Open Access Article
Open Access ArticleHow to improve Nafion with tailor made annealing†
Riccardo Narducci *ac,
Philippe Knauthbc,
Jean-François Chailand and
Maria Luisa Di Vonaac
*ac,
Philippe Knauthbc,
Jean-François Chailand and
Maria Luisa Di Vonaac
aUniversity of Rome Tor Vergata, Department of Industrial Engineering, Via del Politecnico 1, 00133 Roma, Italy. E-mail: riccardo.narducci@uniroma2.it
bAix Marseille Univ, CNRS, Madirel (UMR 7246), Electrochemistry of Materials Group, Campus St Jérôme, 13397 Marseille, France
cInternational Associated Laboratory (L.I.A.), Ionomer Materials for Energy, France and Italy
dUniversité de Toulon, MAPIEM (EA 4323), CS 60584, 83041 Toulon Cedex 9, France
First published on 31st July 2018
Abstract
A tailor-made annealing procedure was developed for Nafion in order to avoid a critical degradation of the mechanical properties associated with a decrease of the ionic conductivity. The formation of layered morphologies, prevalently oriented in the direction parallel to the membrane surface, is responsible of the decay in fuel cell operation conditions. Nafion membranes are annealed at 140 °C over 7 days in the presence of dimethylsulfoxide (DMSO) as a proton-acceptor solvent. The important increase of mechanical stability is related to the formation of a crystalline phase, which acts as a physical cross-linker. The procedure is followed by hydrothermal annealing in liquid water in order to obtain an optimal water uptake at equilibrium (tailor made). To better understand the behavior of these polymers, we use the INCA method (Ionomer nc Analysis) and compare with dynamic mechanical analysis (DMA). The stabilized materials are proposed for use in intermediate temperature fuel cells, where the mechanical stabilization by the annealing procedure plays a fundamental role.
1. Introduction
Both the impelling need for a consistent reduction of pollution in large towns and carbon dioxide in the atmosphere, as well as the continuous increase in petrol cost, have reinforced the interest in efficient and clean systems such as fuel cells (FCs) for the conversion of fuels into electrical energy. In particular, polymer electrolyte fuel cells (PEMFCs) are preferred for automotive applications,1–7 but are also used for co-generation systems,8,9 and sometimes in combination with electrolyzers.10 Perfluorinated sulfonic acid (PFSA) membranes are characterized by excellent chemical inertness, high proton conductivity, and good mechanical and thermal stability.11 However, the mechanical and thermal stability are not high enough for present needs.2,12–14When the relative humidity and temperature (RH/T) exceed certain critical values, irreversible processes take place and provoke a severe decrease of the through-plane conductivity of membranes constrained between electrodes. Measurements of the frequency response show that the bulk-transport properties of the ionomer membrane are modified.15 The conductivity decay does not occur when the membrane is free to swell in all dimensions; an anisotropic deformation only occurs when the membranes are forced to swell in the direction parallel to the electrodes.
A layered morphology has been hypothesized for Nafion more than 35 years ago, even if subject to debates still today.16 The presence of lamellar platelets in Nafion was initially proposed by Fujimura;17 one year later, a layered morphology was reported by Starkweather.18 Lamellar morphologies were described in 1997 by Litt,19 in 2001 by Haubold20 and in 2013 by Kreuer.21 Ribbon morphologies were disclosed by Gebel,22 Rubatat,23 Perrin24 and Termonia.25 The passage from random to in-plane oriented morphologies was assumed to explain the experimental behaviour of this ionomer. In our group, the Nafion conductivity was recorded in function of time for about 150 hours. The irreversible transition was affected by RH values between 95–100% in the temperature range of 70–130 °C; for RH = 100%, the instability begins near 80 °C.15,16,26 Large layered Nafion 117 membrane batches prevalently in-plane oriented and with decayed through-plane conductivity were prepared and characterized with a special device.16 Densities, proton conductivities and counter-osmotic pressure index (nc) were determined vs. temperature (nc/T plots) for the above samples. To avoid the formation of these harmful layered morphologies, we tried to use annealing procedures, well known in polymer technology.27–29 Many attempts for obtaining some mechanical stabilization by thermal procedures have been reported also for pre-formed ionomer membranes.30–32 The concept that the mechanical stabilization was due to a generic decrease of the free volume between ionomer chains when thermally treated33,34 was abandoned and replaced by the consideration that the mechanical stabilization was induced by an increased crystallization of a pre-existing semi-crystalline phase. A certain amount of molecular rearrangement must take place during crystallization, but this rearrangement cannot arise through large-scale molecular diffusion as the process is occurring in the solid state.29 The annealing temperature (Tann) must be chosen between the glass transition temperature (Tg) and the melting temperature (Tm) of the crystalline phase.29 In order to facilitate the crystallization process, the addition of small amounts of proton acceptor solvents with plasticizing effect was considered and experiments in the presence of these substances were carried out. Our choice was dimethyl sulfoxide (DMSO), which is an annealing agent used for the crosslinking of sulfonated poly(ether ether ketone) (SPEEK),35–42 a benchmark of sulfonated aromatic polymers (SAP); SAP are alternative materials to perfluorinated membranes in fuel cells at medium temperature (80–110 °C).42–44 During these studies, the effect of the thermal annealing was evaluated by the use of nc/T plots (INCA method) described in ref. 12, 29, 45 and 46. The improved functional properties are of great interest in electrochemical applications (such as PEMFCs).
In this paper, we describe thermal treatments of Nafion 117 in DMSO to increase the crystallinity and the mechanical stability and allow a better control of the water uptake. The materials treated in this way are compared with the material as received, in various aspects such as water uptake, Young's modulus, DMA tests and with the INCA method (Ionomer nc Analysis). The ionomer can be further processed in a home-made device to obtain the layered material and observe its stability in these critical conditions. Finally, an optimized treatment (tailor made) will be proposed for use in fuel cells.
2. Material and methods
2.1 Chemicals
Nafion 117 membranes (equivalent weight EW = 1100 g eq.−1, thickness 180 μm) were supplied by Sigma-Aldrich. Dimethyl sulfoxide (DMSO) and all the other chemical reagents were Carlo Erba RP products.2.2 Membrane preparations
2.3. Characterization
After this equilibration, membranes were kept at 25 °C for 24 hours in a closed Teflon vessel.12 The excess of water was carefully wiped off and the membrane mass was determined (mwet); then the samples were dried over P2O5 for 3 days and weighed (mdry). The water uptake (WU) was calculated according to eqn (1):
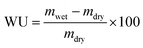 | (1) |
The hydration number was calculated as:
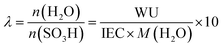 | (2) |
IEC is the ion exchange capacity of Nafion (0.909 meq. g−1). The uncertainty is about 0.5.
The λ values were converted into nc values, as previously described,2 by the eqn (3):
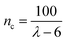 | (3) |
This equation is valid for λ ≥ 10.
| σ = d/R × A | (4) |
3. Results and discussion
3.1 Ionomer nc analysis
The Ionomer nc Analysis (INCA method) uses nc/T plots to get some fundamental properties of an ionomer, such as the degree of crystallinity, Young's modulus12 and the melting temperature. The use of the INCA method allows a quantitative and comparative evaluation of different materials.Figure 1 shows the nc/T plot in a large range of 50 °C to 140 °C for “as received and oriented” Nafion 117 (sample a),16 “as received” Nafion 117 (sample b)12 and “as received and annealed” Nafion 117 (sample c).29 For example, the “as received and annealed, H2O 100 °C” sample corresponds to the sample c equilibrated 600 hours at 100 °C in liquid water. The hydrothermal treatment in liquid water is applied in order to obtain an optimal water uptake at equilibrium (tailor made sample). The formation of oriented ribbon-type phases is harmful in our application because of the low conductivity of such domains. In order to avoid or limit them, the goal is to reinforce the matrix by performing heat treatments in the presence of annealing agents that increase the crystallinity without sacrificing the conductivity. This is why we decided to test the “annealed” sample after orientation, this sample is called “annealed, H2O 100 °C and oriented”.
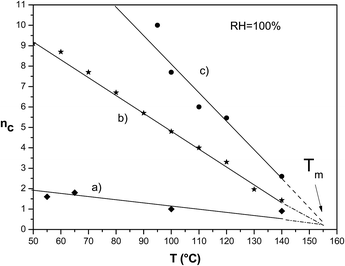 | ||
| Fig. 1 Comparison between nc/T plots for Nafion 117 “as received and oriented” (curve a), “as received” (curve b) and “as received and annealed” (curve c) in the range of temperature 50–140 °C. | ||
In the nc/T plots, a shift towards right is linked to an increase of mechanical properties; from these plots, one can also get the glass transition (Tg) and melting temperature (Tm) of the material and decide which treatment to perform (tailor made annealing). We remember that one nc unit is equivalent to an increase of Young's modulus by about 6.5 MPa;12,13,48 it is clear that the “as received and oriented” material has a low elastic modulus in the whole range of temperatures. The decrease with respect to the “as received” material (Fig. 1) is between 7 units of nc for 50 °C and about 1 nc unit for 140 °C. The “as received and annealed” Nafion presents instead high values of nc due to the increased crystallinity of the material. However, all of them have a melting temperature of about 160 °C (see the extrapolated nc plots);29 obviously the crystalline phase is already present in the “as received” material, remains, but probably with a lower content, in the “as received and oriented” material and increases in “as received and annealed” samples.
The mechanical properties were measured for Nafion 117 “as received and annealed, H2O 100 °C” to compare its properties with those of pristine Nafion (Table 1). In liquid water, the elastic modulus is higher for the annealed sample with respect to pristine Nafion due to the increase of the crystalline phase.29 This stabilization is important for the application in FCs working in fully humidified conditions. In ambient humidity, the increase of the elastic modulus can be observed if the residual DMSO, which acts as plasticizer, is removed with concentrated sulfuric acid. The elongation at break after annealing increases compared to pristine Nafion especially when the residual DMSO was removed.
| Sample | Measurement conditions | E (MPa) | Sigma (MPa) | σmax (MPa) | εBreak (%) |
|---|---|---|---|---|---|
| a Washed: 2 h H2SO4 1 M + 24 h H2O; after washing, 3 days under P2O5 and then stabilized for 10 days at RH = 36 ± 3%.b https://www2.dupont.com/FuelCells/en_US/assets/downloads/dfc101.pdf. | |||||
| N117 “as received and annealed, H2O 100 °C” | RH = 36 ± 3%, 20 °C | 255 ± 20 | 10.7 ± 0.5 | 27 ± 3 | 230 ± 20 |
| Liquid water, 20 °C | 190 ± 20 | 10.5 ± 0.5 | 31 ± 3 | 280 ± 20 | |
| aWashed, RH = 36 ± 3%, 20 °C | 290 ± 20 | 10.5 ± 0.5 | 36 ± 3 | 320 ± 20 | |
| N117b | RH = 50%, 23 °C | 249 | — | 43 | 225 |
| N117b | Liquid water, 23 °C | 114 | — | 34–26 | 200 |
Recent DSC results confirm that thermal treatments in the presence of DMSO increase the amount of crystalline phase, which improves the Young's modulus, due to its greater compactness.29
In Table 2 are shown data of various Nafion samples treated 600 h at 100 °C and kept at 25 °C for 24 hours in a closed Teflon vessel. From the conductivity data shown in Table 2, it can be noted that the annealing treatment does not affect the conductivity; the data are very close to that of as received samples, even if the water uptake has a lower value. We can assume that the increased crystallinity reduces the tortuosity of the material. From Table 2, the treatment with DMSO does not entirely prevent the formation of the low conductivity phase after orientation, checked by the density measurements, however, the conductivity decrease between “as received and annealed” and “annealed and oriented” samples is limited only to 15%.
| Sample | σ (mS cm−1) | λ | nc | dwet (g cm−3) | ddry (g cm−3) |
|---|---|---|---|---|---|
| As received | 30 | 25 | 5.3 | — | 2.0 ± 0.1 |
| As received and annealed, H2O 100 °C | 31 | 18 | 8.3 | 1.7 ± 0.1 | 2.0 ± 0.1 |
| As received and oriented | 10 | 70 | 1.6 | — | 1.4 ± 0.1 |
| Annealed H2O 100 °C and oriented | 26 | 17 | 9.1 | 1.6 ± 0.1 | 1.7 ± 0.1 |
The density changes in the dry state are lower when compared with the large change observed in Nafion 117 “as received and oriented” (Table 2), where the density reaches a very low value around 1.4 g cm−3 and the conductivity decreases by a factor 3, in spite of the large water uptake. We can therefore deduce that the thermal treatment in presence of DMSO reduces the formation of non-conductive oriented phases inside the membrane.
The hydration number of “as received and oriented” Nafion is much higher than the other data due to the conformational modifications that occur after orientation (density lowering, large equivalent volume, and swelling along the c axis, perpendicular to the plates of the device). One can observed that the annealing reduce the effect of the orientation.
Alberti and coworkers16 demonstrated that the swelling of the oriented materials takes place along the c direction, perpendicular to the surface. The conductivity decrease can be observed in Table 2, which is accompanied by a dramatic decrease of the ionomer density (1.4 g cm−3). We concluded that lamellar morphologies are present in “oriented” Nafion samples. This ionomer presents a low degree of crystallinity and low Young's modulus; a consequence is the large water uptake across the temperature range (Fig. 1). The changes in density are very likely due to a disordered packing of in-plane oriented morphologies.16 To prevent the formation of these non-conductive phases, we treated the Nafion 117 membranes at an annealing temperature Tann = 140 °C with a small amount of DMSO, a proton acceptor solvent with plasticizing effect to increase the amount and/or the size of the preexisting semicrystalline phase.29 Gebel has shown with wide-angle (WAXS) and small angle X-ray scattering (SAXS)49 that the heat treatment increases the degree of crystallinity of Nafion.50
3.2 Dynamic mechanical analysis (DMA)
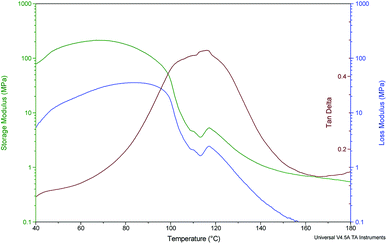
Using sinusoidal solicitations of the sample, DMA analysis allows a separation of the storage modulus E′, describing the elastic response of the polymer, and the loss modulus E′′, related to the viscous response of the polymer. Given that in most cases the viscous part of the polymer modulus is much lower than the elastic part, the storage modulus is very close to the static elastic modulus obtained from mechanical measurements. The damping (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ), which is defined as the ratio of the loss modulus and the storage modulus E′, is a good parameter to evidence dynamic mechanical relaxations and especially the glass transition of the polymer Tg. Those phenomena appear as damping peaks during temperature ramp experiments. In an earlier publication, Kyu and Eisenberg51 discussed such relaxations for the acid form of Nafion and assigned greek letters based on the sequence of observation from low to high temperature. The first relaxation γ (around −100 °C) was assigned to short-range molecular motions in the tetrafluoroethylene backbone (crankshaft motions). The second one β around 20 °C was assigned to large chain motions of amorphous fluorocarbon units separated from the ionic aggregates. Finally, the largest peak α around 110 °C was assigned to the main relaxation process, which is associated to the glass transition, as in most polymers. In the Nafion case, this relaxation mainly takes place in the polar regions, because it is sensitive to the ion type (in Na+ form the α peak is around 250 °C), the degree of neutralization, and the water content. It is due to chain motions within or near the ion-rich domains.52,53 More recently, correlations of DMA data with molecular dynamics studies and morphological relaxations observed using variable temperature solid state NMR and SAXS experiments, allows attributing the α relaxation to the onset of long-range mobility of both the main and side chains as a result of a destabilization of the electrostatic network of ion-rich domains.1 Fig. 2 presents the DMA results for “as received and oriented” Nafion 117 in the 40–180 °C temperature range. The tan
δ), which is defined as the ratio of the loss modulus and the storage modulus E′, is a good parameter to evidence dynamic mechanical relaxations and especially the glass transition of the polymer Tg. Those phenomena appear as damping peaks during temperature ramp experiments. In an earlier publication, Kyu and Eisenberg51 discussed such relaxations for the acid form of Nafion and assigned greek letters based on the sequence of observation from low to high temperature. The first relaxation γ (around −100 °C) was assigned to short-range molecular motions in the tetrafluoroethylene backbone (crankshaft motions). The second one β around 20 °C was assigned to large chain motions of amorphous fluorocarbon units separated from the ionic aggregates. Finally, the largest peak α around 110 °C was assigned to the main relaxation process, which is associated to the glass transition, as in most polymers. In the Nafion case, this relaxation mainly takes place in the polar regions, because it is sensitive to the ion type (in Na+ form the α peak is around 250 °C), the degree of neutralization, and the water content. It is due to chain motions within or near the ion-rich domains.52,53 More recently, correlations of DMA data with molecular dynamics studies and morphological relaxations observed using variable temperature solid state NMR and SAXS experiments, allows attributing the α relaxation to the onset of long-range mobility of both the main and side chains as a result of a destabilization of the electrostatic network of ion-rich domains.1 Fig. 2 presents the DMA results for “as received and oriented” Nafion 117 in the 40–180 °C temperature range. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve shows a main peak around 110 °C attributed to the α relaxation phenomenon. Although the arrangement is probably different (the ribbons are present both in the “as received” and “as received and oriented” Nafion), this result indicates that the ionic interactions are similar in the layered and in the as received sample. This main relaxation process of the polymer is in line with that reported in the literature.1 One can see a shoulder probably due to the loss of water during the temperature ramp. This last phenomenon can also explain the increase in storage modulus below 60 °C, which is compensated above this temperature by the decrease of modulus during the α relaxation process. Most likely, the decrease in proton conductivity is due to a lower percolation also attributable to the new matrix arrangements. The question still deserves further study.
δ curve shows a main peak around 110 °C attributed to the α relaxation phenomenon. Although the arrangement is probably different (the ribbons are present both in the “as received” and “as received and oriented” Nafion), this result indicates that the ionic interactions are similar in the layered and in the as received sample. This main relaxation process of the polymer is in line with that reported in the literature.1 One can see a shoulder probably due to the loss of water during the temperature ramp. This last phenomenon can also explain the increase in storage modulus below 60 °C, which is compensated above this temperature by the decrease of modulus during the α relaxation process. Most likely, the decrease in proton conductivity is due to a lower percolation also attributable to the new matrix arrangements. The question still deserves further study.
Fig. 3 shows the DMA results for Nafion 117 “as received and annealed” after washing in sulfuric acid and water. The Tα value of 110 °C is the same as for oriented samples. A small shoulder is also visible around 40–50 °C leading to a non-symmetric α peak and probably due again to some water loss. Given that the DMA technique is very sensitive to local hindrance of macromolecular chains, further studies will be performed to understand how the increased crystallinity affects the position of this peak.
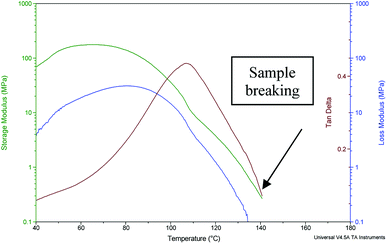 | ||
| Fig. 3 DMA analysis for “as received and annealed” Nafion 117. Sample parameters: length 18.51 mm, width 5.24 mm, thickness 0.18 mm. This sample broke at 140 °C. | ||
Fig. 4 presents the DMA results for Nafion 117 “annealed and oriented”. The main tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak attributed to the α relaxation phenomenon is around 130 °C, 20 °C higher compared to literature data and previous oriented or crystallised samples. In this case, orientation and crystallisation are combined and strongly hinder chains, which need more energy to move, leading to a higher relaxation temperature.54 In addition, one can note that at 160 °C, in the rubbery state, the storage modulus is higher for the “annealed and oriented” sample than for the “as received and oriented” one.
δ peak attributed to the α relaxation phenomenon is around 130 °C, 20 °C higher compared to literature data and previous oriented or crystallised samples. In this case, orientation and crystallisation are combined and strongly hinder chains, which need more energy to move, leading to a higher relaxation temperature.54 In addition, one can note that at 160 °C, in the rubbery state, the storage modulus is higher for the “annealed and oriented” sample than for the “as received and oriented” one.
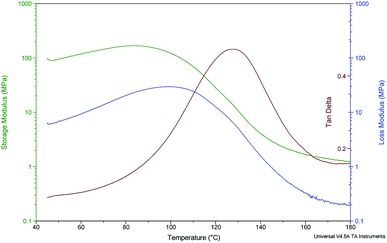 | ||
| Fig. 4 DMA analysis for Nafion 117 “annealed, H2O 100 °C and oriented”. Sample parameters: length 13.47 mm, width 5.75 mm, thickness 0.20 mm. | ||
A limitation of the DMA technique is the drying of the samples, which levels the effect on the main relaxation process due to its temperature close to 100 °C. The INCA method measures the mechanical properties at the working temperature and in liquid H2O and can be a valid complement to DMA analysis in order to distinguish different ionomer samples.
Summarizing these results, we can say that the treatment at 140 °C in the presence of dimethyl sulfoxide is effectively limiting the formation of low conductivity oriented ribbon-type phases. The resulting formation of semi-crystalline phases with physical crosslinking increases the thermal stability. Then, the mechanical properties do not collapse at Tg, but at the melting temperature of the semi-crystalline phase (Tm). We remember that the knowledge of Tm is essential for using the appropriate annealing temperature to increase the crystallinity of ionomers, especially those having a low EW.46 The “annealed” samples are useful for applications in fuel cells with operating temperatures close to 100 °C. At such temperature, it is not necessary to pressurize the system and increase the cooling equipment for vehicles, which would lead to greater weight and greater use of energy. The increase of the working temperature of PEMFC by 15–20 °C can significantly improve their efficiency.
4. Conclusions
The through-plane conductivity decay, accompanied by an important matrix rearrangement with a consistent decrease of the ionomer density and nc, is due to the formation of “oriented ribbon-type” morphologies with semi-crystalline and amorphous layers parallel for the most part to the membrane surface. The formation of the in-plane-oriented layered morphologies must be avoided for membranes used as proton conductors, due to their low through-plane conductivity essentially due to a scarce connection between aqueous pools of the inner proton solution. Annealing in the presence of DMSO is a promising method for the stabilization of membranes under the operating conditions of fuel cells, preventing the formation of low conductive phases. With the INCA method it is easy to find important properties of the material such as the Tg, also verified by DMA, and Tm; moreover it is possible to establish the most suitable treatment of Nafion 117 (tailor made annealing). These results are promising for the use of these treated ionomers in a highly efficient medium temperature fuel cell.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are indebted to the late Prof. G. Alberti (May 1930–October 2017) for having initiated this work. The authors thank Dr A. Fahs for his help for the DMA measurements.References
- K. A. Mauritz and R. B. Moore, Chem. Rev., 2004, 104, 4535–4585 CrossRef PubMed.
- G. Alberti, R. Narducci and M. L. Di Vona, Solid State Proton Conductors: Properties and Applications in Fuel Cells, Wiley, 2012 Search PubMed.
- A. Paciaroni, M. Casciola, E. Cornicchi, M. Marconi, G. Onori, M. Pica and R. Narducci, J. Phys. Chem. B, 2006, 110, 13769–13776 CrossRef PubMed.
- A. Paciaroni, M. Casciola, E. Cornicchi, M. Marconi, G. Onori, M. Pica, R. Narducci, A. De Francesco and A. Orecchini, J. Phys.: Condens. Matter, 2006, 18, S2029–S2038 CrossRef.
- E. Roberti, G. Carlotti, S. Cinelli, G. Onori, A. Donnadio, R. Narducci, M. Casciola and M. Sganappa, J. Power Sources, 2010, 195, 7761–7764 CrossRef.
- G. Napoli, S. Micari, G. Dispenza, S. Di Novo, V. Antonucci and L. Andaloro, Int. J. Hydrogen Energy, 2017, 42, 28034–28047 CrossRef.
- j. Yuan, L. Yang and Q. Chen, Int. J. Hydrogen Energy, 2018, 43, 8063–8078 CrossRef.
- S. Bose, T. Kuila, X. L. N. Thi, N. H. Kim, K. T. Lau and J. H. Lee, Prog. Polym. Sci., 2011, 36, 813–843 CrossRef.
- Z. B. Wang, G. P. Yin, Y. Y. Shao, B. Q. Yang, P. F. Shi and P. X. Feng, J. Power Sources, 2007, 165, 9–15 CrossRef.
- H. Ito, T. Maeda, A. Nakano and H. Takenaka, Int. J. Hydrogen Energy, 2011, 36, 10527–10540 CrossRef.
- A. Kusoglu and A. Z. Weber, Chem. Rev., 2017, 117, 987–1104 CrossRef PubMed.
- G. Alberti, R. Narducci and M. Sganappa, J. Power Sources, 2008, 178, 575–583 CrossRef.
- G. Alberti and R. Narducci, Fuel Cells, 2009, 9, 410–420 CrossRef.
- M. Haghayegh, M. H. Eikani and S. Rowshanzamir, Int. J. Hydrogen Energy, 2017, 42, 21944–21954 CrossRef.
- M. Casciola, G. Alberti, M. Sganappa and R. Narducci, J. Power Sources, 2006, 162, 141–145 CrossRef.
- G. Alberti, R. Narducci, M. L. Di Vona and S. Giancola, Ind. Eng. Chem. Res., 2013, 52, 10418–10424 CrossRef.
- M. Fujimura, T. Hashimoto and H. Kawai, Macromolecules, 1981, 14, 1309–1315 CrossRef.
- H. W. Starkweather, Macromolecules, 1982, 15, 320–323 CrossRef.
- M. H. Litt, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 1997, 38, 80–81 Search PubMed.
- H. G. Haubold, T. Vad, H. Jungbluth and P. Hiller, Electrochim. Acta, 2001, 46, 1559–1563 CrossRef.
- K. D. Kreuer, Solid State Ionics, 2013, 252, 93–101 CrossRef.
- G. Gebel, Polymer, 2000, 41, 5829–5838 CrossRef.
- L. Rubatat, G. Gebel and O. Diat, Macromolecules, 2004, 37, 7772–7783 CrossRef.
- J. C. Perrin, S. Lyonnard, A. Guillermo and P. Levitz, J. Phys. Chem. B, 2006, 110, 5439–5444 CrossRef PubMed.
- Y. Termonia, Polymer, 2007, 48, 1435–1440 CrossRef.
- K. V. Peinemann and S. P. Nunes, Membranes for Energy Conversion, Wiley, 2008 Search PubMed.
- J. E. Hensley, J. D. Way, S. F. Dec and K. D. Abney, J. Membr. Sci., 2007, 298, 190–201 CrossRef.
- Z. Y. Zhang and H. M. Zeng, Polymer, 1993, 34, 3648–3652 CrossRef.
- G. Alberti, R. Narducci, M. L. Di Vona and S. Giancola, Fuel Cells, 2013, 13, 42–47 CrossRef.
- X. Y. Ding, S. Didari, T. F. Fuller and T. A. L. Harris, J. Electrochem. Soc., 2013, 160, F793–F797 CrossRef.
- Y. Xiao, K. K. Poornesh, L. Wendling and C. Cho, Mater. Lett., 2014, 124, 293–295 CrossRef.
- H. B. Park, H. S. Shin, Y. M. Lee and J. W. Rhim, J. Membr. Sci., 2005, 247, 103–110 CrossRef.
- M. K. Hassan, A. Abukmail and K. A. Mauritz, Eur. Polym. J., 2012, 48, 789–802 CrossRef.
- L. A. Zook and J. Leddy, Anal. Chem., 1996, 68, 3793–3796 CrossRef PubMed.
- B. Maranesi, H. Hou, R. Polini, E. Sgreccia, G. Alberti, R. Narducci, P. Knauth and M. L. Di Vona, Fuel Cells, 2013, 13, 107–117 CrossRef.
- M. L. Di Vona, L. Pasquini, R. Narducci, K. Pelzer, A. Donnadio, M. Casciola and P. Knauth, J. Power Sources, 2013, 243, 488–493 CrossRef.
- J. A. Kerres, Fuel Cells, 2005, 5, 230–247 CrossRef.
- J. A. Kerres, D. M. Xing and F. Schonberger, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 2311–2326 CrossRef.
- A. Bhattacharya, J. W. Rawlins and P. Ray, Polymer Grafting and Crosslinking, Wiley, 2008 Search PubMed.
- R. Y. M. Huang, P. H. Shao, C. M. Burns and X. Feng, J. Appl. Polym. Sci., 2001, 82, 2651–2660 CrossRef.
- P. Knauth, E. Sgreccia and M. L. Di Vona, J. Power Sources, 2014, 267, 692–699 CrossRef.
- R. Narducci, M. L. Di Vona and P. Knauth, J. Membr. Sci., 2014, 465, 185–192 CrossRef.
- M. L. Di Vona, E. Sgreccia, R. Narducci, L. Pasquini, H. Hou and P. Knauth, Front. Energy Res., 2014, 2, 1–7 Search PubMed.
- M. L. Di Vona and P. Knauth, Z. Phys. Chem., 2013, 227, 595–614 CrossRef.
- G. Alberti, M. L. Di Vona and R. Narducci, Int. J. Hydrogen Energy, 2012, 37, 6302–6307 CrossRef.
- G. Alberti, R. Narducci, M. L. Di Vona and S. Giancola, Int. J. Hydrogen Energy, 2017, 42, 15908–15912 CrossRef.
- R. Narducci, J. F. Chailan, A. Fahs, L. Pasquini, M. L. Di Vona and P. Knauth, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 1180–1187 CrossRef.
- R. Narducci, G. Alberti and M. L. Di Vona, Solid State Proton Conductors, Wiley, New York, 2012 Search PubMed.
- G. Gebel, P. Aldebert and M. Pineri, Macromolecules, 1987, 20, 1425–1428 CrossRef.
- R. J. Young and P. A. Lovell, Introduction to Polymers, Third Edition, Taylor & Francis, 2011 Search PubMed.
- T. Kyu and A. Eisenberg, Perfluorinated Ionomer Membranes, American Chemical Society, 1982, vol. 180, pp. 79–112 Search PubMed.
- S. J. Osborn, M. K. Hassan, G. M. Divoux, D. W. Rhoades, K. A. Mauritz and R. B. Moore, Macromolecules, 2007, 40, 3886–3890 CrossRef.
- K. A. Page, K. M. Cable and R. B. Moore, Macromolecules, 2005, 38, 6472–6484 CrossRef.
- A. N. Frone, S. Berlioz, J. F. Chailan, D. M. Panaitescu and D. Donescu, Polym. Compos., 2011, 32, 976–985 CrossRef.
Footnote |
| † In memoriam Prof. Giulio Alberti. |
| This journal is © The Royal Society of Chemistry 2018 |