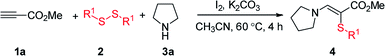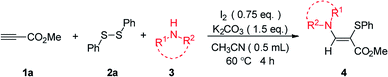Open Access Article
Open Access ArticleIodine-promoted stereoselective amidosulfenylation of electron-deficient alkynes†
Fuhong Xiao *,
Dahan Wang,
Shanshan Yuan,
Huawen Huang
*,
Dahan Wang,
Shanshan Yuan,
Huawen Huang and
Guo-Jun Deng
and
Guo-Jun Deng
Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, Key Laboratory for Green Organic Synthesis and Application of Hunan Province, College of Chemistry, Xiangtan University, Xiangtan 411105, China. E-mail: fhxiao@xtu.edu.cn
First published on 27th June 2018
Abstract
Iodine-promoted three-component synthesis of substituted β-amino sulfides has been developed starting from a propargyl ester, aliphatic secondary amine, and disulfide. This protocol provides a step-economic and highly regioselective entry to trisubstituted olefins with good substrate scope and functional group tolerance.
Polysubstituted olefins are ubiquitous structural units among biologically important drugs such as Tamoxifen or Vioxx,1 and natural products such as Stemona alkaloids and Nileprost analogues.2 Importantly, functionalized alkenes also contribute extensively to materials science and as building blocks in organic synthesis.3 In this context, the development of green and efficient methods for olefin synthesis has attracted much attention in recent years. Thereby, difunctionalization of alkynes is one of the most powerful and reliable procedures.4 On the other hand, multi-component reactions (MCRs) have been found to show great advantages as efficient protocols because of their general features of convergence, operational simplicity, facile automation, and so forth.5 However, regio- and stereoselective synthesis of polysubstituted olefins is still one of the most challenging tasks in organic synthesis, especially in the process of multicomponent reactions.
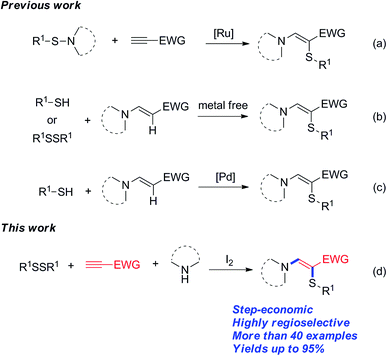
Organosulfur compounds are widespread in organic and biological research.6 Among them, β-amino vinylsulfides are well known to be particularly interesting chemical entities, which can be converted to many other useful compounds.7 Due to their importance, various synthetic strategies have been developed to construct these sulfur-containing molecules. For example, in 2004, Mitsudo reported the ruthenium-catalyzed addition of sulfenamides to alkynes, which provides a series of β-amino sulfides under mild reaction conditions (Scheme 1a).8 Recently, the groups of Du, Wan, and Prabhu independently disclosed their results of the enaminone sulfenylation with disulfides or thiophenols under metal-free conditions (Scheme 1b).9 Loh and co-workers developed a similar transformation through palladium-catalyzed C–H functionalization of enamines by using simple thiols (Scheme 1c).10 As a continuation of our research interest in functionalization of alkenes/alkynes,11 we herein disclose a novel protocol for the preparation of β-amino sulfides through iodine-promoted amidosulfenylation of electron-deficient alkynes involving C–S and C–N bond formation (Scheme 1d).
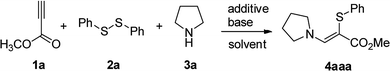
In our initial study, the reaction of methyl propiolate (1a), 1,2-diphenyldisulfane (2a), and pyrrolidine (3a) was selected as the model reaction to optimize the reaction conditions (Table 1). To our delight, the desired product 4aaa was obtained in 67% yield when the reaction was performed with NIS (1.5 equiv.) and K2CO3 (3.5 equiv.) in CH3CN at 60 °C for 4 h (entry 1). Lower yields were obtained when NH4I, KI, and tetrabutylammonium iodide (TBAI) were used as the additive (entries 2–4). The use of molecular I2 (0.75 equiv.) further enhanced the reaction yield to 90% (entry 5). Other solvents were also investigated and showed less efficiency (entries 6–9). The product was observed in 27% yield when H2O was used as the sole solvent (entry 9). Instead of K2CO3, other bases such as KOH, K3PO4, Cs2CO3, Na2CO3, t-BuOK and Li2CO3 all decreased the reaction yield (entries 10–15). Decreasing the amounts of I2 or K2CO3 or the reaction temperature all could not improve the reaction yield (entries 16–18). We have also studied the optimization of reaction conditions when using catalytic amount of iodine with oxidants (see Table S1 in ESI†).
| Entry | Additive | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Conditions: 1a (0.2 mmol), 2a (0.15 mmol), 3a (0.3 mmol), additive (1.5 equiv., for I2 0.75 equiv.), base (1.5 equiv.), solvent (0.5 mL), 60 °C, 4 h, under air.b Isolated yield based on 1a.c I2 (0.5 equiv.).d K2CO3 (1.0 equiv.).e 50 °C.f I2 (10 mol%), TBHP (2.0 equiv.). | ||||
| 1 | NIS | K2CO3 | CH3CN | 67 |
| 2 | NH4I | K2CO3 | CH3CN | 33 |
| 3 | KI | K2CO3 | CH3CN | 37 |
| 4 | TBAI | K2CO3 | CH3CN | 30 |
| 5 | I2 | K2CO3 | CH3CN | 90 |
| 6 | I2 | K2CO3 | DMSO | 67 |
| 7 | I2 | K2CO3 | DMF | 65 |
| 8 | I2 | K2CO3 | Toluene | 43 |
| 9 | I2 | K2CO3 | H2O | 27 |
| 10 | I2 | KOH | CH3CN | 56 |
| 11 | I2 | K3PO4 | CH3CN | 73 |
| 12 | I2 | Cs2CO3 | CH3CN | 65 |
| 13 | I2 | Na2CO3 | CH3CN | 83 |
| 14 | I2 | n-BuOK | CH3CN | 10 |
| 15 | I2 | Li2CO3 | CH3CN | 65 |
| 16c | I2 | K2CO3 | CH3CN | 67 |
| 17d | I2 | K2CO3 | CH3CN | 77 |
| 18e | I2 | K2CO3 | CH3CN | 73 |
| 19f | I2 | K2CO3 | CH3CN | 74 |
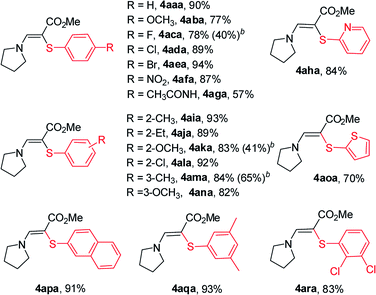
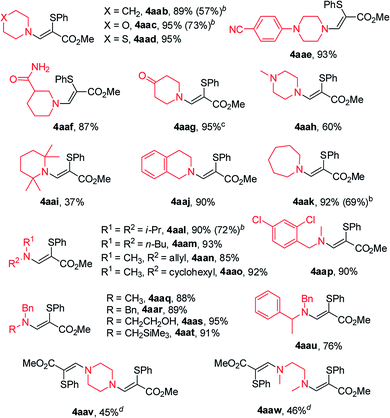
With the optimized results in hand, we applied this amidosulfenylation conditions to other disulfides and the results are illustrated in Table 2. All of the tested disulfides performed well and gave the desired products 4 in moderate to excellent yields. When disulfides with para-substitutions were used, the corresponding β-amino sulfides were obtained in excellent yields in most cases (4aba–4afa), while the one with an acetylamino group showed modest activity and gave 4aga in 57% yield. Steric effect of the substituent has no obvious impact on this amidosulfenylation reaction since o-MeO- (2k), m-MeO- (2n), and p-MeO- (2b) disulfides afforded the corresponding products 4aka, 4ana, and 4aba, respectively, in almost the same yields (77–82%). 1,2-Di(naphthalen-2-yl)disulfane (2p) was well suited to this amidosulfenylation protocol, and delivered the corresponding product 4apa in 91% yield. Disulfides 2q and 2v with two functional groups were also suitable substrates and afforded the desired products in 93% and 83% yields (4aqa and 4ara), respectively. Furthermore, heteroaromatic disulfides bearing pyridin-2-yl (2h) and thiophen-2-yl (2o) functionalities were accommodated, further demonstrating the broad functional-group tolerance of this method. It is noteworthy that ethyl propiolate (1b) and but-3-yn-2-one (1c) also proved to be suitable coupling partners to provide the corresponding products in high yields.
The scope with respect to the amine was then investigated (Table 3). Under the standard reaction conditions, a broad range of substituted aliphatic secondary amine was successfully coupled with methyl propiolate (1a) and 1,2-diphenyldisulfane (2a) to give the respective β-amino sulfides in moderate to very good yields (4aab–4aau). Simple cyclic secondary amines, such as piperidine (3b), morpholine (3c), thiomorpholine (3d) and azepane (3k) proceeded smoothly to afford the corresponding β-amino sulfides in excellent yields. When 4-(piperazin-1-yl)benzonitrile (3e) and piperidine-3-carboxamide (3f) were used as the coupling partner, the desired products were obtained in 93% and 86% yields, respectively. 1-Methylpiperazine (3h) and 2,2,6,6-tetramethylpiperidine (3i) furnished the corresponding products in lower yields. Notably, piperidin-4-one (3g) and 1,2,3,4-tetrahydroisoquinoline (3j) were well tolerated in the present system, delivering the corresponding products in high yields. Other chain secondary amines (3l–3o) were also evaluated. Thereby, N-methylallylamine (3n) was tolerated, and the desired product (4aan) was obtained in 85% yield. To our delight, this reaction system could also be applied to the direct sulfenylation of N-substituent benzylamine derivatives (3p–3u). Interestingly, the reaction of diamine, such as piperazine (3v) and N,N′-dimethylethylenediamine (1w) with methyl propiolate (1a) and 1,2-diphenyldisulfane (2a) worked well to afford the corresponding products in moderate yields.
| a Conditions: 1a (0.2 mmol), 2 (0.15 mmol), 3a (0.3 mmol), I2 (0.15 mmol), K2CO3 (0.3 mmol), CH3CN (0.5 mL), 60 °C, 4 h, air.b Conditions: 1a (0.2 mmol), 2 (0.15 mmol), 3a (0.3 mmol), I2 (10 mol%), TBHP (2.0 equiv.), K2CO3 (0.3 mmol), CH3CN (0.5 mL), 60 °C, 4 h, air. Isolated yield based on 1a.c K2CO3 (0.6 mmol).d Amine (0.3 mmol). |
|---|
 |
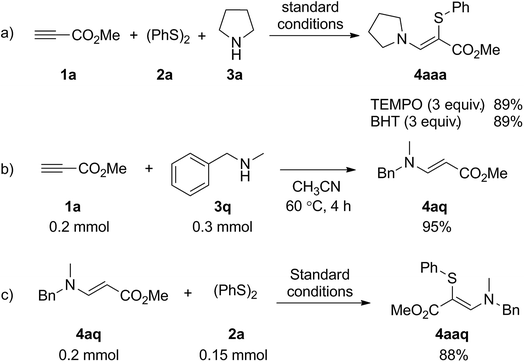
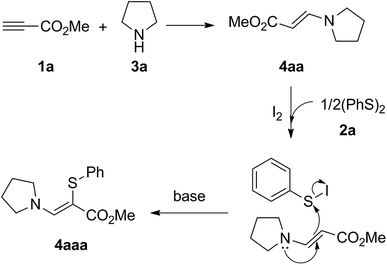
Some control experiments were carried out to gain preliminary insight into the reaction mechanism. First, the addition of a radical scavenger such as 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) or butylated hydroxytoluene (BHT) to the reaction system did not inhibit the reaction (Scheme 2a), which suggested that the reaction may not proceed through a radical pathway. Second, the coupling of methyl propiolate (1a) with N-methyl-1-phenylmethanamine (3q) occurred well to afford enamine 4aq under the standard reaction conditions (Scheme 2b). The product 4aaq was obtained in 88% yield when 4aq was used as the substrate with 1,2-diphenyldisulfane 2a (Scheme 2c). On the basis of these results, a plausible mechanism to rationalize this transformation was proposed (Scheme 3). Initially, Michael-type addition of pyrrolidine (3a) to methyl propiolate (1a) generates the enamine intermediate 4aa. Meanwhile, the interaction of molecular iodine with disulfide 2a generated electrophilic species Ph–SI. Finally, nucleophilic displacement of an iodo group by enaminone and subsequent deprotonation form the product 4aaa.
In summary, we have developed a simple and efficient method for the preparation of β-amino sulfides from propargyl ester, aliphatic secondary amine, and disulfide with moderate to high yields. Halogen, nitro, carbonyl, alkenyl, hydroxyl and amide functional groups were well tolerated under the mild reaction conditions. This method affords an efficient alternative approach for the synthesis of biologically important nitrogen- and sulfur-functionalized olefins.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21502160, 21572194, and 21372187), the Collaborative Innovation Center of New Chemical Technologies for Environmental Benignity and Efficient Resource Utilization, Hunan Provincial Natural Science Foundation of China (16JJ3112), and the Scientific Research Fund of Hunan Provincial Education Department (YB2016B024).References
-
(a) G. Likhtenshtein, Stilbenes: Applications in Chemistry, Life Sciences and Materials Science, Wiley-VCH, Weinheim, 2010 Search PubMed
; (b) H. G. Richey, in The Chemistry of Alkenes, ed. J. Zabichy, Wiley, NY, 1970, vol. 2, pp. 39–114 Search PubMed
; (c) A. S. Levenson and V. C. Jordan, Eur. J. Cancer, 1999, 35, 1628 CrossRef PubMed
; (d) N. F. McKinley and D. F. O'Shea, J. Org. Chem., 2006, 71, 9552 CrossRef PubMed
; (e) M. Wadman, Nature, 2006, 440, 277 CrossRef PubMed
; (f) B. M. Trost, J. P. N. Papillon and T. Nussbaumer, J. Am. Chem. Soc., 2005, 127, 17921 CrossRef PubMed
.
-
(a) M. R. Elliott, A. L. Dhimane and M. Malacria, J. Am. Chem. Soc., 1997, 119, 3427 CrossRef
; (b) G. A. Molander and D. J. Jean Jr, J. Org. Chem., 2002, 67, 3861 CrossRef PubMed
; (c) R. B. Williams, A. Norris, C. Slebodnick, J. Merola, J. S. Miller, R. Andriantsiferana, V. E. Rasamison and D. G. Kingston, J. Nat. Prod., 2005, 68, 1371 CrossRef PubMed
.
-
(a) W. Tang, S. Wu and X. Zhang, J. Am. Chem. Soc., 2003, 125, 9570 CrossRef PubMed
; (b) C. Dobler, G. M. Mehltretter, U. Sundermeier and M. Beller, J. Am. Chem. Soc., 2000, 122, 10289 CrossRef
; (c) J. Waser, B. Gaspar, H. Nambu and E. M. Carreira, J. Am. Chem. Soc., 2006, 128, 11693 CrossRef PubMed
.
- For reviews, see:
(a) N. Franssen, H. Reek and B. de Bruin, Chem. Soc. Rev., 2013, 42, 5809 RSC
; (b) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236 CrossRef PubMed
; (c) Z. Shao and H. Zhang, Chem. Soc. Rev., 2012, 41, 560 RSC
; (d) Y. Xia and J. Wang, Chem. Soc. Rev., 2017, 46, 2306 RSC
.
-
(a) J. Zhu and H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005 CrossRef
; (b) B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef PubMed
; (c) S. Brauch, S. S. van Berkel and B. Westermann, Chem. Soc. Rev., 2013, 42, 4948 RSC
; (d) C. M. Marson, Chem. Soc. Rev., 2012, 41, 7712 RSC
; (e) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef
; (f) J. D. Sunderhaus and S. F. Martin, Chemistry, 2009, 15, 1300 CrossRef PubMed
; (g) V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2014, 43, 4633 RSC
; (h) L. Levi and T. J. J. Müller, Chem. Soc. Rev., 2016, 45, 2825 RSC
; (i) V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2010, 39, 4402 RSC
; (j) C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969 RSC
; (k) B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323 CrossRef PubMed
; (l) M. Haji, Beilstein J. Org. Chem., 2016, 12, 1269 CrossRef PubMed
.
-
(a) F. Bernardi, I. G. Csizmadia and A. Mangini, Organic Sulfur Chemistry. Theoretical and Experimental Advances, Elsevier, Amsterdam, 1985, vol. 19 Search PubMed
; (b) P. Kielbasinski, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 1104 CrossRef
; (c) Sulfur Compounds: Advances in Research and Application, ed. A. Q. Acton, Scholarly Editions, Atlanta and GA, 2012 Search PubMed
.
-
(a) S. Chen, R. Gopalakrishnan, T. Schaer, F. Marger, R. Hovius, D. Bertrand, F. Pojer and C. Heinis, Nat. Chem., 2014, 6, 1009 CrossRef PubMed
; (b) J. S. Zheng, H. N. Chang, F. L. Wang and L. Liu, J. Am. Chem. Soc., 2011, 133, 11080 CrossRef PubMed
.
- T. Kondo, A. Baba, Y. Nishi and T. Mitsudo, Tetrahedron Lett., 2004, 45, 1469 CrossRef
.
-
(a) J. Sun, D. Zhang-Negrerie and Y. Du, Adv. Synth. Catal., 2016, 358, 2035 CrossRef
; (b) J.-P. Wan, S. Zhong, L. Xie, X. Cao, Y. Liu and L. Wei, Org. Lett., 2016, 18, 584 CrossRef PubMed
; (c) Y. Siddaraju and K. R. Prabhu, J. Org. Chem., 2017, 82, 3084 CrossRef PubMed
.
- Y. Jiang, G. Liang, C. Zhang and T.-P. Loh, Eur. J. Org. Chem., 2016, 3326 CrossRef
.
-
(a) X. Zhou, J. Luo, J. Liu, S. Peng and G.-J. Deng, Org. Lett., 2011, 13, 1432 CrossRef PubMed
; (b) Y. Liao, S. Chen, P. Jiang, H. Qi and G.-J. Deng, Eur. J. Org. Chem., 2013, 6878 CrossRef
; (c) S. Liu, Y. Bai, X. Cao, F. Xiao and G.-J. Deng, Chem. Commun., 2013, 49, 7501 RSC
; (d) L. Yang, Q. Wen, F. Xiao and G.-J. Deng, Org. Biomol. Chem., 2014, 12, 9519 RSC
; (e) S. Liu, L. Tang, H. Chen, F. Zhao and G.-J. Deng, Org. Biomol. Chem., 2014, 12, 6076 RSC
; (f) B. Li, P. Ni, H. Huang, F. Xiao and G.-J. Deng, Adv. Synth. Catal., 2017, 359, 4300 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04374d |
| This journal is © The Royal Society of Chemistry 2018 |