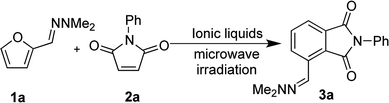
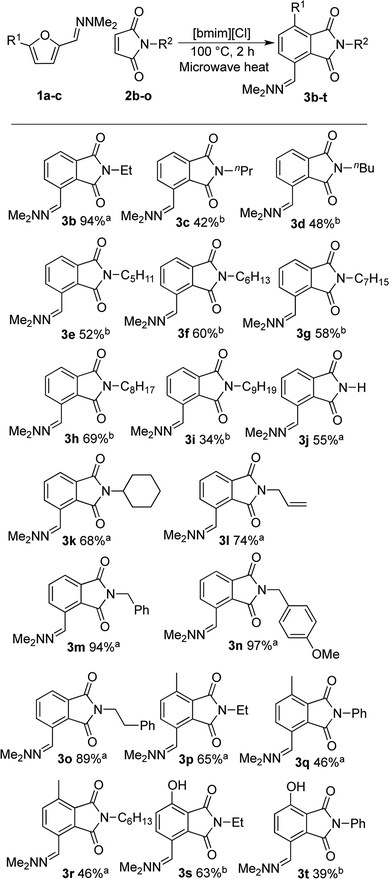
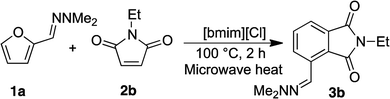
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of a microwave-assisted sustainable conversion of furfural hydrazones to functionalised phthalimides in ionic liquids†
Valerija Karaluka‡
a,
Kengo Murata‡a,
Shinto Masudaa,
Yuto Shiramatsua,
Takuji Kawamoto a,
Helen C. Hailes
a,
Helen C. Hailes *b,
Tom D. Sheppard
*b,
Tom D. Sheppard *b and
Akio Kamimura
*b and
Akio Kamimura *a
*a
aDepartment of Applied Chemistry, Yamaguchi University, Ube 755-8611, Japan. E-mail: ak10@yamaguchi-u.ac.jp
bDepartment of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK
First published on 20th June 2018
Abstract
A sustainable synthetic procedure to convert furfural hydrazones into functionalised phthalimides was developed. The reaction was performed in a microwave using a hydrophilic ionic liquid, [bmim][Cl], as the solvent which could be readily recovered by a simple extraction. The ionic liquid was successfully recycled with no significant loss in product yields.
Introduction
Increasing awareness of the negative impact that the use of non-renewable petrochemicals has on the environment has led the scientific community to investigate greener, more sustainable alternatives. For this reason, the use of biomass waste has been proposed as a potential feedstock for the generation of valuable chemicals and fuel.1–5 Furfural and HMF can be obtained from lignocellulosic biomass through the acid-catalysed dehydration of sugars.2,3,6 They have been reported as precursors to a number of valuable chemical products ranging from medicinally relevant compounds with furan scaffolds to cycloaddition products including poly-substituted benzenes.4–9 The use of furan and its derivatives in Diels–Alder cyclisations to access commodity aromatics (e.g. toluene, xylene, benzoic acid) has been widely investigated.5,10,11 Although the cycloaddition–aromatisation reaction of furans represents an atom economical strategy, as well as a green route to aromatic compounds because it only releases water as a waste by-product, many of the procedures reported to date employ relatively harsh conditions such as high temperatures/pressures, or require a large excess of the furan to achieve high conversions.4,5,10 Recently, procedures employing biomass-derived compounds in tandem with green solvents have been reported.8,12–15 Amongst them, we developed a one-pot protocol for the synthesis of polysubstituted aromatic compounds from furfural in water.4 This procedure is completely performed in water and avoids the use of any organic solvent in the extraction or purification steps. However, in some cases direct crystallisation of the product from the reaction mixture did not readily take place, and purification after extraction with organic solvents was necessary. In addition, where substrates are poorly soluble in water the reaction does not proceed efficiently. In this new study, we wished to explore the development of a more general protocol applicable to more hydrophobic compounds using ionic liquids as the medium for the reaction.Ionic liquids are recognized as new green solvents for chemical transformations,16–18 because of their low volatility, compatibility with catalysts, ability to dissolve reactants, and their unique physical properties which often enable them to catalyse organic reactions.16,19–21 Additionally, it has been reported that reactions in ionic liquids were efficiently accelerated by microwave heating because very rapid heating of IL to high temperatures was easily achieved. This advantage is mainly because of dipolar rotation and ionic conduction effects derived from the use of ionic liquids.22–30 For example, we have recently demonstrated the successful utilisation of ionic liquids for; (i) depolymerisation of plastics,31,32 (ii) cellulolysis33 using the hydrophobic IL [PP13][NTf2], and (iii) conversion of sorbitol to isosorbide using [TMPA][NTf2].34
The ultimate goal of this current study is to develop a sustainable route for the conversion of biomass waste to useful aromatic compounds using ionic liquids. Herein, we report the use of an ionic liquid, [bmim][Cl], as an alternative green solvent for the synthesis of substituted phthalimides from furfurals. We also demonstrate the recyclability of [bmim][Cl] in the reaction.
Results and discussion
We initially sought to identify suitable ionic liquids for the synthesis of phthalimides from furfural hydrazone 3a (Table 1). A variety of hydrophilic (entries 1, 2, 7, 8) and hydrophobic (entries 3–6) ionic liquids were examined. We first examined conventional heating of the reaction, but the reaction progressed slowly and the desired product 3a was obtained in only 32% yield (entry 1). To increase the reaction rate, microwave heating was employed. The IL [bmim][BF4] was found to be unsuitable for the reaction due to difficulties observed in the isolation of the product (entry 2), and the low recovery of the IL. We envisaged that [bmim][NTf2] might be effective at mediating the reaction due to its high ionicity.35,36 Although [bmim][NTf2] was found to give the best yield of 61% (entry 3), separation of the IL from the product was difficult because of its hydrophobicity, and this required copious washes with organic solvents, which was against the purpose of developing a sustainable environmentally friendly procedure. Similarly, [TMPA][NTf2] and [PP13][NTf2] resulted in good yields of 3a, but the recovery of the IL was not satisfactory (entries 4 and 5, respectively). The IL [DEME][NTf2] was a poor solvent for the reaction with only 32% yield of the product obtained (entry 6). We then examined the hydrophilic ionic liquid [bmim][Cl]. The reaction progressed well with microwave heating, and complete separation of the IL was accomplished by washing the organic layers three times with water, although the yield of 3a remained at 35% (entries 7). To improve the yield of 3a, we examined the use of freshly prepared hydrazone 1a. Compound 3a was then isolated in 83% yield without contamination of the IL (entry 8). Pleasingly, 99% of the IL was recovered after the work-up. The product 3a was isolated by recrystallisation from an acetone/water mixture without the need for chromatographic purification. It was considered that the lower yields initially observed during the formation of 3a could be attributed to contamination with N,N-dimethylaminohydrazine, liberated from hydrazone 1a on storage. Indeed, the cycloaddition reaction became sluggish when 10 mol% of dimethlhydrazine was added and the yield of compound 3a decreased to less than 10% (Table 1 footnote d). We think that contamination with small amounts of N,N-dimethylhydrazine in ionic liquids could progress some other reactions, possibly conjugate addition to 2a, preventing the formation of 3a in high yield.| Entry | Ionic liquids | Temp (°C) | Time (min) | Yield (%) | Recovery of IL (%) |
|---|---|---|---|---|---|
| a Conventional heating was employed instead of microwave irradiation.b Product partially contaminated with IL.c IL partially contaminated with product 3a.d Freshly prepared hydrazone 1a was used. When the reaction was carried out in the presence of 10 mol% of N,N-dimethylhydrazine, the yield of 3a dropped to less than 10%. | |||||
| 1 | [bmim][Cl] | 100 | 120 | 32a | 99 |
| 2 | [bmim][BF4] | 120 | 60 | 50b | 46c |
| 3 | [bmim][NTf2] | 120 | 60 | 61b | 97c |
| 4 | [TMPA][NTf2] | 100 | 120 | 57 | 88 |
| 5 | [PP13][NTf2] | 100 | 120 | 60 | 90 |
| 6 | [DEME][NTf2] | 100 | 120 | 32 | 92 |
| 7 | [bmim][Cl] | 100 | 120 | 35 | 97 |
| 8 | [bmim][Cl] | 100 | 120 | 83d | 99 |
With satisfactory reaction conditions shown in Table 1, entry 8, we examined the applicability of the reaction conditions to a variety of furfural hydrazone derivatives 1 and maleimides 2 (Scheme 1). The reaction gave good yields of phthalimide products with aliphatic-substituted maleimides (3b–3i), including many examples of highly hydrophobic compounds that are unsuitable for reactions in water. The Diels–Alder reaction between 1a and 2b in IL gave phthalidimide 3b in 94% yield along with a 96% recovery of [bmim][Cl]. Longer aliphatic substituents on the maleimide were also tolerated, giving phthalimides 3c–3i in moderate to good yields. The reaction with a bulky maleimide such as 2k also progressed smoothly to give N-cyclohexylphthalimide 3k in 68% yield. Substituted benzyl maleimides similarly afforded the products 3m and 3n in excellent yields (94% and 97%, respectively). Note that the N-unsubstituted maleimide gave the cycloaddition product 3j in a good yield, which opens up the opportunity to further functionalisation at the nitrogen atom. N-Allyl substituted maleimide 2l underwent the cycloaddition to give the product 3l in 74% yield. We also examined the cycloaddition reaction using 5-methylfurfural hydrazone 1b, giving 3p, 3q and 3r in reasonable yields.
Treatment of 5-bromofurfural hydrazone 1c with maleimide 2a and 2b resulted in the formation of phenol derivative 3s and 3t, respectively. During the formation of these compounds, elimination of hydrogen bromide instead of dehydration occurred to aromatise the adduct. Unfortunately, the yield of 3t remained low because of its water solubility which caused loss of the product during the extraction/washing procedure.
To investigate the recyclability of the ionic liquids, we examined the reaction between furfural hydrazone 1a and N-ethylmaleimide 2b further (Table 2).
The yields of 3b remained high in each of the five experiments, and an excellent recovery of [bmim][Cl] was achieved each time. 1H NMR spectroscopic data of the recovered IL indicated that the compound was identical to unused IL. These results clearly highlighted that [bmim][Cl] is stable under the reaction conditions used. Thus, the ionic liquids are useful for this conversion, and the efficient recovery and reuse of the ionic liquid is possible under these reaction conditions. Overall, a green conversion of furfurals into phthalimides using ionic liquids has been achieved.
Conclusions
We have developed a successful protocol employing recyclable ionic liquids for the synthesis of substituted phthalimides from furfurals. The reaction proceeds efficiently under microwave heating with products isolated via a simple work-up procedure requiring a minimal amount of solvent to recover the ionic liquid. The products were recovered by recrystallisation from an acetone/water mixture without the need for chromatographic purification. The recyclability of the ionic liquid was >95% with no loss in efficiency over five cycles of use in the reaction. With such straightforward manipulations and the use of ionic liquids as a green solvent, the present protocol will contribute to the development of green chemical syntheses.Experimental section
General methods
All reagents and solvents were purchased and used as supplied unless otherwise stated. All reactions were carried out under the atmosphere of nitrogen unless otherwise stated. All reactions were monitored by thin layer chromatography (TLC) or 1H NMR spectroscopy. TLC plates used were pre-coated with silica gel 60 F254 on aluminium (Merck KGaA) and visualised by UV light (254 nm) or chemically stained (KMnO4). Flash column chromatographic purification was performed using silica gel (Wako Co. Wakosil C-300). 1H NMR and 13C NMR spectra were recorded at 500 MHz (for 1H) 125 MHz on JEOL lamda-500 or JNM-ECA 500 Delta2 at ambient temperature, unless otherwise indicated. Deuterated solvents used for NMR spectroscopic characterisation were CDCl3, CD3OD, or DMSO-d6, as stated. Peaks are assigned as singlet (s), doublet (d), triplet (t), quintet (qn), sextet (sx), septet (sept), or multiplet (m). 1H and 13C shifts are reported in parts per million (ppm) and compared against residual solvent signals: CDCl3 (δ = 7.26 ppm, s; 77.2 ppm, t), CD3OD (δH 4.87, s and 3.31, qn; δC 49.1 ppm, sept), DMSO-d6 (δH 2.56 ppm, qn; δC 39.5 ppm, sept); 1H and 13C NMR shifts relative to TMS were calibrated using residual solvents peak. Infra-red spectra were obtained using a SHIMADZU FTIR-8400S spectrometer, all frequencies were given in reciprocal centimetres (cm−1). Melting points were measured on a YANACO MP-J3. High-resolution mass spectra were recorded on a JEOL JMS-T100LP mass spectrometer. Microwave heating was carried out using a SMW-087 microwave reactor, made by Shikoku Instrument Co. Ltd., 2.45 GHz, output 700 W, multimode. The reaction vessel (a 50 mL glass flask) was placed in the microwave oven (temperature was set at 100 °C or 120 °C). The reaction temperature was monitored by a thermocouple inserted into the reaction mixture and the reaction was stirred under microwave irradiation. Hydrazones 1 were prepared as described in the ESI.†Yellow solid; mp 221–222 °C; 1H NMR (500 MHz, CDCl3) δ 8.31 (d, J = 8.1 Hz, 1H, NNCHCCH), 8.15 (s, 1H, NNCH), 7.72 (d, J = 7.2 Hz, 1H, NCHCCHCHCH), 7.64 (t, J = 7.8 Hz, 1H, NCHCCHCH), 7.53–7.46 (m, 2H, Ph), 7.44–7.37 (m, 3H, Ph), 3.13 (s, 6H, 2 × CH3); 13C NMR (125 MHz, CDCl3) δ 168.5, 167.5, 137.0, 133.8, 132.1, 131.8, 129.4, 129.2, 128.1, 126.8, 124.3, 124.1, 121.3, 42.7; HMRS: found (ESI): [M + H]+ 294.12390 C17H16N3O2, requires 294.12425; data in agreement with the literature.4
Yellow solid; mp 141–143 °C [lit.4 142–143 °C]; 1H NMR (500 MHz, CDCl3) δ 8.22 (d, J = 7.7 Hz, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCCH), 8.11 (s, 1H, N
CHCCH), 8.11 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.61 (d, J = 7.7 Hz, 1H, N
CH), 7.61 (d, J = 7.7 Hz, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCCHCHCH), 7.55 (t, J = 7.7 Hz, 1H, N
CHCCHCHCH), 7.55 (t, J = 7.7 Hz, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCCHCH), 3.72 (q, J = 7.3 Hz, 2H, CH3CH2), 3.12 (s, 6H, 2 × CH3), 1.26 (t, J = 7.3 Hz, 3H, CH3CH2); 13C NMR (125 MHz, CDCl3) δ 169.5, 168.5, 136.2, 133.4, 132.6, 129.0, 124.9, 124.7, 120.9, 42.7, 32.9, 14.1; HMRS: found (ESI): [M + H]+ 268.1068 C13H15N3NaO2, requires 268.1062; data in agreement with the literature.4
CHCCHCH), 3.72 (q, J = 7.3 Hz, 2H, CH3CH2), 3.12 (s, 6H, 2 × CH3), 1.26 (t, J = 7.3 Hz, 3H, CH3CH2); 13C NMR (125 MHz, CDCl3) δ 169.5, 168.5, 136.2, 133.4, 132.6, 129.0, 124.9, 124.7, 120.9, 42.7, 32.9, 14.1; HMRS: found (ESI): [M + H]+ 268.1068 C13H15N3NaO2, requires 268.1062; data in agreement with the literature.4
Yellow solid; mp 121–122 °C; νmax (solid per cm) 2964, 1703, 1552; 1H NMR (500 MHz, CDCl3) δ 8.20 (d, J = 8.0 Hz, 1H, NNCHCCH), 8.10 (s, 1H, NNCH), 7.59 (d, J = 7.2 Hz, 1H, NNCCHCHCH), 7.54 (t, J = 7.6 Hz, 1H, NNCHCCHCH), 3.61 (t, J = 7.3 Hz, 2H, NCH2CH2CH3), 3.11 (s, 6H, 2 × CH3), 1.72–1.65 (m, 2H, NCH2CH2CH3), 0.94 (t, J = 7.3 Hz, 3H, NCH2CH2CH3); 13C NMR (125 MHz, CDCl3) δ 169.7, 168.7, 136.3, 133.3, 132.4, 128.9, 124.7, 124.6, 120.8, 42.7, 39.5, 22.1, 11.5; HRMS: found (ESI): [M + H]+ 260.13883 C14H18N3O2, requires 260.13990.
Yellow solid; mp 91–92 °C; 1H NMR (500 MHz, CDCl3) δ 8.22 (d, J = 8.0 Hz, 1H, NNCHCCH), 8.12 (s, 1H, NNCH), 7.61 (d, J = 7.2 Hz, 1H, NNCCHCHCH), 7.59–7.50 (m, 1H, NNCCHCH), 3.66 (t, J = 7.3 Hz, 2H, NCH2CH2CH2CH3), 3.12 (s, 6H, 2 × CH3), 1.68–1.62 (m, 2H, NCH2CH2CH2CH3), 1.38–1.32 (m, 2H, NCH2CH2CH2CH3), 0.94 (t, J = 7.4 Hz, 3H, NCH2CH2CH2CH3); 13C NMR (125 MHz, CDCl3) δ 169.6, 168.6, 136.3, 133.3, 132.4, 128.9, 124.8, 124.6, 120.8, 42.7, 37.7, 31.1, 20.2, 13.8; HRMS: found (ESI): [M + H]+ 274.15652 C15H20N3O2, requires 274.15555.
Yellow solid; mp 74–75 °C; νmax (solid per cm) 2995, 1697, 1548; 1H NMR (500 MHz, CDCl3) δ 8.20 (d, J = 8.0 Hz, 1H, NNCHCCH), 8.09 (s, 1H, NNCH), 7.61–7.56 (m, 1H, NNCCHCHCH), 7.53 (t, J = 7.7 Hz, 1H, NNCCHCH), 3.66–3.58 (m, 2H, NCH2CH2CH2CH2CH3), 3.10 (s, 6H, 2 × CH3), 1.68–1.60 (m, 2H, NCH2CH2CH2CH2CH3), 1.37–1.29 (m, 4H, NCH2 CH2CH2CH2CH3), 0.87 (t, J = 7.1 Hz, 3H, NCH2CH2CH2CH2CH3); 13C NMR (125 MHz, CDCl3) δ 169.6, 168.6, 136.3, 133.3, 132.4, 128.9, 124.8, 124.6, 120.8, 42.7, 38.0, 29.1, 28.4, 22.4, 14.1; HRMS: found (ESI): [M + H]+ 288.17265 C16H22N3O2, requires 288.17120.
Yellow solid; mp 73–74 °C; νmax (solid per cm) 2929, 1699, 1550; 1H NMR (500 MHz, CDCl3) δ 8.18 (d, J = 7.8 Hz, 1H, NNCHCCH), 8.08 (s, 1H, NN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.57 (d, J = 8.2 Hz, 1H, NNCCHCHCH), 7.52 (t, J = 7.6 Hz, 1H, NNCCHCH), 3.62 (d, J = 7.4 Hz, 2H, NCH2CH2CH2CH2CH2CH3), 3.09 (s, 6H, 2 × CH3), 1.63 (p, J = 7.4 Hz, 2H, NCH2CH2CH2CH2CH2CH3), 1.36–1.13 (m, 6H, NCH2 CH2CH2CH2CH2CH3), 0.85 (t, J = 6.9 Hz, 3H, N–(CH2)5–CH3); 13C NMR (125 MHz, CDCl3) δ 169.6, 168.6, 136.3, 133.3, 132.4, 128.9, 124.8, 124.6, 120.8, 42.7, 38.0, 31.5, 28.7, 26.7, 22.6, 14.1; HRMS: found (ESI): [M + Na]+ 324.16711 C17H23N3O2Na, requires 324.16880.
CH), 7.57 (d, J = 8.2 Hz, 1H, NNCCHCHCH), 7.52 (t, J = 7.6 Hz, 1H, NNCCHCH), 3.62 (d, J = 7.4 Hz, 2H, NCH2CH2CH2CH2CH2CH3), 3.09 (s, 6H, 2 × CH3), 1.63 (p, J = 7.4 Hz, 2H, NCH2CH2CH2CH2CH2CH3), 1.36–1.13 (m, 6H, NCH2 CH2CH2CH2CH2CH3), 0.85 (t, J = 6.9 Hz, 3H, N–(CH2)5–CH3); 13C NMR (125 MHz, CDCl3) δ 169.6, 168.6, 136.3, 133.3, 132.4, 128.9, 124.8, 124.6, 120.8, 42.7, 38.0, 31.5, 28.7, 26.7, 22.6, 14.1; HRMS: found (ESI): [M + Na]+ 324.16711 C17H23N3O2Na, requires 324.16880.
Yellow solid; mp 55–56 °C; νmax (solid per cm) 2928, 1699, 1550; 1H NMR (500 MHz, CDCl3) δ 8.21 (d, J = 8.0 Hz, 1H, NNCHCCH), 8.12 (s, 1H, NNCH), 7.60 (d, J = 7.3 Hz, 1H, NNCHCCHCHCH), 7.56 (d, J = 7.6 Hz, 1H, NNCHCCHCH), 3.68–3.60 (m, 2H, NCH2(CH2)5CH3), 3.11 (s, 6H, 2 × CH3), 1.70–1.61 (m, 2H, NCH2CH2(CH2)4CH3), 1.36–1.29 (m, 4H, 2 × CH2), 1.29–1.18 (m, 4H, 2 × CH2), 0.86 (t, J = 6.8 Hz, 3H, N(CH2)6CH3); 13C NMR (125 MHz, CDCl3) δ 169.4, 168.4, 136.2, 133.1, 132.4, 128.7, 124.6, 124.4, 120.6, 42.5, 37.9, 31.8, 28.9, 28.7, 26.9, 22.6, 14.1.; HRMS: found (ESI): [M + H]+ 316.20250 C18H26N3O2, requires 316.20250.
Yellow solid; mp 50–51 °C; νmax (solid per cm) 2926, 1703, 1550; 1H NMR (500 MHz, CDCl3) δ 8.21 (d, J = 7.9 Hz, 1H), 8.11 (s, 1H), 7.60 (d, J = 7.1 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 3.63 (t, J = 7.3 Hz, 2H), 3.11 (s, 6H), 1.65 (m, J = 7.3 Hz, 2H), 1.34–1.22 (m, 10H), 0.85 (t, J = 6.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 169.6, 168.6, 136.3, 133.3, 132.5, 128.9, 124.8, 124.7, 120.8, 42.7, 38.0, 31.9, 29.3, 29.3, 28.8, 27.0, 22.7, 14.2; HRMS: found (ESI): [M + Na]+ 352.19881 C19H27N3O2Na, requires 352.20010.
Yellow solid; mp 42–43 °C; νmax (solid per cm) 2924, 1701, 1550; 1H NMR (500 MHz, CDCl3) δ 8.21 (d, J = 8.0 Hz, 1H, NNCHCCH), 8.11 (s, 1H, NNCH), 7.60 (d, J = 7.2 Hz, 1H, NNCHCCHCHCH), 7.55 (t, J = 7.6 Hz, 1H, NNCHCCHCH), 3.64 (t, J = 7.3 Hz, 2H, NCH2(CH2)8CH3), 3.11 (s, 6H, 2 × CH3), 1.66–1.62 (m, 2H, NCH2CH2(CH2)6CH3), 1.37–1.22 (m, 4H, 2 × CH2), 1.27–1.22 (m, 8H, 4 × CH2), 0.86 (t, J = 6.9 Hz, 3H, NCH2(CH2)8CH3); 13C NMR (125 MHz, CDCl3) δ 169.6, 168.6, 136.3, 134.1, 133.3, 132.4, 128.9, 124.6, 120.8, 42.7, 38.0, 31.9, 29.5, 29.3, 29.2, 28.8, 27.0, 22.8, 14.2; HRMS: found (ESI): [M + Na]+ 366.21742 C20H29N3O2Na, requires 366.21575.
Yellow solid; mp 240–241 °C; 1H NMR (500 MHz, CDCl3) δ 8.27 (d, J = 7.2 Hz, 1H, NNCHCCH), 8.06 (s, 1H, NCH), 7.63–7.58 (m, 3H, NNCHCCHCH, NNCHCCHCHCH and N–H), 3.13 (s, 6H, 2 × CH3); 13C NMR (125 MHz, CDCl3) δ 169.0, 168.0, 137.0, 133.8, 133.0, 129.3, 125.1, 124.3, 121.2, 42.8; HRMS found (ESI): [M + Na]+ 240.0743 C11H11N3NaO2, requires 240.0749.
Yellow solid; mp 187–189 °C; νmax (solid per cm) 2951, 2918, 2851, 1755, 1697, 1549, 1464; 1H NMR (500 MHz, CDCl3) δ 8.20 (d, J = 7.5 Hz, 1H, NNCHCCH), 8.12 (s, 1H, NCH), 7.58 (d, J = 7.5 Hz, 1H, NNCCHCHCH), 7.54 (t, J = 7.5 Hz, 1H, NNCCHCH), 4.13–4.03 (m, 1H, CONCH), 3.11 (s, 6H, 2 × CH3), 2.25–2.13 (m, 2H, CyCH2), 1.87–1.84 (m, 2H, CyCH2), 1.73–1.71 (m, 2H, CyCH2), 1.67 (s, 1H, CyCH2), 1.43–1.17 (m, 2H, CyCH2), 0.98–0.74 (m, 1H, CyCH2); 13C NMR (125 MHz, CDCl3) δ 169.8, 168.6, 136.1, 133.2, 132.3, 128.9, 124.7, 120.7, 50.8, 42.7, 30.0, 29.8, 26.2, 25.3; HRMS: found (ESI): [M + H]+ 300.1727 C17H22N3O2, requires 300.1707.
Yellow solid; mp 128–129 °C; νmax (solid per cm) 2986, 2924, 2889, 2359, 2332, 1759, 1701, 1547, 1427; 1H NMR (500 MHz, CDCl3) δ 8.22 (d, J = 8.0 Hz, 1H, NNCHCCH), 8.08 (s, 1H, NNCH), 7.61 (d, J = 7.2 Hz, 1H, NNCHCCHCHCH), 7.55 (t, J = 7.7 Hz, 1H, NNCHCCHCH), 5.94–5.82 (m, 1H, CHH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.30–5.21 (m, 1H, CHH
CH), 5.30–5.21 (m, 1H, CHH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.18 (d, J = 10.2 Hz, 1H, CH2
CH), 5.18 (d, J = 10.2 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.26 (d, J = 5.5 Hz, 2H, NCH2), 3.11 (s, 6H, 2 × CH3); 13C NMR (125 MHz, CDCl3) δ 169.1, 168.1, 136.5, 133.4, 132.4, 131.9, 129.0, 124.6, 124.4, 121.0, 117.6, 42.7, 40.0; HRMS: found (ESI): [M + H]+ 258.1256 C14H16N3O2, requires 258.1237.
CH), 4.26 (d, J = 5.5 Hz, 2H, NCH2), 3.11 (s, 6H, 2 × CH3); 13C NMR (125 MHz, CDCl3) δ 169.1, 168.1, 136.5, 133.4, 132.4, 131.9, 129.0, 124.6, 124.4, 121.0, 117.6, 42.7, 40.0; HRMS: found (ESI): [M + H]+ 258.1256 C14H16N3O2, requires 258.1237.
Yellow solid; mp 48–49 °C; 1H NMR (500 MHz, CDCl3) δ 8.22 (d, J = 8.0 Hz, 1H, NNCHCCH), 8.08 (s, 1H, NNCH), 7.61 (d, J = 7.0 Hz, 1H, NNCHCCHCHCH), 7.55 (t, J = 7.7 Hz, 1H, NNCHCCHCH), 7.42 (d, J = 7.3 Hz, 2H, Ph), 7.35–7.22 (m, 3H, Ph), 4.82 (s, 2H, PhCH2), 3.11 (s, 6H, 2 × CH3); 13C NMR (125 MHz, CDCl3) δ 169.2, 168.3, 136.7, 136.5, 133.4, 132.4, 129.1, 128.8, 128.6, 127.8, 124.7, 124.4, 121.0, 42.7, 41.5; HRMS: found (ESI): [M + H]+ 308.14024 C18H18N3O2, requires 308.13990; data in agreement with the literature.4
Yellow solid; mp 129–131 °C; νmax (solid per cm) 2949, 2833, 1755, 1697, 1611, 1549, 1510; 1H NMR (500 MHz, CDCl3) δ 8.21 (d, J = 8.0 Hz, 1H, NNCHCCH), 8.08 (s, 1H, NNCH), 7.63–7.57 (m, 1H, NNCHCCHCHCH), 7.54 (t, J = 7.6 Hz, 1H, NNCHCCHCH), 7.37 (d, J = 8.6 Hz, 2H, Ar), 6.86–6.80 (m, 2H, Ar), 4.76 (s, 2H, ArCH2), 3.76 (s, 3H, OCH3), 3.11 (s, 6H, 2 × CH3); 13C NMR (125 MHz, CDCl3) δ 169.2, 168.3, 159.2, 136.5, 133.4, 132.4, 130.1, 129.0, 128.9, 124.7, 124.5, 121.0, 114.1, 55.4, 42.7, 41.0; HRMS: found (ESI): [M + H]+ 338.15041 C19H20N3O3, requires 338.15047.
Yellow solid; mp 106–107 °C; νmax (solid per cm) 2931, 2864, 1763, 1694, 1541, 1454; 1H NMR (500 MHz, CDCl3) δ 8.22 (d, J = 7.9 Hz, 1H, Ar), 8.09 (s, 1H, NCH), 7.62–7.58 (m, 1H, Ar), 7.55 (t, J = 7.6 Hz, 1H, Ar), 7.31–7.25 (m, 4H, Ph), 7.24–7.19 (m, 1H, Ph), 3.94–3.83 (m, 2H, PhCH2CH2), 3.12 (s, 6H, 2 × CH3), 3.02–2.92 (m, 2H, PhCH2); 13C NMR (125 MHz, CDCl3) δ 169.4, 168.3, 138.2, 136.4, 133.4, 132.4, 129.0, 128.9, 128.7, 126.7, 124.7, 124.5, 120.9, 42.7, 39.3, 34.9; HRMS: found (ESI): [M + H]+ 322.15728 C19H20N3O2, requires 322.15555.
Yellow solid; mp 142–143 °C; 1H NMR (500 MHz, CDCl3) δ 8.14 (s, 1H, NNCH), 8.08 (d, J = 8.3 Hz, 1H, NNCHCCH), 7.30 (d, J = 8.3 Hz, 1H, NNCHCCHCH), 3.68 (d, J = 7.1 Hz, 2H, CH2CH3), 3.08 (s, 6H, 2 × CH3), 2.63 (s, 3H, CH3), 1.24 (t, J = 7.1 Hz, 3H, CH2CH3);13C NMR (125 MHz, CDCl3) δ 169.3, 169.1, 136.2, 135.8, 134.1, 128.8, 128.7, 125.6, 125.2, 42.7, 32.6, 17.6, 14.1; HRMS: found (ESI): [M + H]+ 260.1398 C14H18N3O3, requires 260.1399; data in agreement with the literature.4
Yellow solid; mp 201–203 °C; νmax (solid per cm) 1753, 1694, 1541, 1489; 1H NMR (500 MHz, CDCl3) δ 8.18–8.17 (m, 2H, NNCH and NNCHCCH), 7.49 (t, J = 7.8 Hz, 2H, Ph), 7.45–7.36 (m, 4H, Ph and NNCHCCHCH), 3.09 (s, 6H, 2 × CH3), 2.69 (s, 3H, CH3);13C NMR (125 MHz, CDCl3) δ 168.4, 168.1, 136.7, 136.4, 134.7, 131.9, 129.2, 129.1, 128.2, 128.0, 126.9, 125.3, 124.5, 42.7, 17.8; HRMS: found (ESI): [M + H]+ 308.1341 C18H28N3O3, requires; 308.1399.
Yellow solid; mp 80–81 °C; νmax (solid per cm) 2929, 1693, 1548; 1H NMR (500 MHz, CDCl3) δ 8.13 (s, 1H, NNCH), 8.07 (d, J = 8.2 Hz, 1H, NNCHCCH), 7.29 (d, J = 8.2 Hz, 1H, NNCHCCHCH), 3.64–3.56 (m, 2H, NCH2(CH2)4CH3), 3.07 (s, 6H, 2 × CH3), 2.63 (s, 3H, CH3), 1.66–1.60 (m, 2H, CH2), 1.34–1.28 (m, 6H, 3 × CH2), 0.86 (t, J = 6.9 Hz, 3H, NCH2(CH2)4CH3);13C NMR (125 MHz, CDCl3) δ 169.5, 169.3, 136.2, 135.8, 134.0, 128.7, 128.6, 125.6, 125.1, 42.7, 37.8, 31.5, 28.7, 26.7, 22.6, 17.6, 14.1; HRMS: found (ESI): [M + H]+ 316.20474 C18H26N3O2, requires 316.20250.
Red-brown solid; mp 147–148 °C; 1H NMR (500 MHz, CDCl3) δ 8.15 (d, J = 8.7 Hz, 1H, NNCHCCH), 8.00 (s, 1H, NNCH), 7.73 (br s, 1H, OH), 7.07 (d, J = 8.7 Hz, 1H, NNCHCCHCH), 3.69 (q, J = 7.3 Hz, 2H, CH2), 3.07 (s, 6H, 2 × CH3), 1.27 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (125 MHz, CDCl3) δ 170.5, 168.6, 153.7, 132.4, 129.8, 125.3, 124.2, 123.1, 113.5, 42.8, 32.7, 14.2; HRMS: found (ESI): [M + Na]+ 284.1018 C13H15N3NaO3, requires 284.1011.
Red-brown solid; mp 227–229 °C; νmax (KBr/cm−1) 3246, 1763, 1686, 1557, 1493; 1H NMR (500 MHz, CDCl3) δ 8.24 (d, J = 8.8 Hz, 1H, NNCHCCH), 8.03 (s, 1H, NNCH), 7.88 (s, 1H, OH), 7.49 (d, J = 7.8 Hz, 2H, Ph), 7.46–7.34 (m, 3H, Ph), 7.15 (d, J = 8.8 Hz, 1H, NNCHCCHCH), 3.07 (s, 6H, 2 × CH3); 13C NMR (125 MHz, CDCl3) δ 169.5, 167.6, 154.2, 132.9, 131.4, 130.3, 129.2, 128.2, 126.5, 124.9, 123.6, 123.2, 113.0, 42.76; HRMS: found (ESI): [M + H]+ 301.1184 C17H16N3O2, requires 310.1192.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by KAKENHI for Challenging Exploratory Research 17K19139. The authors gratefully acknowledge JSPS for doctoral fellowship funding provided to V. K. (PE17018).Notes and references
- A. Kamimura, T. Okagawa, N. Oyama, T. Otsuka and M. Yoshimoto, Green Chem., 2012, 14, 2816–2820 RSC.
- N. Shi, Q. Liu, T. Wang, L. Ma, Q. Zhang and Q. Zhang, ACS Sustainable Chem. Eng., 2014, 2, 637–642 CrossRef.
- J. Cui, J. Tan, T. Deng, X. Cui, Y. Zhu and Y. Li, Green Chem., 2016, 18, 1619–1624 RSC.
- S. Higson, F. Subrizi, T. D. Sheppard and H. C. Hailes, Green Chem., 2016, 18, 1855–1858 RSC.
- A. E. Settle, L. Berstis, N. A. Rorrer, Y. Roman-Leshkóv, G. T. Beckham, R. M. Richards and D. R. Vardon, Green Chem., 2017, 19, 3468–3492 RSC.
- E. Ramírez, R. Bringué, C. Fité, M. Iborra, J. Tejero and F. Cunill, J. Chem. Technol. Biotechnol., 2017, 92, 2775–2786 CrossRef.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef PubMed.
- S. A. Sanchez-Vazquez, H. C. Hailes and J. R. G. Evans, Polym. Rev., 2013, 53, 627–694 CrossRef.
- S. Shylesh, A. A. Gokhale, C. R. Ho and A. T. Bell, Acc. Chem. Res., 2017, 50, 2589–2597 CrossRef PubMed.
- J. M. Fraile, J. I. García, M. A. Gomez, A. de la Hoz, J. A. Mayoral, A. Moreno, P. Prieto, L. Salvatella and E. Vázquez, Eur. J. Org. Chem., 2001, 2891–2899 CrossRef.
- G. V. Kumar and A. Rajendran, Synth. Commun., 2016, 46, 483–496 CrossRef.
- B. H. Shanks and P. L. Keeling, Green Chem., 2017, 19, 3177–3185 RSC.
- J. J. Lee and G. A. Kraus, Green Chem., 2014, 16, 2111–2116 RSC.
- E. Mahmoud, J. Yu, R. J. Gorte and R. F. Lobo, ACS Catal., 2015, 5, 6946–6955 CrossRef.
- R. J. Van Putten, J. C. Van Der Waal, E. De Jong, C. B. Rasrendra, H. J. Heeres and J. G. De Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef PubMed.
- R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC.
- R. Fu, Y. Yang, W. Feng, Q. Ge, Y. Feng, X. Zeng, W. Chai, J. Yi and R. Yuan, Tetrahedron, 2016, 72, 8319–8326 CrossRef.
- B. Yuan, J. Guan, J. Peng, G. Zhou Zhu and J. Hong Jiang, Chem. Eng. J., 2017, 330, 109–119 CrossRef.
- R. Ratti, Adv. Chem., 2014, 2014, 1–16 CrossRef.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef PubMed.
- E. Dresler, E. Jasinska, A. Kryger-Lapczuk, E. Nowakowska-Bogdan and R. Jasinski, Chemik, 2015, 69, 288–296 Search PubMed.
- R. Martínez-Palou, Mol. Diversity, 2010, 14, 3 CrossRef PubMed.
- E. Van der Eycken, P. Appukkuttan, W. De Borggraeve, W. Dehaen, D. Dallinger and C. O. Kappe, J. Org. Chem., 2002, 67, 7904 CrossRef PubMed.
- J. Hoffmann, M. Nuchter, B. Ondruschka and P. Wasserscheid, Green Chem., 2003, 5, 296 RSC.
- M. M. Dharman, H.-Y. Ju, H.-L. Shim, M.-K. Lee, K.-H. Kim and D.-W. Park, J. Mol. Catal. A: Chem., 2009, 303, 91 CrossRef.
- N. E. Leadbeater and H. M. Torenius, J. Org. Chem., 2002, 67, 3145 CrossRef PubMed.
- K. S. A. Vallin, P. Emilsson, M. Larhed and A. Hallberg, J. Org. Chem., 2002, 67, 6243 CrossRef PubMed.
- N. Z. Kiss and G. Keglevich, Tetrahedron Lett., 2016, 57, 971 CrossRef.
- J. Tharun, A. C. Kathalikkattil, R. Roshan, D.-H. Kang, H.-C. Woo and D.-W. Park, Catal. Commun., 2014, 54, 31 CrossRef.
- Y. Chen, Y. Zu, Y. Fu, X. Zhang, P. Yu, G. Sun and T. Efferth, Molecules, 2010, 15, 9486 CrossRef PubMed.
- A. Kamimura and S. Yamamoto, Org. Lett., 2007, 9, 2532–2535 CrossRef PubMed.
- A. Kamimura, S. Yamamoto and K. Yamada, ChemSusChem, 2011, 4, 644–649 CrossRef PubMed.
- A. Kamimura, T. Okagawa, N. Oyama, T. Otsuka and M. Yoshimoto, Green Chem., 2012, 14, 2816–2820 RSC.
- A. Kamimura, K. Murata, Y. Tanaka, T. Okagawa, H. Matsumoto, K. Kaiso and M. Yoshimoto, ChemSusChem, 2014, 7, 3257–3259 CrossRef PubMed.
- D. R. MacFarlane, M. Forsyth, E. I. Izgorodina, A. P. Abbott, G. Annata and K. Fraser, Phys. Chem. Chem. Phys., 2009, 11, 4962–4967 RSC.
- K. Ueno, H. Tokuda and M. Watanabe, Phys. Chem. Chem. Phys., 2010, 21, 1649–1658 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures for the preparation of hydrazones 1; 1H and 13C NMR spectra. See DOI: 10.1039/c8ra03895c |
| ‡ Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2018 |