Open Access Article
Open Access ArticleCharacteristics of wear particles and wear behavior of retrieved PEEK-on-HXLPE total knee implants: a preliminary study†
Xiangchao Meng‡
a,
Zhe Du‡ab and
You Wang *a
*a
aDepartment of Bone and Joint Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, 145 Middle Shandong Road, Shanghai, China. E-mail: drwangyou@126.com; Fax: +86 21 53882181; Tel: +86 21 53882181
bDepartment of Trauma and Emergency Center, Peking University People's Hospital, Beijing, China
First published on 28th August 2018
Abstract
Polyether-ether-ketone (PEEK) has been used clinically for intervertebral fusion and internal fixators in spine and trauma surgery because of its mechanical properties and bioinertness. The present study aimed to assess the suitability of PEEK as an alternative material to cobalt–chromium–molybdenum alloy in total knee arthroplasty (TKA) and evaluate the in vivo wear property on the contact surface of the PEEK-on-highly cross-linked polyethylene (HXLPE). PEEK prosthesis was designed and manufactured using injection molding based on the computed tomography data of a standard goat right hind limb. Fifteen goats underwent TKA using PEEK-on-HXLPE prosthesis on the right hind limb. The goats were sacrificed at 12, 24, and 48 weeks postoperatively. The mean surface roughness (Ra) of the retrieved components, proinflammatory cytokines in the synovial fluid, and characteristics of wear particles in the synovial membrane were investigated using laser confocal microscopy, ELISA and polarized light microscopy. The Ra of the femoral component was about 0.08, 0.1, 0.2, and 0.26 μm at pre-study, 12-, 24-, and 48 weeks in the retrievals, respectively. The Ra of the HXLPE bearing samples was approximately 0.38, 0.4, 0.1, and 0.42 μm at pre-study, 12-, 24-, and 48 weeks in the retrievals, respectively. The median size of the particles was 2.63 μm, 1.98 μm, and 3.00 μm at 12, 24, and 48 weeks, respectively. The particles ranged in size from 0.4 μm to 15 μm, and particles <1 μm accounted for 7–13%, those of size 2–5 μm accounted for 67–76%, and those >5 μm accounted for 11–22%. Levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-α (TNF-α) were significantly increased in synovial fluids at 24- and 48 weeks after surgery. Wear occurred on the surfaces of the PEEK and HXLPE material and the size of most wear particles was 1–5 μm. This induced an inflammatory response in the synovial membrane and release of proinflammatory cytokines. A high polishing process may be necessary to lengthen the life of the PEEK prosthesis by reducing the wear and the generation of debris. The PEEK prosthesis as a new generation of artificial joints is promising to be used clinically in the future.
Introduction
At present, cobalt–chromium–molybdenum (Co–Cr–Mo) alloy is the most commonly used in vivo metallic biomaterial in the sliding part of a joint prosthesis.1 However, because of its high elastic modulus and poor mechanical compatibility with human bone, it can easily produce stress occlusion and stress concentration, which lead to bone resorption and atrophy and eventually cause prosthesis loosening and sinking.2,3 In addition, meeting the strict surface quality requirements in the manufacturing process and the time-consuming process of polishing the surface of Co–Cr–Mo alloy femoral component are challenging.4However, there has been growing interest in polyether-ether-ketone (PEEK) material as an arthroplasty-bearing material, such as PEEK-on-PEEK articulations in the spine5,6 as well as total knee implants,3,7 and it is regarded as an alternative to Co–Cr–Mo alloy in knee arthroplasty. PEEK is a thermoplastic polymer known to be resistant to fatigue strain. The characteristics of PEEK include a high melting temperature (approx. 335 °C) and a high glass transition temperature (approx. 135 °C), and its mechanical properties match more closely those of bone compared to titanium.8 It is radiologically transparent and used clinically in orthopedic applications because of its mechanical properties and bioinertness, as it does not demonstrate toxicity or mutagenicity, teratogenicity, and carcinogenicity.5 Basically, the toxicity always induced by the wear debris which generated from the wear between the contacted materials surface, and these wear debris also lead to osteolysis around the prosthesis and, eventually, prosthesis loosening.9 Therefore, wear resistance in vivo and generation of wear particles are the issues of greatest concern for joint prosthesis. Previous studies had proved PEEK material resists fatigue strain and wear in vitro well,7,10,11 but there has been few in vivo study as yet of PEEK-on- highly cross-linked polyethylene (HXLPE) TKA implants to investigate the wear behavior under the complicated mechanical and chemical environment of knee joints.
In the present study, PEEK was manufactured by injection molding to a geometry for use as femoral components and tibia trays in total knee replacement. The aim of the present study was to assess the suitability of PEEK as an alternative sliding component material to Co–Cr–Mo alloy in total knee replacements and evaluate the change in surface roughness of the retrieved components and the properties of the wear particles.
Materials and methods
PEEK material
The PEEK material used is Solvay's Zeniva® PEEK (Zeniva PEEK ZA-500) which was obtained from Solvay S.A., Brussels, Belgium. The chemical structure and characteristics of PEEK are provided on Fig. S1,† and the characteristics of the PEEK material used can be obtained from the website (http://www.matweb.com/search/datasheettext.aspx?matguid=5f6774aeb3ff4d739709a7579c5a4c8d).Live subject statement
Fifteen adult female standardized goats (weight, 30 kg; height, 66–70 cm) were obtained from Jiagan Biotechnology Company (Shanghai, China) and randomly assigned into three groups (n = 15). All animals were raised in accordance with Shanghai Jiaotong University School of Medicine Animal Care and Use Committee Guidelines. All animal studies were performed following the appropriate guidelines (no. 2017021) and approved by the animal ethics committee of the Renji Hospital, School of Medicine, Shanghai Jiaotong University (Shanghai, China).Design and manufacture prosthesis of goat
The goat prosthesis and surgical instruments were designed based on computed tomography (CT) data (Light speed 16; GE Medical Systems, Milwaukee, WI, USA) of a standardized goat right hind limb. The PEEK component and the HXLPE insert (Zeniva PEEK ZA-500, Chirulen HXLPE 1020X) were provided by Jiangsu Okani Medical Technology Co., Ltd. (Soochow, JS, China). The femoral prosthesis was sterilized by irradiation, and the tibial prosthesis was sterilized by ethylene oxide.Surgical procedures
Surgery was performed on the right hind limb, and the incision was made by a lateral para-patellar approach under general anesthesia. Bone cuts were completed using appropriate surgical instruments, and bone cement was applied to the femoral and tibial sides to fix the implants, some synovial membranes were removed, and then closed the incision. Antibiotics (penicillin, 60 mg kg−1) were administered for 5 days after surgery. Five goats were sacrificed at each post-operative interval: 12, 24, and 48 weeks.Cytokine detection by ELISA
All animals underwent synovial fluid examinations (cytokine detection) at 12, 24, and 48 weeks after surgery. The synovial fluids were obtained from the joint when the goats were sacrificed. The cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α from synovial fluids were measured using enzyme-linked immunoassay kit specific for goat IL-1β, IL-6, and TNF-α (Duoset R&D Systems, Abingdon, UK).Surface topography measurement of implants in retrievals
Implant components, including femoral and tibial components, were retrieved when the goat was sacrificed. Fifteen pairs of implants retrieved at 12, 24, and 48 weeks were chosen for testing, and five pairs of femoral and tibial components, manufactured in the same batch, were chosen as the control. The regions of interest in surface topography measurement were the part of the lateral posterior condyle of the femoral component and the recess of the lateral plateau of the tibial tray. The surface was assessed by laser confocal microscopy (Leica TCS SP8 MP, Wetzlar, Germany) and the mean surface roughness (Ra) was investigated using measurement software (Keyence VK H1XP, Osaka, Japan).Histological analysis and measurement of polymer particles
The goat knee synovial membrane was obtained from the sacrificed animal and fixed in 4% paraformaldehyde for 24 hours and then washed with phosphate buffered saline and dehydrated following a standard process. After embedding in paraffin, the specimens were sliced into 3 μm thick sections and stained with hematoxylin and eosin. Pathology and wear particles were observed under white light microscope and polarized light microscope, respectively. Pathological assessment of the tissues included tissue architecture, inflammatory reactions, and degenerative changes. The particles were measured by using Photoshop CS6 software with polarized light images.Tribological study in vitro
One preliminary tribological study of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles test in vitro was carried out on a six-station knee simulator testing machine (Simulation Solutions, UK). The axial force and flexion extension measurements were taken from the international standard for wear testing (ISO 14243-3). The test was carried out using the conventional methods published previously.7
000 cycles test in vitro was carried out on a six-station knee simulator testing machine (Simulation Solutions, UK). The axial force and flexion extension measurements were taken from the international standard for wear testing (ISO 14243-3). The test was carried out using the conventional methods published previously.7
Statistical analysis
A Kruskal–Wallis test was used for the nonparametric data and a Student's t-test was used for the parametric data. P < 0.05 was considered statistically significant.Results
Design, manufacturing, implementation, and retrieval of PEEK prosthesis and HXLPE tray
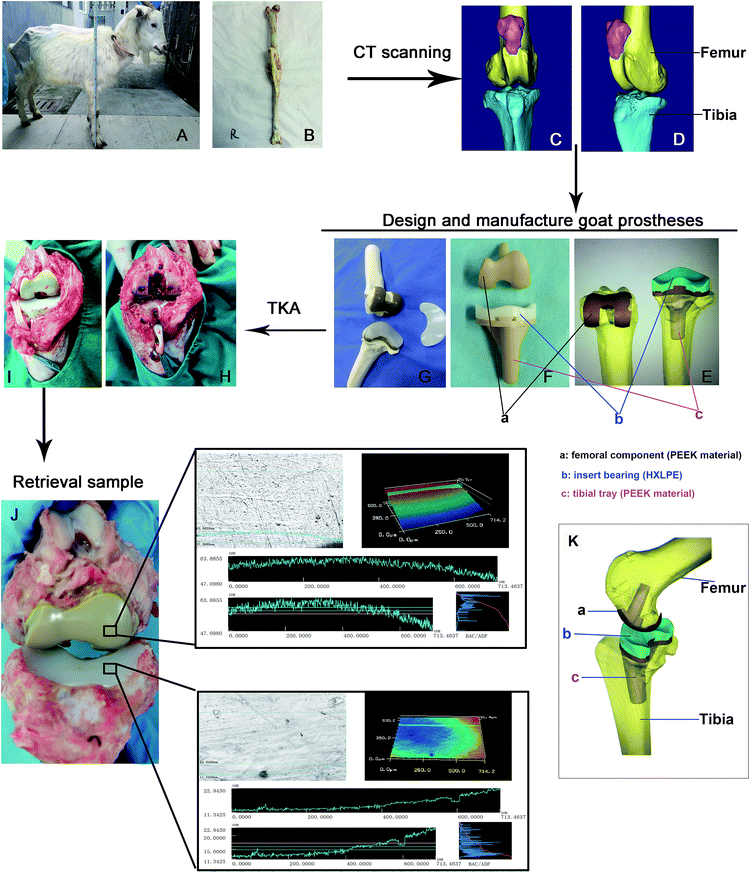
We had established a three-dimensional model of the knee joint (Fig. 1A–D) based on the CT data. Then, the model of the corresponding knee joint prosthesis was designed by computer and manufactured by injection molding using PEEK material (Fig. 1E and F). The goat prosthesis consists of a femoral component (injection-molded PEEK material) and a tibial tray (PEEK material) with an insert (HXLPE). The tray and insert bearing were embedded tightly together and locked using 1 mm metal wires (G). After assembly and testing of the PEEK implant component, we performed TKA on goats according to the traditional TKA operation (Fig. 1H and I). In the sham-operation group, the knee capsule was only incised and part of the synovium was resected. These animals were raised under the same conditions and sacrificed at different intervals to retrieve the PEEK prosthesis (Fig. 1J). A 3D-sketch of the knee prosthesis indicating the femoral component and the tibial tray and the respective materials for each that were used in the study is provided as Fig. 1E, F and K.Laser confocal profile of the manufacturing samples and retrievals and Ra changes in vivo and vitro
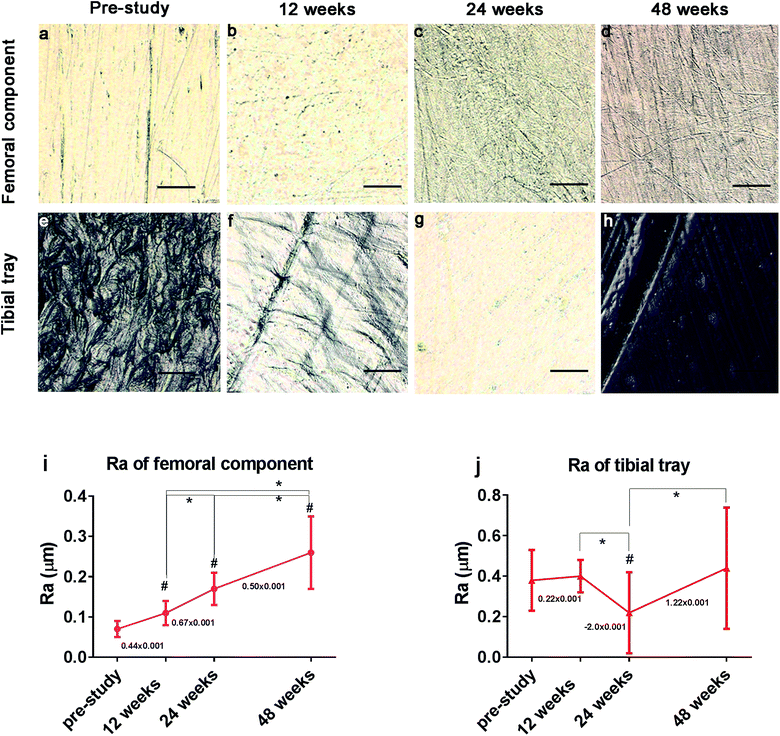
The surface of the implant components was detected by laser confocal microscope, and Ra values were obtained using VK analyzer software. Compared with the initial surface of the sample, the relatively shallow grooves on the surface of the PEEK component increased with time (Fig. 2a–d). The grooves on the PE-bearing surface increased and deepened at 12 weeks but disappeared at 24 weeks (Fig. 2f and g). This phenomenon indicated that the polishing effect of friction occurred during the wear and friction between PEEK and HXLPE. The grooves of the specimen deepened and increased at 48 weeks (Fig. 2h). The Ra of the injection-molded prosthetic femoral component is about 0.08 μm at pre-study and 0.1, 0.2, and 0.26 μm at 12, 24, and 48 weeks in the retrievals, respectively (Fig. 2i). The continuously increasing trend of Ra indicated that the wear of the PEEK material gradually increased. The Ra of the HXLPE was about 0.38 μm at pre-study and 0.4, 0.1, and 0.42 μm at 12, 24, and 48 weeks in the retrievals, respectively (Fig. 2, j). There was switching of Ra value in the HXLPE component, indicating that the wear polishing effect occurred on the initial rough surface. One preliminary tribological study of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles test in vitro revealed similar tribological changes: the surface of the PEEK component tended to be rougher while that of HXLPE tended to be smoother (Fig. S2†).
000 cycles test in vitro revealed similar tribological changes: the surface of the PEEK component tended to be rougher while that of HXLPE tended to be smoother (Fig. S2†).
Morphological observation of wear particles in the synovial membrane and characterization of polymer particles
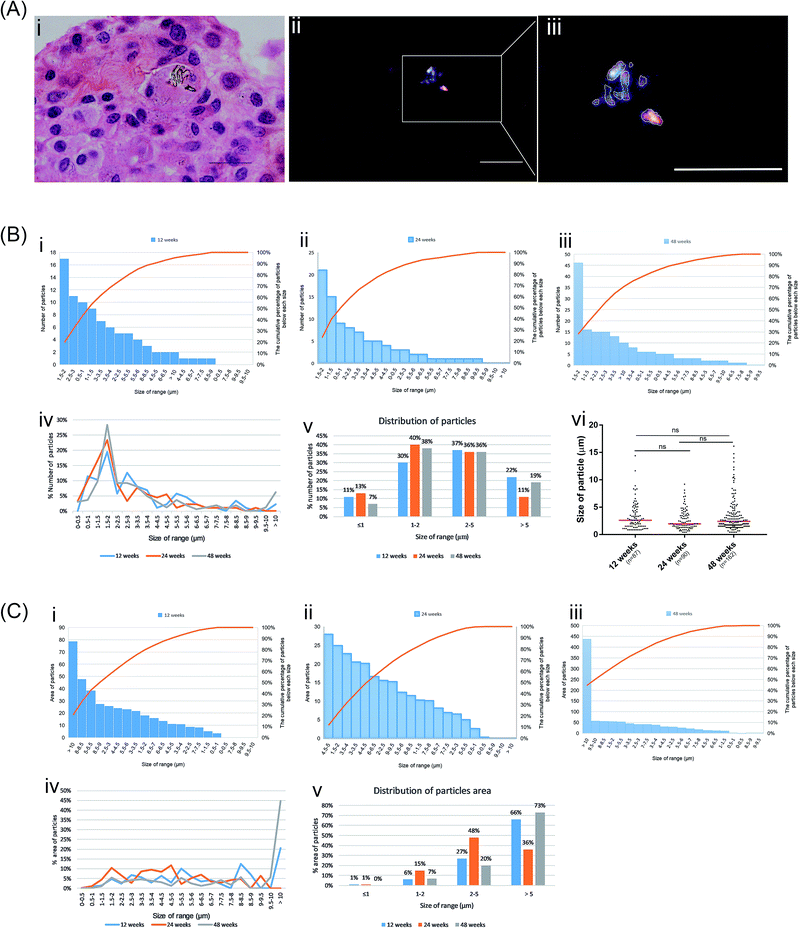
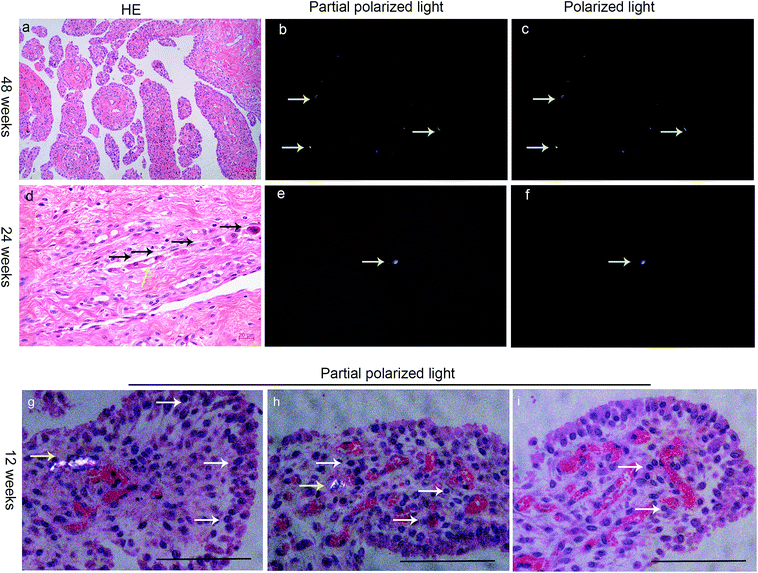
Based on the characteristics of PEEK and HXLPE particles, which show a strong birefringence under polarized light, we found wear particles in the synovial membrane of the postoperative knee joint samples (Fig. 3). In the present study, there were two types of wear particles in the synovial membrane as the prosthesis is composed of PEEK and HXLPE materials. As both polymer particles show a strong birefringence under polarized light, the number, shape, and size of the particles could be evaluated (Fig. 3A and 4a–c). The wear particles had rod-shaped, lumpy, or flake-like morphologies. The distribution of debris size, which is our point of interest, ranged from <1 μm to >15 μm, and most wear particles were close to 1.5 μm (Fig. 3B). The distribution trends of the three groups were comparable with regards to the size and area distribution of the particles (Fig. 3B and C) which was skewed: particles <1 μm accounted for 7–13%, between 2–5 μm accounted for 67–76%, and >5 μm accounted for 11–22% (Fig. 3B). The median size of each group was 2.63 μm, 1.98 μm, and 3.00 μm, respectively, with no significance between them (Fig. 3B). Particles >5 μm represented only approximately 17% of the number of particles (Fig. 3B); however, these particles accounted for about 60% of the area of particles (Fig. 3C).The particles were engulfed by macrophages (Fig. S3†). At 12 weeks post-surgery, we observed that particles in the synovial tissue were aggregated into strips and a large number of inflammatory cells infiltrated into the surrounding tissue (Fig. 4g–i). This is comparable to previous studies that confirmed that wear particles can stimulate synovial tissue proliferation, synovial tissue inflammation, and capillary proliferation. In the synovial tissue of the 24 week group, we found several eosinophilic stained cells near the particles and gather along the site of the particles (Fig. 4d–f), which suggests that the particles may cause the local macrophages to express chemokines which recruited many eosinophilic staining cells.
Inflammatory cytokine detection in the synovial fluid
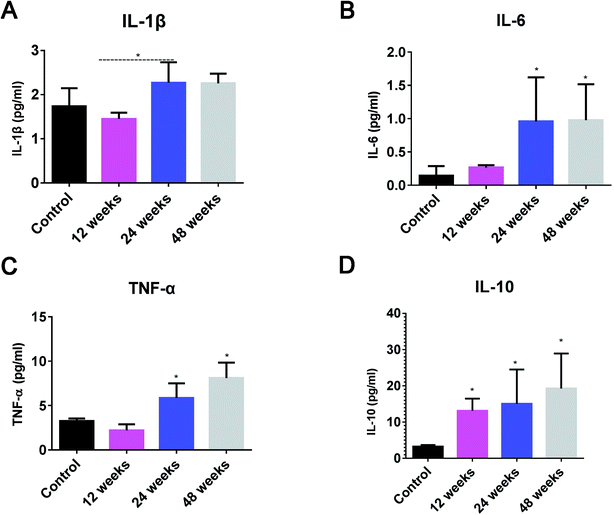
We also detected inflammatory cytokines in the synovial fluid of the experimental animals. The results show that IL-6 and TNF-α were lower in normal synovial fluid but significantly increased at 24 and 48 weeks after surgery (Fig. 5B and C). IL-1β tended to increase at 24 and 48 weeks after surgery but was not significantly increased compared with the pre-surgery value (Fig. 5A). IL-10 significantly increased at the postoperative intervals (Fig. 5D).Discussion
PEEK materials are expected to be used in the new generation of knee prostheses as they may resolve complications such as periprosthetic osteoporosis and fracture, caused by the stress-shielding effect of traditional metal prostheses.3,12,13 Before the PEEK prosthesis is used in patients, more in vivo experimental data are needed to prove its suitability and safety. However, there have been few in vivo studies regarding the wear characteristics of the PEEK prosthesis and the characteristics of the associated wear particles and subsequent tissue inflammatory response. In the present study, we used injection-molded PEEK material as a component of a TKA prosthesis in a goat model to assess the suitability of PEEK as an alternative material to conventional metallic biomaterial. In addition, we retrieved the PEEK-on-HXLPE prosthesis at different intervals to evaluate the change in surface roughness of the retrieved components and the properties of the wear particles.Wear resistance is an important evaluation of prosthesis material as it relates to the life of the prosthesis and disease associated with wear particles, which cause osteolysis around the prosthesis and prosthesis loosening.8,9 Therefore, less wear and fewer wear particles are goals of the development of new prosthesis materials. Wear resistance is mainly determined by several factors, such as surface hardness, surface smoothness, and resistance to fatigue and fracture, and, basically, the higher the hardness or smoothness, the lower the wear.14–17 The Co–Cr–Mo alloy has a higher elastic modulus (200 GPa) than PEEK (3.84–17.94 GPa) and PE (1.0–1.39 GPa), which means the Co–Cr–Mo alloy has a greater hardness,18–20 but previous studies have confirmed that the biotribology of PEEK-on-HXLPE bearings is comparable to traditional bearings of metal-on-PE in vitro.9 In this study, we found that the surface of PEEK becomes rougher over time, but the HXLPE tended to be smoother at 24 weeks and then became rougher after 24 weeks. Given the same change of surface roughness in the wear behavior of previous studies,10 the phenomenon by which the HXLPE surface tended to be smoothed and then roughened over time indicates that wear was generally greater on the HXLPE bearing. The arithmetic mean for surface roughness (Ra) is one of the most important factors affecting the wear rate.21 The surface roughness of typical prosthetics varied in the range Ra = 0.005–0.04 μm; this was representative of femoral heads and femoral knee components currently used clinically.22 Studies showed that wear increased obviously with the increased Ra value, but less rapidly with decreased counterface roughness Ra values < 0.05 μm.23 Nevertheless, the researchers also hold the view that there were significant advantages to be gained from improving counterface roughness to below 0.01 μm Ra.22 In this study, the initial Ra of PEEK was 0.08 μm and PE was 0.4 μm, which is much larger than the standard range. On the other hand, the elastic modulus of the PE was lower than the PEEK, which indicates that the hardness of PE was lower than that of the PEEK material and PE experienced more wear.
There was a 24 week running-in period between the two friction pairs for the high Ra of these femoral components and bearings that is comparable to the results of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles test in vitro. This behavior has also been described in previous tribological studies in ductile metallic samples and brittle ceramic materials.24,25 Studies have confirmed that the reduction in surface roughness may reduce the wear of materials and reduce the generation of debris.8,26 Polished surfaces may decrease the wear baseline at the same time compared with that rougher components. Therefore, a highly polished bearing surface is one of the ways to reduce the wear rate and reduce production of wear debris. The polishing process may shorten the breaking-in period, decrease the wear baseline, and reduce the wear rate and generation of debris mainly produced by wear.
000 cycles test in vitro. This behavior has also been described in previous tribological studies in ductile metallic samples and brittle ceramic materials.24,25 Studies have confirmed that the reduction in surface roughness may reduce the wear of materials and reduce the generation of debris.8,26 Polished surfaces may decrease the wear baseline at the same time compared with that rougher components. Therefore, a highly polished bearing surface is one of the ways to reduce the wear rate and reduce production of wear debris. The polishing process may shorten the breaking-in period, decrease the wear baseline, and reduce the wear rate and generation of debris mainly produced by wear.
The wear particles have been associated with a locally aggressive biologic response that can lead to synovitis, periprosthetic bone loss, and aseptic loosening of the implants.9 Both metal alloy and PEEK material produces numerous wear particles from the wear of the artificial implant. The degree of particle disease would be determined by the functional biological activity of the particles, which can be estimated from the biological activity of debris, size distribution, and the total wear volume over the particle size range.27 In previous studies, no obvious difference between PEEK particles and HXLPE particles and Co–Cr–Mo particles was observed in terms of immunological and inflammatory responses and osteolysis, but the PEEK particles could activate CD8+ T-cells.28,29 However, the mechanisms for activating CD8+ T-cells needs more research; it may stimulate antigen presenting cells which then activate the class-I MHC molecular pathways, resulting in elevated CD8+ T-cell responses. In the present study, the wear debris in the synovial membrane of postoperative goats was identified by a polarized light microscope. The size ranged from 0.4–15 μm, and median size was close to 1.5 μm. The size and distribution of particles are comparable to the characteristics of the particles in previous studies that investigated the biologic reactivity to orthopedic implant debris.28,29 There is a consensus that particles between 0.1 and 10 μm would be easily engulfed by macrophages, causing an inflammatory response,30,31 so these particles would easily induce a biological response in the synovial membrane. Previous studies have shown that CFR-PEEK particles can stimulate the proliferation of vascular synovial tissue and may even enter the circulatory system through the capillaries, stimulating the tissue reaction of other organs.32 In the present study, synovial tissue hyperplasia, small blood vessel hyperplasia, and inflammatory cell infiltration were found in the postoperative synovial tissue and several particles were phagocytosed by macrophages of the synovial membrane. These results indicate that the wear debris, including the PEEK and HXLPE particles, induces the immunological and inflammatory responses in synovial membranes. However, the particles may interact with the outer receptors of the macrophage membranes, as cluster of differentiation (CD) 11b, CD14, and the Toll-like receptor (TLR) family, and then activate macrophages to release proinflammatory cytokines.12,33
Proinflammatory agents of TNF-α, IL-1β, IL-6, and prostaglandin E2 are always released in the inflammatory response and among these factors, TNF-α and IL-1β appear to have the most significant roles in a cascade that causes osteolysis.34,35 In this study, TNF-α, IL-10, and IL-6 were increased in the synovia at 24- and 48 week after TKA. These cytokines reached the osteoblasts, thereby increasing RANKL activation, which increases nuclear transcription factor-kappa B gene expression by binding to RANK and promotes an influx of osteoclast precursors and drives osteoclastic differentiation and activity, thereby promoting osteolysis.9,33,34 The results indicated that a decrease of wear particles or depression of the inflammatory response to particles would help to improve particle-induced osteolysis. Considering that PEEK particles and HXLPE particles had comparable functional biological activity,29 the best way to improve particle-induced osteolysis is highly polished surfaces (decreased Ra) of the PEEK and HXLPE components.
Some limitations of this study should be noted. It is difficult to measure and count nanoscale particles <0.4 μm, which are at the limitations of optical microscopy. Therefore, the distribution statistics of the particles have certain limitations. In addition, many particles aggregate into clumps or overlap, which results in measurement errors. In addition, the goat model could not completely imitate the mechanical environment of the human body. The biomechanical and tribological evaluations of PEEK prosthesis should be further investigated to determine influencing factors, such as surface roughness, sliding speed, pressure, and so on.
Conclusion
The results of this pre-investigation did not show any harmful influences of PEEK-on-HXLPE prosthesis in goats. However, wear did occur on the surface of the materials and the size of most particles was 1–5 μm, which induced an inflammatory response of the synovial membrane and release of TNF-α, IL-1β, IL-6, and IL-10. A high polishing process, aimed at reducing abrasive wear, may negligibly lengthen the life of the PEEK artificial joint by shortening the breaking-in period, thereby reducing the wear rate and generation of debris. Unlike traditional implants made of metal alloys, PEEK implants exhibit a modulus similar to cortical bone, which may improve patient comfort, provide a more stable fixation over time, and extend implant lifetime by reducing wear of the TKA prosthesis. In the future, the development of PEEK production and polishing technologies and 3D printing would make PEEK-on-HXLPE prothesis more applicable to human medicine.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This study was supported by the National Key R&D Program of China (2016YFC1101802).Many thanks to Zhonglin Zhu and the Jiangsu Okani Medical Technology Company for providing the PEEK prosthesis.References
- R. Civinini, C. Carulli, F. Matassi, A. C. Lepri, L. Sirleo and M. Innocenti, The survival of total knee arthroplasty: Current data from registries on tribology: Review article, HSS J., 2017, 13, 28–31 CrossRef PubMed.
- D. D. Robertson, C. M. Mintzer, B. N. Weissman, F. C. Ewald, M. LeBoff and M. Spector, Distal loss of femoral bone following total knee arthroplasty. Measurement with visual and computer-processing of roentgenograms and dual-energy x-ray absorptiometry, J. Bone Jt. Surg., Am. Vol., 1994, 76, 66–76 CrossRef.
- K. E. Rankin, A. S. Dickinson, A. Briscoe and M. Browne, Does a peek femoral tka implant preserve intact femoral surface strains compared with cocr? A preliminary laboratory study, Clin. Orthop. Relat. Res., 2016, 474, 2405–2413 CrossRef PubMed.
- Y. Song, C.-H. Park and T. Moriwaki, Mirror finishing of Co–Cr–Mo alloy using elliptical vibration cutting, Precis. Eng., 2010, 34, 784–789 CrossRef.
- I. V. Panayotov, V. Orti, F. Cuisinier and J. Yachouh, Polyetheretherketone (peek) for medical applications, J. Mater. Sci., 2016, 27, 118 Search PubMed.
- T. Brown and Q. B. Bao, The use of self-mating peek as an alternative bearing material for cervical disc arthroplasty: A comparison of different simulator inputs and tribological environments, Eur. Spine J., 2012, 21(suppl 5), S717–S726 CrossRef PubMed.
- R. M. Cowie, A. Briscoe, J. Fisher and L. M. Jennings, Peek-optima™ as an alternative to cobalt chrome in the femoral component of total knee replacement: A preliminary study, Proc. Inst. Mech. Eng., Part H, 2016, 230, 1008–1015 CrossRef PubMed.
- M. Becker, S. Lorenz, D. Strand, C. F. Vahl and M. Gabriel, Covalent grafting of the rgd-peptide onto polyetheretherketone surfaces via schiff base formation, TheScientificWorld, 2013, 2013, 616535 Search PubMed.
- D. D. Naudie and C. H. Rorabeck, Sources of osteolysis around total knee arthroplasty: Wear of the bearing surface, Instr. Course Lect., 2004, 53, 251–259 Search PubMed.
- H. E. Rebecca, B. Adam and U. Anthony, Wear of peek-optima® and peek-optima®-wear performance articulating against highly cross-linked polyethylene, Proc. Inst. Mech. Eng., Part H, 2015, 229, 187–193 Search PubMed.
- D. Baykal, R. S. Siskey, R. J. Underwood, A. Briscoe and S. M. Kurtz, The biotribology of peek-on-hxlpe bearings is comparable to traditional bearings on a multidirectional pin-on-disk tester, Clin. Orthop. Relat. Res., 2016, 474, 2384–2393 CrossRef PubMed.
- L. Cavalli and M. L. Brandi, Periprosthetic bone loss: Diagnostic and therapeutic approaches, F1000Research, 2013, 2, 266 Search PubMed.
- S. Roux and P. Orcel, Bone loss. Factors that regulate osteoclast differentiation: An update, Arthritis Res., 2000, 2, 451–456 CrossRef PubMed.
- A. C. Faria, U. M. Benassi, R. C. Rodrigues, R. F. Ribeiro and M. G. Mattos, Analysis of the relationship between the surface hardness and wear resistance of indirect composites used as veneer materials, Braz. Dent. J., 2007, 18, 60 CrossRef PubMed.
- S. D. Heintze, A. Cavalleri, M. Forjanic, G. Zellweger and V. Rousson, Wear of ceramic and antagonist-a systematic evaluation of influencing factors in vitro, Dent. Mater., 2008, 24, 433–449 CrossRef PubMed.
- M. N. Mandikos, G. P. McGivney, E. Davis, P. J. Bush and J. M. Carter, A comparison of the wear resistance and hardness of indirect composite resins, J. Prosthet. Dent., 2001, 85, 386–395 CrossRef PubMed.
- A. Peutzfeldt, F. Garcia-Godoy and E. Asmussen, Surface hardness and wear of glass ionomers and compomers, Am. J. Dent., 1997, 10, 15–17 Search PubMed.
- N. M. S. A. Malek, S. R. Mohamed, S. A. C. Ghani and W. S. W. Harun, Critical evaluation on structural stiffness of porous cellular structure of cobalt chromium alloy, IOP Conf. Ser.: Mater. Sci. Eng., 2015, 100, 012019 Search PubMed.
- R. F. Heary, N. Parvathreddy, S. Sampath and N. Agarwal, Elastic modulus in the selection of interbody implants, Journal of spine surgery, 2017, 3, 163–167 CrossRef PubMed.
- L. A. Pruitt, Deformation, yielding, fracture and fatigue behavior of conventional and highly cross-linked ultra high molecular weight polyethylene, Biomaterials, 2005, 26, 905–915 CrossRef PubMed.
- R. M. Hall, A. Unsworth, P. Siney and M. Wroblewski B, The surface topography of retrieved femoral heads, 1996 Search PubMed.
- J. G. Lancaster, D. Dowson, G. H. Isaac and J. Fisher, The wear of ultra-high molecular weight polyethylene sliding on metallic and ceramic counterfaces representative of current femoral surfaces in joint replacement, Proc. Inst. Mech. Eng., Part H, 1997, 211, 17–24 CrossRef PubMed.
- A. Wang, V. K. Polineni, C. Stark and J. H. Dumbleton, Effect of femoral head surface roughness on the wear of ultrahigh molecular weight polyethylene acetabular cups, J. Arthroplasty, 1998, 13, 615–620 CrossRef PubMed.
- A. A. Goldsmith, D. Dowson, G. H. Isaac and J. G. Lancaster, A comparative joint simulator study of the wear of metal-on-metal and alternative material combinations in hip replacements, Proc. Inst. Mech. Eng., Part H, 2000, 214, 39–47 CrossRef PubMed.
- M. Kalin and S. Jahanmir, Influence of roughness on wear transition in glass-infiltrated alumina, 2003 Search PubMed.
- F. Billi, S. N. Sangiorgio, S. Aust and E. Ebramzadeh, Material and surface factors influencing backside fretting wear in total knee replacement tibial components, J. Biomech., 2010, 43, 1310–1315 CrossRef PubMed.
- N. J. Hallab, K. Mcallister, M. Brady and M. Jarmansmith, Macrophage reactivity to different polymers demonstrates particle size- and material-specific reactivity: Peek-optima(®) particles versus uhmwpe particles in the submicron, micron, and 10 micron size ranges, J. Biomed. Mater. Res., Part B, 2012, 100, 480–492 CrossRef PubMed.
- Z. Du, S. Wang, B. Yue, Y. Wang and Y. Wang, Effects of wear particles of polyether-ether-ketone and cobalt-chromium-molybdenum on cd4- and cd8-t-cell responses, Oncotarget, 2017, 11197–11208 Search PubMed.
- Z. Du, S. Wang and Y. Wang, Preferential cd8 rather than cd4 t-cell response to wear particles of polyether-ether-ketone and highly cross-linked polyethylene, RSC Adv., 2018, 8, 1866–1874 RSC.
- M. Schramm, D. C. Wirtz, U. Holzwarth and R. P. Pitto, The morse taper junction in modular revision hip replacement-a biomechanical and retrieval analysis, Biomed. Tech., 2000, 45, 105–109 Search PubMed.
- A. Liu, L. Richards, C. L. Bladen, E. Ingham, J. Fisher and J. L. Tipper, The biological response to nanometre-sized polymer particles, Acta Biomater., 2015, 23, 38–51 CrossRef PubMed.
- A. C. Paulus, S. Haßelt, V. Jansson, A. Giurea, H. Neuhaus, T. M. Grupp and S. Utzschneider, Histopathological analysis of peek wear particle effects on the synovial tissue of patients, BioMed Res. Int., 2016, 2016, 2198914 Search PubMed.
- J. Gallo, S. B. Goodman, Y. T. Konttinen, M. A. Wimmer and M. Holinka, Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms, Acta Biomater., 2013, 9, 8046–8058 CrossRef PubMed.
- P. Wojdasiewicz, Ł. A. Poniatowski and D. Szukiewicz, The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis, Mediators Inflammation, 2014, 2014, 561459 Search PubMed.
- N. Zini, G. Lisignoli, L. Solimando, A. Bavelloni, F. Grassi, L. Guidotti, C. Trimarchi, A. Facchini and N. M. Maraldi, Il1-beta and tnf-alpha induce changes in the nuclear polyphosphoinositide signalling system in osteoblasts similar to that occurring in patients with rheumatoid arthritis: An immunochemical and immunocytochemical study, Histochem. Cell Biol., 2003, 120, 243–250 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04661a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |