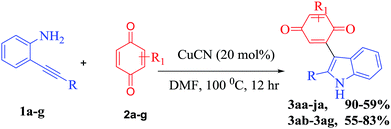Open Access Article
Open Access ArticleCopper-catalyzed tandem reaction of 2-alkynylanilines with benzoquinones: efficient access to 3-indolylquinones†
Raveendra Jillella and
Chang Ho Oh *
*
Department of Chemistry and Research Institute of Natural Science, Hanyang University, Seoul 04763, South Korea. E-mail: changho@hanyang.ac.kr
First published on 15th June 2018
Abstract
A simple, mild, catalytic and efficient method for the straightforward synthesis of an interesting class of 2-aryl/alkyl-substituted-3-indolyl quinones in good to high yields is reported for the first time. This atom-efficient method proceeds via copper-catalyzed one-pot sequential intramolecular hydroamination (C–N bond formation) of 2-alkynylanilines followed by oxidative C–C coupling with benzoquinones.
Introduction
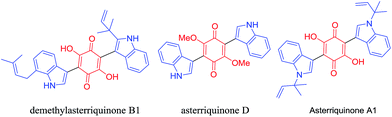
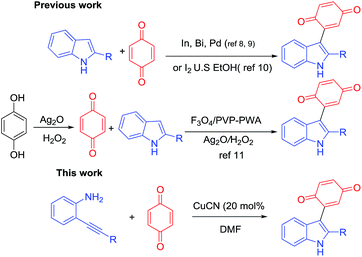
Indole scaffolds represent one of the privileged structural motifs in biologically active natural products and drug molecules which have vital medicinal value.1 Among the family of indole derivatives, 3-indolylquinones, mainly bis(indolyl) quinone, are a significant structural unit in many natural products because of their numerous biological activities.2 Among these indolylquinones, asterriquinones show the most potential as pharmacophores, and could be used in a wide range of biological activities, including the inhibition of human immunodeficiency virus reverse transcriptase3 (Fig. 1). These asterriquinones mostly possess a symmetrical benzoquinone unit containing two oppositely N1-prenylated tryptophanyl moieties which are isolated from Aspergillus terreus, Chaetomium sp., and Pseudomassaria sp.4 It is worth mentioning demethylasterriquinone B1, described as a non-peptidyl mimetic of insulin with excellent antidiabetic activity in recent studies.5For the synthesis of 3-indolylquinones, in the past, several approaches have been reported from indolic starting materials as efficient methodologies. For instance, in 1911, Mohlau et al. described this for the first time using the reaction of benzoquinone and indole, however the product was not isolated.6 Later, Bu’Lock reinvestigated and isolated 3-indolylquinones with a very low yield.7 Subsequently, various catalytic routes were developed in this scenario. Yadav et al.8 reported these products from indoles and benzoquinones using various Lewis acids such as InBr3 and Bi(OTf)3 under mild reaction conditions. Likewise, Park and co-workers synthesized 3-indolylquinones with indoles and benzoquinones using mineral acids like Zn(OTf)2 and mercury reagents along with Pd(II)/Cu(OAc)2.9 In addition, 3-indolylquinones were also prepared using ultrasound activation between the indole and quinone using molecular iodine as a catalyst.10 Very recently, Kamble et al.11 developed 3-indolylquinones using the in situ oxidation of hydroquinone followed by C–C bonding with indole using a combination of Ag2O in H2O2 and an Fe3O4/PVP–PWA catalytic system. These are all reported in the literature, mainly from indolic starting materials. Therefore, it is highly necessary to develop an efficient catalytic and high yielding tandem protocol that allows the quick preparation of 3-indolylquinones from simple 2-alkynylanilines and benzoquinone as raw materials in the presence of copper cyanide as a catalyst (Scheme 1).
Interestingly, the transition metal-catalyzed tandem one-pot annulation of o-alkynylanilines followed by in situ trapping with suitable electrophiles has become an extremely useful protocol for the construction of 2,3-difunctionalized indoles.12 Previously, we reported an efficient silver catalyzed13 domino process for the synthesis of 2,3-disubstituted indoles from alkyne imino ethers. Furthermore, the copper catalysed three-component synthesis of 2-[(2-alkyl-1H-indol-3-yl)methylene]malonates from o-alkynylanilines, triethyl orthoformate and diethyl malonoate followed by Sc-catalyzed intramolecular Friedel–Crafts reactions to the corresponding heterocyclic compounds was also reported.14 Based on these studies, we believe that it is possible to access 3-indolylquinones using the same protocol with benzoquinone as a good electrophile. Despite considerable progress observed in the synthesis of 3-indolylquinones, nobody has reported on the transition-metal-catalyzed heterocyclization reaction between 2-alkyanilines and quinones. Nevertheless, this combination constitutes an interesting class of 3-quinone-substituted indole scaffolds.
However, the cyclization of 2-alkynylanilines followed by functionalization at the C3-position of free (N–H) indoles is a highly complex step because it often suffers from competing reactions of amines15 and alkynes with benzoquinones which are predominantly expected to form the addition products of benzoquinone and amines under acidic conditions. However, the present study primarily exploits indole formation instead of side reactions with benzoquinone and this concept is reported for the first time in the presence of benzoquinone.
Results and discussion
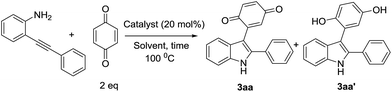
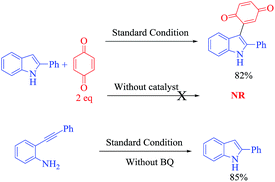
We commenced optimization studies using readily available o-alkynylaniline 1a and benzoquinone 2a (1 eq.) as coupling partners. The tandem heterocyclization reaction was performed in the presence of 20 mol% CuCN as a Lewis acid catalyst at a temperature of 100 °C in DMF under an air atmosphere. Pleasingly, a new class of 3-indolyl quinone (3aa, 20%) and 3-indolyl hydroquinone (3aa′, 55%) were obtained as a mixture of products with moderate yield, after 12 h (entry 1, Table 1). Pleasantly, when the same reaction was conducted with 1.5 equivalents of 2a, surprisingly, the yield of 3aa was 52% and that of 3aa′ was 30%. These products (3aa & 3aa′) can be distinguished by proton & carbon NMR spectroscopy: 3aa shows two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signals in the carbon NMR spectrum but for 3aa′, the corresponding C
O signals in the carbon NMR spectrum but for 3aa′, the corresponding C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks disappear.
O peaks disappear.
| Entry | Catalyst | Solvent | Time [h] | Yieldb% 3aa +3aa′ |
|---|---|---|---|---|
| a All the reactions were performed with 1a (38.6 mg, 0.2 mmol), 2a (2 equiv.) at 100 °C under air.b All the yields are isolated yields.c Reaction with 1 eq. of 2a.d Reaction with 1.5 equiv. of 2a.e Reaction with 2 mol% gold catalyst.f Reaction with 5 mol% palladium catalyst.g ND denotes not determined. | ||||
| 1 | CuCN | DMF | 12 | (20 + 55)c |
| 2 | CuCN | DMF | 12 | (52 + 30)d |
| 3 | CuCN | DMF | 12 | 80 + <5 |
| 4 | CuI | DMF | 24 | 64 + trace |
| 5 | CuCl | DMF | 24 | 68 + trace |
| 6 | Cu(OTf)2 | DMF | 24 | <5 |
| 7 | Cu(NO2)3 | DMF | 24 | NDg |
| 8 | Cu(OAc)2·H2O | DMF | 24 | 58 |
| 9 | Cu(OAc)2 | DMF | 24 | 60 |
| 10 | InCl3 | DMF | 24 | NDg |
| 11 | NaAuCl4·3H2O | DMF | 24 | 57e |
| 12 | Pd(OAc)2 | DMF | 24 | 60f |
| 13 | CuCN | DCE | 24 | NDg |
| 14 | CuCN | DMSO | 24 | <5 |
| 15 | CuCN | Toluene | 24 | NDg |
| 16 | CuCN | H2O | 24 | NDg |
| 17 | CuCN | MeOH | 24 | <5 |
| 18 | CuCN | ACN | 24 | NDg |
| 19 | Iodine | DMF | 24 | 25 |
| 20 | PTSA | DMF | 24 | NDg |
| 21 | NaAuCl4·3H2O | DCM | 12 | 78e |
| 22 | Pd(OAc)2 | DCM | 12 | 65f |
From this study, we observed that formed 3-indolylhydroquinone undergoes oxidation in the presence of excess benzoquinone. Hence, in the same reaction repeated with 2eq. of 2a, the yield of 3aa significantly improved to 80% with a trace amount of 3aa′ (entry 3). These results prompted us to examine various Lewis-acid promoted tandem heterocyclization reactions in more detail. In this context, a variety of transition-metal salts such as CuI, CuCl, Cu(OTf)2, Cu(NO2)3, Cu(OAc)2, Cu(OAc)2·H2O, InCl3, NaAuCl4·3H2O and Pd(OAc)2, as well-known alkyne bond activators, were tested for this tandem cyclization process as shown in Table 1 (entries 4–12) using DMF as a solvent. Among them, CuCN is observed as the best catalyst to form the desired product with 80%, yield (entry 3). The obtained results from screening reactions of 2-alkynylaniline (1a) and benzoquinone (2a) with common organic solvents such as toluene, DCE, MeCN, THF, DMSO and MeOH revealed that none of the solvents (except DMF) could promote the tandem process significantly (entries 13–18, Table 1). However, NaAuCl4·3H2O (2 mol%) and Pd(OAc)2 (5 mol%) afford 72% and 60% yields, respectively, at room temperature in 12 h, with DCM as the solvent (entry 21, 22, Table 1). After optimization of the reaction conditions, we established an efficient route to the formation of 3-indolylquinones. The optimal reaction conditions are 1a (0.2 mmol) and 2a (0.4 mmol) as the reactants with CuCN (0.04 mmol) and DMF (2 mL) at 100 °C for 12 h.
With the optimized reaction conditions in hand, we next explored the efficiency and generality of this tandem heterocyclization process using the reactions of several 2-(aryl/alkylethynyl) aniline derivatives with various quinones using CuCN as an efficient Lewis acid catalyst. These results are summarized in Table 2. First, various 2-alkyl-substituted (phenyl, ethyl benzene, cyclohexyl, hydroxyl alkane, biphenyl, benzyl) ethynylanilines (1a–f) were reacted efficiently with benzoquinone to give the desired products 3aa–3fa in good to excellent yields (52–90%). Contrarily, N-acylated 2-alkynyl aniline (2j) does not undergo the domino cyclization due to the low efficiency of N-acyl amine donation of a loan pair of electrons to the pi activated electron deficient alkyne. Halogen substitute alkynyl anilines like chlorine and fluorine have little influence under optimized reaction conditions on the formation of the corresponding products 3ga and 3ha in moderate yields (59% & 63%). Both alkyl and aryl substituted ethynylaniline (1i) produce 3ia in 82% yield. In the case of lower yields of the final compounds, the annulated indole is the main by-product along with trace amounts of the 3-indolyl hydroquinone.
| a The reaction was carried out with 1 (0.2 mmol), 2 (0.4 mmol) and CuCN (20 mol%) in DMF (2 mL) at 100 °C, 12 h. |
|---|
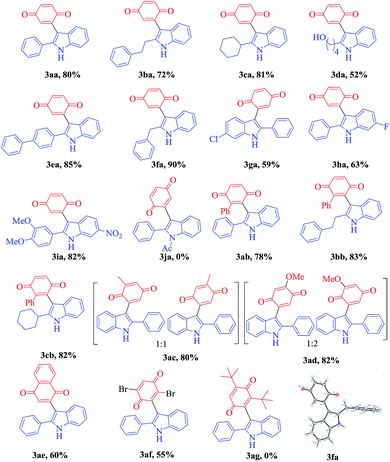 |
To further investigate the substrate scope, we turned our attention to examine various unsymmetrical benzoquinones such as methyl, phenyl and methoxy benzoquinone (2b–2e) and symmetrical benzoquinones such as 2,6-ditertiary butyl and 2,5-dibromo benzoquinone (2g and 2h). The tandem coupling of 2-alkynyl aniline with aliphatic substituted unsymmetrical benzoquinones (methyl, methoxy) affords a mixture of isomers with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 3ac and 3ad in 80% and 82% yields, respectively. However, phenyl substituted unsymmetrical benzoquinone (2b) under the present reaction conditions exclusively gives the regio selective and more substituted side coupling product as shown in Table 2. Thus, these unprecedented results motivated us to work on different 2-alkynyl anilines with 2-phenyl benzoquinones under the present conditions and good to excellent yields were attained (3ab–3cb) and the results are shown in Table 2. Additionally, reactions with 1,4-naphthoquinone show moderate yields (3ae, 60%). Furthermore, the structure of compound 3fa was unambiguously confirmed by X-ray analysis (CCDC 1844842†).
1 ratio of 3ac and 3ad in 80% and 82% yields, respectively. However, phenyl substituted unsymmetrical benzoquinone (2b) under the present reaction conditions exclusively gives the regio selective and more substituted side coupling product as shown in Table 2. Thus, these unprecedented results motivated us to work on different 2-alkynyl anilines with 2-phenyl benzoquinones under the present conditions and good to excellent yields were attained (3ab–3cb) and the results are shown in Table 2. Additionally, reactions with 1,4-naphthoquinone show moderate yields (3ae, 60%). Furthermore, the structure of compound 3fa was unambiguously confirmed by X-ray analysis (CCDC 1844842†).
However, due to the highly bulky nature of 2,6-ditertiary butyl substituted symmetrical benzoquinones (2ag), they produce only the indole derivative without undergoing C–C bond formation. On the other hand, 2,5-dibromo benzoquinone achieves a 55% yield of 3af as shown in Table 2, along with the intermediate indole.
At this stage, to gain an understanding of the detailed mechanism of this reaction, several control experiments were carried out, as demonstrated in Scheme 2. Initially, we believed that the reaction proceeds via a 2-substituted indole derivative (through an aminocuprate step), which then adds to benzoquinone in a 1,4-fashion to generate the 3-indolyl hydroquinones followed by oxidation. To get a better understanding, a couple of control experiments were performed in which 2-phenylindole (1 equiv.) was treated with benzoquinone (2 equiv.) in the presence or absence of the Cu catalyst at 100 °C in DMF. In the presence of the Cu catalyst, the product 3aa is obtained in 82% quantitative yield after 12 h whereas in the absence of the Cu catalyst, the present reaction does not proceed and no products are observed even after 24 h in DMF at 100 °C.
In addition, we also investigated the standard reaction conditions in the absence of benzoquinone and surprisingly, a quantitative amount of anticipated products formed after 24 h.
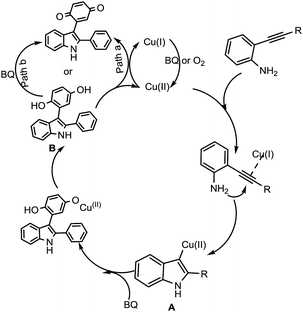
Based on these control experiments, a plausible mechanism for the formation of 3-indolyl quinones was proposed as shown in Scheme 3. At first, copper(I) gets oxidised in the presence of oxygen or benzoquinone to form Cu(II), which reacts with 2-alkynyl anilines to afford a vinyl cuprate intermediate (A). This subsequently undergoes a 1,2-migratory reaction with benzoquinone, which inserts into the C–Cu bond to produce the intermediate 3-indolyl hydroquinone (B).
The formed intermediate 3-indolyl hydroquinone (B) has two possible ways to get oxidised to the final product, 3-indolyl quinone; path B,8b which involves oxidation in the presence of excess benzoquinone, or path A,16 where Cu(II) is reduced to Cu(I) in the presence of 3-indolyl hydroquinone, finally to access the desired product 3aa.
Conclusions
In summary, we report a novel catalytic method for the synthesis of 3-indolylquinones from 2-ethynylanilines and benzoquinones through a tandem-type cyclization followed by a C–C bond formation sequence. The use of a copper catalyst enabled the process to be performed efficiently under mild reaction conditions. In addition, aromatic unsymmetrical benzoquinones give regio-selective substituted 3-indolylquinones with moderate to good yields. Outstandingly, we are extending this protocol to the construction of other heterocyclic compounds, the details of which will be reported in due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Korean Government, through the Center for New Directions in Organic Synthesis (NRF-2014R1A5A1011165).Notes and references
- (a) R. J. Sundberg, The Chemistry of Indoles, Academic Press, San Diego, 1996 Search PubMed; (b) M. Toyota and N. Ihara, Nat. Prod. Rep., 1998, 15, 327–340 RSC; (c) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef PubMed; (d) M. Bandini and A. Eichholzer, Angew. Chem. Int. Ed., 2009, 48, 9608 (Angew. Chem., 2009, 121, 9786) CrossRef PubMed; (e) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449 RSC; (f) P. S. Bhadury and J. Pang, Curr. Org. Chem., 2014, 18, 2108 CrossRef.
- D. H. Jr, A. Nguyen, A. Harald, P. Hirth, G. McMahon and C. Tang, Org. Lett., 1999, 1, 431 CrossRef.
- (a) Y. Yamamoto, N. Kiriyama, S. Shimizu and S. Koshimura, Jpn. J. Cancer Res., 1976, 67, 623 Search PubMed; (b) K. Ono, H. Nakane, S. Shimizu and S. Koshimura, Biochem. Biophys. Res. Commun., 1991, 174, 56 CrossRef PubMed; (c) A. Fredenhagen, F. Petersen, B. M. Tintelnot, J. Rosel, H. Mett and P. Hug, J. Antibiot., 1997, 50, 395 CrossRef PubMed.
- (a) D. Brewer, W. Jerram and A. Taylor, Can. J. Microbiol., 1968, 14, 861 CrossRef PubMed; (b) S. Sekita, Chem. Pharm. Bull., 1983, 31, 2998 CrossRef; (c) A. Kaji, R. Saito, Y. Hata and N. Kiriyama, Chem. Pharm. Bull., 1999, 47, 77 CrossRef.
- B. Zhang, G. Salituro, D. Szalkowski, Z. Li, Y. Zhang, I. Royo, D. Vilella, M. Diez, F. Pelaez, C. Ruby, R. Kendall, X. Mao, P. Griffin, J. Calaycay, J. Zierath, J. Heck, R. Smith and D. Moller, Science, 1999, 284, 974 CrossRef PubMed.
- R. Mohlau and R. Redlich, Ber. Dtsch. Chem. Ges., 1911, 44, 3605 CrossRef.
- J. BuLock and H. Mason, J. Chem. Soc., 1951, 703 RSC.
- (a) J. S. Yadav, B. V. S. Reddy and T. Swamy, Tetrahedron Lett., 2003, 44, 9121 CrossRef; (b) J. S. Yadav, B. V. S. Reddy and T. Swamy, Synthesis, 2004, 1, 106 Search PubMed.
- (a) M. Pirrung, L. Deng, Z. Li and K. Park, J. Org. Chem., 2002, 67, 8374 CrossRef PubMed; (b) M. C. Pirrung, Y. Liu, L. Deng, D. Halstead, Z. Li, J. May, M. edel, D. Austin and N. Webster, J. Am. Chem. Soc., 2005, 127, 4609 CrossRef PubMed; (c) M. C. Pirrung, K. Fujita and K. Park, J. Org. Chem., 2005, 70, 2537 CrossRef PubMed.
- B. Liu, S. Ji, J. Su and S. Wang, Synth. Commun., 2008, 38, 1279 CrossRef.
- S. B. Kamble, P. V. Praneet, V. J. Radha and V. R. Chandrashekhar, ACS Omega, 2017, 2, 2238 CrossRef.
- (a) Y. Chen, C. H. Cho and R. O. Larock, Org. Lett., 2009, 11, 173 CrossRef PubMed; (b) K. Hiroya, S. Itoh and T. Sakamoto, J. Org. Chem., 2004, 69, 1126 CrossRef PubMed; (c) M. Alfonsi, A. Arcadi, M. Aschi, G. Bianchi and F. Marinelli, J. Org. Chem., 2005, 70, 2265 CrossRef PubMed; (d) I. Ambrogio, A. Arcadi, S. Cacchi, G. Fabrizi and F. Marinelli, Synlett, 2007, 1775 Search PubMed; (e) Y. J. Guo, R. Y. Tang, J. H. Li, P. Zhong and X. G. Zhang, Adv. Synth. Catal., 2009, 351, 2615 CrossRef; (f) N. K. Swamy, A. Yazici and S. G. Pyne, J. Org. Chem., 2010, 75, 3412 CrossRef PubMed; (g) J. P. Brand, C. Chevalley and J. B. Waser, J. Org. Chem., 2011, 7, 565 Search PubMed; (h) B. Gabriele, L. Veltri, R. Mancuso, G. Salerno and M. Costa, Eur. J. Org. Chem., 2012, 2549 CrossRef; (i) C. Xu, V. K. Murugan and S. A. Pullarkat, Org. Biomol. Chem., 2012, 10, 3875 RSC; (j) Q. Wang, L. Huang, X. Wu and H. Jiang, Org. Lett., 2013, 15, 5940 CrossRef PubMed; (k) A. Arcadi, E. Pietropaolo, A. Alvino and V. Michelet, Org. Lett., 2013, 15, 2766 CrossRef PubMed; (l) D. Janreddy, V. Kavala, C. W. Kuo, T. S. Kuo, C. H. He and C. F. Yao, Tetrahedron, 2013, 69, 3323 CrossRef; (m) Z. Hu, S. Luo and Q. Zhu, Adv. Synth. Catal., 2015, 357, 1060 CrossRef.
- C. H. Oh, S. Karmakar, H. Park, Y. Ahn and J. W. Kim, J. Am. Chem. Soc., 2010, 132, 1792 CrossRef PubMed.
- C. H. Oh, H. S. Park, N. Park, S. Y. Kim and L. Piao, Synlett, 2014, 0579 Search PubMed.
- (a) K. T. Vishnu and K. M. Hardesh, Tetrahedron Lett., 2009, 50, 5896 CrossRef; (b) J. S. Yadav, B. V. S. Reddy, T. Swamy and K. S. Shankar, Monatsh. Chem., 2008, 139, 1317 CrossRef; (c) K. A. MacGregor, M. K. Abdel-Hamid, L. R. Odell, N. Chau, A. Whiting, P. J. Robinson and A. Mcluskey, Eur. J. Med. Chem., 2014, 85, 191 CrossRef PubMed.
- X. Yuan, A. N. Pham, C. J. Miller and T. D. Waite, Environ. Sci. Technol., 2013, 47, 8355 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1844842. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra03712d |
| This journal is © The Royal Society of Chemistry 2018 |