptosis Detection Kit, Thermo Fisher Scientific, Waltham, USA) following the manufacturer protocols. Then the apoptosis rates were analyzed using the FACS Caliber II sorter and Cell Quest FACS system (BD Bio-sciences, San Jose, CA, USA) according to the manufacturer's protocols. The flow cytometry analysis was repeated at least three times.Western blot
Cells were lysed in lysis buffer supplemented with phenylmethanesulfonyl fluoride (PMSF) to extract proteins. The protein concentration was detected by using the BCA protein assay (Tiangen, China). 30 μg proteins per lane were separated by 10% SDS-PAGE and transferred to PDVF membrane (Millipore, Billerica, USA). The PVDF membrane was placed in 5% skimmed milk and blocked, and the primary (PCNA (ab18197, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), MMP-3 (ab53015, 1
1000), MMP-3 (ab53015, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), Bax (ab53154, 1
1000), Bax (ab53154, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), LC3 II/I (ab48394, 1
1000), LC3 II/I (ab48394, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), P62, Beclin1 (ab91526, 1
500), P62, Beclin1 (ab91526, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), AMPK (ab3760, 1
500), AMPK (ab3760, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), p-AMPK (ab109402, 1
1000), p-AMPK (ab109402, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), Mtor (ab2732, 1
1000), Mtor (ab2732, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000), p-mTOR (ab84400, 1
2000), p-mTOR (ab84400, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400)) and secondary antibodies (ab205718, 1
400)) and secondary antibodies (ab205718, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) were incubated (all primary antibodies were purchased from Abcam, Cambridge, MA) and tested with a ChemiDoc XRS imaging system.
2000) were incubated (all primary antibodies were purchased from Abcam, Cambridge, MA) and tested with a ChemiDoc XRS imaging system.
Immunofluorescence staining
After 8 h, cells were fixed in 4% paraformaldehyde in PBS at room temperature for 10 min, then permeabilized with 0.3% Triton X-100 for 10 min at room temperature. After washing in PBS, the cells were incubated with primary antibody (ab51520, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) and secondary antibodies (ab205718, 1
2000) and secondary antibodies (ab205718, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) (all antibodies were purchased from Abcam, Cambridge, MA). Then cells were counterstained with 10 mg ml−1 DAPI. The cells were examined under a Nikon fluorescence microscope (Image Systems, Columbia, MD).
2000) (all antibodies were purchased from Abcam, Cambridge, MA). Then cells were counterstained with 10 mg ml−1 DAPI. The cells were examined under a Nikon fluorescence microscope (Image Systems, Columbia, MD).
Statistical analysis
SPSS 19.0 software was used for statistical analysis. The data were expressed as mean ± standard deviation. T test was used between the two groups, and more groups were compared with ANOVA, with P < 0.05 indicating a statistically significant difference.Results
Expression of MALAT1 in bladder cancer tissues and cells
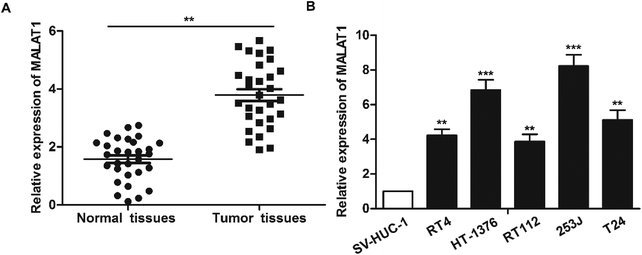
The expression of LncR-MALAT1 in bladder cancer tissues, adjacent healthy tissues, bladder cancer cell lines and normal urinary epithelial cells was detected through qRT-PCR. The results showed that the expression of LncR-MALAT1 in bladder cancer tissues was significantly higher than that in normal healthy bladder tissues (Fig. 1A, **P < 0.01). Besides, the expression of LncR-MALAT1 in bladder cancer cell lines was also up-regulated compared with the normal urinary epithelial cells (Fig. 1B, **P < 0.01, ***P < 0.01). The results showed that LncR-MALAT1 was highly expressed in bladder cancer tissues and cells.Effect of MALAT1 shRNA on bladder cancer cell proliferation and invasion
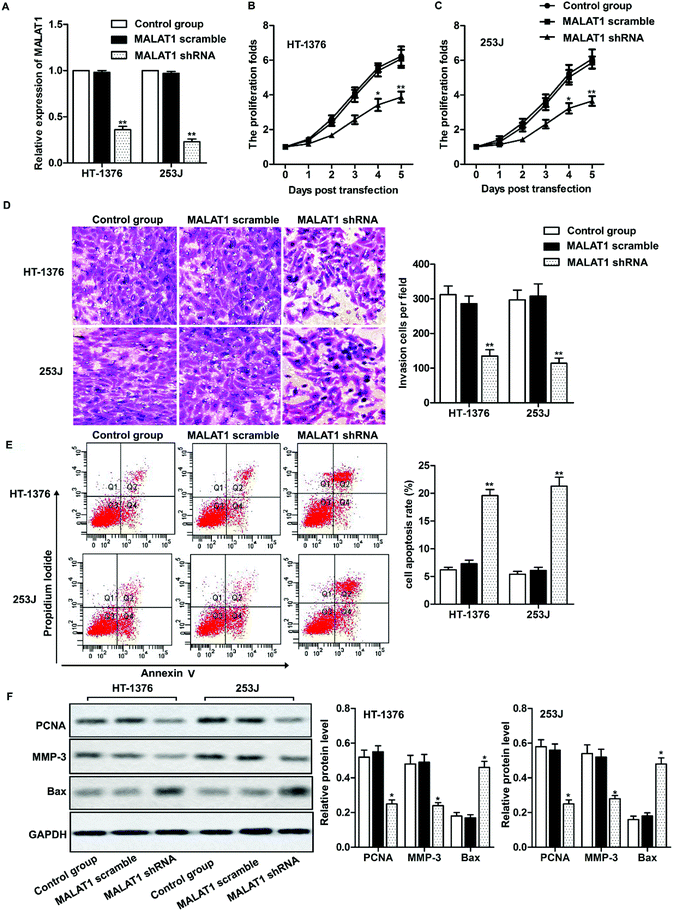
Two kinds of cell lines (HT-1376 cells and 253J cells) with the highest expression of LncR-MALAT1 were selected for the following experiments. After transfection of MALAT1 shRNA or MALAT1 scramble for 48 h, qRT-PCR was used to detect the transfection efficiency. The expression of MALAT1 was measured by qRT-PCR. The transfection efficiency was quantified by calculating the percentage of reduced expression of MALAT1. As shown in Fig. 2A, the expression of MALAT1 in HT-1376 cells and 253J cells was significantly decreased by 80% after MALAT1 shRNA transfection, indicating successful transfection (P < 0.01). The results of CCK8 assay showed that MALAT1 shRNA significantly inhibited the proliferation of HT-1376 cells and 253J cells and the expression of proliferating cell nuclear antigen (PCNA) compared with the control group (Fig. 2B, C and F, *P < 0.05, **P < 0.01). What is more, transwell invasion assay and western blot showed that MALAT1 shRNA significantly inhibited the invasion of bladder cancer cells and decreased the expression of matrix metalloprotein-3 (MMP-3) (Fig. 2D and F, *P < 0.05, **P < 0.01). At the same time, cell apoptosis was largely promoted by MALAT1 shRNA detected through flow cytometry (Fig. 2E, **P < 0.01). The expression of pro-apoptotic protein Bax was also obviously elevated by MALAT1 shRNA (Fig. 2F, *P < 0.05). In summary, MALAT1 shRNA inhibited the proliferation and invasion of bladder cancer cells.MALAT1 shRNA inactivated autophagy and inactivated the AMPK/mTOR pathway
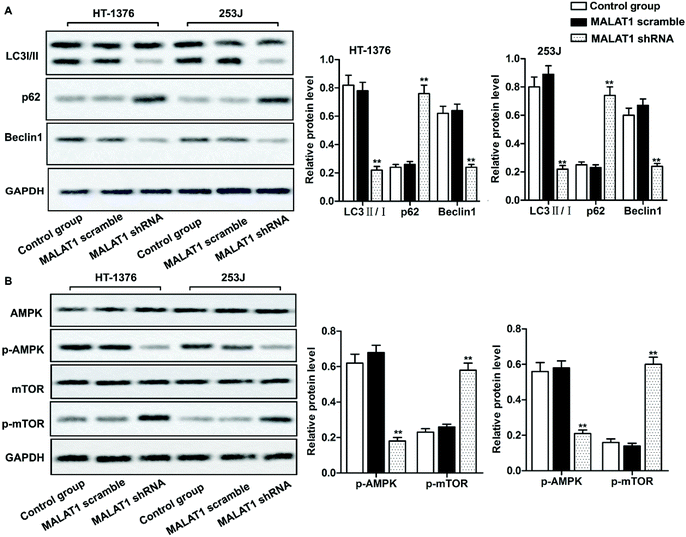
Investigations have shown that regulation of autophagy can affect the development of bladder cancer, and AMPK/mTOR signaling pathway plays an important role in regulating cell autophagy.22,23 We detected the phosphorylation of autophagy-related protein (AMPK and mTOR) by western blot, in order to investigate whether MALAT1 shRNA affects the autophagy of bladder cancer by regulating AMPK/mTOR signaling pathway. The results showed that MALAT1 shRNA significantly inhibited autophagy by down-regulating the expression of Beclin1 in HT-1376 cells and 253J cells, increasing the expression of p62 and decreasing the ratio of LC3 II/I (Fig. 3A, **P < 0.01) compared with control group. At the same time, MALAT1 shRNA significantly inactivated the AMPK/mTOR pathway by suppressing the phosphorylation level of AMPK in bladder cancer cells and increasing the phosphorylation level of mTOR (Fig. 3B, **P < 0.01). It indicated that down-regulation of MALAT1 expression may inhibit autophagy via inactivating AMPK/mTOR pathway.Suppression of MALAT1 inhibited cell proliferation and invasion by inactivating autophagy via the AMPK/mTOR pathway
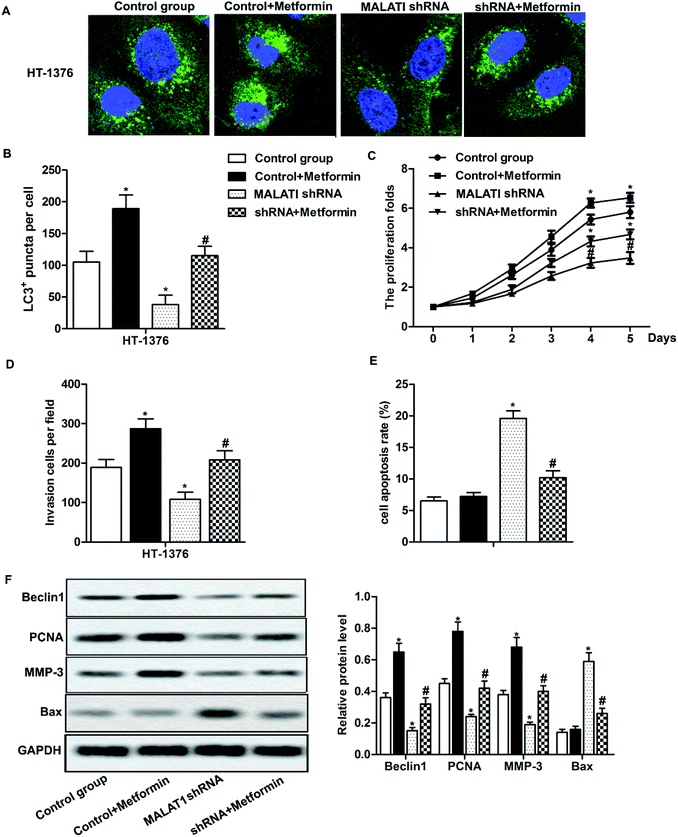
Previous research has shown that autophagy can promote the proliferation and migration of pancreatic cancer cells.24 In our previous investigations, we have shown that MALAT1 shRNA inhibited the proliferation and invasion of bladder cancer cells and may inhibit autophagy through the AMPK/mTOR pathway. To further investigate that whether MALAT1 regulated the proliferation and invasion of bladder cancer cells by regulating autophagy, anAMPK/mTOR pathway activator metformin was used to activate the AMPK/mTOR pathway. The results showed that the adding of metformin abolished the inhibiting effect of MALAT1 shRNA on autophagy and significantly increased the number of LC3 fluorescence focus and Beclin1 expression compared with MALAT1 shRNA group (Fig. 4A, B and F, *P < 0.05, #P < 0.05). At the same time, the addition of metformin reversed the effect of MALAT1 shRNA on cell proliferation and invasion by significantly promoting cell proliferation, suppressing cell apoptosis and elevating the number of invasive cells (Fig. 4C–E, *P < 0.05, #P < 0.05). Besides, the activation of AMPK/mTOR pathway increased the expression of PCNA and MMP-3 and decreased the expression of Bax (Fig. 4F, *P < 0.05, #P < 0.05). These results above suggest that down-regulation of LncR-MALAT1 inhibits autophagy via the AMPK/mTOR pathway, thereby inhibiting the proliferation and invasion of cancer cells.Discussion
LncR-MALAT1 is one of the earliest discovered LncRNAs and it was firstly discovered in lung cancer.25 LncR-MALAT1 affects the occurrence and development of cancer by affecting the viability and mobility of cancer cells, and the aberrant expression of MALAT1 is considered as one of the most important prognostic indicators in cancers.26 Similarly, in our investigation, we found that LncR-MALAT1 was highly expressed in bladder cancer tissues and cell lines, which was consistent with previous investigations.27 Then we set to explore the effect of MALAT1 on the progression of bladder cancer and related mechanism.Investigations have shown that silenced LncR-MALAT1 inhibits the proliferation and migration of cervical cancer cells.28 Yang L. et al. also reported that high MALAT1 expression predicted a poor prognosis of cervical cancer and promoted cancer cell growth and invasion.29 In accordance with previous reports, the results of our research showed that shRNA-mediated MALAT1 silencing significantly inhibited the proliferation and invasion ability of bladder cancer cells, and induced obvious cell apoptosis at the same time. The decreased expression of PNCA and MMP-3 and increased expression of Bax also demonstrated that MALAT1 shRNA inhibited cell proliferation and invasion. In summary, inhibition of LncR-MALAT1 expression can play a role in inhibiting bladder cancer cell proliferation and invasion, but the mechanism remains to be further investigated.
Autophagy plays a “double-edged sword” in the process of tumor development. Autophagy can inhibit the expression of cancer genes and destroy cancer cell organelles in the early stages of cancer, thereby preventing tumor development.30 Autophagy is beneficial to the dormancy of tumor cells, which can be prevented by chemotherapy and radiotherapy, and can maintain cell activity during metastasis.31 It has been demonstrated that inhibition of autophagy enhances the therapeutic effect of radiotherapy and chemotherapy on breast cancer.32 But autophagy can provide nutrients for cancer cell survival, maintaining the function of cancer cell mitochondria, and promoting the proliferation and metastasis of malignant tumors when cancer worsens.20,24 In recent decades, there has been a growing body of evidence demonstrated that LncRNA can influence cancer progression by modulating autophagy. As reported in pancreatic cancer, LncR-MALAT1 was able to promote the proliferation and migration of pancreatic cancer cells by activating autophagy.33 Others also reported that MALAT1 activated autophagy and promoted cell proliferation by sponging miR-101 in glioma.34 But whether LncR-MALATI can influence the development of bladder cancer through autophagy is unknown. Our research showed that silencing LncR-MALAT1 significantly inhibited the expression of Beclin1 and the rate of LC3 II/I in bladder cancer cells and increased the expression of p62. So, it suggests that MALAT1 shRNA may affect the formation or degradation of autophagosomes, thereby reducing the bladder cancer cell autophagy and thus affect the pathological process of bladder cancer.
The AMPK/mTOR signaling pathway is one of the most classic autophagy regulatory pathways. MTOR consists mainly of two complexes, mTORC1 and mTORC2. MTORC1 mainly plays a role in inhibiting the formation of autophagy, and mTORC2 regulate autophagy through phosphorylation of PKCα and Akt indirectly.35 AMPK is thought to be an “energy regulator” of eukaryotic cells which is activated when cell energy is reduced, whereas phosphorylation-activated AMPK enhances autophagy by inhibiting mTORC1.36 Our investigation showed that LncR-MALAT1 shRNA reduced the phosphorylation level of AMPK in bladder cancer cells and increased the phosphorylation level of mTOR, thus inactivating the AMPK/mTOR signaling pathway. It suggests that down-regulated LncR-MALAT1 inhibits autophagy may be associated with the inhibition of AMPK/mTOR pathway. Further research found that the activation of the AMPK/mTOR signaling pathway by metformin partially reversed the autophagic inhibition and restored the proliferation and invasion ability of cancer cells elevated by MALAT1 shRNA. The further research demonstrated that the effect of LncR-MALAT1 on proliferation and invasion of bladder cancer cells is partly due to the regulation of autophagy via AMPK/mTOR signaling pathway.
In summary, down-regulation of LncR-MALAT1 expression can inhibit cell proliferation, promote cell apoptosis and suppress cell invasion in bladder cancer by inhibiting autophagy via regulating AMPK/mTOR signaling pathway. Thus, we suggest that LncR-MALAT1 may serve as a prognostic and prognostic target for bladder cancer. There is clearly much work to be done about the target gene of MALAT1 and the associated regulation mechanism, so our research provides a foundation for the treatment of bladder cancer and other related cancers.
Conflicts of interest
None.Abbreviations
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript |
| LncRNA | Long non-coding RNA |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| shRNA | short hairpin RNA |
| AMPK/mTOR | Adenosine monophasphate activated protein kinase/mammalian target of rapamycin |
| PCNA | Proliferating cell nuclear antigen |
| MMP | Matrix metallo protein |
References
- F. L. Egerod, A. Bartels, N. Fristrup, M. Borre, T. F. Orntoft, M. B. Oleksiewicz, N. Brunner and L. Dyrskjot, High frequency of tumor cells with nuclear Egr-1 protein expression in human bladder cancer is associated with disease progression, BMC Cancer, 2009, 9, 385 CrossRef PubMed.
- S. Antoni, J. Ferlay, I. Soerjomataram, A. Znaor, A. Jemal and F. Bray, Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends, Eur. Urol., 2017, 71, 96–108 CrossRef PubMed.
- P. E. Clark, N. Agarwal, M. C. Biagioli, M. A. Eisenberger, R. E. Greenberg, H. W. Herr, B. A. Inman, D. A. Kuban, T. M. Kuzel, S. M. Lele, J. Michalski, L. C. Pagliaro, S. K. Pal, A. Patterson, E. R. Plimack, K. S. Pohar, M. P. Porter, J. P. Richie, W. J. Sexton, W. U. Shipley, E. J. Small, P. E. Spiess, D. L. Trump, G. Wile, T. G. Wilson, M. Dwyer and M. Ho, Bladder cancer, J. Natl. Compr. Cancer Network, 2013, 11, 446–475 CrossRef.
- Q. Zhang, M. Su, G. Lu and J. Wang, The complexity of bladder cancer: long noncoding RNAs are on the stage, Mol. Cancer, 2013, 12, 101 CrossRef PubMed.
- Y. Fan, B. Shen, M. Tan, X. Mu, Y. Qin, F. Zhang and Y. Liu, TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12, Clin. Cancer Res., 2014, 20, 1531–1541 CrossRef PubMed.
- P. Bertone, V. Stolc, T. E. Royce, J. S. Rozowsky, A. E. Urban, X. Zhu, J. L. Rinn, W. Tongprasit, M. Samanta, S. Weissman, M. Gerstein and M. Snyder, Global identification of human transcribed sequences with genome tiling arrays, Science, 2004, 306, 2242–2246 CrossRef PubMed.
- U. A. Orom, T. Derrien, M. Beringer, K. Gumireddy, A. Gardini, G. Bussotti, F. Lai, M. Zytnicki, C. Notredame, Q. Huang, R. Guigo and R. Shiekhattar, Long noncoding RNAs with enhancer-like function in human cells, Cell, 2010, 143, 46–58 CrossRef PubMed.
- M. Szymanski, M. Z. Barciszewska, V. A. Erdmann and J. Barciszewski, A new frontier for molecular medicine: noncoding RNAs, Biochim. Biophys. Acta, 2005, 1756, 65–75 Search PubMed.
- P. Ji, S. Diederichs, W. Wang, S. Boing, R. Metzger, P. M. Schneider, N. Tidow, B. Brandt, H. Buerger, E. Bulk, M. Thomas, W. E. Berdel, H. Serve and C. Muller-Tidow, MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer, Oncogene, 2003, 22, 8031–8041 CrossRef PubMed.
- L. H. Schmidt, T. Spieker, S. Koschmieder, S. Schaffers, J. Humberg, D. Jungen, E. Bulk, A. Hascher, D. Wittmer, A. Marra, L. Hillejan, K. Wiebe, W. E. Berdel, R. Wiewrodt and C. Muller-Tidow, The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth, J. Thorac. Oncol., 2011, 6, 1984–1992 CrossRef PubMed.
- F. Guerrieri, Long non-coding RNAs era in liver cancer, World. J. Hepatol., 2015, 7, 1971–1973 CrossRef PubMed.
- M. C. Lai, Z. Yang, L. Zhou, Q. Q. Zhu, H. Y. Xie, F. Zhang, L. M. Wu, L. M. Chen and S. S. Zheng, Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation, Med. Oncol., 2012, 29, 1810–1816 CrossRef PubMed.
- Z. Zhao, C. Chen, Y. Liu and C. Wu, 17beta-Estradiol treatment inhibits breast cell proliferation, migration and invasion by decreasing MALAT-1 RNA level, Biochem. Biophys. Res. Commun., 2014, 445, 388–393 CrossRef PubMed.
- Y. Dong, G. Liang, B. Yuan, C. Yang, R. Gao and X. Zhou, MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway, Tumour Immunobiol., 2015, 36, 1477–1486 CrossRef PubMed.
- C. Li, Y. Cui, L. F. Liu, W. B. Ren, Q. Q. Li, X. Zhou, Y. L. Li, Y. Li, X. Y. Bai and X. B. Zu, High Expression of Long Noncoding RNA MALAT1 Indicates a Poor Prognosis and Promotes Clinical Progression and Metastasis in Bladder Cancer, Clin. Genitourin. Cancer, 2017, 15, 570–576 CrossRef PubMed.
- L. Galluzzi, F. Pietrocola, B. Levine and G. Kroemer, Metabolic control of autophagy, Cell, 2014, 159, 1263–1276 CrossRef PubMed.
- N. Martinez-Lopez, D. Athonvarangkul and R. Singh, Autophagy and aging, Adv. Exp. Med. Biol., 2015, 847, 73–87 CrossRef PubMed.
- R. A. Nixon, The role of autophagy in neurodegenerative disease, Nat. Med., 2013, 19, 983–997 CrossRef PubMed.
- J. M. M. Levy, C. G. Towers and A. Thorburn, Targeting autophagy in cancer, Nat. Rev. Cancer, 2017, 17, 528–542 CrossRef PubMed.
- E. White, J. M. Mehnert and C. S. Chan, Autophagy, Metabolism, and Cancer, Clin. Cancer Res., 2015, 21, 5037–5046 CrossRef PubMed.
- P. Yuan, W. Cao, Q. Zang, G. Li, X. Guo and J. Fan, The HIF-2alpha-MALAT1-miR-216b axis regulates multi-drug resistance of hepatocellular carcinoma cells via modulating autophagy, Biochem. Biophys. Res. Commun., 2016, 478, 1067–1073 CrossRef PubMed.
- M. Kang, C. W. Jeong, J. H. Ku, C. Kwak and H. H. Kim, Inhibition of autophagy potentiates atorvastatin-induced apoptotic cell death in human bladder cancer cells in vitro, Int. J. Mol. Sci., 2014, 15, 8106–8121 CrossRef PubMed.
- X. Fan, J. Wang, J. Hou, C. Lin, A. Bensoussan, D. Chang, J. Liu and B. Wang, Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway, J. Transl. Med., 2015, 13, 92 CrossRef PubMed.
- E. White, Deconvoluting the context-dependent role for autophagy in cancer, Nat. Rev. Cancer, 2012, 12, 401–410 CrossRef PubMed.
- C. Xu, M. Yang, J. Tian, X. Wang and Z. Li, MALAT-1: a long non-coding RNA and its important 3′ end functional motif in colorectal cancer metastasis, Int. J. Oncol., 2011, 39, 169–175 Search PubMed.
- X. Tian and G. Xu, Clinical value of lncRNA MALAT1 as a prognostic marker in human cancer: systematic review and meta-analysis, BMJ Open, 2015, 5, e008653 CrossRef PubMed.
- K. Kohls, D. Schmidt, S. Holdenrieder, S. C. Muller and J. Ellinger, [Detection of cell-free lncRNA in serum of cancer patients], Urologe A, 2015, 54, 819–825 CrossRef PubMed.
- R. Sun, C. Qin, B. Jiang, S. Fang, X. Pan, L. Peng, Z. Liu, W. Li, Y. Li and G. Li, Down-regulation of MALAT1 inhibits cervical cancer cell invasion and metastasis by inhibition of epithelial-mesenchymal transition, Mol. Biosyst., 2016, 12, 952–962 RSC.
- L. Yang, H. S. Bai, Y. Deng and L. Fan, High MALAT1 expression predicts a poor prognosis of cervical cancer and promotes cancer cell growth and invasion, Eur. Rev. Med. Pharmacol. Sci., 2015, 19, 3187–3193 Search PubMed.
- D. S. Arroyo, E. A. Gaviglio, J. M. Peralta Ramos, C. Bussi, M. C. Rodriguez-Galan and P. Iribarren, Autophagy in inflammation, infection, neurodegeneration and cancer, Int. Immunopharmacol., 2014, 18, 55–65 CrossRef PubMed.
- D. A. Gewirtz, Autophagy, senescence and tumor dormancy in cancer therapy, Autophagy, 2009, 5, 1232–1234 CrossRef PubMed.
- M. L. Bristol, X. Di, M. J. Beckman, E. N. Wilson, S. C. Henderson, A. Maiti, Z. Fan and D. A. Gewirtz, Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D3, Autophagy, 2012, 8, 739–753 CrossRef PubMed.
- L. Li, H. Chen, Y. Gao, Y. W. Wang, G. Q. Zhang, S. H. Pan, L. Ji, R. Kong, G. Wang, Y. H. Jia, X. W. Bai and B. Sun, Long Noncoding RNA MALAT1 Promotes Aggressive Pancreatic Cancer Proliferation and Metastasis via the Stimulation of Autophagy, Mol. Cancer Ther., 2016, 15, 2232–2243 CrossRef PubMed.
- Z. Fu, W. Luo, J. Wang, T. Peng, G. Sun, J. Shi, Z. Li and B. Zhang, Malat1 activates autophagy and promotes cell proliferation by sponging miR-101 and upregulating STMN1, RAB5A and ATG4D expression in glioma, Biochem. Biophys. Res. Commun., 2017, 492, 480–486 CrossRef PubMed.
- Y. Luo, L. Liu, Y. Wu, K. Singh, B. Su, N. Zhang, X. Liu, Y. Shen and S. Huang, Rapamycin inhibits mSin1 phosphorylation independently of mTORC1 and mTORC2, Oncotarget, 2015, 6, 4286–4298 Search PubMed.
- S. Sciarretta, M. Volpe and J. Sadoshima, Mammalian target of rapamycin signaling in cardiac physiology and disease, Circ. Res., 2014, 114, 549–564 CrossRef PubMed.
Footnote |
| † Jiude Qi and Yanfeng Chu are the co-first authors. |
| This journal is © The Royal Society of Chemistry 2018 |