Open Access Article
Open Access ArticleCarboxymethylcellulose ammonium-derived nitrogen-doped carbon fiber/molybdenum disulfide hybrids for high-performance supercapacitor electrodes†
Yanyan Lva,
Yi Zhou a,
Ziqiang Shao
a,
Ziqiang Shao *a,
Jie Weia,
Lei Li
*a,
Jie Weia,
Lei Li a and
Yiping Wangb
a and
Yiping Wangb
aBeijing Engineering Research Centre of Cellulose and Its Derivatives, School of Materials Science and Engineering, Beijing Institute of Technology, Beijing, 100081, P. R. China. E-mail: shaoziqiang@263.net
bNantong Tailida Chemical Co., Ltd, Jiangsu 226000, P. R. China
First published on 14th August 2018
Abstract
In this paper, a new type of nitrogen-doped carbon fiber/molybdenum disulfide (N-CFs/MoS2) hybrid electrode materials are prepared via a certain concentration in solvothermal synthesis followed by a high-temperature carbonization process and using the carboxymethylcellulose ammonium (CMC-NH4) as a structure-directing agent for MoS2 nanosheet growth during the solvothermal synthesis process. The addition of CMC-NH4 effectively prevents the agglomeration of MoS2 nanosheets to increase the specific surface area. Moreover, it not only serves as a carbon source to provide conductive pathways, but also introduces N atoms to improve the conductivity of the CFs and promote the transfer of electrons and ions. This ultimately increases the conductivity of the electrode materials. Thus, the as-prepared N-CFs/MoS2 hybrids exhibit excellent electrochemical performance. The specific capacitance is up to 572.6 F g−1 under a current density of 0.75 A g−1 and the specific capacitance retained 98% of the initial capacitance after 5000 cycles of charge–discharge tests at a current density of 2.5 A g−1. Moreover, the hybrids show a maximum energy density of 19.5 W h kg−1 at a power density of 94 W kg−1. Therefore, the as-prepared N-CFs/MoS2 hybrids with remarkable electrochemical properties, low cost and environment protection show potential for practical application in the development of high-performance electrochemical energy storage devices.
Introduction
The diminishing reserves of non-renewable fossil fuels and the changing global climate have led to efforts to find sustainable, renewable energy and conversion and storage devices that possess the advantages of high efficiency, environmental friendliness and reutilization.1–3 Lithium-ion batteries and supercapacitors are the two common energy systems with excellent performance of high-capacity and fast charge/discharge rates.4,5 As one kind of important electrochemical energy storage device, supercapacitors with high power density, low cost, high reliability, environment-friendly and long life and other advantages have aroused people's widespread interest with the rapid development of electric vehicles, clean energy storage and other electronic products.6–10 Their performance mainly relies on physicochemical processes at the electrode–electrolyte interface such as ion transfer, charge transfer and electrodes wetted by electrolyte. Therefore, electrode materials are one of the main factors that affect the electrochemical performance of the supercapacitor and the current research focus is mainly on the electrode material with excellent electrochemical properties and appropriate microstructure.11–13As a typical 2D transition metal dichalcogenides (TMDs) layered material, molybdenum disulfide (MoS2) shows graphene-like structure and has a layered structure held together by weak van der Waals forces, and in which molybdenum atoms are sandwiched between two layers of sulfur atoms. Its researches on energy storage devices have achieved improved interest in recent years because of they can provide high specific surface area for charge storage of electric double layer.14,15 However, it is reported that the MoS2 electrode can exhibit a fast capacity decaying and inferior rate capability during charge–discharge cycling because of its large volume change and poor electrical/ionic conductivity between the two adjacent S–Mo–S sheets.16–18 Moreover, during the synthesis, the MoS2 nanosheets can grow and self-assemble together ascribe to its 2D structure. Consequently, hybridization of MoS2 with other conductive materials to form hierarchical nanostructures is an effective method to improve the dispersion of MoS2 nanosheets and enhance its conductivity to achieve optimal specific capacitance. Currently, MoS2-based hybrids such as MoS2/RGO, MoS2/PANI, MoS2/carbon fibers, and cellulose nanofibers/MoS2/RGO as electrode materials for supercapacitors have been reported in the articles.19–22 Although the hybrids' electrochemical performances are not poor, the substrates are not environmentally friendly and renewable.
Cellulose ether is an important cellulose derivative.23 It is the generic terms of a variety of derivatives obtained after alkalization, etherification, purification and drying the natural cellulose which as raw material and it is widely used in many fields such as agriculture, industry, daily chemical industry, aerospace and defence, environmental protection, biomedicine, nanomaterials, optoelectronic materials and so on for its abundant resource, low cost, and eco-friendliness. Carboxymethylcellulose ammonium (CMC-NH4) is a kind of ionic cellulose ether which can easily dissolve in water for it has a number of ammonium carboxymethyl groups (–CH2COONH4). Using it as a carbon source can obtain nitrogen-doped carbon fibers (N-CFs) by high-temperature carbonization process. The introduction of nitrogen atoms can effectively enhance the electron-donating ability of the surface of carbon material and reduce the valence orbital energy levels to induce synergistically enhanced conductive capacity.24
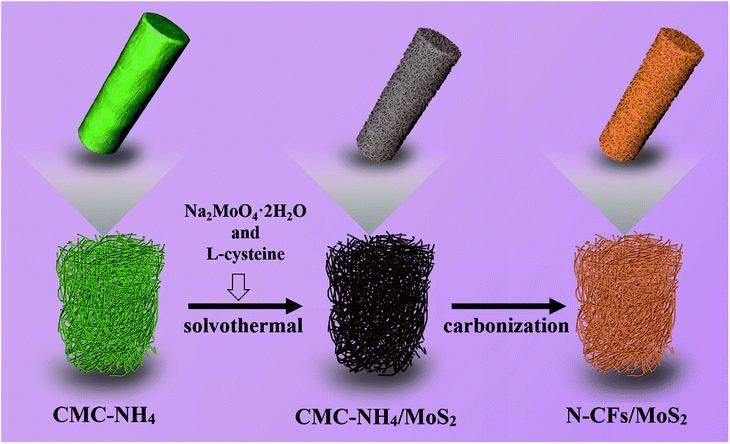
A novel, low-cost and environmentally friendly N-CFs/MoS2 hybrids are successfully prepared via solvothermal synthesis followed by a high-temperature carbonization process (Fig. 1). The CMC-NH4 serves as a structure-directing agent in the solvothermal synthesis of MoS2 nanosheets. However, CMC-NH4 is a water-soluble cellulose ether that contains many ammonium groups (–CH2COONH4). It maintains its molecular form when dissolved in water, but it is insoluble in anhydrous ethanol. Therefore, ethanol aqueous solution with certain concentration is first prepared, so that the CMC-NH4 is slowly released into the aqueous medium and still exists as fibers forms. It thus can serve as a structure-directing agent for the growth of MoS2 nanosheets during the solvothermal synthesis process. The addition of CMC-NH4 effectively prevents the self-assembly agglomeration of the MoS2 nanosheets and thus helps to increase the specific surface area of MoS2, which further provides large numbers of active sites for electrochemical reactions, offers more contact area for electrolyte and electrode materials, and reduces the distance for ion diffusion. Moreover, it not only serves as a carbon source to provide conductive pathways, but also introduces N atoms to effectively improve the electron-donating ability of the carbon material from its surface, lower the valence orbital energy, and ultimately improve the conductivity of the electrode materials. The as-prepared low-cost electrode materials not only exhibit excellent electrochemical performance but also are environmentally friendly, making it a candidate for practical applications in the energy field.
Experimental
Materials
CMC-NH4, with a degree of substitution of 0.9, was purchased from Beijing North Century Cellulose Technology Research&Development Co., Ltd. Ethanol (EtOH), sodium molybdate (Na2MoO4·2H2O), L-cysteine, potassium hydroxide (KOH) and N-methylpyrrolidone (NMP) were analytic grade and purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. Polyvinylidene fluoride (PVDF) was provided by Sigma-Aldrich, Co., Ltd. Acetylene black and nickel foam were obtained from Changsha Liyuan New Material Co., Ltd.Preparation of CMC-NH4/MoS2 hybrid materials
Ethanol aqueous solutions (70 g) of different concentrations (0%, 25%, 50%, 75%, and 100%) were first prepared, after which the CMC-NH4 (0.4 g) was magnetically stirred for 6 h and dispersed in the above solutions. Then, Na2MoO4·2H2O (0.4 g) was magnetically stirred and ultrasonically dispersed in the dispersions. L-cysteine (1 g) was added via magnetic stirring and ultrasonic dispersion. The as-prepared dispersions were then transferred into a 100 mL Teflon-lined stainless-steel autoclave and heated at 200 °C for 24 h. After cooling naturally, the black CMC-NH4/MoS2 hybrid materials were collected by filtration, washed with 85% ethanol solutions three to five times and cleaned with anhydrous ethanol, and then dried under vacuum at 60 °C for 24 h. For comparison, pure MoS2 powder was prepared via hydrothermal synthesis in a similar way.Preparation of N-CFs/MoS2 hybrid electrode materials
The above-prepared CMC-NH4/MoS2 hybrid materials were carbonized in a tube furnace under flowing nitrogen atmosphere at a constant temperature of 800 °C for 2 hours with a heating rate of 5 °C min−1 and then cooled to room temperature with a cooling rate of 10 °C min−1 to obtain N-CFs/MoS2 with CMC-NH4 as carbon and nitrogen source. The preparation process is shown in Fig. 1.For comparison, N-CFs were also prepared from CMC-NH4 by high temperature carbonization under the same conditions.
Characterization
The microstructure and morphology of the samples were conducted using a Hitachi S-4800 field-emission-gun scanning electron microscope (SEM) at 5 kV and 15 kV and a JEM-200CX transmission electron microscope (TEM). The chemical composition was performed by the energy dispersive X-ray spectroscopy (EDS). The high-resolution transmission electron microscopy (HRTEM) observations were investigated on Tecnai F20 TEM under 200 kV acceleration voltage. X-ray diffraction (XRD) analysis was characterized with a Bruker AXS D8 Discover diffractometer (Germany) using Cu-Kα radiation (λ = 1.5406 Å) and the 2θ used in the measurements was range from 5 to 90°. Fourier transform infrared spectroscopy (FT-IR) was measured with a Magna-IR 750 spectrophotometer. X-ray photoelectron spectroscopy (XPS) of the samples were characterized with a PHI 5300 Photoelectron Spectrometer (Perkin Elmer Instruments Co. Ltd., USA). Thermogravimetric analysis (TG-DTA 6200 LAB SYS) was carried out in air from 50 to 800 °C at a heating rate of 10 °C min−1.Electrochemical measurements
The electrochemical properties of the as-synthesized N-CFs/MoS2 hybrids, MoS2 and N-CFs were investigated on an electrochemical workstation (on a CHI 660D, CH Instruments, Inc). PVDF was added to NMP and magnetically stirred until it was completely dissolved and the mixture turned transparent. The concentration of the PVDF/NMP solution was 0.01 g mL−1. The as-synthesized three materials were each ground in an agate mortar and mixed with acetylene black and the PVDF/NMP solutions by magnetic stirring until uniformly mixed. The mass ratio of electroactive material to acetylene black and PVDF was 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was then evenly coated onto a Ni foam current collector (1 cm × 3 cm) and the coating area measured 1 × 1 cm2. The electrode was dried in an oven at 60 °C for 18 h until all the NMP was evaporated, after which the Ni foam coated with the electrode material was pressed into thin sheet. For the three-electrode system tests, the thin Ni foam sheet with electrode material was used as a working electrode. Pt and Ag/AgCl were used as counter and reference electrodes, respectively. The electrode clips were respectively clamped on the corresponding working, counter, and reference electrodes for electrochemical testing and a KOH aqueous solution (6 M) was used as an electrolyte. For the two-electrode system, two identically sized electrode sheets were used as symmetrical electrodes tested in the 6 M KOH electrolyte.
1. The mixture was then evenly coated onto a Ni foam current collector (1 cm × 3 cm) and the coating area measured 1 × 1 cm2. The electrode was dried in an oven at 60 °C for 18 h until all the NMP was evaporated, after which the Ni foam coated with the electrode material was pressed into thin sheet. For the three-electrode system tests, the thin Ni foam sheet with electrode material was used as a working electrode. Pt and Ag/AgCl were used as counter and reference electrodes, respectively. The electrode clips were respectively clamped on the corresponding working, counter, and reference electrodes for electrochemical testing and a KOH aqueous solution (6 M) was used as an electrolyte. For the two-electrode system, two identically sized electrode sheets were used as symmetrical electrodes tested in the 6 M KOH electrolyte.
The cyclic voltammograms (CV) were performed at scan rates of 10, 20, 50, 100, 200 and 400 mV s−1 with a potential range of −0.9 to 0.1 V. The galvanostatic charge–discharge (GCD) properties were recorded in a potential window of −0.9 to 0.1 V at the current densities of 0.75, 1.5, 2.5, 5 and 10 A g−1. The electrochemical impedance spectroscopy (EIS) was assessed using a sinusoidal signal of 5 mV within the frequency range of 0.01 Hz to 105 Hz under open circuit potential. Cyclic stability was measured using GCD measurement for over 5000 cycles at the current density of 2.5 A g−1.
All the electrochemical parameters are calculated as follows,
The gravimetric capacitance (Cg, F g−1):
| Cg = (∫idV)/(v × m × V) (CV curves) |
| or Cg = I × Δt/(ΔV × m) (GCD curves) |
The energy density (E, W h kg−1) and power density (P, W kg−1):
| E = 1/2 × C × ΔV2 |
| P = E/Δt |
Results and discussion
Characterization of N-CFs/MoS2 hybrid electrode materials
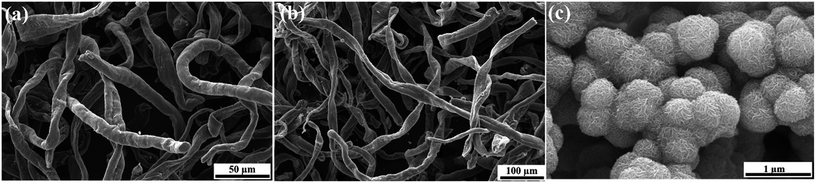
Fig. 2 shows the scanning electron microscope (SEM) images of CMC-NH4, N-CFs and MoS2. The observed diameter of the intertwined cylindrical and ribbon-like CMC-NH4 fibers, which are several hundred micrometers in length as shown in Fig. 2a, is 10–15 μm. After water loss due to carbonization, almost all the N-CFs exhibit ribbon-like fibers with virtually no change in length (Fig. 2b). The SEM image of MoS2 powder prepared via hydrothermal synthesis is displayed in Fig. 2c. The MoS2 powder exhibits a relatively uniform and spherical shape with an average diameter of approximately 350 nm. These spheres consisted of MoS2 nanosheets, indicating that the as-prepared MoS2 nanosheets would easily self-assemble into a flower-like structure.The SEM images of CMC-NH4 and CMC-NH4/MoS2 prepared via solvothermal synthesis under ethanol solutions with different concentrations are shown in Fig. S1†. CMC-NH4 is a polymer whose substituent group (–CH2COONH4) is hydrophilic, so it is insoluble in anhydrous ethanol but readily soluble in water. Generally, polymers swell first and then dissolve in the dissolution process. They are normally composed of flexible chains that are easily curled into spherical shapes in solution. Therefore, after CMC-NH4 is dissolved in water, treated hydrothermally, and washed with ethanol, its microscopic morphology changes from fibrous to a smooth spherical shape with a diameter of 1–5 μm, as shown in Fig. S1a.† Therefore, the MoS2 nanosheets are coated on the surface of the CMC-NH4 spheres (Fig. S1b†) in the CMC-NH4/MoS2 which is synthesized on the CMC-NH4 matrix in the aqueous medium, and the self-aggregation could not be prevented effectively. When the synthesis is conducted under ethanol solution with a concentration of 25%, the rate of dissolution for CMC-NH4 is slightly lower than that in water. Therefore, CMC-NH4 is not completely dissolved, and a small number of short-length CMC-NH4 fibers remained at the end of the reaction (Fig. S1c†). Fig. S1d† shows the microscopic morphology of CMC-NH4/MoS2 hybrids prepared via solvothermal synthesis in 25% ethanol solution. The MoS2 nanosheets are uniformly attached on the surface of CMC-NH4 fibers that are not dissolved completely, while the remaining dissolved spherical CMC-NH4 are tightly coated by self-assembled MoS2 nanosheets. The self-agglomeration is still not effectively prevented. When the concentration of ethanol solution is further increased to 50%, the dissolution rate of CMC-NH4 is even slower. The morphology of CMC-NH4 still maintains its fibrous structure, but it changes from long-length fibers to short-length fibers and rods (Fig. S1e†). The SEM image of the CMC-NH4/MoS2 hybrids prepared under the same conditions is displayed in Fig. S1f.† Obviously, the CMC-NH4 serves as a structure-directing agent for the growth of MoS2 nanosheets so that they are evenly grown on the surfaces of the CMC-NH4 fibers. Thus, the self-assembly agglomeration of MoS2 nanosheets is effectively avoided. Moreover, the CMC-NH4 is almost insoluble as the concentration of ethanol solution increased to 75%, so the CMC-NH4 exhibits a fibrous structure almost similar to its initial morphology (Fig. S1g†). However, MoS2 nanosheets can only be formed when Na2MoO4·2H2O and L-cysteine react in aqueous solutions. The amount of water is not sufficient for the synthesis of MoS2 nanosheets in 75% ethanol solution. Consequently, only a small amount of MoS2 nanosheets are formed and sparsely adhered to the surface of CMC-NH4 fibers (Fig. S1h†). Furthermore, no MoS2 nanosheets are formed in anhydrous ethanol, in which the CMC-NH4 is insoluble and Na2MoO4·2H2O does not react with L-cysteine. Hence, only CMC-NH4 fibers are observed in Fig. S1i and j.†
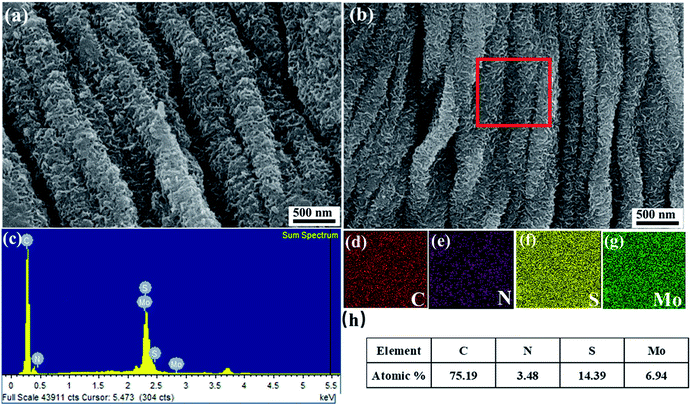
Fig. 3 shows magnified SEM images of CMC-NH4/MoS2 hybrids prepared solvothermally in 50% ethanol solution, N-CFs/MoS2 hybrid electrode materials prepared by high-temperature carbonization, the EDS spectra, and the corresponding EDS mappings of elements. As shown in Fig. 3a, the CMC-NH4 successfully serves as the structure-directing agent for the growth of MoS2 nanosheets in 50% ethanol solution. The as-synthesized MoS2 nanosheets are evenly grown on the surfaces of the CMC-NH4 fibers, indicating that the CMC-NH4 successfully prevents the self-accumulation of MoS2 nanosheets during the synthesis process. After high-temperature carbonization, the MoS2 nanosheets are still uniformly attached on the surfaces of CMC-NH4 fibers (Fig. 3b). The EDS spectra (Fig. 3c) and mass percentage of the elements (Fig. 3h) further demonstrate the co-existence of the elements as follows: C (49.78%), N (4.71%), S (21.48%), and Mo (24.03%); and the C and N atoms are derived from N-CFs in N-CFs/MoS2 obtained by high-temperature carbonization of CMC-NH4/MoS2. In addition, the EDS mappings of elements shown in Fig. 3d–g provide more evidence for the co-existence of C, N, S, and Mo atoms, respectively. This further confirms that MoS2 nanosheets are uniformly grown on the surfaces of CMC-NH4 fibers. Moreover, the atomic percentages of S and Mo in N-CFs/MoS2 electrode materials are 14.39% and 6.94%, respectively, which is consistent with the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in MoS2.
1 in MoS2.
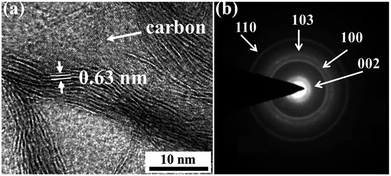
The morphology of the N-CFs/MoS2 hybrid materials is further investigated by high resolution transmission electron microscopy (HRTEM). In Fig. 4a, five to eight layers of MoS2 nanosheets can be observed with a spacing of 0.63 nm which corresponds to the (002) lattice of hexagonal MoS2. Fig. 4b shows selected-area electron diffraction (SAED) spectra of the MoS2. The diffraction rings correspond to the characteristic diffraction peaks of the (110), (103), (100), and (002) crystal planes of MoS2 respectively, which are consistent with the XRD pattern of MoS2.
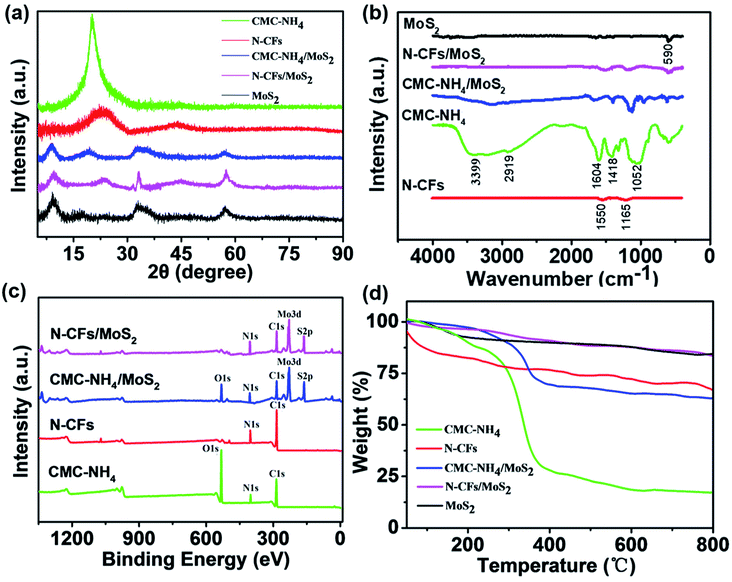
The crystalline structures of the CMC-NH4, MoS2, N-CFs, CMC-NH4/MoS2, and N-CFs/MoS2 are characterized by X-ray diffraction (XRD) as shown in Fig. 5a. A sharp characteristic diffraction peak at a 2θ angle of 19.5° is detected in the XRD pattern for CMC-NH4, corresponding to the (110) crystal plane of a typical cellulose II crystal structure.25 In the XRD pattern for pure MoS2 powder, the characteristic diffraction peaks at 2θ angles of 14° and 59° are assigned to the (002) and (110) crystal planes, respectively, whereas the stacked diffraction peaks around 2θ angles of 33–40° are assigned to the (100) and (103) planes, respectively.26 The XRD pattern of CMC-NH4/MoS2 exhibits characteristic diffraction peaks similar to those of CMC-NH4 and MoS2, indicating that the MoS2 nanosheets are successfully grown on the surfaces of CMC-NH4 fibers. However, the characteristic diffraction peak at a 2θ angle of 19.5° is weaker than that of the pure CMC-NH4. This is because part of the crystalline structure of CMC-NH4 is destroyed by water molecules during the solvothermal synthesis process, thus converting the long-length fibers to short-length fibers and rods. The XRD pattern of N-CFs/MoS2 shows the characteristic diffraction peaks of MoS2 and N-CFs. The peaks at a 2θ angle of 19.5° disappeared completely for N-CFs/MoS2 and N-CFs, while two new characteristic diffraction peaks are observed at 2θ angles of 24.6° and 44°, which correspond to the (002) and (100) crystal planes of graphene, respectively, indicating that the N-CFs/MoS2 and N-CFs have a low degree of graphitization.27
The Fourier transform infrared spectroscopy (FTIR) spectra of pure MoS2 powder, CMC-NH4, N-CFs, CMC-NH4/MoS2, and N-CFs/MoS2 hybrids are shown in Fig. 5b. The weak peak at 590 cm−1 for pure MoS2 powder is attributed to the Mo–S vibrations.28 In the FTIR spectrum of CMC-NH4, the strong and broad absorption peak at 3399 cm−1 corresponds to its O–H stretching vibrations,29 the characteristic absorption peak at 2919 cm−1 corresponds to the stretching vibrations of C–H, and the characteristic absorption peaks at 1604 and 1418 cm−1 are due to the vibrations of –COO– in the carboxylate groups.30 Moreover, the characteristic absorption peak at 1052 cm−1 is attributed to the stretching vibrations of C–O–C, which proves the existence of ether linkage in six-membered rings.31 Compared with CMC-NH4, the characteristic absorption peaks of the above functional groups almost disappear in the FTIR spectra of N-CFs, while two new absorption peaks appear at 1550 and 1165 cm−1, which can be assigned to the characteristic absorption peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N bonds, respectively. It is further proved that the CMC-NH4 is successfully carbonized and nitrogen-doped. Additionally, the CMC-NH4/MoS2 maintains the characteristic absorption peaks of CMC-NH4 and MoS2, and N-CFs/MoS2 contains the characteristic peaks of N-CFs and MoS2. These also confirm that the MoS2 nanosheets are successfully and evenly attached on the surfaces of CMC-NH4 and N-CFs. Fig. 5c shows the X-ray photoelectron spectroscopy (XPS) spectra for the as-prepared samples. The characteristic absorption peaks of O 1s, C 1s, N 1s, S 2p, and Mo 3d are detected, while characteristic peaks for only C 1s, N 1s, S 2p, and Mo 3d are observed for N-CFs/MoS2 hybrid electrode material. This is also consistent with the EDS results shown in Fig. 3.
N and C–N bonds, respectively. It is further proved that the CMC-NH4 is successfully carbonized and nitrogen-doped. Additionally, the CMC-NH4/MoS2 maintains the characteristic absorption peaks of CMC-NH4 and MoS2, and N-CFs/MoS2 contains the characteristic peaks of N-CFs and MoS2. These also confirm that the MoS2 nanosheets are successfully and evenly attached on the surfaces of CMC-NH4 and N-CFs. Fig. 5c shows the X-ray photoelectron spectroscopy (XPS) spectra for the as-prepared samples. The characteristic absorption peaks of O 1s, C 1s, N 1s, S 2p, and Mo 3d are detected, while characteristic peaks for only C 1s, N 1s, S 2p, and Mo 3d are observed for N-CFs/MoS2 hybrid electrode material. This is also consistent with the EDS results shown in Fig. 3.
Fig. 5d shows the thermogravimetric analysis (TG) analysis graph for the as-prepared samples. Both physical and chemical changes are involved in the thermal decomposition process of CMC-NH4, as shown in Fig. 5d. The slight weight loss from 50 °C to 220 °C is due to the evaporation of the physically absorbed water. There is a relative large weight loss in the range of 220–290 °C, while a very large and rapid weight loss is detected in the range of 290–380 °C due to the decomposition of CMC-NH4. The weight loss is mainly caused by the removal of volatile gases generated from the decomposition of organic compounds. As the temperature continued to increase, the rate of weight loss gradually decreased, and a constant weight is obtained after 600 °C when only a small amount of sodium salts are the major residuals. The weight loss of N-CFs is also mainly caused by its decomposition, with a small quantity of sodium salts as residuals. However, the weight loss of MoS2 comes from the oxidation of MoS2 to MoO3 and SO2 when heated to 315 °C. Thus, the weight losses in CMC-NH4/MoS2 and N-CFs/MoS2 are not only attributed to the decomposition of CMC-NH4 and N-CFs substrates, but also to the oxidation reaction of MoS2. Therefore, the main residues are dominated by a small amount of sodium salts and MoO3.
The specific surface area and porous structure of the samples are characterized by measuring the N2 adsorption–desorption isotherms. It can be seen from the isotherm curves (Fig. S2a†) and the pore size distribution (Fig. S2b†), the samples of MoS2, CMC-NH4, N-CFs, CMC-NH4/MoS2 and N-CFs/MoS2 are mainly characteristic of micropores and mesoporous, and their specific surface areas are in order of 9.3 m2 g−1, 14.73 m2 g−1, 18.76 m2 g−1, 40.47 m2 g−1, 32.78 m2 g−1. These results demonstrate the addition of the CMC-NH4 effectively prevents the agglomeration of MoS2 nanosheets during the solvothermal synthesis of MoS2 and thus increasing the specific surface area.
Electrochemical characteristics of N-CFs/MoS2 hybrid electrode materials
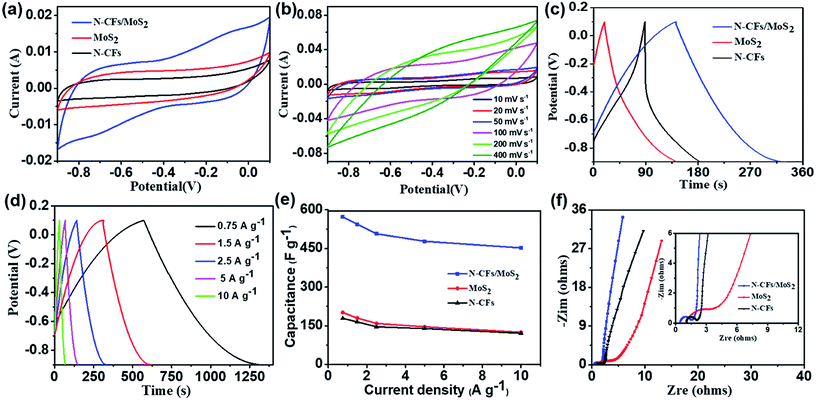
The N-CFs/MoS2 hybrids prepared above are then evaluated as an electrode material for supercapacitors. For comparison, the electrochemical performance of pure MoS2 and N-CFs are also tested. All tests are conducted under room-temperature conditions.Fig. 6a shows the cyclic voltammograms (CV) curves of the pure MoS2, N-CFs, and N-CFs/MoS2 hybrids at a scan rate of 50 mV s−1 with a potential window range of −0.9 to 0.1 V. All the CV curves exhibit a nearly symmetrical rectangular shape, indicating ideal capacitive behaviors of the three materials. The difference is the N-CFs/MoS2 hybrids display a much higher capacitance than the other two for the larger CV enclosed area which can also be demonstrated by a longer discharge time in the GCD curves at a current density of 2.5 A g−1 with voltage between −0.9 and 0.1 V (Fig. 6c). Fig. 6b exhibits the CV curves of the N-CFs/MoS2 hybrids at scan rates of 10 to 400 mV s−1. As can be seen from the figure, even the scan rate is increased to 400 mV s−1, the CV curve still maintains an almost symmetrical rectangular shape without significant deformation. This provides evidence for the N-CFs/MoS2 hybrids' fast response to current when the voltage reverses. Furthermore, the specific capacitance (Cg) of the N-CFs/MoS2 hybrids calculated from the CV curve at a scan rate of 10 mV s−1 is 588.5 F g−1, which is higher than those reported in the literature for MoS2/C based electrodes, such as CF@MoS2 nanocomposites synthesized by Liu et al. (503.7 F g−1 at a scan rate of 50 mV s−1 in 1 M Na2SO4 aqueous electrolyte),21 and porous tubular C/MoS2 nanocomposites by Hu et al. (210 F g−1 at a current density of 1 A g−1).32 Moreover, it is higher than other MoS2-based hybrids, such as MoS2/RGO (148 F g−1 at a scan rate of 10 mV s−1),19 and MoS2/PANI (575 F g−1 at a current density of 1 A g−1).20
Fig. 6d displays the galvanostatic charge–discharge (GCD) curves of the N-CFs/MoS2 hybrid electrode materials under different current densities (0.75, 1.5, 2.5, 5, and 10 A g−1). An almost symmetrical triangular shape is observed in all of the charge–discharge curves in the designated potential range, indicating that the N-CFs/MoS2 hybrids exhibit excellent capacitance performance and fast current–voltage response.33 Fig. 6e presents the Cg of the three materials which are calculated from the discharge curves at various current densities. As can be clearly seen from the figure, the Cg of the N-CFs/MoS2 hybrids are higher than the Cg of the other two at the same current density. And under a current density of 0.75 A g−1, the Cg of N-CFs/MoS2 hybrids, pure MoS2 and N-CFs are 572.6 F g−1, 198.7 F g−1 and 178.6 F g−1, respectively. In addition, the Cg of them are maintained up to 456.4 F g−1, 125.2 F g−1 and 121.1 F g−1 in order, which are 79.7%, 63.0% and 67.8% of the initial Cg even when the current density is increased to 10 A g−1. This illuminates the excellent capacitance retention property of the N-CFs/MoS2 hybrids when working as an electrode material. Furthermore, for the large volume change and poor electrical/ionic conductivity between the two adjacent S–Mo–S sheets of MoS2 (ref. 16–18) and the introduction of nitrogen atoms to the carbonized CMC-NH4, the N-CFs has a higher capacitance retention than MoS2.
The electrochemical impedance spectroscopy (EIS) is one of the most important methods for investigating the electrochemical behavior of electrodes and excellent electrode materials should possess high capacitance and low resistance.34 Fig. 6f shows the Nyquist impedance spectra of the three materials at a frequency of 0.01 Hz to 100 kHz under open-circuit voltage. As shown in the Fig. 6f, in the low-frequency region, the Nyquist plots display straight and almost vertical lines, indicating that the three materials exhibit ideal capacitance behaviors.35 The diffusion process of electrolyte ions in the electrode material can be evaluated in the mid-frequency region from the projected length of the Warburg curve with a slope of 45° on the real axis.36 As displayed in the figure, the Warburg-type line of N-CFs/MoS2 hybrids is relatively shorter than that of the other two, demonstrating the electrolyte ions diffuse faster in the N-CFs/MoS2 hybrid materials. This is because the N-CFs in the hybrid materials not only provides conductive pathways, but also improves the conductivity of the electrodes, thus promoting the diffusion of electrons and ions. In the high-frequency region, the equivalent series resistance (RESR) values can be obtained from the real axis intersection of the Nyquist plots as presented in the inset of Fig. 6f. The value of N-CFs/MoS2 hybrid materials is only 0.42 Ω, lower than the values of N-CFs and pure MoS2 which are 1.1 Ω and 0.9 Ω, respectively. In addition, from the absence of semicircles (due to the charge transfer resistance) of the Nyquist plots, the N-CFs/MoS2 hybrid materials display lower charge transfer resistance than the other two materials. Thus, the N-CFs/MoS2 hybrid materials possess excellent ion-migration ability as an electrode material.
The cyclic stability is also an important feature of electrodes. The Fig. 7 shows the decay of the specific capacitance of the N-CFs/MoS2 hybrids for 5000 cycles of galvanostatic charge–discharge tests at a current density of 2.5 A g−1. After cycles, the specific capacitance of the N-CFs/MoS2 hybrids is 561.8 F g−1, which is 98% of the initial specific capacitance, indicating the outstanding electrochemical cyclic stability of the N-CFs/MoS2 hybrids as an electrode material.
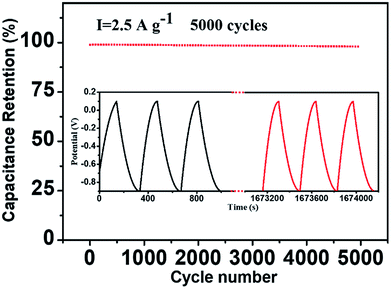 | ||
| Fig. 7 Cycling stability of the N-CFs/MoS2 hybrid electrode materials over 5000 cycles at the current density of 2.5 A g−1, the inset is the galvanostatic charge–discharge curves. | ||
The energy and power densities are also calculated due to the two parameters are important as well for evaluating the electrochemical performance of supercapacitors. Fig. S3† is GCD curves and Cg of the pure MoS2, N-CFs, and N-CFs/MoS2 hybrids based symmetric supercapacitors at different current densities. Fig. 8 presents the Ragone plots corresponding to the relationship between energy density and power density for the hybrids-based symmetric supercapacitors. It can be seen from the figure, as the power density increases from 94 to 1250 W kg−1, the energy densities of N-CFs and MoS2 decrease from 6.1 and 6.9 W h kg−1 to 4.1 and 4.3 W h kg−1, respectively. Comparatively, the N-CFs/MoS2 hybrids show energy density of 19.5 W h kg−1 at a power density of 94 W kg−1, and still retain 15.8 W h kg−1 at a power density of 1250 W kg−1. These results illustrate the N-CFs/MoS2 hybrid electrode materials have higher energy density and power output.
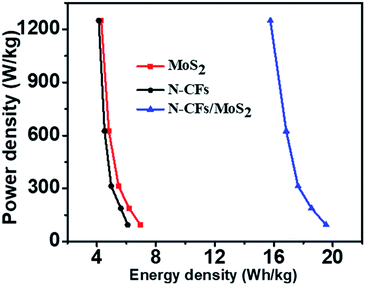 | ||
| Fig. 8 Ragone plots (energy density vs. power density) of the MoS2, N-CFs, and N-CFs/MoS2 hybrids based symmetric supercapacitors. | ||
The excellent electrochemical properties of the N-CFs/MoS2 hybrids as electrode materials are mainly attributed to the following two aspects. First, CMC-NH4 serves as a structure-directing agent during the solvothermal synthesis of MoS2, which effectively prevents the agglomeration of MoS2 nanosheets. In addition, the MoS2 nanosheets are evenly grown on the surfaces of CMC-NH4 thus increasing the specific surface area and providing large numbers of active sites for electrochemical reactions, so that the electrolyte and electrode materials can fully contact with each other to reduce the distance for ion diffusion. Second, using the CMC-NH4 as a carbon source to obtain the N-CFs via high-temperature carbonization not only provide conductive pathways, but also introduce N atoms that would effectively improve the electron-donating ability of the CFs, lower the valence orbital energy, improve the conductivity of the CFs, and promote the transfer of electrons and ions. This ultimately increases the conductivity of the electrode materials. Consequently, the as-prepared N-CFs/MoS2 hybrids exhibit remarkable electrochemical performance as electrode materials.
Conclusions
Novel N-CFs/MoS2 hybrid materials were prepared via solvothermal synthesis in 50% ethanol solution followed by a high-temperature carbonization process, using CMC-NH4 as a structure-directing agent in the solvothermal synthesis of MoS2 nanosheets. The as-prepared N-CFs/MoS2 hybrid electrode materials exhibit excellent electrochemical performance. The measured specific capacitance is up to 572.6 F g−1 at current density of 0.75 A g−1. Additionally, the N-CFs/MoS2 hybrids show high rate capability and great electrochemical cyclic stability that the specific capacitance retained 98% of the initial capacitance after 5000 cycles of charge–discharge tests at a current density of 2.5 A g−1. In addition, the hybrids display a maximum energy density of 19.5 W h kg−1 at a power density of 94 W kg−1. Therefore, due to the excellent electrochemical properties, low cost, and environmental friendliness, the N-CFs/MoS2 hybrids show potential for application in the energy field.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the support of the Beijing Engineering Research Center of Cellulose and Its Derivatives.Notes and references
- D. Zhu, Y. Wang, W. Lu, H. Zhang, Z. Song, D. Luo, L. Gan, M. Liu and D. Sun, Carbon, 2017, 111, 667–674 CrossRef.
- Y. Yang, F. Yang, H. Hu, S. Lee, Y. Wang, H. Zhao, D. Zeng, B. Zhou and S. Hao, Chem. Eng. J., 2017, 307, 583–592 CrossRef.
- M. Liu, X. Wang, D. Zhu, L. Li, H. Duan, Z. Xu, Z. Wang and L. Gan, Chem. Eng. J., 2017, 308, 240–247 CrossRef.
- C. Wang, J. Xu, M.-F. Yuen, J. Zhang, Y. Li, X. Chen and W. Zhang, Adv. Funct. Mater., 2014, 24, 6372–6380 CrossRef.
- M. X. Liu, X. M. Ma, L. H. Gan, Z. J. Xu, D. Z. Zhu and L. W. Chen, J. Mater. Chem. A, 2014, 2, 17107–17114 RSC.
- I. Oh, M. Kim and J. Kim, Chem. Eng. J., 2015, 273, 82–91 CrossRef.
- X. Zhu, H. Dai, J. Hu, L. Ding and L. Jiang, J. Power Sources, 2012, 203, 243–249 CrossRef.
- Y.-H. Lin, T.-Y. Wei, H.-C. Chien and S.-Y. Lu, Adv. Energy Mater., 2011, 1, 901–907 CrossRef.
- W. Lu, M. Liu, L. Miao, D. Zhu, X. Wang, H. Duan, Z. Wang, L. Li, Z. Xu, L. Gan and L. Chen, Electrochim. Acta, 2016, 205, 132–141 CrossRef.
- D. Zhu, Y. Wang, L. Gan, M. Liu, K. Cheng, Y. Zhao, X. Deng and D. Sun, Electrochim. Acta, 2015, 158, 166–174 CrossRef.
- X. Yang, J. Zhu, L. Qiu and D. Li, Adv. Mater., 2011, 23, 2833–2838 CrossRef PubMed.
- C. X. Guo and C. M. Li, Energy Environ. Sci., 2011, 4, 4504 RSC.
- B. Xu, S. Yue, Z. Sui, X. Zhang, S. Hou, G. Cao and Y. Yang, Energy Environ. Sci., 2011, 4, 2826–2830 RSC.
- M. Pumera, Z. Sofer and A. Ambrosi, J. Mater. Chem. A, 2014, 2, 8981–8987 RSC.
- M.-R. Gao, Y.-F. Xu, J. Jiang and S.-H. Yu, Chem. Soc. Rev., 2013, 42, 2986–3017 RSC.
- Q. Sun, Q.-Q. Ren, H. Li and Z.-W. Fu, Electrochem. Commun., 2011, 13, 1462–1464 CrossRef.
- D. Su, S. Dou and G. Wang, Chem. Commun., 2014, 50, 4192–4195 RSC.
- Y. Sun, X. Hu, W. Luo and Y. Huang, ACS Nano, 2011, 5, 7100–7107 CrossRef PubMed.
- E. G. d. S. Firmiano, C. J. Dalmaschio, A. N. Pinheiro, E. C. Pereira, W.H. Schreiner and E. R. Leite, Adv. Energy Mater., 2014, 4, 1301380 CrossRef.
- K.-J. Huang, L. Wang, Y.-J. Liu, H.-B. Wang, Y.-M. Liu and L.-L. Wang, Electrochim. Acta, 2013, 109, 587–594 CrossRef.
- X. Liu, K. Liu, W. Zhou, L. Li, K. Zhou and S. Chen, Sci. Adv. Mater., 2015, 7, 2336–2342 CrossRef.
- Y. Y. Lv, L. Li, Y. Zhou, M. Yu, J. Q. Wang, J. X. Liu, J. G. Zhou, Z. Q. Fan and Z. Q. Shao, RSC Adv., 2017, 7, 43512–43520 RSC.
- W. Li, R. Wang and S. X. Liu, Prog. Chem., 2010, 22, 2060–2070 Search PubMed.
- J. Yamashita, T. Ojima, M. Shioya, H. Hatori and Y. Yamada, Carbon, 2003, 41, 285–294 CrossRef.
- Y. Yang, Z. Tong, T. Ngai and C. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 6351–6360 CrossRef PubMed.
- A. G. A. Di Fabio, M. Mastragostino and F. Soavi, J. Electrochem. Soc., 2001, 148, A845–A850 CrossRef.
- H. R. Nie, M. Z. Liu, F. L. Zhan and M. Y. Guo, Carbohydr. Polym., 2004, 58, 185–189 CrossRef.
- S. Liu, X. Zhang, H. Shao, J. Xu, F. Chen and Y. Feng, Mater. Lett., 2012, 73, 223–225 CrossRef.
- X. Wu and W. Jia, Chem. Eng. J., 2014, 245, 210–216 CrossRef.
- M. Xu, Y. Ao, S. Wang, J. Peng, J. Li and M. Zhai, Carbohydr. Polym., 2015, 128, 171–178 CrossRef PubMed.
- E. Y. L. Teo, L. Muniandy, E.-P. Ng, F. Adam, A. R. Mohamed, R. Jose and K. F. Chong, Electrochim. Acta, 2016, 192, 110–119 CrossRef.
- B. L. Hu, X. Y. Qin, A. M. Asiri, K. A. Alamry, A. O. Al-Youbi and X. P. Sun, Electrochim. Acta, 2013, 100, 24–28 CrossRef.
- X. Wang, X. Wang, L. Yi, L. Liu, Y. Dai and H. Wu, J. Power Sources, 2013, 224, 317–323 CrossRef.
- A. Di Fabio, A. Giorgi, M. Mastragostino and F. Soavi, J. Electrochem. Soc., 2001, 148, A845–A850 CrossRef.
- J. Yan, J. P. Liu, Z. J. Fan, T. Wei and L. J. Zhang, Carbon, 2012, 50, 2179–2188 CrossRef.
- W. Shi, J. Zhu, D. H. Sim, Y. Y. Tay, Z. Lu, X. Zhang, Y. Sharma, M. Srinivasan, H. Zhang, H. H. Hng and Q. Yan, J. Mater. Chem., 2011, 21, 3422 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The SEM images of CMC-NH4 and CMC-NH4/MoS2 prepared via solvothermal synthesis under ethanol solutions with different concentrations, and the calculation formula of the electrochemical parameters. See DOI: 10.1039/c8ra04492a |
| This journal is © The Royal Society of Chemistry 2018 |