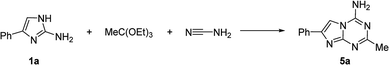Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A one-pot, multicomponent reaction for the synthesis of novel 2-alkyl substituted 4-aminoimidazo[1,2-a][1,3,5]triazines†‡
Felicia Phei Lin Lim a,
Lin Yuing Tan
a,
Lin Yuing Tan a,
Edward R. T. Tiekinkb and
Anton V. Dolzhenko
a,
Edward R. T. Tiekinkb and
Anton V. Dolzhenko *ac
*ac
aSchool of Pharmacy, Monash University Malaysia, Jalan Lagoon Selatan, Bandar Sunway, Selangor Darul Ehsan 47500, Malaysia. E-mail: dolzhenkoav@gmail.com; anton.dolzhenko@monash.edu; Fax: +60-3-5514-6364; Tel: +60-3-5514-5867
bResearch Centre for Crystalline Materials, School of Science and Technology, Sunway University, Bandar Sunway, Selangor Darul Ehsan 47500, Malaysia
cSchool of Pharmacy and Biomedical Sciences, Curtin Health Innovation Research Institute, Faculty of Health Sciences, Curtin University, GPO Box U1987 Perth, Western Australia 6845, Australia
First published on 12th June 2018
Abstract
A highly selective, one-pot, three-component synthesis of novel 2-alkyl-substituted 4-aminoimidazo[1,2-a][1,3,5]triazines has been developed. The scope of the method was explored in two dimensions, varying the structures of trialkyl orthoesters and 2-aminoimidazoles in their reactions with cyanamide. Conveniently performed under microwave irradiation, this method was also proved to be efficient under conventional heating. A library of 24 novel compounds was prepared in high purity using this multicomponent approach. Molecular and crystal structures of representative molecules were studied using X-ray crystallography.
Introduction
The imidazo[1,2-a][1,3,5]triazine ring system is recognised as an important scaffold in medicinal chemistry.2 The well-defined targets for imidazo[1,2-a][1,3,5]triazines include a variety of enzymes, such as focal adhesion kinase,3 activated Cdc42-associated tyrosine kinase 1,4 dipeptidyl peptidase IV,5 and phosphodiesterase 5.6 This scaffold has been successfully used for the design of ligands for μ-opioid7 and A1 adenosine8 receptors. The pharmacological potential of imidazo[1,2-a][1,3,5]triazines as anti-cancer,9 anti-viral10 and anti-diabetic11 agents has also been well documented in the literature. The broad spectrum of biological activities of imidazo[1,2-a][1,3,5]triazines results in ongoing demand for the development of new practical methods for the synthesis of diversely functionalized imidazo[1,2-a][1,3,5]triazines.Since the first report12 on the synthesis of the imidazo[1,2-a][1,3,5]triazine ring system 50 years ago, a number of methods for the preparation of compounds with this heterocyclic core have been reported. They typically involve triazine ring construction onto substituted imidazoles13 or, more often, the annulation of the imidazole ring onto substituted 1,3,5-triazines.14 The two most common approaches utilize: (1) reactions of 2-amino-1,3,5-triazines with α-haloaldehydes/ketones3,8,12,14a,b or chalcones,14c and (2) intramolecular cyclisations of 2-amino-1,3,5-triazines substituted at the amino group.11,14d–f Reports on the preparation of imidazo[1,2-a][1,3,5]triazines via the formation of the 1,3,5-triazine ring onto imidazoles are limited and usually utilize addition of 2-aminoimidazoles to N-acyliso(thio)cyanates followed by the intramolecular closure of the 1,3,5-triazine ring.13
Multicomponent reactions (MCRs) represent an efficient strategy in modern synthetic chemistry.15 Minimising the number of synthetic steps in obtaining targeted compounds from available starting reagents is highly desirable in the fields of organic synthesis and drug discovery. Due to their diversity and combinatorial potential, MCRs have become highly appreciated tools for the preparation of libraries of compounds for biological screening.16 However, issues surrounding chemo- and regio-selectivity of MCRs often pose a challenge and require fine-tuning during method development. The need for rapid and selective construction of biologically active compounds for drug discovery has stimulated intense development in the use of microwave technology. Microwave irradiation has been effectively used as an alternative source of energy, improving efficiency and selectivity of MCRs.1,17,18
We have been exploring the potential for new one-pot, multicomponent, microwave-assisted protocols for the synthesis of azolo[1,3,5]triazines.18 After the development of the MCRs utilizing aminoazoles, orthoformates and cyanamide, we have been refining the scope of this reaction using a variety of aminoazoles. In particular, the molecular diversity of compounds prepared has been achieved by varying the structures of the aminoazole precursors used in this three-component condensation and included libraries of pyrazolo[1,5-a][1,3,5]triazines,1,18a–c 1,2,4-triazolo[1,5-a][1,3,5]triazines,18d,e and imidazo[1,2-a][1,3,5]triazines.18f Herein, we report our attempts to further expand the scope of this MCR by exploring its capacity to accommodate various orthoesters and therefore, introduce a new point of diversity in the reaction products. 2-Aminoimidazoles were selected as aminoazole substrates for the reaction in order to explore the regio- and chemo-selectivity of the process for these challenging substrates bearing endocyclic nitrogen atoms of similar reactivity.
Results & discussion
Synthesis
The starting materials used in our three-component reaction, viz. 2-aminoimidazoles (1), were prepared using the previously reported method developed by Van der Eycken's group.19 Optimization of the conditions for our proposed MCR was performed using a model reaction of 2-amino-4-phenylimidazole (1a), triethyl orthoacetate, and cyanamide under microwave irradiation (Table 1). The initial attempt to carry out this reaction at 150 °C for 20 min in ethyl acetate led to 4-amino-2-methyl-7-phenylimidazo[1,2-a][1,3,5]triazine (5a) obtained in 42% yield. Despite the moderate yield, the product 5a was isolated in a chromatography-free process as the sole product. We found that the yield could be improved to 60% by performing the reaction at 160 °C for 35 min. However, despite attempts to further increase the yields by manipulating the reaction conditions, no improvement in the outcome was achieved.| Entry | Temperature (°C) | Time (min) | Isolated yield (%) |
|---|---|---|---|
| a The reaction was performed using a Discover SP CEM microwave synthesizer with 1 mmol of 1a, 2.5 mmol of triethyl orthoacetate and 2.5 mmol of cyanamide in 2 mL of the ethyl acetate.b The reaction was performed using 1 mmol of 1a, 3 mmol of triethyl orthoacetate and 3 mmol of cyanamide. | |||
| 1 | 150 | 20 | 42 |
| 2 | 150 | 30 | 47 |
| 3 | 160 | 20 | 47 |
| 4 | 170 | 20 | 41 |
| 5 | 160 | 30 | 51 |
| 6 | 160 | 35 | 60 |
| 7 | 160 | 40 | 47 |
| 8b | 160 | 35 | 45 |
The developed method was tested using two different models of microwave synthesizers: Discover SP (CEM) and Monowave 400 (Anton Paar). The three-component reaction of 1a, triethyl orthoacetate and cyanamide under the optimized conditions was conducted in both systems and resulted in the formation of 5a in 60% and 56% yields, respectively.
Attempts to perform the reaction under reflux in ethyl acetate for 12 h led to the recovery of the starting material 1a without traces of 5a. However, an attempt to carry out the MCR of 1a, triethyl orthoacetate and cyanamide in the Monowave 50 (Anton Paar) reactor using sealed vessels under fast conventional heating imitating the optimised microwave irradiation conditions was successful, resulting in the 54% yield of equally pure 5a. Therefore, we could exclude any significant contribution of non-thermal microwave effects in promoting this MCR.
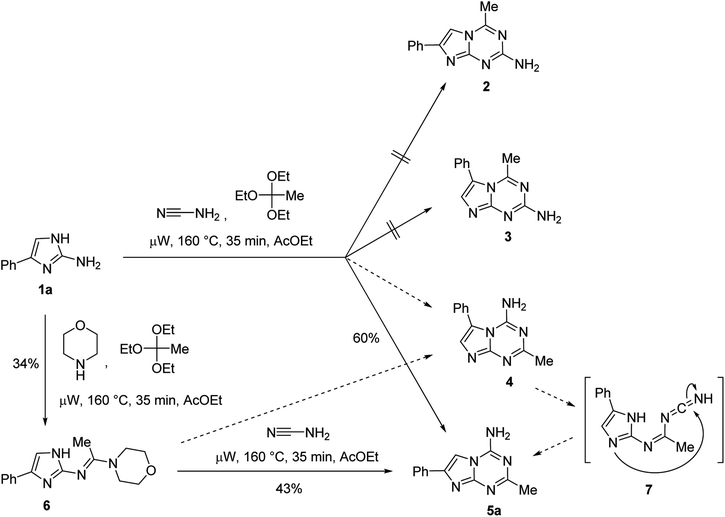
In principle, the annulation of the 1,3,5-triazine ring onto the 2-aminoimidazole 1a in the three-component reaction could proceed with the formation of four regioisomeric structures viz. 2-aminoimidazo[1,2-a][1,3,5]triazines 2 and 3 as well as 4-aminoimidazo[1,2-a][1,3,5]triazines 4 and 5a (Scheme 1). However, only one product was isolated from the MCR. The structure of this product was assigned on the basis of spectral data and an alternative synthesis of 5a adopting our previously reported step-wise synthesis of azolo[1,3,5]triazines via the 1,3,5-triazine ring annulation onto aminoazoles with a predisposed position of the amino group on the triazine ring.20 This method converted aminoazoles into formamidine intermediates via the reaction with orthoformates and morpholine followed by the triazine ring closing reaction of these intermediates with cyanamide. The conversion of 2-amino-4-phenylimidazole (1a) to the corresponding acetamidine (6) was achieved successfully using the reaction with triethyl orthoacetate and morpholine under microwave irradiation. The subsequent reaction of 6 with cyanamide led to the formation of a product identical to the one obtained from the MCR (Scheme 1). While the step-wise method excluded formation of compounds 2 and 3, a ring-closing reaction could still afford compounds 4 and/or 5a. The steric hindrance between the amino group and the phenyl ring in 4 would affect the thermodynamic stability of this potential product, therefore decreasing chances for its formation. Moreover, we could not exclude a rearrangement of 4 into 5a under the reaction conditions. The rearrangement would proceed via intermediate 7 via the mechanism similar to the one reported21 for the rearrangement of another fused heterocycle with the amino-1,3,5-triazine ring. NOESY experiments performed on the product did not revealed any through-space interactions between the phenyl and methyl groups of the compound thus supporting assignment of structure 5a as the MCR product. X-ray crystal structure determinations for analogous compounds 5g and 5p (vide infra) further confirmed the regioselective closure of the triazine ring to the more sterically accessible endocyclic nitrogen of the imidazole.
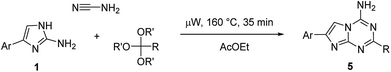
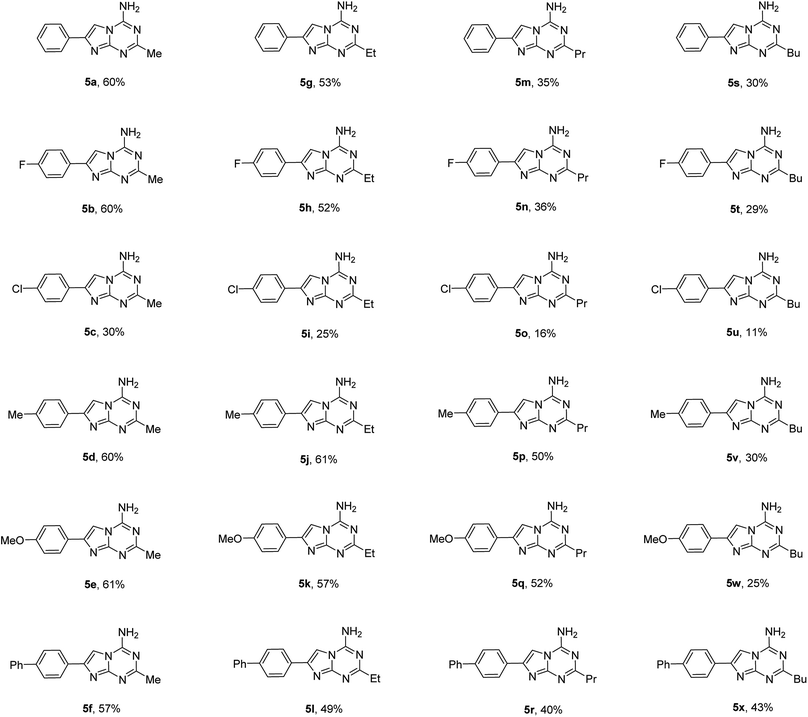
Using the optimized conditions from the model reaction, we explored the scope of our three-component reaction using a variety of trialkyl orthoesters and 4-aryl substituted 2-aminoimidazoles (1). The method was successfully applied for the synthesis of a library of 24 new 2-alkyl-4-amino-7-arylimidazo[1,2-a][1,3,5]triazines (5) (Table 2). The reaction was found to proceed similarly for the full range of tested 2-aminoimidazoles accommodating aryl substituents in the position 4 of the imidazole ring. However, variations in the alkyl orthoester type affected product yields more significantly. In general, an increase in length of alkyl chain substituent on compound 5 led to a decrease of isolated yields. The yields of compounds 5a–f and 5g–l obtained from triethyl orthoacetate and triethyl orthopropionate, respectively, were substantially higher than those of 5m–r and 5s–x prepared using triethyl orthobutyrate and trimethyl orthovalerate. The decrease of yields in the homologous series of compounds 5 could be attributed to the relatively lower reactivity of long chain orthoesters used for their preparation. This assumption found a confirmation in the isolation of compounds 5 exclusively from all the reactions. No traces of other isomers 2–4 were detected. Besides products 5, only unreacted 2-aminoimidazoles (1) could be isolated from the reaction mixtures.
| a The reaction was performed using a Discover SP CEM microwave synthesizer with 1 mmol of 2-aminoimidazoles (1), 2.5 mmol of trialkyl orthoesters and 2.5 mmol of cyanamide at 160 °C for 35 min in 2 mL of ethyl acetate. |
|---|
 |
X-ray crystallography
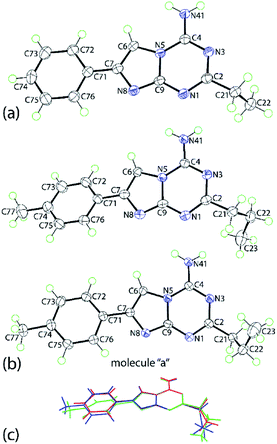
Crystals were obtained for two representative compounds, namely 5g and 5p, and their crystal and molecular structures determined by X-ray crystallography. The molecular structure of 5g is shown in Fig. 1a and comprises an essentially planar nine-membered ring with the r.m.s. deviation of the fitted atoms being 0.0129 Å; the maximum deviations above and below the least-square plane are found for the C2 [0.0215(8) Å] and C9 [0.0232(9) Å] atoms, respectively; the N41 and C71 atoms lie, respectively, 0.0464(13) and 0.0413(13) Å to the same side of the molecule.Indeed, the entire molecule, with the exception of the C2-bound ethyl group, is essentially co-planar as seen in the dihedral between the imidazo[1,2-a][1,3,5]triazine and phenyl rings of 2.31(5)°. The ethyl group has an +anti-clinal (+ac) disposition with respect to the fused-ring system as seen in the torsion angle of 97.41(12)° for N1–C2–C21–C22.
The crystallographic asymmetric of 5p comprises two independent molecules, illustrated in Fig. 1b. The molecules present features that differ from those described for 5g. The r.m.s. of the nine atoms of the fused-ring system is 0.0247 Å [0.0103 Å for the second independent molecule] with the N41 and C71 atoms lying 0.0263(15) and 0.0837(14) Å [0.0333(15) and 0.0113(14) Å] to the same side of the plane. A twist in the molecule is evident as seen in the dihedral angle of 18.78(6)° [10.45(3)°] between the rings. The C2-bound n-propyl groups have very similar conformations with the C2–C21–C22–C23 torsion angles being −68.78(14) and −72.27(13)°, indicating a −syn-clinal (−sc) disposition in each case. The difference is that in the first independent molecule, the terminal methyl group is orientated away from the amino substituent whereas in the second molecule, it is orientated towards the amino group. An overlay diagram of the three molecules of 5g and 5p is shown in Fig. 1c. The difference in the co-planarity of the aromatic residues of 5g and 5p is clearly evident, as are the different orientations of the n-propyl substituents in 5p.
The molecular packing arrangements in the crystals of 5g and 5p feature directional amino-N-H⋯N(ring) hydrogen bonding. In 5g, centrosymmetrically-related molecules are connected via amino-N-H⋯N3(triazine) hydrogen bonds to form eight-membered {⋯HNCN}2 synthons. The second amino-N-H hydrogen atom forms a hydrogen bond to the triazine-N1 atom. The result is a supramolecular layer parallel to (1 0 0) and with a zig-zag topology with the phenyl groups lying to either side, Fig. 2a. The layers stack along the a-axis with the primary interactions between them being of the type π⋯π. The closest such interactions [3.4699(6) Å] occur between imidazo rings, and between phenyl rings [3.8142(7) Å]. A view of the unit cell contents is shown in ESI Fig. S1‡ and geometric parameters characterizing the intermolecular interactions are given in the figure caption.
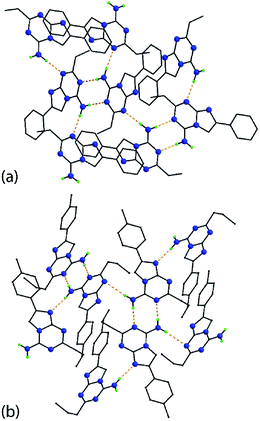 | ||
| Fig. 2 Supramolecular layers sustained by amino-N-H⋯N(ring) hydrogen bonding (see ESI Fig. S1‡), shown as orange dashed lines, in the crystals of (a) 5g and (b) 5p. Non-participating hydrogen atoms have been omitted and the carbon atoms are represented as small spheres. | ||
A supramolecular layer sustained by amino-N-H⋯N(ring) hydrogen bonding is also found in the crystal of 5p. The eight-membered {⋯HNCN}2 synthons arising from amino-N-H⋯N3(triazine) hydrogen bonds is formed by each of the independent molecules which self-associate. For the first independent molecule, rather than an amino-N-H⋯N3(triazine) hydrogen bond as formed by the second independent molecule (and in the crystal of 5g), an amino-N-H⋯N8(imidazo) hydrogen bond is formed instead; the latter pairs of interactions involve both independent molecules. As in 5g, the supramolecular layer, which is approximately parallel to (1 0 1), has a jagged topology with the 4-tolyl rings projecting to either side, Fig. 2b. The connections between layers to consolidate the three-dimensional architecture are of the type π(imidazo)⋯π(imidazo) [3.5695(7) Å] and π(triazine)⋯π(phenyl) [3.8946(7) and 3.9224(7) Å]. A view of the unit cell contents is shown in ESI Fig. S2‡ with geometric parameters given in the chart caption.
Conclusion
In summary, we have successfully developed a three component reaction for the synthesis of novel 2-alkyl substituted 4-aminoimidazo[1,2-a][1,3,5]triazines 5 using easily accessible starting materials and a simple, catalyst- and chromatography-free protocol. The distinct advantage of the proposed approach was found to be high chemo- and regio-selectivity with the exclusive formation of desired products in high purity.Experimental section
General information
Melting points (uncorrected) were determined on a Stuart™ SMP40 automatic melting point apparatus. 1H and 13C NMR spectra were recorded on a Bruker Fourier 300 spectrometer (300 MHz) using DMSO-d6 as a solvent and TMS as an internal reference. Microwave-assisted reactions were carried out in the closed vessel focused single mode using a Discover SP microwave synthesizer (CEM, USA) monitoring reaction temperature by equipped IR sensor. The model reaction was also carried out using Monowave 400 (Anton Paar, Austria) and Monowave 50 (Anton Paar, Austria) reactors.4-Amino-2-methyl-7-phenylimidazo[1,2-a][1,3,5]triazine (5a). Light yellow solid, yield 60%, mp 303–305 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 2.38 (3H, s, CH3), 7.35 (1H, t, 3J = 7.4 Hz, H-4′), 7.47 (2H, t, 3J = 7.5 Hz, H-3′ and H-5′), 7.85 (2H, d, 3J = 7.0 Hz, H-2′ and H-6′), 8.15 (1H, s, H-6), 8.35 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 25.3 (CH3), 101.7 (C-6), 125.2 (C-2′ and C-6′), 127.9 (C-4′), 128.7 (C-3′ and C-5′), 133.3 (C-1′), 143.2 (C-7), 150.3 & 150.6 (C-4 and C-8a), 164.7 (C-2). Anal. Calcd for C12H11N5: C, 63.99; H, 4.92; N, 31.09. Found: C, 63.82; H, 5.08; N, 30.91.
4-Amino-2-methyl-7-(4-fluorophenyl)imidazo[1,2-a][1,3,5]triazine (5b). White solid, yield 60%, mp > 350 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 2.38 (3H, s, CH3), 7.30 (2H, dd, 3JHF = 8.9 Hz, 3JHH = 8.9 Hz, H-3′ and H-5′), 7.87 (2H, dd, 4JHF = 5.5 Hz, 3JHH = 8.9 Hz, H-2′ and H-6′), 8.11 (1H, s, H-6), 8.37 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 25.3 (CH3), 101.5 (C-6), 115.7 (d, 2JCF = 21.7 Hz, C-3′ and C-5′), 127.2 (d, 3JCF = 8.3 Hz, C-2′ and C-6′), 129.8 (d, 4JCF = 2.9 Hz, C-1′), 142.3 (C-7), 150.4 & 150.6 (C-4 and C-8a), 161.9 (d, 1JCF = 244.7 Hz, C-4′), 164.8 (C-2). Anal. Calcd for C12H10FN5: C, 59.25; H, 4.14; N, 28.79. Found: C, 59.02; H, 4.30; N, 28.63.
4-Amino-2-methyl-7-(4-chlorophenyl)imidazo[1,2-a][1,3,5]triazine (5c). Brown solid, yield 30%, mp > 350 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 2.37 (3H, s, CH3), 7.53 (2H, d, 3J = 8.6 Hz, H-3′ and H-5′), 7.9 (2H, d, 3J = 8.58 Hz, H-2′ and H-6′), 8.16 (1H, s, H-6), 8.38 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 25.3 (CH3), 102.1 (C-6), 126.9 (C-2′ and C-6′), 128.8 (C-3′ and C-5′), 132.1 (C-1′), 132.3 (C-4′), 142.1 (C-7), 150.6 & 150.4 (C-4 and C-8a), 164.9 (C-2). Anal. Calcd for C12H10ClN5: C, 55.50; H, 3.88; N, 26.97. Found: 55.42; H, 4.01; N, 26.75.
4-Amino-2-methyl-7-(4-methylphenyl)imidazo[1,2-a][1,3,5]triazine (5d). White solid, yield 60%, mp > 350 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 2.34 (3H, s, CH3), 2.37 (3H, s, CH3), 7.27 (2H, d, 3J = 7.9 Hz, H-3′ and H-5′), 7.73 (2H, d, 3J = 8.1 Hz, H-2′ and H-6′), 8.09 (1H, s, H-6), 8.30 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 20.8 (CH3), 25.3 (CH3), 101.1 (C-6), 125.2 (C-2′ and C-6′), 129.3 (C-3′ and C-5′), 130.5 (C-1′), 137.3 (C-4′), 143.4 (C-7), 150.2 & 150.5 (C-4 and C-8a), 164.5 (C-2). Anal. Calcd for C13H13N5: C, 65.25; H, 5.48; N, 29.27. Found: 65.18; H, 5.60; N, 29.18.
4-Amino-2-methyl-7-(4-methoxyphenyl)imidazo[1,2-a][1,3,5]triazine (5e). Light yellow solid, yield 61%, mp 338–339 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 2.37 (3H, s, CH3), 3.80 (3H, s, OCH3), 7.03 (2H, d, 3J = 8.9 Hz, H-3′ and H-5′), 7.77 (2H, d, 3J = 8.9 Hz, H-2′ and H-6′), 8.02 (1H, s, H-6), 8.29 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 25.3 (CH3), 55.1 (OCH3), 100.4 (C-6), 114.2 (C-3′ and C-5′), 125.8 (C-1′), 126.6 (C-2′ and C-6′), 143.3 (C-7), 150.3 & 150.5 (C-4 and C-8a), 159.2 (C-4′), 164.4 (C-2). Anal. Calcd for C13H13N5O: C, 61.17; H, 5.13; N, 27.43. Found: C, 61.05; H, 5.23; N, 27.32.
4-Amino-2-methyl-7-(4-biphenyl)imidazo[1,2-a][1,3,5]triazine (5f). Light brown solid, yield 57%, mp > 350 °C (DMF). 1H NMR (300 MHz, DMSO-d6): δ 2.38 (3H, s, CH3), 7.38 (1H, t, 3J = 7.3 Hz, H-4′′), 7.49 (2H, t, 3J = 7.5 Hz, H-3′′ and H-5′′), 7.73 (2H, d, 3J = 7.1 Hz, H-2′′ and H-6′′), 7.79 (2H, d, 3J = 8.4 Hz, H-3′ and H-5′), 7.94 (2H, d, 3J = 8.4 Hz, H-2′ and H-6′), 8.20 (1H, s, H-6), 8.36 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 25.3 (CH3), 101.8 (C-6), 125.8 (C-3′ and C-5′), 126.4 (C-3′′ and C-5′′), 127.0 (C-2′ and C-6′), 127.4 (C-4′′), 128.9 (C-2′′ and C-6′′), 132.4 (C-1′), 139.5 (C-4′ and C-1′′), 142.9 (C-7), 150.4 & 150.6 (C-4 and C-8a), 164.7 (C-2). Anal. Calcd for C18H15N5: C, 71.74; H, 5.02; N, 23.24. Found: C, 71.68; H, 5.11; N, 23.10.
4-Amino-2-ethyl-7-phenylimidazo[1,2-a][1,3,5]triazine (5g). Light yellow solid, yield 53%, mp 299–301 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 1.25 (3H, t, 3J = 7.6 Hz, CH3), 2.66 (2H, q, 3J = 7.5 Hz, CH2), 7.35 (1H, t, 3J = 7.4 Hz, H-4′), 7.47 (2H, t, 3J = 7.5 Hz, H-3′ and H-5′), 7.86 (2H, d, 3J = 7.0 Hz, H-2′ and H-6′), 8.16 (1H, s, H-6), 8.36 (2H, br s, NH2); 13C NMR (75 MHz, DMSO-d6): δ 11.7 (CH3), 31.5 (CH2), 101.7 (C-6), 125.2 (C-2′ and C-6′), 127.9 (C-4′), 128.7 (C-3′ and C-5′), 133.3 (C-1′), 143.3 (C-7), 150.4 & 150.8 (C-4 and C-8a), 168.6 (C-2). Anal. Calcd for C13H13N5: C, 65.25; H, 5.48; N, 29.27. Found: C, 65.22; H, 5.52; N, 29.12.
4-Amino-2-ethyl-7-(4-fluorophenyl)imidazo[1,2-a][1,3,5]triazine (5h). White solid, yield 52%, mp 339–340 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 1.25 (3H, t, 3J = 7.6 Hz, CH3), 2.65 (2H, q, 3J = 7.5 Hz, CH2), 7.31 (2H, dd, 3JHF = 8.9 Hz, 3JHH = 8.9 Hz, H-3′ and H-5′), 7.88 (2H, dd, 4JHF = 5.5 Hz, 3JHH = 8.9 Hz, H-2′ and H-6′), 8.12 (1H, s, H-6), 8.35 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 11.7 (CH3), 31.5 (CH2), 101.5 (C-6), 115.7 (d, 2JCF = 21.8 Hz, C-3′ and C-5′), 127.2 (d, 3JCF = 8.3 Hz, C-2′ and C-6′), 129.9 (d, 4JCF = 3.0 Hz, C-1′), 142.4 (C-7), 150.4 & 150.8 (C-4 and C-8a), 161.9 (d, 1JCF = 245.1 Hz, C-4′), 168.7 (C-2). Anal. Calcd for C13H12FN5: C, 60.69; H, 4.70; N, 27.22. Found: C, 60.62; H, 4.83; N, 27.11.
4-Amino-2-ethyl-7-(4-chlorophenyl)imidazo[1,2-a][1,3,5]triazine (5i). Brown solid, yield 25%, mp 340–342 °C (EtOH).1H NMR (300 MHz, DMSO-d6): δ 1.24 (3H, t, 3J = 7.6 Hz, CH3), 2.65 (2H, q, 3J = 7.5 Hz, CH2), 7.53 (2H, d, 3J = 8.7 Hz, H-3′ and H-5′), 7.85 (2H, d, 3J = 8.7 Hz, H-2′ and H-6′), 8.17 (1H, s, H-6), 8.36 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 11.7 (CH3), 31.5 (CH2), 102.1 (C-6), 126.9 (C-2′ and C-6′), 128.8 (C-3′ and C-5′), 132.2 (C-1′), 132.3 (C-4′), 142.1 (C-7), 150.5 & 150.8 (C-4 and C-8a), 168.8 (C-2). Anal. Calcd for C13H12ClN5: C, 57.04; H, 4.42; N, 25.59. Found: C, 56.97; H, 4.50; N, 25.49.
4-Amino-2-ethyl-7-(4-methyphenyl)imidazo[1,2-a][1,3,5]triazine (5j). Light yellow solid, yield 61%, mp > 350 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 1.25 (3H, t, 3J = 7.6 Hz, CH3), 2.34 (3H, s, CH3), 2.65 (2H, q, 3J = 7.5 Hz, CH2), 7.27 (2H, d, 3J = 8.0 Hz, H-3 and H-5), 7.75 (2H, d, 3J = 8.1 Hz, H-2′ and H-6′), 8.10 (1H, s, H-6), 8.31 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 11.7 (CH3), 20.8 (CH3), 31.5 (CH2), 101.2 (C-6), 125.2 (C-2′ and C-6′), 129.3 (C-3′ and C-5′), 130.6 (C-1′), 137.3 (C-4′), 143.5 (C-7), 150.3 & 150.7 (C-4 and C-8a), 168.5 (C-2). Anal. Calcd for C14H15N5: C, 66.38; H, 5.97; N, 27.65. Found: C, 66.33; H, 6.08; N, 27.58.
4-Amino-2-ethyl-7-(4-methoxyphenyl)imidazo[1,2-a][1,3,5]triazine (5k). White solid, yield 57%, mp 328–330 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 1.24 (3H, t, 3J = 7.6 Hz, CH3), 2.64 (2H, q, 3J = 7.6 Hz, CH2), 3.80 (3H, s, OCH3), 7.04 (2H, d, 3J = 8.9 Hz, H-3′ and H-5′), 7.78 (2H, d, 3J = 8.9 Hz, H-2′ and H-6′), 8.02 (1H, s, H-6), 8.29 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 11.7 (CH3), 31.4 (CH2), 100.4 (C-6), 114.2 (C-2′ and C-6′), 125.9 (C-4′), 126.6 (C-3′ and C-5′), 143.4 (C-7), 150.3 & 150.6 (C-4 and C-8a), 159.2 (C-1′), 168.3 (C-2). Anal. Calcd for C14H15N5O: C, 62.44; H, 5.61; N, 26.01. Found: C, 62.38; H, 5.74; N, 25.90.
4-Amino-2-ethyl-7-(4-biphenyl)imidazo[1,2-a][1,3,5]triazine (5l). White solid, yield 49%, mp 348–350 °C (DMF).1H NMR (300 MHz, DMSO-d6): δ 1.26 (3H, t, 3J = 7.6 Hz, CH3), 2.66 (2H, q, 3J = 7.6 Hz CH2), 7.38 (1H, t, 3J = 7.3 Hz, H-4′′), 7.49 (2H, t, 3J = 7.5 Hz, H-3′′ and H-5′′), 7.73 (2H, d, 3J = 7.2 Hz, H-2′′ and H-6′′), 7.79 (2H, d, 3J = 8.4 Hz, H-3′ and H-5′), 7.95 (2H, d, 3J = 8.3 Hz, H-2′ and H-6′), 8.21 (1H, s, H-6), 8.36 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 11.7 (CH3), 31.5 (CH2), 101.9 (C-6), 125.8 (C-3′ and C-5′), 126.4 (C-3′′and C-5′′), 127.0 (C-2′ and C-6′), 127.4 (C-4′′), 128.9 (C-2′′ and C-6′′), 132.4 (C-1′), 139.5 & 139.6 (C-4′ and C-1′′), 143.0 (C-7), 150.5 & 150.8 (C-4 and C-8a), 168.7 (C-2). Anal. Calcd for C19H17N5: C, 72.36; H, 5.43; N, 22.21. Found: C, 72.31; H, 5.52; N, 22.14.
4-Amino-2-propyl-7-phenylimidazo[1,2-a][1,3,5]triazine (5m). Light yellow solid, yield 35%, mp 272–274 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 0.94 (3H, t, 3J = 7.4 Hz, CH3), 1.77 (2H, m, 3J = 7.4 Hz, CH2), 2.60 (2H, t, 3J = 7.4 Hz, CH2), 7.35 (1H, t, 3J = 7.3 Hz, H-4′), 7.47 (2H, t, 3J = 7.5 Hz, H-3′ and H-5′), 7.85 (2H, d, 3J = 7.1 Hz, H-2′ and H-6′), 8.15 (1H, s, H-6), 8.35 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.6 (CH3), 20.4 (CH2), 40.2 (CH2), 101.7 (C-6), 125.2 (C-2′ and C-6′), 127.9 (C-4′), 128.7 (C-3′ and C-5′), 133.3 (C-1′), 143.3 (C-7), 150.3 & 150.8 (C-4 and C-8a), 167.6 (C-2). Anal. Calcd for C14H15N5: C, 66.38; H, 5.97; N, 27.65. Found: C, 66.31; H, 6.02; N, 27.60.
4-Amino-2-propyl-7-(4-fluorophenyl)imidazo[1,2-a][1,3,5]triazine (5n). White solid, yield 36%, mp 290–292 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 0.94 (3H, t, 3J = 7.4 Hz, CH3), 1.76 (2H, m, 3J = 7.4 Hz, CH2), 2.60 (2H, t, 3J = 7.4 Hz, CH2), 7.31 (2H, dd, 3JHF = 8.9 Hz, 3JHH = 8.9 Hz, H-3′ and H-5′), 7.87 (2H, dd, 4JHF = 5.5 Hz, 3JHH = 8.9 Hz, H-2′ and H-6′), 8.11 (1H, s, H-6), 8.34 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.6 (CH3), 20.4 (CH2), 40.2 (CH2), 101.5 (C-6), 115.7 (d, 2JCF = 21.7 Hz, C-3′ and C-5′), 127.2 (d, 3JCF = 8.3 Hz, C-2′ and C-6′), 129.9 (d, 4JCF = 3.5 Hz, C-1′), 142.4 (C-7), 150.4 & 150.8 (C-4 and C-8a), 161.9 (d, 1JCF = 244.5 Hz, C-4′), 167.7 (C-2). Anal. Calcd for C14H14FN5: C, 61.98; H, 5.20; N, 25.81. Found: C, 61.92; H, 5.29; N, 25.72.
4-Amino-2-propyl-7-(4-chlorophenyl)imidazo[1,2-a][1,3,5]triazine (5o). Light brown solid, yield 16%, mp 294–295 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 0.94 (3H, t, 3J = 7.4 Hz, CH3), 1.76 (2H, m, 3J = 7.4 Hz, CH2), 2.60 (2H, t, 3J = 7.4 Hz, CH2), 7.53 (2H, d, 3J = 8.7 Hz, H-3′ and H-5′), 7.85 (2H, d, 3J = 8.9 Hz, H-2′ and H-6′), 8.17 (1H, s, H-6), 8.36 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.6 (CH3), 20.4 (CH2), 40.2 (CH2), 102.1 (C-6), 126.9 (C-2′ and C-6′), 128.8 (C-3′ and C-5′), 132.2 (C-1′), 132.3 (C-4′), 142.1 (C-7), 150.4 & 150.8 (C-4 and C-8a), 167.8 (C-2). Anal. Calcd for C14H14ClN5: C, 58.44; H, 4.90; N, 24.34. Found: C, 58.37; H, 5.03; N, 24.22.
4-Amino-2-propyl-7-(4-methyphenyl)imidazo[1,2-a][1,3,5]triazine (5p). Light yellow solid, yield 50%, mp 305–307 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 0.94 (3H, t, 3J = 7.4 Hz, CH3), 1.77 (2H, m, 3J = 7.4 Hz, CH2), 2.34 (3H, s, CH3), 2.60 (2H, t, 3J = 7.4 Hz, CH2), 7.27 (2H, d, 3J = 7.3 Hz, H-3′ and H-5′), 7.75 (2H, d, 3J = 8.0 Hz, H-2′ and H-6′), 8.10 (1H, s, H-6), 8.30 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.6 (CH3), 20.4 (CH2), 20.8 (CH3), 40.2 (CH2), 101.2 (C-6), 125.2 (C-3′ and C-5′), 129.3 (C-2′ and C-6′), 130.6 (C-1′), 137.3 (C-4′), 143.5 (C-7), 150.3 & 150.7 (C-4 and C-8a), 167.5 (C-2). Anal. Calcd for C15H17N5: C, 67.39; H, 6.41; N, 26.20. Found: C, 67.32; H, 6.53; N, 26.06.
4-Amino-2-propyl-7-(4-methoxyphenyl)imidazo[1,2-a][1,3,5]triazine (5q). White solid, yield 52%, mp 295–297 °C (MeOH). 1H NMR (300 MHz, DMSO-d6): δ 0.94 (3H, t, 3J = 7.4 Hz, CH3), 1.76 (2H, m, 3J = 7.4 Hz, CH2), 2.59 (2H, t, 3J = 7.4 Hz, CH2), 3.80 (3H, s, OCH3), 7.04 (2H, d, 3 J = 8.9 Hz, H-3′ and H-5′), 7.78 (2H, d, 3J = 8.9 Hz, H-2′ and H-6′), 8.03 (1H, s, H-6), 8.30 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.6 (CH3), 20.4 (CH2), 40.2 (CH2), 55.1 (OCH3), 100.4 (C-6), 114.2 (C-3′ and C-5′), 125.9 (C-1′), 126.6 (C-2′ and C-6′), 143.4 (C-7), 150.3 & 150.6 (C-4 and C-8a), 159.2 (C-4′), 167.3 (C-2). Anal. Calcd for C15H17N5O: C, 63.59; H, 6.05; N, 24.72. Found: C, 63.52; H, 6.20; N, 24.59.
4-Amino-2-propyl-7-(4-biphenyl)imidazo[1,2-a][1,3,5]triazine (5r). Light brown solid, yield 40%, mp 337–338 °C (DMF). 1H NMR (300 MHz, DMSO-d6): δ 0.95 (3H, t, 3J = 7.4 Hz, CH3), 1.78 (2H, m, 3J = 7.4 Hz, CH2), 2.62 (2H, t, 3J = 7.4 Hz, CH2), 7.38 (1H, t, 3J = 7.3 Hz, H-4′′), 7.49 (2H, t, 3J = 7.5 Hz, H-3′′ and H-5′′), 7.73 (2H, d, 3J = 7.2 Hz, H-2′′ and H-6′′), 7.79 (2H, d, 3J = 8.4 Hz, H-3′ and H-5′), 7.95 (2H, d, 3J = 8.3 Hz, H-2′ and H-6′), 8.21 (1H, s, H-6), 8.36 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.7 (CH3), 20.4 (CH2), 40.2 (CH2), 101.9 (C-6), 125.8 (C-3′ and C-5′), 126.4 (C-3′′ and C-5′′), 127.0 (C-2′ and C-6′), 127.4 (C-4′′), 128.9 (C-2′′ and C-6′′), 132.4 (C-1′), 139.5 & 139.6 (C-4′ and C-1′′), 143.0 (C-7), 150.4 & 150.8 (C-4 and C-8a), 167.7 (C-2). Anal. Calcd for C20H19N5: C, 72.92; H, 5.81; N, 21.26. Found: C, 72.88; H, 5.94; N, 21.13.
4-Amino-2-butyl-7-phenylimidazo[1,2-a][1,3,5]triazine (5s). Light yellow solid, yield 30%, mp 267–269 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 0.92 (3H, t, 3J = 7.3 Hz, CH3), 1.36 (2H, m, 3J = 7.4 Hz, CH2), 1.72 (2H, m, 3J = 7.5 Hz, CH2), 2.62 (2H, t, 3J = 7.5 Hz, CH2), 7.35 (1H, t, 3J = 7.3 Hz, H-4′), 7.47 (2H, t, 3J = 7.5 Hz, H-3′ and H-5′), 7.85 (2H, d, 3J = 7.0 Hz, H-2′ and H-6′), 8.15 (1H, s, H-6), 8.35 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.7 (CH3), 21.7 (CH2), 29.3 (CH2), 37.9 (CH2), 101.7 (C-6), 125.2 (C-2′ and C-6′), 127.9 (C-4′), 128.7 (C-3′ and C-5′), 133.3 (C-1′), 143.3 (C-7), 150.3 & 150.8 (C-4 and C-8a), 167.8 (C-2). Anal. Calcd for C15H17N5: C, 67.39; H, 6.41; N, 26.20. Found: C, 67.32; H, 6.55; N, 26.04.
4-Amino-2-butyl-7-(4-fluorophenyl)imidazo[1,2-a][1,3,5]triazine (5t). White solid, yield 29%, mp 296–298 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 0.92 (3H, t, 3J = 7.3 Hz, CH3), 1.36 (2H, m, 3J = 7.4 Hz, CH2), 1.72 (2H, m, 3J = 7.5 Hz, CH2), 2.62 (2H, t, 3J = 7.5 Hz, CH2), 7.31 (2H, dd, 3JHF = 8.9 Hz, 3JHH = 8.9 Hz, H-3′ and H-5′), 7.88 (2H, dd, 4JHF = 5.6 Hz, 3JHH = 8.9 Hz, H-2′ and H-6′), 8.12 (1H, s, H-6), 8.34 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.8 (CH3), 21.7 (CH2), 29.3 (CH2), 37.9 (CH2), 101.5 (C-6), 115.7 (d, 2JCF = 21.7 Hz, C-3′ and C-5′), 127.2 (d, 3JCF = 8.3 Hz, C-2′ and C-6′), 129.9 (d, 4JCF = 3.0 Hz, C-1′), 142.4 (C-7), 150.4 & 150.8 (C-4 and C-8a), 161.9 (d, 1JCF = 244.8 Hz, C-4′), 167.9 (C-2). Anal. Calcd for C15H16FN5: C, 63.14; H, 5.65; N, 24.55. Found: C, 63.05; H, 5.77; N, 24.42.
4-Amino-2-butyl-7-(4-chlorophenyl)imidazo[1,2-a][1,3,5]triazine (5u). Light brown solid, yield 11%, mp 287–288 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 0.91 (3H, t, 3J = 7.3 Hz, CH3), 1.35 (2H, m, 3J = 7.4 Hz, CH2), 1.72 (2H, m, 3J = 7.5 Hz, CH2), 2.62 (2H, t, 3J = 7.5 Hz, CH2), 7.53 (2H, d, 3J = 8.6 Hz, H-3′ and H-5′), 7.84 (2H, d, 3J = 8.6 Hz, H-2′ and H-6′), 8.16 (1H, s, H-6), 8.36 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.7 (CH3), 21.7 (CH2), 29.3 (CH2), 37.9 (CH2), 102.1 (C-6), 126.9 (C-2′ and C-6′), 128.8 (C-3′ and C-5′), 132.2 & 132.33 (C-1′ and C-4′), 142.1 (C-7), 150.4 & 150.8 (C-4 and C-8a), 168.1 (C-2). Anal. Calcd for C15H16ClN5: C, 59.70; H, 5.34; N, 23.21. Found: C, 59.63; H, 5.41; N, 23.15.
4-Amino-2-butyl-7-(4-methylphenyl)imidazo[1,2-a][1,3,5]triazine (5v). Light yellow solid, yield 25%, mp 287–289 °C (EtOH). 1H NMR (300 MHz, DMSO-d6): δ 0.92 (3H, t, 3J = 7.3 Hz, CH3), 1.36 (2H, m, 3J = 7.4 Hz, CH2), 1.72 (2H, m, 3J = 7.5 Hz, CH2), 2.34 (3H, s, CH3), 2.62 (2H, t, 3J = 7.5 Hz, CH2), 7.27 (2H, d, 3J = 7.9 Hz, H-3′ and H-5′), 7.74 (2H, d, 3J = 8.1 Hz, H-2′ and H-6′), 8.09 (1H, s, H-6), 8.30 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.7 (CH3), 20.8 (CH3), 21.7 (CH2), 29.3 (CH2), 37.9 (CH2), 101.2 (C-6), 125.2 (C-2′ and C-6′), 129.3 (C-3′ and C-5′), 130.5 (C-1′), 137.3 (C-4′), 143.5 (C-7), 150.3 & 150.7 (C-4 and C-8a), 167.7 (C-2). Anal. Calcd for C16H19N5: C, 68.30; H, 6.81; N, 24.89. Found: C, 68.24; H, 6.90; N, 24.81.
4-Amino-2-butyl-7-(4-methoxyphenyl)imidazo[1,2-a][1,3,5]triazine (5w). Light yellow solid, yield 25%, mp 279–281 °C (MeOH). 1H NMR (300 MHz, DMSO-d6): δ 0.92 (3H, t, 3J = 7.3 Hz, CH3), 1.36 (2H, m, 3J = 7.4 Hz, CH2), 1.72 (2H, m, 3J = 7.5 Hz, CH2), 2.62 (2H, t, 3J = 7.5 Hz, CH2), 3.81 (3H, s, OCH3), 7.04 (2H, d, 3J = 8.9 Hz, H-3′ and H-5′), 7.78 (2H, d, 3J = 8.8 Hz, H-2′ and H-6′), 8.03 (1H, s, H-6), 8.30 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.8 (CH3), 21.7 (CH2), 29.3 (CH2), 37.9 (CH2), 55.1 (OCH3), 100.4 (C-6), 114.2 (C-3′ and C-5′), 125.9 (C-1′), 126.6 (C-2′ and C-6′), 143.4 (C-7), 150.3 & 150.6 (C-4 and C-8a), 159.2 (C-4′), 167.6 (C-2). Anal. Calcd for C16H19N5O: C, 64.63; H, 6.44; N, 23.55. Found: C, 64.55; H, 6.54; N, 23.43.
4-Amino-2-butyl-7-(4-biphenyl)imidazo[1,2-a][1,3,5]triazine (5x). Light brown solid, yield 43%, mp 327–329 °C (DMF). 1H NMR (300 MHz, DMSO-d6): δ 0.92 (3H, t, 3J = 7.3 Hz, CH3), 1.37 (2H, m, 3J = 7.4 Hz, CH2), 1.73 (2H, m, 3J = 7.5 Hz, CH2), 2.63 (2H, t, 3J = 7.5 Hz, CH2), 7.38 (1H, t, 3J = 7.3 Hz, H-4′′), 7.49 (2H, t, 3J = 7.5 Hz, H-3′′ and H-5′′), 7.73 (2H, d, 3J = 7.5 Hz, H-2′′ and H-6′′), 7.79 (2H, d, 3J = 8.4 Hz, H-3′ and H-5′), 7.94 (2H, d, 3J = 8.4 Hz, H-2′ and H-6′), 8.20 (1H, s, H-6), 8.36 (2H, br s, NH2). 13C NMR (75 MHz, DMSO-d6): δ 13.7 (CH3), 21.7 (CH2), 29.3 (CH2), 37.9 (CH2), 101.8 (C-6), 125.8 (C-3′ and C-5′), 126.4 (C-3′′ and C-5′′), 127.0 (C-2′ and C-6′), 127.4 (C-4′′), 128.9 (C-2′′ and C-6′′), 132.4 (C-1′), 139.5 (C-4′ and C-1′′), 143.0 (C-7), 150.4 & 150.7 (C-4 and C-8a), 167.9 (C-2). Anal. Calcd for C21H21N5: C, 73.44; H, 6.16; N, 20.39. Found: C, 73.38; H, 6.26; N, 20.31.
N′,N′-Morpholino-N-[3(5)-phenylimidazolo-5(3)yl acetamidine (6). A mixture of 2-amino-1H-imidazole 1a (159 mg, 1 mmol) with triethyl orthoacetate (0.46 mL, 2.5 mmol) and morpholine (0.22 mL, 2.5 mmol) in ethyl acetate (2 mL) were irradiated in a 10 mL seamless pressure vial using the Discover SP (CEM) microwave system operating at maximal microwave power up to 150 W at 160 °C for 35 min. After cooling, the precipitate was filtered and recrystallised from EtOH to give pure formamidine 6. Light yellow solid, yield 34%, mp 217–219 °C (EtOH).1H NMR (300 MHz, DMSO-d6): δ 2.28 (3H, s, CH3), 3.55–3.56 (4H, m, (CH2)2), 3.62–3.65 (4H, m, (CH2)2), 7.10 (1H, t, 3J = 7.3 Hz, H-4′), 7.22 (1H, s, CH), 7.29 (2H, t, 3J = 7.7 Hz, H-3′ and H-5′), 7.66 (2H, d, 3J = 6.9 Hz, H-2′ and H-6′), 11.08 & 11.44 (1H, br s, NH). Anal. Calcd for C15H18N4O: C, 66.64; H, 6.71; N, 20.73. Found: C, 66.46; H, 6.94; N, 20.55.
4-Amino-2-methyl-7-phenylimidazo[1,2-a][1,3,5]triazine (5a). A mixture of 6 (270 mg, 1 mmol) with cyanamide (105 mg, 2.5 mmol) in ethyl acetate (2 mL) were irradiated in a 10 mL seamless pressure vial using the Discover SP (CEM) microwave system operating at maximal microwave power up to 150 W at 160 °C for 35 min. After cooling, the precipitate was filtered to obtain a compound, which was identical to 5a prepared using the multicomponent reaction. Yield 43%.
Crystal data for 4-amino-2-ethyl-7-phenylimidazo[1,2-a][1,3,5]triazine (5g). M = 239.28, orthorhombic, Pccn, a = 16.6173(2), b = 11.17070(10), c = 12.4074(2) Å, V = 2303.15(5) Å3, Z = 8, Dx = 1.380 g cm−3, F(000) = 1008, μ = 0.709 mm−1, no. reflns meas. = 14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762, no. unique reflns = 2060 (Rint = 0.026), no. reflns with I ≥ 2σ(I) = 1935, no. parameters = 170, R(obs. data) = 0.032, wR2(all data) = 0.085. CCDC deposition number: 1840247.
762, no. unique reflns = 2060 (Rint = 0.026), no. reflns with I ≥ 2σ(I) = 1935, no. parameters = 170, R(obs. data) = 0.032, wR2(all data) = 0.085. CCDC deposition number: 1840247.
Crystal data for 4-amino-2-propyl-7-(4-methyphenyl)imidazo[1,2-a][1,3,5]triazine (5p). M = 267.33, triclinic, P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.7534(2), b = 11.8995(2), c = 12.1124(3) Å, α = 71.918(2), β = 68.962(2), γ = 78.712(2)°, V = 1368.93(5) Å3, Z = 4, Dx = 1.297 g cm−3, F(000) = 568, μ = 0.650 mm−1, no. reflns meas. = 32
, a = 10.7534(2), b = 11.8995(2), c = 12.1124(3) Å, α = 71.918(2), β = 68.962(2), γ = 78.712(2)°, V = 1368.93(5) Å3, Z = 4, Dx = 1.297 g cm−3, F(000) = 568, μ = 0.650 mm−1, no. reflns meas. = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816, no. unique reflns = 4893 (Rint = 0.029), no. reflns with I ≥ 2σ(I) = 4530, no. parameters = 377, R(obs. data) = 0.034, wR2(all data) = 0.092. CCDC deposition number: 1840248.
816, no. unique reflns = 4893 (Rint = 0.029), no. reflns with I ≥ 2σ(I) = 4530, no. parameters = 377, R(obs. data) = 0.034, wR2(all data) = 0.092. CCDC deposition number: 1840248.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Ministry of Higher Education, Malaysia under Fundamental Research Grant Scheme (FRGS). We would like to thank Anton Paar Malaysia Sdn. Bhd. for their technical support. The Research Centre for Crystalline Materials (Sunway University) is thanked for the X-ray intensity data.Notes and references
- F. P. L. Lim, N. R. Halcovitch, E. R. T. Tiekink and A. V. Dolzhenko, Tetrahedron, 2018, 74, 1868–1879 CrossRef
.
- F. P. L. Lim and A. V. Dolzhenko, Eur. J. Med. Chem., 2014, 85, 371–390 CrossRef PubMed
.
- P. Dao, N. Smith, C. Tomkiewicz-Raulet, E. Yen-Pon, M. Camacho-Artacho, D. Lietha, J.-P. Herbeuval, X. Coumoul, C. Garbay and H. Chen, J. Med. Chem., 2015, 58, 237–251 CrossRef PubMed
.
- X. Jiao, D. J. Kopecky, J. Liu, J. Liu, J. C. Jaen, M. G. Cardozo, R. Sharma, N. Walker, H. Wesche, S. Li, E. Farrelly, S.-H. Xiao, Z. Wang and F. Kayser, Bioorg. Med. Chem. Lett., 2012, 22, 6212–6217 CrossRef PubMed
.
- J. Feng, S. L. Gwaltney, J. A. Stafford, M. B. Wallace and Z. Zhang, US Pat., 20060135767, 2006
.
- H. Haning, U. Niewoehner, T. Schenke, T. Lampe, A. Hillisch and E. Bischoff, Bioorg. Med. Chem. Lett., 2005, 15, 3900–3907 CrossRef PubMed
.
-
(a) M. Rzadkowska, E. Szacon, D. Matosiuk, E. Kedzierska and S. Fidecka, Acta Pol. Pharm., 2012, 69, 1270–1275 Search PubMed
; (b) D. Matosiuk, S. Fidecka, L. Antkiewicz-Michaluk, J. Lipkowski, I. Dybala and A. E. Koziol, Eur. J. Med. Chem., 2002, 37, 761–772 CrossRef PubMed
.
- E. Novellino, E. Abignente, B. Cosimelli, G. Greco, M. Iadanza, S. Laneri, A. Lavecchia, M. G. Rimoli, F. Da Settimo, G. Primofiore, D. Tuscano, L. Trincavelli and C. Martini, J. Med. Chem., 2002, 45, 5030–5036 CrossRef PubMed
.
-
(a) F. Saczewski, M. Maruszak and P. J. Bednarski, Arch. Pharm., 2008, 341, 121–125 CrossRef PubMed
; (b) I. Lakomska, B. Golankiewicz, J. Wietrzyk, M. Pelczynska, A. Nasulewicz, A. Opolski, J. Sitkowski, L. Kozerski and E. Szlyk, Inorg. Chim. Acta, 2005, 358, 1911–1917 CrossRef
.
-
(a) D. Dukhan, F. Leroy, J. Peyronnet, E. Bosc, D. Chaves, M. Durka, R. Storer, P. La Colla, F. Seela and G. Gosselin, Nucleosides, Nucleotides Nucleic Acids, 2005, 24, 671–674 CrossRef PubMed
; (b) J. O. Ojwang, S. Ali, D. F. Smee, J. D. Morrey, C. D. Shimasaki and R. W. Sidwell, Antiviral Res., 2005, 68, 49–55 CrossRef PubMed
.
- P. Cui, T. L. Macdonald, M. Chen and J. L. Nadler, Bioorg. Med. Chem. Lett., 2006, 16, 3401–3405 CrossRef PubMed
.
- J. Kobe, B. Stanovnik and M. Tisler, Chem. Commun., 1968, 1456 RSC
.
-
(a) Z. Wang, H. K. Huynh, B. Han, R. Krishnamurthy and A. Eschenmoser, Org. Lett., 2003, 5, 2067–2070 CrossRef PubMed
; (b) P. Rao and S. A. Benner, J. Org. Chem., 2001, 66, 5012–5015 CrossRef PubMed
; (c) E. J. Prisbe, J. P. H. Verheyden and J. G. Moffatt, J. Org. Chem., 1978, 43, 4784–4794 CrossRef
; (d) J. J. Voegel, M. M. Altorfer and S. A. Benner, Helv. Chim. Acta, 1993, 76, 2061–2069 CrossRef
.
-
(a) P. Dao, C. Garbay and H. Chen, Tetrahedron, 2013, 69, 3867–3871 CrossRef
; (b) T. Hanami, H. Oda, A. Nakamura, H. Urata, M. Itoh and Y. Hayashizaki, Tetrahedron Lett., 2007, 48, 3801–3803 CrossRef
; (c) J. J. Li, C. Song, D.-M. Cui and C. Zhang, Org. Biomol. Chem., 2017, 15, 5564–5570 RSC
; (d) L. B. Krasnova, J. E. Hein and V. V. Fokin, J. Org. Chem., 2010, 75, 8662–8665 CrossRef PubMed
; (e) B. Han, B. Jaun, R. Krishnamurthy and A. Eschenmoser, Org. Lett., 2004, 6, 3691–3694 CrossRef PubMed
; (f) Z. Wang, H. K. Huynh, B. Han, R. Krishnamurthy and A. Eschenmoser, Org. Lett., 2003, 5, 2067–2070 CrossRef PubMed
.
-
(a) J. F. Allochio Filho, B. C. Lemos, A. S. de Souza, S. Pinheiro and S. J. Greco, Tetrahedron, 2017, 73, 6977–7004 CrossRef
; (b) S. Maddila, S. B. Jonnalagadda, K. K. Gangu and S. N. Maddila, Curr. Org. Synth., 2017, 14, 634–653 Search PubMed
; (c) J.-P. Wan, L. Gan and Y. Liu, Org. Biomol. Chem., 2017, 15, 9031–9043 RSC
; (d) I. A. Ibarra, A. Islas-Jacome and E. Gonzalez-Zamora, Org. Biomol. Chem., 2018, 16, 1402–1418 RSC
; (e) P. Patil, B. Mishra, G. Sheombarsing, K. Kurpiewska, J. Kalinowska-Tluscik and A. Doemling, ACS Comb. Sci., 2018, 20, 70–74 CrossRef PubMed
; (f) E. A. Muravyova, S. M. Desenko, R. V. Rudenko, S. V. Shishkina, O. V. Shishkin, Y. V. Sen'ko, E. V. Vashchenko and V. A. Chebanov, Tetrahedron, 2011, 67, 9389–9400 CrossRef
; (g) P. V. Santhini, R. A. Krishnan, S. A. Babu, B. S. Simethy, G. Das, V. K. Praveen, S. Varughese and J. John, J. Org. Chem., 2017, 82, 10537–10548 CrossRef PubMed
.
-
(a) R. Pathoor and D. Bahulayan, New J. Chem., 2018, 42, 6810–6816 RSC
; (b) E. Ezzatzadeh and Z. Hossaini, Nat. Prod. Res., 2018 DOI:10.1080/14786419.2018.1428598
; (c) P. Sathiyachandran, P. Manogaran, V. N. Nesterov, V. V. Padma and K. J. Rajendra Prasad, Eur. J. Med. Chem., 2018, 150, 851–863 CrossRef PubMed
; (d) P. R. Mali, P. K. Shirsat, N. Khomane, L. Nayak, J. B. Nanubolu and H. M. Meshram, ACS Comb. Sci., 2017, 19, 633–639 CrossRef PubMed
.
-
(a) A. J. Ansari, S. Sharma, R. S. Pathare, K. Gopal, D. M. Sawant and R. T. Pardasani, ChemistrySelect, 2016, 1, 1016–1021 CrossRef
; (b) A. S. Medvedeva, M. M. Demina, T. V. Kon'kova, T. L. H. Nguyen, A. V. Afonin and I. A. Ushakov, Tetrahedron, 2017, 73, 3979–3985 CrossRef
; (c) N. M. Drosos, C. Kakoulidou, M. Raftopoulou, J. Stephanidou-Stephanatou, C. A. Tsoleridis and A. G. Hatzidimitriou, Tetrahedron, 2017, 73, 1–7 CrossRef
; (d) F. B. Souza, D. C. Pimenta, H. A. Stefani and A. Helio, Tetrahedron Lett., 2016, 57, 1592–1596 CrossRef
; (e) P. Vincetti, A. Brianza, N. Scalacci, G. Costantino, D. Castagnolo and M. Radi, Tetrahedron Lett., 2016, 57, 1464–1467 CrossRef
; (f) M. Veeranarayana Reddy, B. Siva Kumar, K. T. Lim, B. G. Cho and Y. T. Jeong, Tetrahedron Lett., 2016, 57, 476–478 CrossRef
; (g) A. Kumbhar, S. Jadhav, R. Shejwal, G. Rashinkar and R. Salunkhe, RSC Adv., 2016, 6, 19612–19619 RSC
; (h) R. Mishra and L. H. Choudhury, RSC Adv., 2016, 6, 24464–24469 RSC
; (i) B. Jiang, T.-S. Zhang, R. Fu, W.-J. Hao, S.-L. Wang and S.-J. Tu, Tetrahedron, 2016, 72, 5652–5658 CrossRef
; (j) M. Kumar, T. Kaur, V. K. Gupta and A. Sharma, RSC Adv., 2015, 5, 17087–17095 RSC
; (k) P. Wadhwa, T. Kaur and A. Sharma, RSC Adv., 2015, 5, 44353–44360 RSC
.
-
(a) F. P. L. Lim, G. Luna and A. V. Dolzhenko, Tetrahedron Lett., 2014, 55, 5159–5163 CrossRef
; (b) F. P. L. Lim, G. Luna and A. V. Dolzhenko, Tetrahedron Lett., 2015, 56, 7016–7019 CrossRef
; (c) F. P. L. Lim, G. Luna and A. V. Dolzhenko, Tetrahedron Lett., 2015, 56, 521–524 CrossRef
; (d) A. V. Dolzhenko, S. A. Kalinina and D. V. Kalinin, RSC Adv., 2013, 3, 15850–15855 RSC
; (e) S. A. Kalinina, D. V. Kalinin and A. V. Dolzhenko, Tetrahedron Lett., 2013, 54, 5537–5540 CrossRef
; (f) F. P. L. Lim, S. T. Low, E. L. K. Ho, N. R. Halcovitch, E. R. T. Tiekink and A. V. Dolzhenko, RSC Adv., 2017, 7, 51062–51068 RSC
.
-
(a) D. S. Ermolatev, E. P. Svidritsky, E. V. Babaev and E. Van der Eycken, Tetrahedron Lett., 2009, 50, 5218–5220 CrossRef
; (b) D. S. Ermolat'ev and E. V. Van der Eycken, J. Org. Chem., 2008, 73, 6691–6697 CrossRef PubMed
; (c) D. S. Ermolat'ev, B. Savaliya, A. Shah and E. Van der Eycken, Mol. Diversity, 2011, 15, 491–496 CrossRef PubMed
.
- D. V. Kalinin, S. A. Kalinina and A. V. Dolzhenko, Heterocycles, 2013, 87, 147–154 CrossRef
.
- Y. S. Agasimundin, F. T. Oakes and N. J. Leonard, J. Org. Chem., 1984, 50, 2474–2480 CrossRef
.
- Rigaku Oxford Diffraction, CrysAlis PRO, Yarnton, Oxfordshire, England, 2017 Search PubMed
.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef PubMed
.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef
.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 CrossRef PubMed
.
- K. Brandenburg, DIAMOND, Crystal Impact GbR, Bonn, Germany, 2006 Search PubMed
.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148–155 CrossRef PubMed
.
Footnotes |
| † Part 33 in the series “Fused heterocyclic systems with an s-triazine ring.” For part 32 see ref. 1. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1840247 and 1840248. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra03703e |
| This journal is © The Royal Society of Chemistry 2018 |