Open Access Article
Open Access ArticlePalladium-catalyzed direct C(sp3)–H arylation of indole-3-ones with aryl halides: a novel and efficient method for the synthesis of nucleophilic 2-monoarylated indole-3-ones†
Yong-Long Zhao *a,
Yong-Qin Tanga,
Xing-Hai Feia,
Tao Xiaoa,
Ya-Dong Lua,
Xiao-Zhong Fua,
Bin Hea,
Meng Zhoua,
Chun Lia,
Peng-Fei Xu
*a,
Yong-Qin Tanga,
Xing-Hai Feia,
Tao Xiaoa,
Ya-Dong Lua,
Xiao-Zhong Fua,
Bin Hea,
Meng Zhoua,
Chun Lia,
Peng-Fei Xu *b and
Yuan-Yong Yang*a
*b and
Yuan-Yong Yang*a
aState Key Laboratory of Functions and Applications of Medicinal Plants, School of Pharmacy, Engineering Research Center for the Development and Application of Ethnic Medicine and TCM (Ministry of Education), Guizhou Medical University, Guiyang 550004, P. R. China. E-mail: zhaoyl05@lzu.edu.cn; 2211452786@qq.com; 1156320953@qq.com; 643904784@qq.com; 1536408086@qq.com; 2660860532@qq.com; binhe@gmc.edu.cn; qingfeng476281051@163.com; 1784193313@qq.com; 312854015@qq.com
bState Key Laboratory of Applied Organic Chemistry, College of Chemistry & Chemical Engineering, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: xupf@lzu.edu.cn
First published on 16th July 2018
Abstract
A novel and efficient method for the synthesis of nucleophilic 2-monoarylated indole-3-ones via palladium-catalyzed direct C(sp3)–H arylation of indole-3-ones with aryl halides has been developed. Various 2-monoarylated indole-3-ones were readily obtained with yields up to 95%. As a class of important nucleophilic intermediates, 2-monoarylated indole-3-ones can be used for the construction of C2-quaternary indolin-3-one skeletons.
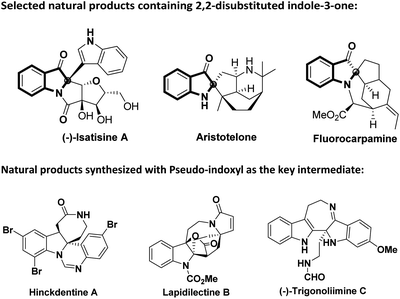
2,2-Disubstituted indole-3-ones (trivially known as pseudo-indoxyl) are privileged core heterocyclic structural motifs that occur in a great number of biologically active natural products.
(Fig. 1, (−)Isatisine A,1a–c Aristotelone,1e Fluorocarpamine1d).1 In addition, they can be used as a key synthetic intermediate in the synthesis of many natural products (Fig. 1, Hinckdentine A,2a–c Lapidilectine B2d,e and (−)-Trigonoliimine C2f–j).2 Owing to its interesting structure and biological activity, the pseudo-indoxyl scaffold has attracted extensive attention from both synthetic and medicinal chemists. Numerous elegant synthetic protocols have been developed for the construction of the 2,2-disubstituted indole-3-one scaffold.2c,3,4 Recently, nucleophilic indole-3-ones have been demonstrated to be very reliable building blocks for the enantioselective or racemic construction of C2-quaternary indolin-3-one skeletons.3d,4 Among these significant advances, one of the key substrates was nucleophilic 2-monoarylated indole-3-ones (Scheme 1a).3d,4a
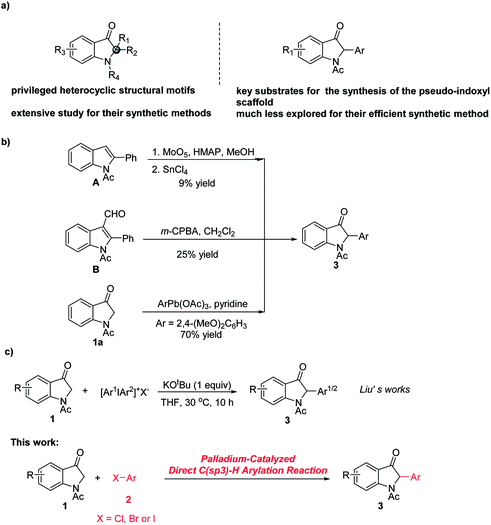 | ||
| Scheme 1 Reported approaches toward the synthesis of C2-quaternary indolin-3-one skeletons and palladium-catalyzed direct C(sp3)–H arylation reaction of indole-3-ones with aryl halides. | ||
So far, much of the effort has been focused on the synthesis of C2-quaternary indolin-3-one skeletons,2c,3,4 however, the routes for the synthesis of nucleophilic 2-monoarylated indole-3-ones have been challenging and are rare (Scheme 1a).3d,4a,5 Although some useful methods were developed via Baeyer–Villiger oxidation of C-3 phenyl substituted indole derivatives (A and B) and direct arylation of indolin-3-one 1a with aryllead triacetate, it should be noted that the application of these methods is restricted by the limited number of available substrates and the low yields (Scheme 1b).5 In 2015, a method for the potassium tert-butoxide mediated direct C2-arylation of indolin-3-ones 1 was reported by Liu et al., however, diaryliodonium salts were required as the arylating agents and lower yields (up to 70%) were obtained.3d In addition, the “Selective Problem” arose due to the use of unsymmetric diaryliodonium salts as the arylating agents and the mixtures of two C2-arylation products were produced (Scheme 1c).3d Therefore, more efficient methods for the synthesis of nucleophilic 2-monoarylated indole-3-ones are highly desired.
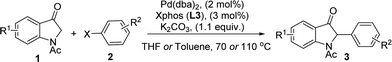
Palladium-catalyzed α-arylation of carbonyl and related compounds has served as a powerful tool for the quick construction of C–C bonds.6 Although palladium-catalyzed C-3 arylation of 2-oxindole has been reported by Willis et al.,6c palladium-catalyzed direct C(sp3)–H arylation of indole-3-ones with aryl halides remains unexplored.3d With our ongoing interest in the study of palladium-catalyzed coupling reactions6d,7 and indolin-3-one chemistry,4d,e we developed an efficient procedure for this transformation for the synthesis of nucleophilic 2-monoarylated indole-3-ones 3 (Scheme 1c).
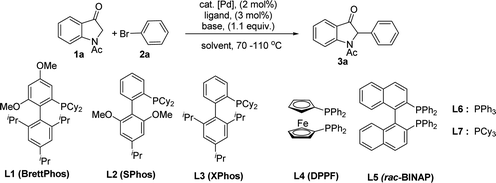
1-Acetylindolin-3-one 1a and bromobenzene 2a were used as the model substrate for the initial study and the results are summarized in Table 1. It was found that 1-acetylindolin-3-one 1a and bromobenzene 2a reacted in the presence of the Brettphos ligand L1 (3 mol%), K2CO3 (1.1 equiv.) and Pd(dba)2 (2 mol%) in THF under an high pure nitrogen atmosphere at 70 °C to furnish the desired product 3a with a low yield (Table 1, entry 1). Various ligands was then screened and Xphos ligand L3 was found to be the best ligand for this α-arylation reaction (Table 1, entry 3). For ligands L4 and L5, no desired product was obtained (Table 1, entries 4 and 5). For ligands L2, L6 and L7, desired product 3a was obtained albeit with lower yields (Table 1, entry 2, 6 and 7). Next, Pd(OAc)2, Pd(TFA)2, PdCl2 and Pd(dba)2 were also tested for this α-arylation reaction using K2CO3 as the base in the presence of Xphos ligand L3 (Table 1, entry 3, 8, 9 and 10), and Pd(dba)2 was found to be the optimal choice (Table 1, entry 3). Finally, various bases such as K2CO3, KHMDS, tBuOK, tBuONa, CsCO3, K3PO4 and AcONa were also tested (Table 1, entry 3, 12, 13, 14, 15 and 16), and K2CO3 was found to be the most promising base (Table 1, entry 3). We then tested the effect of high boiling-point solvents on this α-arylation process, some common solvents such as THF, toluene and dioxane were used, and THF was found to be the optimal choice (Table 1, entry 3). Finally, we examined the effect of different concentrations and reaction time on this α-arylation process, and found this process could be finished in 14 hours in the presence of the Pd(dba)2 (2 mol%) and Xphos ligand L3 (3 mol%) under 2.0 ml THF. Accordingly, Pd(dba)2 (2 mol%), Xphos ligand L3 (3 mol%) and K2CO3 (1.1 equiv.) with THF as the solvent under an high pure nitrogen atmosphere at 70 °C are the optimal conditions for this α-arylation reaction of indole-3-ones with aryl halides.
| Entry | Cat. [Pd] | Ligand | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions performed on a 0.25 mmol scale using 1a (1.0 equiv.) and 2a (1.1 equiv.) in 2.0 ml of the solvent for 14 h, reaction performed at 70 °C under an high pure nitrogen atmosphere.b Isolated yield.c NR = no reaction.d Decomposition observed based on TLC.f 1.0 ml THF was used as the solvent.g 3.0 ml THF was used as the solvent.h Reaction time = 10 h.i Reaction performed at 100–110 °C and 1.0 ml solvent was used.j Reaction time = 18 h. | |||||
| 1 | Pd(dba)2 | L1 | K2CO3 | THF | <10 |
| 2 | Pd(dba)2 | L2 | K2CO3 | THF | 26 |
| 3 | Pd(dba)2 | L3 | K2CO3 | THF | 87 |
| 4 | Pd(dba)2 | L4 | K2CO3 | THF | NRc |
| 5 | Pd(dba)2 | L5 | K2CO3 | THF | NRc |
| 6 | Pd(dba)2 | L6 | K2CO3 | THF | <10 |
| 7 | Pd(dba)2 | L7 | K2CO3 | THF | <10 |
| 8 | Pd(OAc)2 | L3 | K2CO3 | THF | <10 |
| 9 | PdCl2 | L3 | K2CO3 | THF | <10 |
| 10 | Pd(TFA)2 | L3 | K2CO3 | THF | 24 |
| 11 | Pd(dba)2 | L3 | KHMDS | THF | <10 |
| 12 | Pd(dba)2 | L3 | tBuOK | THF | —d |
| 13 | Pd(dba)2 | L3 | CsCO3 | THF | <10 |
| 14 | Pd(dba)2 | L3 | K3PO4 | THF | 32 |
| 15 | Pd(dba)2 | L3 | AcONa | THF | NRc |
| 16 | Pd(dba)2 | L3 | tBuONa | THF | —d |
| 17 | Pd(dba)2 | L3 | K2CO3 | Dioxane | 53 |
| 18i | Pd(dba)2 | L3 | K2CO3 | Toluene | 24 |
| 19f | Pd(dba)2 | L3 | K2CO3 | THF | 80 |
| 20g | Pd(dba)2 | L3 | K2CO3 | THF | 73 |
| 21h | Pd(dba)2 | L3 | K2CO3 | THF | 66 |
| 22j | Pd(dba)2 | L3 | K2CO3 | THF | 84 |
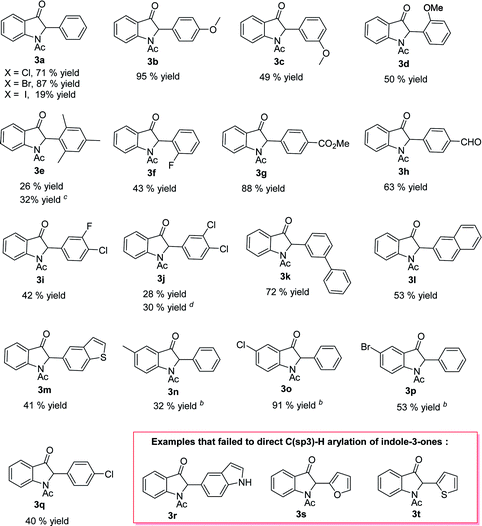
With the reaction conditions optimized, we next investigated the substrate scopes of indole-3-ones 1 and aryl halides 2 (Table 2). In most cases, the reactions afforded the corresponding 2-monoarylated indole-3-one products 3a–f with moderate to excellent yields (19–95%). First, we examined the reactions of 1-acetylindolin-3-one 1a with various substituted aryl halides 2. Bromobenzene, chlorobenzene and iodobenzene were all effective substrates for the synthesis of 3a and bromobenzene was the best substrate for the α-arylation of indole-3-one 1a. The structural variation of aryl bromides 2 could be well tolerated in this reaction irrespective of the electronic nature or the positions of the substituents on the aromatic ring. Compared with the electron-withdrawing aryl bromides (3g–h), the electron-donating aryl bromides (3b) gave higher yields. Generally speaking, the steric effect of aryl bromides decreased the yields of products (3c–d vs. 3b) and 3e was obtained in only 26% yield. When both bromo- and chloro-substituents were present in the arenes, selective reactions at the bromo positions were always observed (3i–j, 3q). It is noteworthy that this α-arylation process could also be successfully extended to others complex aryl bromides and corresponding desired products 3k–m were obtained with 41–72% yields, however, the examples for 5-bromo-1H-indole, 2-bromofuran and 2-bromothiophene are failed (3r–t). The variation on the indolin-3-one scaffold could also be tolerated in this α-arylation process and the desired products 3n–p were afforded with 32–91% yields. Furthermore, when 5-bromo- and chloro-substituted of indole-3-ones were used, corresponding products 3o and 3p were also obtained.
| a Unless otherwise specified, all reactions were carried out with using 1 (0.25 mmol, 1.0 equiv.) and 2 (1.1 equiv.) in 2.0 mL of the THF for 14 h at 70 °C under an high pure nitrogen atmosphere and all the yields were isolated yield.b Reaction performed at 110 °C and 1.0 ml PhMe was used as the solvent.c Pd(dba)2 (10 mol%) and Xphos (10 mol%) was used.d Aryl bromides (2.0 equiv.) and K2CO3 (2.0 equiv.) was used. |
|---|
 |
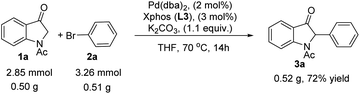
To investigate the potential utility of this strategy, the large-scale synthesis of 3a was also performed under the optimized conditions. The reaction proceeded smoothly to afford the corresponding 2-monoarylated indole-3-one 3a product albeit with the actual yield decreased to 72% (Scheme 2). In addition, the 2-monoarylated indole-3-one 3 could be used as a kind of key nucleophilic substrate for the chiral or achiral synthesis of C2-quaternary indolin-3-one skeletons.3d,4a
As shown in Scheme 3, a possible reaction mechanism for this palladium-catalyzed α-arylation of indole-3-ones was proposed based on the reported mechanisms of the similar palladium-catalyzed α-arylation of carbonyl and related compounds with aryl halides.6 The oxidative addition of a palladium complex into the C–X bond of aryl halides first occurs and the palladium complex intermediate 4 is formed. At the same time the enolate intermediate 5 is also produced by indole-3-one 1 in the presence of a base, which reacts with intermediate 4 to get the arylpalladium enolate complex 6 and its isomerism intermediate 6′. Finally, anionic palladium intermediate 7 is formed by above isomerism intermediate 6′ in the presence of a base,6e,f which then undergoes a reductive elimination to form the desired 2-monoarylated indole-3-one 3, at the same time restores the original palladium catalyst and completes the catalytic cycle.
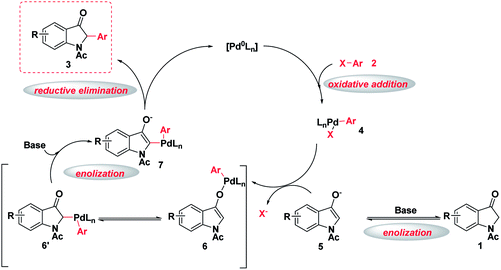 | ||
| Scheme 3 A proposed mechanism for the palladium-catalyzed direct C(sp3)–H arylation of indole-3-ones with aryl halides. | ||
In conclusion, we have developed an efficient method for the synthesis of 2-monoarylated indole-3-ones via palladium-catalyzed direct C(sp3)–H arylation of indole-3-ones with aryl halides. The nucleophilic 2-monoarylated indole-3-ones were obtained in moderate to good yields (up to 95%). The products could be used as building blocks for the enantioselective or racemic synthesis of C2-quaternary indolin-3-one skeletons. Further investigation and application of the nucleophilic 2-monoarylated indole-3-one derivatives are ongoing in our laboratories.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (grants. 21502030, 81660348, 21601039 and 81460523) and the PhD early development program of Guizhou Medical University (J[2014]017). Financial support from the Innovation Team of Nature Science Foundation of Departement of Education of Guizhou Province (No. QJHRCTDZ[2015]57) is also acknowledged.Notes and references
- (a) A. Wetzel and F. Gagosz, Angew. Chem., Int. Ed., 2011, 50, 7354 CrossRef PubMed; (b) B. Lu, Y. Luo, L. Liu, L. Ye, Y. Wang and L. Zhang, Angew. Chem., Int. Ed., 2011, 50, 8358 CrossRef PubMed; (c) Y. Q Zhang, D. Y. Zhu, Z. W. Jiao, B. S. Li, F. M. Zhang, Y. Q. Tu and Z. Bi, Org. Lett., 2011, 13, 3458 CrossRef PubMed; (d) A. J. Birch and J. J. Wright, J. Chem. Soc., Chem. Commun., 1969, 644 RSC; (e) D. S. Bhakuni, M. Silvan, S. A. Matlin and P. G. Sammes, Phytochemistry, 1976, 15, 574 CrossRef; (f) R. R. Liu, S. C. Ye, C. J. Lu, G. L. Zhuang, J. R. Gao and Y. X. Jia, Angew. Chem., Int. Ed., 2015, 54, 11205 CrossRef PubMed; (g) W. Wang, Q. Q. Zhou, J. Xuan, T. R. Li, L. Q. Lu and W. J. Xiao, Tetrahedron Lett., 2014, 55, 4648 CrossRef; (h) S. K. Guchhait, V. Chaudhary, V. A. Rana, G. Priyadarshani, S. Kandekar and M. Kashyap, Org. Lett., 2016, 18, 1534 CrossRef PubMed; (i) Y. Goriya and C. V. Ramana, Chem. Commun., 2013, 49, 6376 RSC.
- (a) K. Higuchi, Y. Sato, M. Tsuchimochi, K. Sugiura, M. Hatori and T. Kawasaki, Org. Lett., 2009, 11, 197 CrossRef PubMed; (b) Y. H. Liu and W. W. McWhorter, J. Am. Chem. Soc., 2003, 125, 4240 CrossRef PubMed; (c) R. O. Torres-Ochoa, T. Buyck, Q. Wang and J. P. Zhu, Angew. Chem., Int. Ed., 2018, 57, 5679 CrossRef PubMed; (d) W. H. Pearson, Y. Mi, I. Y. Lee and P. Stoy, J. Am. Chem. Soc., 2001, 123, 6724 CrossRef PubMed; (e) C. Y. Wang, Z. L. Wang, X. N. Xie, X. T. Yao, G. Li and L. S. Zu, Org. Lett., 2017, 19, 1828 CrossRef PubMed; (f) X. B. Qi, H. L. Bao and U. K. Tambar, J. Am. Chem. Soc., 2011, 133, 10050 CrossRef PubMed; (g) S. Han and M. Movassaghi, J. Am. Chem. Soc., 2011, 133, 10768 CrossRef PubMed; (h) T. Buyck, Q. Wang and J. P. Zhu, Org. Lett., 2012, 14, 1338 CrossRef PubMed; (i) B. N. Reddy and C. V. Ramana, Chem. Commun., 2013, 49, 9767 RSC; (j) B. Zhao, X. Y. Hao, J. X. Zhang, S. Liu and X. J. Hao, Org. Lett., 2013, 15, 528 CrossRef PubMed.
- Synthesis of C2-tetrasubstituted 3-Oxindoles: (a) J. B. Peng, Y. Qi, A. J. Ma, Y. Q. Tu, F. M. Zhang, S. H. Wang and S. Y. Zhang, Chem.–Asian J., 2013, 8, 883 CrossRef PubMed; (b) Y. Goriya and C. V. Ramana, Chem. Commun., 2013, 49, 6376 RSC; (c) S. R. Mothe, M. L. Novianti, B. J. Ayers and P. W. H. Chan, Org. Lett., 2014, 16, 4110 CrossRef PubMed; (d) Y. X. Zhang, J. W. Han and Z. J. Liu, Synlett, 2015, 26, 2593 CrossRef; (e) J. R. Huang, L. Qin, Y. Q. Zhu, Q. Song and L. Dong, Chem. Commun., 2015, 51, 2844 RSC; (f) N. Li, T. Y. Wang, L. Z. Gong and L. M. Zhang, Chem.–Eur. J., 2015, 21, 3585 CrossRef PubMed; (g) K. S. Guchhait, V. Chaudhary, V. A. Rana, G. Priyadarshani, S. Kandekar and S. Kashyap, Org. Lett., 2016, 18, 1534 CrossRef PubMed; (h) X. X. Zhang, P. Li, C. Lyu, W. X. Yang, J. Li, X. Y. Pan, X. B. Zhu and W. D. Rao, Adv. Synth. Catal., 2017, 359, 4147 CrossRef; (i) K. Pal, R. K. Shukla and C. M. R. Volla, Org. Lett., 2017, 19, 5764 CrossRef PubMed; (j) B. B. Zhang, X. Zhang, B. Hu, D. S. Sun, S. L. Wang, D. Zhang-Negrerie and Y. F. Du, Org. Lett., 2017, 19, 902 CrossRef PubMed; (k) Z. L. Xie, J. D. Hu, Y. Q. Gao, Q. Z. Yao and W. Q. Xie, Chem. Commun., 2017, 53, 7485 RSC; (l) Y. J. Li, C. H. Liu, Y. Yu and Y. L. Zhao, Org. Lett., 2017, 19, 1160 CrossRef PubMed; (m) W. Q. Fu and Q. L. Song, Org. Lett., 2018, 20, 393 CrossRef PubMed.
- (a) J. Guo, Z. H. Lin, K. B. Chen, Y. Xie, A. C. Chan, J. Weng and G. Lu, Org. Chem. Front., 2017, 4, 1400 RSC; (b) S. Yarlagadda, B. Ramesh, C. Ravikumar Reddy, L. Srinivas, B. Sridhar and B. V. Subba Reddy, Org. Lett., 2017, 19, 170 CrossRef PubMed; (c) H. Kazahiro, M. Kouhei, K. Tamami, H. Masahiro, S. Masanori and K. Tomomi, Heterocyles, 2007, 73, 641 CrossRef; (d) C. Y. Jin, Y. Wang, Y. Z. Liu, C. Shen and P. F. Xu, J. Org. Chem., 2012, 77, 11307 CrossRef PubMed; (e) Y. L. Zhao, Y. Wang, J. Cao, Y. M. Liang and P. F. Xu, Org. Lett., 2014, 16, 2438 CrossRef PubMed; (f) T. G. Chen, P. Fang, X. L. Hou and L. X. Dai, Synthesis, 2015, 47, 134 CrossRef.
- (a) C.-S. Chien, T. Suzuki, T. Kawasaki and M. Sakamoto, Chem. Pharm. Bull., 1984, 32, 3945 CrossRef; (b) C.-S. Chien, T. Takanami, T. T. Kawasaki and M. Sakamoto, Chem. Pharm. Bull., 1985, 33, 1843 CrossRef; (c) C.-S. Chien, A. Hasegawa, T. Kawasaki and M. Sakamoto, Chem. Pharm. Bull., 1986, 34, 1493 CrossRef; (d) T. Kawasaki, K. Masuda, Y. Baba, R. Terashima, K. Takada and M. Sakamoto, J. Chem. Soc., Perkin Trans. 1, 1996, 729 RSC; (e) A. S. Bourlot, E. Desarbre and J. Y. Mérour, Synthesis, 1994, 411 CrossRef; (f) J. Y. Mérour, L. Chichereau and J. P. Finet, Tetrahedron Lett., 1992, 33, 3867 CrossRef.
- (a) C. C. C. Johansson and T. J. Colacot, Angew. Chem., Int. Ed., 2010, 49, 676 CrossRef PubMed; (b) F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 1082 CrossRef PubMed; (c) M. J. Durbin and M. C. Willis, Org. Lett., 2008, 10, 1413 CrossRef PubMed; (d) Y. Y. Yang, Y. X. Li, C. Cheng, G. Yang, J. Q. Zhang, Y. Zhang, Y. L. Zhao, L. Zhang, C. Li and L. Tang, J. Org. Chem., 2018, 83, 3348 CrossRef PubMed; (e) A. V. Mitin, A. N. Kashin and I. P. Beletskaya, J. Organomet. Chem., 2004, 689, 1085 CrossRef; (f) A. N. Kashin, A. V. Mitin, I. P. Beletskaya and R. Wife, Tetrahedron Lett., 2002, 43, 2539 CrossRef.
- (a) Y. L. Zhao, Y. Wang, X. Q. Hu and P. F. Xu, Chem. Commun., 2013, 49, 7555 RSC; (b) Y. L. Zhao, Y. Wang, Y. C. Luo, X. Z. Fu and P. F. Xu, Tetrahedron Lett., 2015, 56, 3703 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04807j |
| This journal is © The Royal Society of Chemistry 2018 |