Open Access Article
Open Access ArticleA glucose modified filter paper for effective oil/water separation†
Zhonglin Luo *abc,
Cong Duana,
Yan Lia,
Yanbin Wangab and
Biaobing Wang*ab
*abc,
Cong Duana,
Yan Lia,
Yanbin Wangab and
Biaobing Wang*ab
aSchool of Material Science and Engineering, National Experimental Demonstration Center for Materials Science and Engineering (Changzhou University), Changzhou, 213164, P. R. China. E-mail: zhonglinluo@cczu.edu.cn; biaobing@cczu.edu.cn
bJiangsu Collaborative Innovation Center of Photovolatic Science and Engineering, Changzhou, Jiangsu 213164, P. R. China
cState Key Laboratory of Molecular Engineering of Polymers (Fudan University), Shanghai, 200433, P. R. China
First published on 21st August 2018
Abstract
Efficient and low-cost oil/water separation remains a great challenge for industries. Natural cellulose-based filter paper, because of its abundance, low cost, biodegradability and excellent chemical stability, has been developed as an oil/water separator in recent years. In the present study, a superhydrophilic and underwater superoleophobic filter paper is successfully prepared by an aldol condensation reaction to crosslink glucose molecules with filter paper. The prepared filter paper is characterized by IR-spectroscopy, SEM spectroscopy and wettability measurements, and it has high underwater oil contact angles of over 162° for hexane, toluene and petroleum ether. It is shown that the modified filter paper has high water recovery from various oil/water mixtures, not only in a gentle environment but also in acidic, alkaline, and salty environments and at different temperatures. Moreover, the glucose modified filter paper shows excellent oil/water emulsion separation efficiency (>99%) and good recycling performance. The preparation is economic and could be easily scaled up, suggesting its great potential for large-scale industrial applications.
1. Introduction
With the rapid development of the manufacturing industry and food catering industry, frequent oil spills and oily wastewater have caused serious ecological and environmental problems. Energy efficient and cost-effective separation of oil/water mixtures has been plaguing the world for decades.1–4 Recently, materials with special super-wetting properties for oil or water have attracted increasing attention because of their remarkable surface-active features.5,6Superhydrophobic and superoleophilic mesh films are the first such materials to be used.7 Subsequently, many innovative materials, including metallic meshes,7,8 fabrics,9 filter papers,10–12 polymeric membranes,13 sponges and foams,14,15 etc., have been developed for oil/water separation by filtration and absorption methods. Because of the lipophilic properties of the materials, such “oil-removing” materials are easily fouled or even blocked by oil droplets and impurities. Compared with the “oil-removing” materials, “water-removing” ones with superhydrophilic and superoleophobic properties show great advantages in gravity-driven separation processes because most oils are lighter than water. Moreover, it could prevent materials from being polluted by oils. But it is quite difficult to create a superoleophobic material in air because surface tensions of oils are much lower than water.16 Furthermore, the fabrication of superoleophobic materials is rather sparse due to the high surface energy nature of most solids. Only a few hydrophilic and oleophobic materials have been reported, which are based on polymers consisting of hydro-responsive (hydrophilic) and polymer-fluorosurfactant (oleophobic) components. When these materials are in contact with water, their hydrophilic components move to surface, and the hydrophilicity and oleophobicity are obtained.17–20
Inspired by fish scales,21 another innovative approach to achieve the “water-removing” materials, that is, developing superhydrophilic and underwater superoleophobic materials is proposed recently by researchers. Based on Young's equation, hydrophilic surfaces in air can become oleophobic in water,22 and underwater superoleophobic surfaces could be obtained by design of hydrophilic chemical compositions and micro/nano hierarchical structures.22,23 By utilization of macromolecular layers, nano or micro-particles, nano fibres, and etc., a large number of superhydrophilic and underwater superoleophobic materials have been described and drawn extensive attentions due to their highly efficient filtrations and broad application prospects on oil/water separation.17,24–38
Filter paper, with its porous structure constructed by cotton microfibers, is widely used in solid–liquid separation. Natural cotton fibres consist of numerous fibrils of β-glucose with cuticle covered by 55% cellulose and the other non-cellulosic compounds like pectin, protein, and wax,39 which makes primary filter paper impractical for liquid–liquid separation. Due to their abundance, low cost, biodegradability, easily scalable fabrication and excellent chemical stability, fabricating cellulose filters with capacity for liquid–liquid separation is of great importance to both academics and industries. Numerous efforts have been made to turn these materials into oil/water separators. Recently, “oil-removing” filter papers modified by coating of superhydrophobic materials on surfaces10–12,40,41 have been reported. Such filter papers are usually prepared by modification of nanoparticles with hydrophobic materials, resulting in hierarchical structures with low surface energy and high roughness. However, those rough structures may be destroyed by flow of liquids passing through filters because of poor adhesive interactions between nanoparticles and substrates.
And “water-removing” ones prepared by crosslinking of hydrogels or physical depositing of hydrophilic polymers on filter papers are more favoured because of their energy efficiency, appreciable recyclability and chemical stability.27,28,32 For example, filter paper coated with a layer of nano-fibrillated cellulose hydrogel displays oleophobic behavior in water and good performance in oil-in-water emulsion separation.25 A superoleophobic filter paper prepared by a two-step dip adsorption process with paraffin wax and poly(dimethylsiloxane)-b-poly(ethylene oxide) diblock copolymer exhibits good oil–water separation efficiency for non-stabilized emulsions.32 PVA-coated filter papers show high tolerance in harsh acidic, basic and alkaline environments,28 effective oil–water mixture separation with high flux,27 and high efficiency in separation of oil/water emulsions.27,28 Nevertheless, most of them would be hindered in industrial applications due to the expensive reagents, intricate and time-consuming preparation process. It is still highly demanding for high-performance separators prepared with easily available substances by facile and affordable modification methods.
In this paper, we report a glucose-modified superhydrophilic and underwater superoleophobic filter paper for oil/water separation. Our approach stems from the pioneering studies of coating paper with hydrogels, but it is different in using water-soluble small molecules. β-Glucose, the basic monomer unit of cellulose, is a nature product of plants. As a small molecule, it would absorb on hydrated filter paper and enter the inner layer of paper much faster than hydrogels and polymers. By using glutaraldehyde (GA) as the crosslinker and hydrochloride (HCl) as the catalyst, we demonstrate that glucose and filter paper can be covalently bonded through a simple dip-coating method. The modified filter paper is proven to separate oil/water mixtures in acidic, alkaline, and salty environments. The filter paper is also applied to separate surfactant-stabilized oil/water emulsions successfully with high separation efficiency. The preparation avoids pre-treating polymers, and makes the industrial fabrication more facial and economically efficient. The obtained filter papers are biodegradable and chemical stable, reusable, and allowing for scale-up for large-scale applications.
2. Experimental section
2.1 Materials
GA aqueous solution (50 wt% in water) was obtained from Tianjin Damao Chemical Reagent Factory, China. HCl (37 wt%), Sudan IV, sodium hydroxide (NaOH), sulfuric acid (H2SO4), and toluene were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Hexane was obtained from Jiangsu Qiangsheng Functional Chemical Co., Ltd., China. Petroleum ether was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd., China. The filter papers with particle retention sizes of 2.5 μm, 11 μm and 25 μm were obtained from Hangzhou Whatman Filter Paper Co. Ltd., China. The filter papers were dried under vacuum at 35 °C for 24 h before use. Glucose was purchased from a local market (food grade, Changzhou, China). The rest chemicals were all of analytical grade and used as received.2.2 Fabrication of glucose coated filter paper
Glucose solutions were prepared by dissolving glucose grains in deionised water at room temperature. A filter paper was then immersed in a glucose solution at room temperature for 20 min to make the filter paper completely wet. Next, the wetted filter paper was taken out, blotted the surface with a tissue to remove any excess solution, and again immersed into a 20 mL of GA solution (50 wt% in water) at 25 °C for 48 h. pH value of the solution was corrected to 2 with HCl (37 wt%). Finally, the obtained glucose coated filter paper was washed with deionised water and ethanol for several times to remove potential excess glucose or GA, and then dried under vacuum before use.2.3 Characterization of filter paper
The surface chemical states of filter papers were analyzed by an attenuated total refraction Fourier transform infrared (FTIR-ATR) spectra, using a Nicolet Avatar 370 spectrometer and a SMATR-iTR unit (Thermo Fisher Scientific™, USA) equipped with a diamond crystal prism. Spectra were gathered in the range of 4000–400 cm−1 with a spectral resolution of 0.5 cm−1.The surface morphologies of filer papers were investigated by field emission scanning electron microscopy (FESEM) using a SUPRA55 (Zeiss, Germany) microscope, which was working in high vacuum mode and with an acceleration voltage of 8 kV. Test specimens (0.5 cm × 0.5 cm ca.) were mounted on aluminium stubs with conductive tapes. Before measurements, samples were sputter coated with gold using a high resolution sputter coater for 60 s.
All measurements of contact angles (CA) were conducted using a contact angle equipment (JC2000D1, Shanghai Powereach Digital Technology Equipment Co., Ltd., China) at ambient temperature. For underwater oil CA test, a filter paper was placed on an in-house-made transparent support, which was put in water. An oil droplet (5 μL, dyed with Sudan IV) was dropped under the filter paper and the CA was measured. The average value of at least three measurements performed at different positions on the same filter paper was adopted as the underwater oil CA, and at least three pieces of filter papers were tested. To evaluate underwater oil CAs under acidic, alkaline, and saline conditions, we used 2 M HCl solution, 2 M NaOH solution, and saturated NaCl solution as different aqueous environments respectively. A digital camera was used to record images.
The intrusion pressure of oils flowing through the coated filter paper was characterized by measuring the maximum height of oil that could be supported by the coated filter paper,13 and was calculated by eqn (1),
| p = ρghmax | (1) |
2.4 Oil/water mixture separation
Solely gravity-driven filtration technique was adapted for oil/water separation. The tests were carried out by using a filtration device composed of a filter bowl and a filter head. The bowl and the head were fixed by two clamps, and a piece of filter paper (with a diameter 7 cm) was put between them. Oil/water mixtures were prepared by simply mixing hexane, toluene and petroleum ether respectively into water (oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 30
water = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) and slowly poured onto filter paper which was pre-wetted by water. Oil/water mixtures in acidic, alkaline or saline environments were prepared by mixing toluene with 2 M HCl solution, 2 M NaOH solution, and saturated NaCl solution, respectively. For experiments at different temperature, toluene/water mixtures and filtration device were precooled or preheated to desired temperatures (2°, 30° and 65°, respectively) before measurements. At least three times of oil/water separation experiments were taken to evaluate the separation performance of modified filter papers.
70) and slowly poured onto filter paper which was pre-wetted by water. Oil/water mixtures in acidic, alkaline or saline environments were prepared by mixing toluene with 2 M HCl solution, 2 M NaOH solution, and saturated NaCl solution, respectively. For experiments at different temperature, toluene/water mixtures and filtration device were precooled or preheated to desired temperatures (2°, 30° and 65°, respectively) before measurements. At least three times of oil/water separation experiments were taken to evaluate the separation performance of modified filter papers.
The weight of permeated water was recorded at every 5 seconds until separation was completed. And the water recovery (%) was calculated according to eqn (2),31
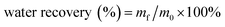 | (2) |
The filtered volume of water through per unit area of paper as a function of time was calculated, and flux of oil/water mixture separation was obtained from slope of the initial linear section (about within 25 seconds) of the volume–time curve25 and calculated according to eqn (3),
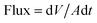 | (3) |
The oil contents of filtrates from toluene/water mixtures were also detected. Hexane was then used to extract toluene from filtrates. The typical UV absorbance characteristic of benzene aromaticity at 262 nm was measured (Fig. S1†) by using an UV-1800 UV spectrometer (Shimadzu Corporation, Japan). The oil content of each sample was acquired by calculating the absorbance and the correction coefficient (Fig. S2†).
2.5 Oil/water emulsion separation
Toluene/water emulsions with different oil contents (toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 5
water = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, 10
95, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 30
80, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v, respectively) were prepared by adding 2.5 mg of sodium dodecylbenzene sulfate (SDBS) in per mL of water as an emulsifier and mixing water with toluene at the stir rate of 1500 rpm for 2 h. The particle sizes of the prepared emulsions were measured by a laser particle size analyzer (BT9300S, Bettersize Instruments Ltd. China). The gravity-driven filtration same as the above description was also adopted in emulsion separation. The separation efficiency was acquired by eqn (4),28
70 v/v, respectively) were prepared by adding 2.5 mg of sodium dodecylbenzene sulfate (SDBS) in per mL of water as an emulsifier and mixing water with toluene at the stir rate of 1500 rpm for 2 h. The particle sizes of the prepared emulsions were measured by a laser particle size analyzer (BT9300S, Bettersize Instruments Ltd. China). The gravity-driven filtration same as the above description was also adopted in emulsion separation. The separation efficiency was acquired by eqn (4),28
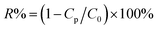 | (4) |
The flow rate for emulsion separation was much slower than that of oil/water mixture separation. The flux for oil/water emulsion separation was also calculated from slope of the initial linear section of the volume–time curve (about within 5 minutes) by eqn (3). Moreover, the stability and recyclability of glucose-modified filter paper were also tested by immersing the filter paper in an ultrasonic washing machine (40 kHz, 800 W, KQ-800KDE, Kunshan ultrasonic instruments Co., Ltd., China) and ultrasonicated for 24 h, and the separation performance after sonication was detected. Also, at least three times of oil/water emulsion separation experiments were taken to evaluate the separation performance of the modified filter paper.
3. Results and discussion
3.1 Chemistry and surface morphology of glucose coated filter paper
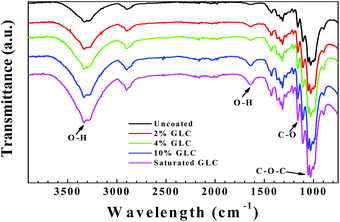
Fig. 1 shows the FTIR-ATR spectra of the pristine and the glucose modified filter papers. Typical FTIR-ATR spectra of the untreated filter paper shows a broad absorption peak at 3328 cm−1, corresponding to the O–H stretching vibrations arising from abundant free hydroxyl and hydrogen-bonded hydroxyl groups, and the peak at 1635 cm−1 is related to the O–H bending vibration of the adsorbed water. It was reported the water adsorbed in cellulose molecules is very difficult to extract even after a carefully drying process due to the cellulose–water interaction.42 The peaks at 2901 cm−1 and 1315 cm−1 are related to C–H stretching and bending vibrations. The absorption band at 1427 cm−1 is assigned to the C–H scissoring motion of cellulose. Finally, the signals of anti-symmetrical bridge C–O stretching and C–O–C stretching vibrations of cellulose appear at 1160 cm−1 and 1030 cm−1, respectively.32,43 As shown in Fig. 1, the absorption bands of the modified filter paper are almost the same as the spectra of the pristine filter paper, but the absorption strength of some vibrations increases obviously. For example, the peaks at 3333 cm−1 and 1635 cm−1 related to O–H stretching vibrations become bigger, which indicates the interactions between cellulose and water are stronger in the modified filter papers.43 The absorption at 1160 cm−1 and 1030 cm−1, related to anti-symmetrical bridge C–O stretching and C–O–C stretching vibrations respectively, also become stronger, indicating glucose molecules are successfully crosslinked to the surface of filter papers.To verify the successful crosslinking of glucose molecules and filter paper, the filter paper treated by saturated glucose solution was chosen for morphology observation. Similar to the pristine filter paper, cellulose fibres are randomly distributed on the surface of the modified filter paper (Fig. 2a and b). The high magnification SEM images indicate that the fibres are covered with a layer of crosslinked glucose molecules and the veins of cellulose fibres become obscured (Fig. 2d and f), also some shallow dents and small pores on the filter paper are disappeared. The thickness of the modified filter paper increases with the increase of glucose concentration (Table S1†), which also proves that filter papers are successfully modified by glucose molecules.
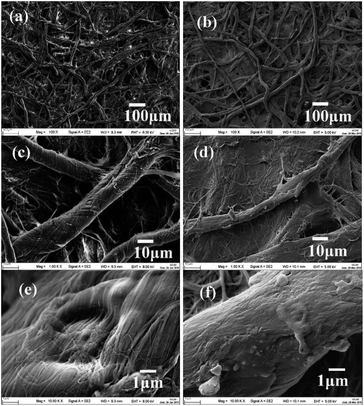 | ||
| Fig. 2 Representative SEM images of (a, c and e) the untreated and (b, d and f) the saturated-glucose-solution treated filter paper (pore size 25 μm) at different magnifications. | ||
When filter papers are immersed into glucose solutions, glucose molecules adsorb on the surface of filter paper through hydrogen bonding interactions with cellulose fibrils. After GA is added, the glucose molecules covalently bond to GA by the aldol condensation reaction between hydroxyl groups and aldehyde groups. The reaction between glucose and GA could be proved by the formation of the crosslinked network when glucose and GA solution were mixed (Fig. S3†). This reaction not only makes glucose molecules crosslinked by GA, but also allows for the co-crosslinking of glucose molecules with cellulose molecules, as well as the crosslinking of cellulose molecules (Fig. 3). Therefore, the crosslinked glucose–GA copolymers covalently bond to cellulose molecules, and cover the surface of fibres.
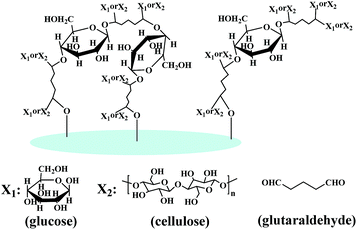 | ||
| Fig. 3 Schematic of a multiple crosslinked network formed by an aldol condensation reaction between cellulose filter paper and glucose, in which GA acts as a crosslinker and HCl as a catalyst. | ||
3.2 Wettability performance
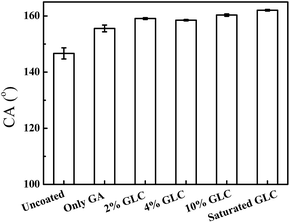
The wettability of the prepared filter papers was tested by putting a 5 μL hexane droplet under filter paper and measuring its underwater CA. As shown in Fig. 4, the underwater oil CA of the unmodified filter paper is 146.7 ± 2.0°. When the filter paper is modified only by GA solution, the underwater oil CA increases to 155.6 ± 1.2°. The result may be caused by the increased surface roughness of the filter paper, which is induced by co-crosslinking of cellulose molecules. With the increase of glucose concentration, the underwater oil CA increases gradually and the filter paper modified by saturated glucose solution presents an underwater oil CA of more than 160° (Fig. 4), indicating the super-repellence to oils.The wettability of the filter paper treated by saturated glucose solution was further tested. As shown in Fig. 5, when a 5 μL water droplet is dripped onto the untreated or treated filter paper, water droplets are absorbed quickly (within 150 milliseconds) on both of them and water CAs in air are 0°, which indicate both the modified and unmodified filter papers are superhydrophilic. When filter papers are immersed in water, a hexane droplet almost keeps a spherical shape under the modified filter paper and the measured CA is 162 ± 0.3°. In comparison, a hexane droplet appears more oblate under the unmodified filter paper, and the oil CA is 146.7 ± 2°. Therefore, the underwater superoleophobic filter papers have been prepared after glucose treatment.
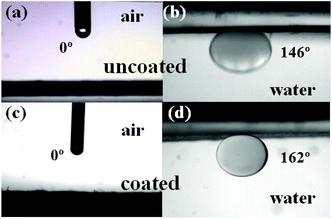 | ||
| Fig. 5 Water CAs and underwater oil (hexane) CAs of the pristine filter paper (a and b) and the saturated-glucose-solution modified filter paper (c and d), respectively. | ||
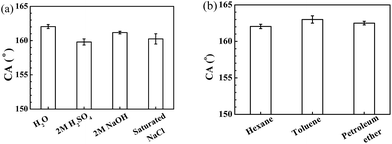
The glucose modified filter papers also show underwater superoleophobicity in acidic, alkaline, and saline environments with hexane CAs larger than 150° (Fig. 6a). The strong oil repellence of the modified filter papers was further examined by other oils, such as toluene and petroleum ether. As shown in Fig. 6b, the modified filter paper displays the excellent underwater superoleophobicity to all oils. These results demonstrate that the affinities of the treated filter paper to oils are negligible. When the modified filter paper is immersed in water, water molecules can be attracted by glucose molecules and cellulose fibres, and would be trapped into the porous structures of the filter paper, which creates a hydration layer and forms an oil–water–solid composite interface.25 Therefore the oil repellent properties of the filter papers are enhanced.
3.3 Oil/water mixture separation
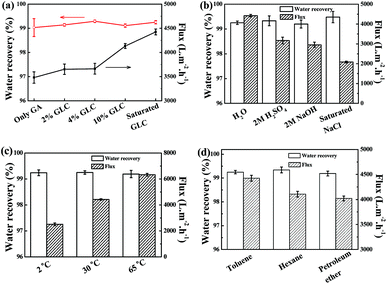
The separation performance of the glucose-modified filter papers for oil/water mixtures was tested. Toluene was used as the oil phase and the oil/water ratio of 30/70 was adopted. When an oil/water mixture was poured onto a pre-wetted filter paper (pore size 25 μm) that was fixed in filtration device, water permeated easily but oil was retained above the filter paper, and no visible oil was observed in the permeated water. As shown in Fig. 7a, with the increase of glucose concentration, the water recovery does not change too much, but only increases slightly. When glucose concentration is more than 2%, the water recovery becomes larger than 99%. The oil contents remained in filtrates were also measured, and they are less than 10 mg L−1 (Table S2†), which is comparable to that of the copper mesh decorated with superhydrophilic nickel nanoparticles.38 Those results indicate the excellent performance of the glucose modified filter papers in oil/water mixture separation. Interesting, the flux increases obviously when glucose concentration is larger than 4%, and the saturated-glucose-solution modified filter paper has a water flux as high as 4424 ± 60 L m−2 h−1. The change of flux with the increase of glucose concentration indicates that the formed network does not block pores of filter papers, and the crosslinked glucose–GA copolymers would swell in water and improve the mobility of the cellulose molecules of fibrils. Moreover, the hydrophilic properties of filter papers are improved after modification, which is beneficial to capture water molecules. The high flux of the saturated-glucose-solution modified filter paper is comparable to that of PVA-coated filter paper27 and poly(vinylidene fluoride) (PVDF) membrane.13 Therefore, it was chosen for further oil/water separation tests.The oil/water mixtures also could be successfully separated with high water recovery (>99%) in acidic, alkaline and saline conditions (Fig. 7b), indicating good tolerance of the modified filter paper for harsh environments. The fluxes for toluene/2 M H2SO4, toluene/2 M NaOH, and toluene/saturated NaCl solutions are 3169 ± 165, 2953 ± 133 and 2082 ± 46 L m−2 h−1, respectively. The decreased fluxes in acidic, alkaline and saline conditions can be contributed to the increased ionic strengths of the solutions, which would depress the swelling of the formed crosslinking network and induce a change in pore size.27,44 The fluxes are even less than that of the only GA crosslinked filter paper in pure water. The residual oil contents for toluene/2 M H2SO4, toluene/2 M NaOH, and toluene/saturated NaCl aqueous mixtures are larger than that of toluene/water solution (Table S3†), which may be caused by the weakened interactions between water and hydroxyl groups on the filter paper because of the increased ionic strength.
The performance of the modified filter paper at different temperatures was also examined. For all cases, the water recoveries are >99% (Fig. 7c), and the oil contents are less than 10 mg L−1 (Table S4†). More interesting, the flux increases from 2509 ± 104 L m−2 h−1 at low temperature (2 °C) to 6327 ± 105 L m−2 h−1 at high temperature (65 °C). This indicates swelling of the modified filter paper only allows water passing through because of its superoleophobicity. Those results indicate oil–water separation capacity will not be strongly influenced by the temperature in the range of 2 °C to 65 °C. Not only for toluene/water separation, the modified filter paper also shows high water recoveries and high fluxes in hexane/water and petroleum ether/water separation processes (Fig. 7d).
Moreover, the intrusion pressures of the saturated-glucose-solution modified filter paper for toluene, hexane, and petroleum ether were tested by measuring the maximum heights of the oils that could be supported by the coated filter paper (Fig. S4†) and calculated by using eqn (1). As shown in Fig. 8, the intrusion pressures for different oils are all larger than 5 kPa, which are much higher than previously reported membranes34 and comparable to the PVA-hydrogel coated filter paper.28 Therefore, the glucose modified filter papers exhibit good stability, tolerance, and promising capacity for separation of the oil/water mixtures.
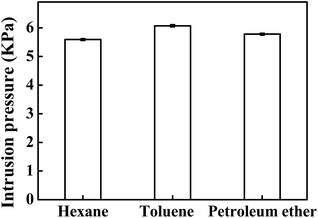 | ||
| Fig. 8 The intrusion pressures of the saturated-glucose-solution treated filter papers supporting different oils. | ||
3.4 Oil/water emulsion separation
The performance of the modified filter papers for oil/water emulsion separation was further tested. The filtration device described above was also used and the gravity-driven filtration was adopted. The surfactant-stabilized oil/water emulsions were prepared by mixing toluene and 0.25% w/v of SDBS of water, and stirring at 1500 rpm for 2 h. As shown in Fig. 9, when toluene/water emulsion (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v) was poured onto the modified filter paper (with pore size 25 μm) which was pre-wetted by water, water permeated through the filter paper but the white emulsion was retained. In comparison, the emulsion could permeate the pristine filter paper and the turbid solution was found in the filtrate. After water permeated the modified filter paper, the above emulsion became viscous, suggesting the demulsification process was happened during the separation. The modified filter paper could extract water from the emulsion due to its superhydrophilicity, and this process would make oil droplets aggregate and coalesce as water passing through filter paper.
70 v/v) was poured onto the modified filter paper (with pore size 25 μm) which was pre-wetted by water, water permeated through the filter paper but the white emulsion was retained. In comparison, the emulsion could permeate the pristine filter paper and the turbid solution was found in the filtrate. After water permeated the modified filter paper, the above emulsion became viscous, suggesting the demulsification process was happened during the separation. The modified filter paper could extract water from the emulsion due to its superhydrophilicity, and this process would make oil droplets aggregate and coalesce as water passing through filter paper.
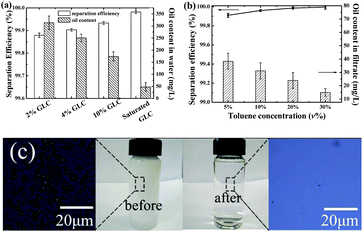
The separation performance of the modified filter paper was estimated. As shown in Fig. 10a, the separation efficiency for the toluene/water emulsion (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v) slightly increases with the glucose concentration. And the oil content decreases gradually with the increase of glucose concentration. For the saturated-glucose-solution modified filter paper, the residual oil content is about 47.5 ± 17.6 mg L−1, which is comparable to the performance of metal foam with nano-pores.45 These results indicate the coated glucose layer would increase the repellence to oils, thus improve the separation efficiency of the modified filter paper.
70 v/v) slightly increases with the glucose concentration. And the oil content decreases gradually with the increase of glucose concentration. For the saturated-glucose-solution modified filter paper, the residual oil content is about 47.5 ± 17.6 mg L−1, which is comparable to the performance of metal foam with nano-pores.45 These results indicate the coated glucose layer would increase the repellence to oils, thus improve the separation efficiency of the modified filter paper.
To test the separation efficiency for emulsions with different particle sizes, SDBS-stabilized toluene/water emulsions with different toluene contents were prepared. Though particle sizes increase with the toluene volume fraction (Fig. S5 and S6†) and the volume-average particle sizes of 5.69 μm, 10.05 μm, 15.66 μm, and 16.84 μm are observed in the emulsions containing 5, 10, 20, and 30 vol% of toluene respectively (Table S5†), there are still many small particles with sizes less than 2 μm present in emulsions (Fig. S6†). It is reported that when pore sizes of separation filters and sizes of emulsion droplets are comparable,45,46 the separation efficiency is satisfactory. Here modified filter paper with average size of 2.5 μm is used to test the separation efficiency for various oil/water emulsions. As shown in Fig. 10b, all of the emulsions could be separated successfully by the glucose modified filter paper. The separation efficiency increases slightly with the toluene concentration and it's over 99.8% for all emulsions. Also, the oil contents in filtrates decrease with the increase of toluene volume fraction, which means separation is more effective for large oil droplets. At all situations, the residual oil contents are less than 50 mg L−1, therefore, the modified filter paper displays good separation performance for oil/water emulsions. The separation performance is comparable to superhydrophobic Ti foam with surface nanocavities.45 As shown in Fig. 10c, no obvious oil droplets are observed in the collected filtrate, further indicating good separation performance of the modified filter paper. The permeate flux as a very important parameter for oil/water emulsion separation was also considered. By measuring the water volume and time of 100 mL SDBS-stabilized toluene-in-water emulsion (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, v/v) permeated through the modified filter paper (Fig. S7†), the flux is determined by the slope of the linear section (about within the first 5 min). The data displays a flux of 37 L m−2 h−1, which is a little smaller than that of PVA hydrogel coated filter papers.28 This may be caused by the smaller sizes of the emulsion droplets prepared with a higher SDBS concentration in this study, which could retard water flow rate and decrease the flux of the filtrate.
95, v/v) permeated through the modified filter paper (Fig. S7†), the flux is determined by the slope of the linear section (about within the first 5 min). The data displays a flux of 37 L m−2 h−1, which is a little smaller than that of PVA hydrogel coated filter papers.28 This may be caused by the smaller sizes of the emulsion droplets prepared with a higher SDBS concentration in this study, which could retard water flow rate and decrease the flux of the filtrate.
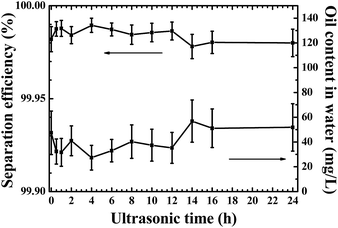
The stability and recyclability of the modified filter paper was also tested. The scrub forces were applied by immersing filter papers in an ultrasonic cleaner and sonication was carried out at a power of 800 W and frequency of 40 kHz. As shown in Fig. 11, with the increase of ultrasonic time, the separation efficiency for toluene/water (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v) emulsion always maintains a high level (>99.95%). The oil content in filtrates slightly increases after 12 h, but is still less than 80 mg L−1 after 24 h sonication. Moreover, no detectable change of the thickness of the dried filter papers is observed after washing for 24 h (0.219 ± 0.006 mm after 24 h washing compared to initial 0.219 ± 0.002 mm of the as-prepared filter paper), indicating the coated GA-glucose layer is stable under ultrasonic washing. In traditional view, filter paper is usually used to retain some solid particles in solutions and it was usually discarded after one-time use. Here, this simple crosslinking process endows the glucose-modified filter paper with good stability and recyclability for oil/water separations.
70 v/v) emulsion always maintains a high level (>99.95%). The oil content in filtrates slightly increases after 12 h, but is still less than 80 mg L−1 after 24 h sonication. Moreover, no detectable change of the thickness of the dried filter papers is observed after washing for 24 h (0.219 ± 0.006 mm after 24 h washing compared to initial 0.219 ± 0.002 mm of the as-prepared filter paper), indicating the coated GA-glucose layer is stable under ultrasonic washing. In traditional view, filter paper is usually used to retain some solid particles in solutions and it was usually discarded after one-time use. Here, this simple crosslinking process endows the glucose-modified filter paper with good stability and recyclability for oil/water separations.
4. Conclusions
In summary, a superhydrophilic and underwater superoleophobic filter paper has been successfully developed by a simple crosslinking reaction between glucose molecules and cellulose filter paper. The modified filter paper can separate various oil/water mixtures under hard environments, such as in acidic, alkaline, and salty environments, and at different temperatures. The water recovery is more than 99% and residual oil content is less than 10 mg L−1. Meanwhile, the modified filter paper plays a high efficiency (>99.8%) with respect to oil/water emulsion separation, and the residual oil content is less than 80 mg L−1. Moreover, the modified filter papers also show good stability and reusability. Those results indicate that filter paper, based on its natural porous structure and surface roughness, could be modified for oil/water separation by simply improving it hydrophilic property. The fabrication is facial and economic, and could be easily scaled up for industrial applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Natural Science Foundation of China (Grant No. 21304012), the Natural Science Foundation of Jiangsu Province (BK20130249), the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and State Key Laboratory of Molecular Engineering of Polymers (Fudan University, K2018-06) is gratefully acknowledged.References
- Y. J. Chan, F. C. Mei, C. L. Law and D. G. Hassell, Chem. Eng. J., 2009, 155, 1–18 CrossRef.
- V. Singh, M. K. Purkait and C. Das, Sep. Sci. Technol., 2011, 46, 1213–1223 CrossRef.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef PubMed.
- S. C. Votier, B. J. Hatchwell, A. Beckerman, R. H. McCleery, F. M. Hunter, J. Pellatt, M. Trinder and T. R. Birkhead, Ecol. Lett., 2005, 8, 1157–1164 CrossRef PubMed.
- Z. Xue, Y. Cao, N. Liu, L. Feng and L. Jiang, J. Mater. Chem. A, 2014, 2, 2445–2460 RSC.
- Q. Ma, H. Cheng, A. G. Fane, R. Wang and H. Zhang, Small, 2016, 12, 2186–2202 CrossRef PubMed.
- L. Feng, Z. Zhang, Z. Mai, Y. Ma, B. Liu, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2004, 43, 2012–2014 CrossRef PubMed.
- C. Wang, T. Yao, J. Wu, C. Ma, Z. Fan, Z. Wang, Y. Cheng, Q. Lin and B. Yang, ACS Appl. Mater. Interfaces, 2009, 1, 2613–2617 CrossRef PubMed.
- A. K. Singh and J. K. Singh, RSC Adv., 2016, 6, 103632–103640 RSC.
- S. Wang, M. Li and Q. Lu, ACS Appl. Mater. Interfaces, 2010, 2, 677–683 CrossRef PubMed.
- B. Ge, X. Zhu, Y. Li, X. Men, P. Li and Z. Zhang, Appl. Phys. A: Mater. Sci. Process., 2015, 121, 1291–1297 CrossRef.
- M. Zhang, C. Wang, S. Wang, Y. Shi and J. Li, Appl. Surf. Sci., 2012, 261, 764–769 CrossRef.
- W. Zhang, Z. Shi, F. Zhang, X. Liu, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2071–2076 CrossRef PubMed.
- C. Yu, C. Yu, L. Cui, Z. Song, X. Zhao, Y. Ma and L. Jiang, Adv. Mater. Interfaces, 2017, 4, 1600862 CrossRef.
- N. Chen and Q. Pan, ACS Nano, 2013, 7, 6875–6883 CrossRef PubMed.
- A. Tuteja, W. Choi, M. Ma, J. M. Mabry, S. A. Mazzella, G. C. Rutledge, G. H. McKinley and R. E. Cohen, Science, 2007, 318, 1618–1622 CrossRef PubMed.
- A. K. Kota, G. Kwon, W. Choi, J. M. Mabry and A. Tuteja, Nat. Commun., 2012, 3, 1025 CrossRef PubMed.
- J. Yang, H. Song, X. Yan, H. Tang and C. Li, Cellulose, 2014, 21, 1851–1857 CrossRef.
- J. Yang, L. Yin, H. Tang, H. Song, X. Gao, K. Liang and C. Li, Chem. Eng. J., 2015, 268, 245–250 CrossRef.
- J. Yang, Z. Zhang, X. Xu, X. Zhu, X. Men and X. Zhou, J. Mater. Chem., 2012, 22, 2834–2837 RSC.
- M. Liu, S. Wang, Z. Wei, Y. Song and L. Jiang, Adv. Mater., 2009, 21, 665–669 CrossRef.
- Z. Xue, M. Liu and L. Jiang, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 1209–1224 CrossRef.
- K. Liu and L. Jiang, Nano Today, 2011, 6, 155–175 CrossRef.
- L. Jiang, Z. Tang, K. J. Park-Lee, D. W. Hess and V. Breedveld, Sep. Purif. Technol., 2017, 184, 394–403 CrossRef.
- K. Rohrbach, Y. Li, H. Zhu, Z. Liu, J. Dai, J. Andreasen and L. Hu, Chem. Commun., 2014, 50, 13296–13299 RSC.
- S. Zhang, F. Lu, L. Tao, N. Liu, C. Gao, L. Feng and Y. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 11971–11976 CrossRef PubMed.
- M. Zeng, B. Peng, C. Ybanez, N. W. Tan, E. A. Deeb, E. Bordovsky, C. H. Choi, I. Echols, A. Nguyen, A. Ye, N. Dendumrongsup, L. Zhang, D. Huang, P. Wang, J. Luo, Y. Situ and Z. Cheng, RSC Adv., 2017, 7, 9051–9056 RSC.
- J. B. Fan, Y. Song, S. Wang, J. Meng, G. Yang, X. Guo, L. Feng and L. Jiang, Adv. Funct. Mater., 2015, 25, 5368–5375 CrossRef.
- X. Zheng, Z. Guo, D. Tian, X. Zhang, W. Li and L. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 4336–4343 CrossRef PubMed.
- M. Rana, J. T. Chen, S. Yang and P. C. Ma, Adv. Mater. Interfaces, 2016, 3, 1600128 CrossRef.
- U. B. Gunatilake and J. Bandara, Chemosphere, 2017, 171, 134–141 CrossRef PubMed.
- U. C. Paul, D. Fragouli, I. S. Bayer and A. Athanassiou, Polymers, 2016, 8, 52 CrossRef.
- L. Zhang, Z. Zhang and P. Wang, NPG Asia Mater., 2012, 4, e8 CrossRef.
- Z. Xue, S. Wang, L. Lin, L. Chen, M. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270–4273 CrossRef PubMed.
- X. Gao, L. P. Xu, Z. Xue, L. Feng, J. Peng, Y. Wen, S. Wang and X. Zhang, Adv. Mater., 2014, 26, 1771–1775 CrossRef PubMed.
- F. Zhang, W. B. Zhang, Z. Shi, D. Wang, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 4192–4198 CrossRef PubMed.
- Z. Y. Luo, K. X. Chen, J. H. Wang, D. C. Mo and S. S. Lyu, J. Mater. Chem. A, 2016, 4, 10566–10574 RSC.
- Z. Y. Luo, K. X. Chen, Y. Q. Wang, J. H. Wang, D. C. Mo and S. S. Lyu, J. Phys. Chem. C, 2016, 120, 12685–12692 CrossRef.
- R. Mitchell, C. M. Carr, M. Parfitt, J. C. Vickerman and C. Jones, Cellulose, 2005, 12, 629–639 CrossRef.
- L. Kong, Q. Wang, S. Xiong and Y. Wang, Ind. Eng. Chem. Res., 2014, 53, 16516–16522 CrossRef.
- T. Yu, G. Xu, X. Wang, J. Yang and J. Hu, BioResources, 2014, 9, 4421–4429 Search PubMed.
- J. Łojewska, P. Miśkowiec, T. Łojewski and L. M. Proniewicz, Polym. Degrad. Stab., 2005, 88, 512–520 CrossRef.
- A. M. Adel, Z. H. A. El-Wahab, A. A. Ibrahim and M. T. Al-Shemy, Carbohydr. Polym., 2011, 83, 676–687 CrossRef.
- S. M. M. Quintero, R. V. Ponce F, M. Cremona, A. L. C. Triques, A. R. d'Almeida and A. M. B. Braga, Polymer, 2010, 51, 953–958 CrossRef.
- Z. Y. Luo, S. S. Lyu, Y. Q. Wang and D. C. Mo, Ind. Eng. Chem. Res., 2017, 56, 699–707 CrossRef.
- Z. Wang, Y. Wang and G. Liu, Angew. Chem., Int. Ed., 2016, 55, 1291–1294 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04328k |
| This journal is © The Royal Society of Chemistry 2018 |