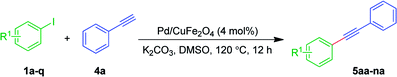Open Access Article
Open Access ArticleRecyclable Pd/CuFe2O4 nanowires: a highly active catalyst for C–C couplings and synthesis of benzofuran derivatives†
Bhairi Lakshminarayana,
Jhonti Chakraborty,
G. Satyanarayana * and
Ch. Subrahmanyam
* and
Ch. Subrahmanyam *
*
Department of Chemistry, Indian Institute of Technology Hyderabad, Kandi, Sangareddy, 502285, Telangana, India. E-mail: gvsatya@iith.ac.in; csubbu@iith.ac.in; Tel: +91(40) 23016054
First published on 7th June 2018
Abstract
Pd/CuFe2O4 nanowire-catalyzed cross coupling transformations are described. Notably, these reactions showed excellent functional group tolerance. Further, the protocol is applied to a one-pot synthesis of benzofurans via a Sonogashira coupling and intramolecular etherification sequence. The catalyst was reused and found to maintain its activity and stability.
Introduction
The ability to form various C–C bonds under transition metal catalysis is important in organic synthesis. Particularly, one-pot construction of heterocyclic core structures of biological relevance is indispensable.1 In this context, Pd is one of the most widely used transition metals for a variety of coupling transformations. When compared with homogeneous counterparts, heterogeneous catalysts enable the formation of less contaminated products and promote green chemistry.1–3 In the recent past, heterogeneous catalysis, for the formation of C–C, C–O, C–N and C–S bonds, became the central part of synthetic chemistry. Amongst them all, carbon–carbon (C–C) bond forming reactions for the preparation of carbon scaffolds are of high importance from the organic synthetic viewpoint.Supported nanocatalysts have become an integral part of heterogeneous catalysis for various organic transformations and industrial applications.4–7 Especially, metal oxide supported heterogeneous nanocatalysts are preferred because of their activity, better selectivity, and high stability over conventional metal based catalyst frameworks.8 Unsupported nanocatalysts face problems due to issues related recovery and recyclability from the reaction mixture.9 This problem can be resolved by immobilizing the active species on a support having high surface area materials such as nano-metal oxides,10 carbon nano-materials,11,12 polymers13 etc. Among all the supporters, nano-metal oxide supported nanocatalysts are easily separable from the reaction mixture.14,15 Especially, the substitution of the copper ion into the ferrite lattice constitutes a well-developed category of the mixed metal ferrites for catalysis in organic reactions.16,17 In this context, we designed CuFe2O4 support for organic transformation reactions (C–C couplings).
The biaryls and diarylacetylenes are useful scaffolds that constitute pharmaceuticals, agrochemicals and biologically active compounds.17,18 The Suzuki–Miyaura cross-coupling reactions of aryl halides with aryl boronic acids and Sonogashira coupling reactions of aryl halides with arylacetylenes are considered to be important carbon–carbon bond forming strategies, for constructing biaryls and internal acetylenes, respectively.19–21 However, most of these reactions made use of toxic ligands in conjunction with Pd-salts or Pd nanoparticles and Pd/Cu nanoparticles.22 Benzofuran derivatives are an important class of heterocyclic compounds, due to their miscellaneous biological profile, such as analgesia, antitumor, antimicrobial, kinase inhibitor and antihyperglycemic activities.23–28 Recently, the research group of Mariusz Jan Bosiak showed the utility of benzofurans in photovoltaic and optoelectronic properties, wherein benzofurans absorb and emit the light.29–32 While, one-pot synthesis of benzofurans, under homogeneous catalysis, is well established. Recently, the synthetic community turned their interest to develop new methods for the synthesis of benzofurans by means of heterogeneous catalytic conversion.33–39 Very recently, there have been various reports described that the catalytic activity of Pd nanoparticles can be retained, the stability of the Pd nanoparticles also enhanced to some extent by using magnetic nanomaterials as a supporter for immobilization of Pd nanoparticles.40–46 Recently, we have developed Pd/CuFe2O4 nanowires and showed its synthetic utility toward Heck couplings.47 Herein, we present the applicability of catalyst for various C–C bond forming reactions (Suzuki, Sonogashira). Further, this catalyst applied for the one-pot synthesis of benzofurans via intermolecular Sonogashira followed by intramolecular cyclization. Initially, a comparative study of as prepared catalyst with other recently reported Pd supported catalysts for Suzuki coupling reactions was shown in Table 1. As shown in Table 1, most of the Pd supported nanocatalysts produces the yields 88–96% (Table 1, entries 1–10). Whereas, with our catalyst Pd/CuFe2O4 nanowires we got near quantitative yields (98%) (Table 2, entry 11).
| Entry | Catalyst | Conditions | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | PdO/GO | K2CO3, DMSO, 120 °C, 10 min | 88 | 48 |
| 2 | Pd-SBT@MCM | K2CO3, PEG, 80 °C, 175 min | 96 | 49 |
| 3 | GO/Fe2O4/Pd composite | K2CO3, H2O/EtOH, 80 °C, 20 min | 95 | 50 |
| 4 | Pd@CQD@Fe3O4 | t-BuOK, EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 120 °C, 60 min 1), 120 °C, 60 min |
95 | 51 |
| 5 | Pd/C@Fe2O4 | K2CO3, H2O, 100 °C, 30 min | 96 | 52 |
| 6 | Pd@PVP | K2PO4, H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), 80 °C, 3–18 h 3), 80 °C, 3–18 h |
93 | 53 |
| 7 | Fe3O4@SiO2–Pd | CaO, H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 85 °C, 20 min 1), 85 °C, 20 min |
93 | 54 |
| 8 | Pd/Celite-PANI | K2CO3, TBAB (10%), dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 90 °C, 4 h 1), 90 °C, 4 h |
94 | 55 |
| 9 | Pd/TiO2 | Na2CO3, NMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 120 °C, 4 h H2O, 120 °C, 4 h |
95 | 56 |
| 10 | HMMS-salpr-Pd | K2CO3, H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 70 °C, 60 min 1), 70 °C, 60 min |
94 | 57 |
| 11 | Pd/CuFe2O4 nanowires | K2CO3, DMSO, 120 °C, 10 min | 98 | this work |
| Entry | Base | Solvent | Yieldb 3aa(%) |
|---|---|---|---|
| a Reaction conditions: aryl iodides 1h (0.25 mmol), arylboronic acid 2a (0.5 mmol), Pd/CuFe2O4 (4 mol%), base (0.5 mmol) and solvent (1 mL) at 120 °C.b Isolated yields of product 3ha. | |||
| 1 | K2CO3 | DMSO | 98 |
| 2 | K2CO3 | DMF | 95 |
| 3 | K2CO3 | DMA | 67 |
| 4 | K2CO3 | Toluene | 52 |
| 5 | K2CO3 | 1,4-Dioxane | 76 |
| 6 | K2CO3 | THF | 72 |
| 7 | K2CO3 | Acetonitrile | 63 |
| 8 | Cs2CO3 | DMSO | 67 |
| 9 | K3PO4 | DMSO | 40 |
| 10 | NaOH | DMSO | 31 |
Results & discussion
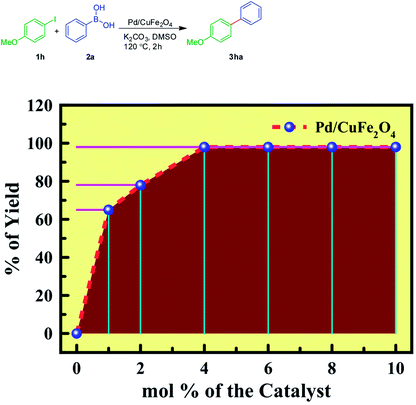
To begin with, the optimization of Suzuki–Miyaura coupling was planned. For this purpose, 4-methoxy iodobenzene 1h and phenylboronic acid 2a were chosen as the model substrates. Recently, we reported that Pd/CuFe2O4 nanowires showed the best catalytic activity for Heck couplings.47 Thus, the reaction was carried out between 4-methoxy iodobenzene 1h and phenylboronic acid 2a in the presence of Pd/CuFe2O4 nanowires (4 mole%), K2CO3 (2 equiv.) and in DMSO (1 mL) at 120 °C for 2 h. Gratifyingly, the reaction was quite successful and furnished the biaryl product 3ha, in near quantitative yield (Table 2, entry 1). On the other hand, other solvents, such as DMF, DMA, toluene, 1,4-dioxane, THF and acetonitrile were inferior (Table 2, entries 2 to 7). While the reaction gave fair, moderate and poor yields of the product 3ha with other bases, such as Cs2CO3, K3PO4, and NaOH respectively (Table 2, entries 8 to 10).Further to optimize the mol% of the Pd/CuFe2O4 catalyst, for the formation of 3ha, the reaction was performed between 4-methoxy iodobenzene 1h and phenylboronic acid 2a with 0, 1, 2, 4, 6, 8, and 10 mol% of Pd in Pd/CuFe2O4, under standard conditions. Among all, it was observed that the reaction yields were more or less same with ≥4 mol% of Pd in Pd/CuFe2O4 (Fig. 1). Thus it was concluded that the 4 mol% of Pd was an optimal load to drive the reaction.
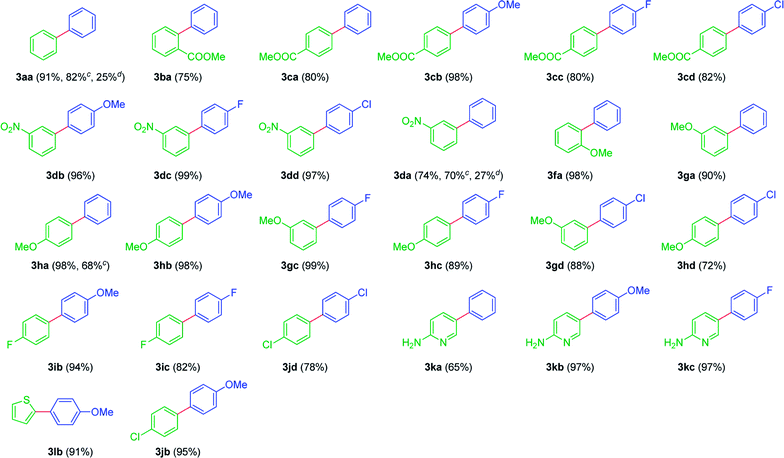
With these best conditions in hand, the scope was extended for the Suzuki–Miyaura coupling with various iodoarenes 1a–l and arylboronic acids 2a–d. Gratifyingly, the reaction was found to be amenable and afforded the corresponding biaryls 3aa–lb, in fair to near quantitative yields (Table 3). Interestingly, the reaction was successful with simple iodobenzene 1a and amenable with abroad range of functional groups (COOMe, NO2, OMe, F & Cl) on aromatic ring 1b–j, which reveals a wide functional group tolerance of this reaction. Notably, heteroaromatic iodide 1l, gave the products 3lb, in excellent yield. Significantly, protecting group free iodopyridine 1k was also tolerable and afforded the products 3ka–kc (Table 3). Interestingly, the reaction was also amenable to simple arylboronic acids 2 bearing broad range of functional moieties on the aromatic ring [i.e. OMe, F and Cl, (Table 3)]. To demonstrate the utility of the strategy, the Suzuki coupling reaction was explored with bromoarenes 1s–u with simple boronic acid 2a, under established conditions. Gratifyingly, biphenyls 3aa, 3da and 3ha were isolated in 82%, 70% and 68% yields (Table 3), respectively. Which ascertains the usefulness of Pd(0)/CuFe2O4 nanowires catalyst. Whereas the coupling with chlorobenzene 1v and meta-chloronitrobenzene 1w as reacting partners with the boronic acid 2a, furnished the desired biaryls 3aa and 3da in poor yields (25% & 27% respectively, Table 3). It is noteworthy to mention that in case of 1v and 1w, the homo coupling of boronic acid 2a was also noticed. In addition, the chloroarenes 1v and 1w were not completely consumed even after 24 h.
| a Reaction conditions: aryl iodides (0.25 mmol), arylboronic acid (0.5 mmol), Pd/CuFe2O4 (4 mol%), K2CO3 (0.5 mmol) and DMSO (1 mL) at 120 °C.b Isolated yields of chromatographically pure products 3aa–lb.c Isolated yields of products when bromoarenes 1s–u were used.d Isolated yields of products when chloroarenes 1v–w were used. |
|---|
 |
The recovery of the catalyst was done by centrifugation and washing with ethyl acetate and acetone followed by drying in a hot air oven at 60 °C for 12 h. The recovered Pd/CuFe2O4 nanowires catalyst was then subjected to the next catalytic cycles. It is worth mentioning that the catalyst retains its activity, which is evident with nearly no loss of activity even after fifth reaction cycle (Fig. 2). The marginal loss of activity after the fifth cycle (<3%) may be due to loss of some amount of the catalyst during the recovery of Pd/CuFe2O4 nanowires. The catalyst was recycled five times without an appreciable change in the product 3ha yield, under the established conditions at 120 °C. Thus, based on the above results it is confirmed that Pd/CuFe2O4 nanowires catalyst is stable enough and can be reused.
The relation between catalytic properties and structure of Pd/CuFe2O4 nanowires
The mechanism for the relation between catalytic properties (Suzuki coupling reaction) and structure of Pd/CuFe2O4 nanowires is shown in Scheme 1. The first step would be the oxidative addition of Pd(0)/CuFe2O4 nanowires to the 4-methoxy iodobenzene 1h to form Pd(II) species A. In most cases the oxidative addition is the rate determining step of a catalytic cycle. During this step, the Pd is oxidized from Pd(0) to Pd(II). Further, the reaction with base gives the intermediate B, which on coupling with the activated boronic acid derivative C (produced by reaction of the boronic acid 2a with K2CO3 base), gives transmetalation complex D. Finally, the reductive elimination of D affords the coupled product 3ha restores the active Pd(0)/CuFe2O4 nanowires, thus completes the catalytic cycle.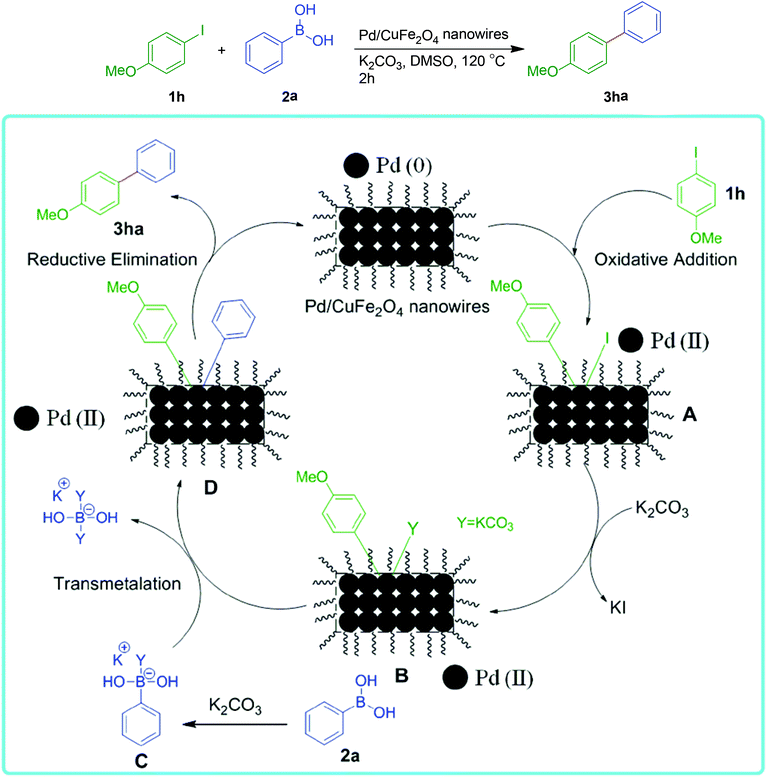 | ||
| Scheme 1 The mechanism for the relation between catalytic properties (Suzuki coupling reaction) and structure of Pd/CuFe2O4 nanowires. | ||
After successful synthesis of biaryls using Suzuki–Miyaura coupling, in order to check the efficiency of the Pd/CuFe2O4 nanowires catalyst, we aimed at Sonogashira coupling between iodoarenes and arylacetylenes. Therefore, initially, the reaction was explored between 2-amino iodobenzene 1m and phenylacetylene 4a, under established conditions as above (see; Table 2, entry 1). The reaction was quite successful and furnished the 2-amino diphenylacetylene product 5ma, in good yield (Table 4, entry 1). On the other hand, the reaction was found to be inferior with the other solvents, such as DMF, DMA, toluene, 1,4-dioxane, THF and acetonitrile (Table 4, entries 2 to 7). While the reaction with bases Cs2CO3 and KOH gave moderate yields of the product 5ma (Table 4, entries 8 and 9).
| Entry | Base | Solvent | Yield (5ma)b | Time (h) |
|---|---|---|---|---|
| a Reaction conditions: aryl iodides 1m (0.5 mmol), phenylacetylene 4a (1 mmol), Pd/CuFe2O4 (4 mol%), base (1 mmol) and solvent (1 mL) at 120 °C.b Isolated yields of product 5ma.c No progress was observed. | ||||
| 1 | K2CO3 | DMSO | 75 | 12 |
| 2 | K2CO3 | DMF | 60 | 12 |
| 3 | K2CO3 | DMA | 35 | 24 |
| 4 | K2CO3 | Toluene | —c | 48 |
| 5 | K2CO3 | 1,4-Dioxane | 36 | 24 |
| 6 | K2CO3 | THF | 30 | 24 |
| 7 | K2CO3 | CH3CN | 32 | 24 |
| 8 | Cs2CO3 | DMSO | 55 | 12 |
| 9 | KOH | DMSO | 48 | 12 |
With the above optimized conditions, for Sonogashira coupling, to demonstrate the utility of the strategy, the activity of Pd/CuFe2O4 catalyst was assessed for the reaction between iodoarenes 1a–q and phenylacetylene 4a. The results of the catalytic reactions are as depicted in Table 5, which showed broad substrate scope and delivered the corresponding internal acetylenes 5aa–qa, in fair to very good yields. For example, iodoarenes bearing electron-withdrawing groups (meta-COOMe, para-COOMe, meta-NO2) were found to be smooth and afforded the products (5ba, 5ca & 5da) moderate yields (Table 5). In addition, the reaction was quite successful with electron releasing groups as well (5fa, 5ga & 5ha, Table 5). Notably, the reaction was tolerable to protecting group free NH2 moiety on pyridine ring (5ka, Table 5). To our delight, 2-iodobenzylalcohol 2n coupled with phenylacetylene 4a and gave the product 5na, in very good yield (Table 5). Further, we have explored Sonogashira coupling between bromobenzene 1s and phenylacetylene 4a. Notably, the reaction was smooth and yielded diphenylacetylene 5aa, in yields 68%. Even the reaction was also successful meta-bromonitrobenzene 1t and para-methoxybromobenzene 1u with 4a. While, the reaction is somewhat sluggish with chloroarenes. Therefore, the present catalyst seems to be active enough and could promote the reactions with bromoarenes as well. To further check the selectivity of the process, it was planned to test the substituent susceptibility. Thus, the reaction was conducted with 1-chloro-4-iodobenzene 1j in the presence of phenylacetylene 4a, in very good yields (Table 5). To our delight, the reaction was found to be smooth and selective and gave the product 5jb, in which relatively more reactive iodo substituent was selectively involved in the coupling reaction. It is worth mentioning that the reaction with ethyl propiolate as coupling partner, did not lead to any product and only starting materials were recovered. This may be due to the fact that less nucleophilicity of acetylenic β-carbon of ethyl propiolate.
| a Reaction conditions: aryl iodides 1a–q (0.5 mmol), phenylacetylene 4a (1 mmol), Pd/CuFe2O4 (4 mol%), K2CO3 (1 mmol) and DMSO (1 mL) at 120 °C.b Isolated yields of product 5aa–5qa.c Isolated yields of products when bromoarenes 1s–u were used.d Isolated yields of products when chloroarenes 1v–w were used. |
|---|
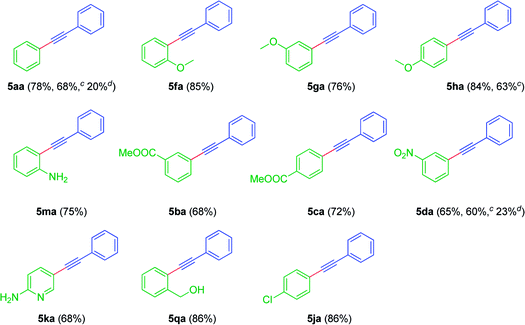 |
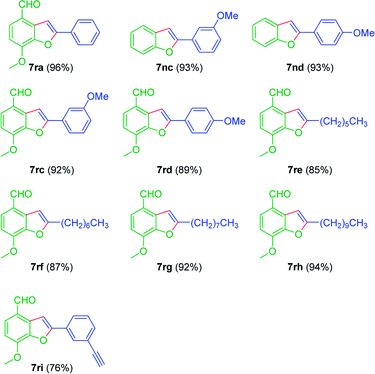
Benzofurans are ubiquitous oxygen-containing heterocyclic motifs that constitute many natural products, pharmaceuticals, biologically important compounds and organic materials. In this context, many synthetic strategies have been established for their synthesis. Notably, transition metal-catalyzed (Pd,58–66 Rh,67 Ru,68–71 Ir,72 Au73 and Cu74–76) annulations proved to be powerful strategies for the synthesis of benzofurans. Quite interestingly, when 2-iodophenols 1n–r were treated with terminal acetylenes 4a–i, benzofurans were obtained as the products (Table 6). For example, the reaction was amenable to different arylacetylenes (4a, 4c & 4d) and furnished the corresponding benzofurans 7ra–rd (Table 6). Notably, the reaction was also successful with terminal alkyl acetylenes 4e–h and afforded the benzofurans 7re–rh, in very good to excellent yields (Table 6). Significantly, the reaction was tolerable to the aldehyde functionality on the aromatic ring of 2-iodophenol derivative 1r. Significantly, 3-hydroxy-2-iodo-4-methoxybenzaldehyde 1r coupled with 1,3-diethynylbenzene 4i, in which only one acetylene group was reacted and gave 7ri as an exclusive product (Table 6).
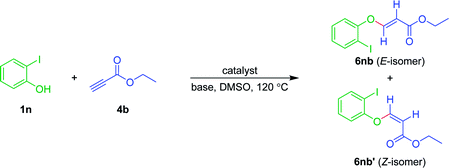
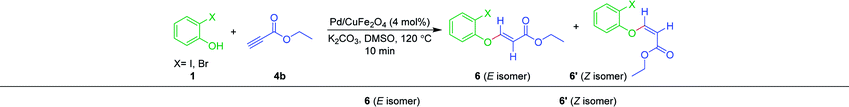
Moreover, to check the scope and generality of Sonogashira coupling, 2-iodophenol was treated with ethyl propiolate 4b. Surprisingly, no Sonogashira product was formed; instead, Michael addition product 6nb was obtained as major diastereomer via nucleophilic attack of the hydroxyl group of phenol (Table 7). This sort of nucleophilic 1,4-addition of phenolic hydroxyl across the activated triple/double bonds is already established in the literature.77–79 Whereas, when 2-iodoaniline was reacted with ethyl propiolate 4b, we could not observe any required product. The reaction was inconclusive from the TLC.
To understand the nature of the reaction and whether or not the palladium is necessary to drive this Michael addition reaction, the reaction was performed under varying conditions, as illustrated in Table 8. The reaction under standard conditions, furnished the product 6nb+6nb′ (57 + 39) (Table 8, entry 1). The reaction was also successful without Pd-catalyst, but with the base and solvent, albeit there was a drop in the yields [6nb+6nb′ (32 + 16), Table 8, entry 2]. In addition, the reaction was smooth, under neat conditions with base and without catalyst [6nb+6nb′ (35 + 18), Table 8, entry 3]. While the yield of the product was good with both catalyst and base and without solvent [6nb+6nb′ (53+35), Table 8, entry 4]. Only with solvent and without base and catalyst, no progress was noted (Table 8, entry 5). Similarly, with the catalyst and solvent, without base, no progress was seen (Table 8, entry 6). The reaction did not proceed without the catalyst, base and solvent (Table 8, entry 7).
| Entry | Catalyst (Pd/CuFe2O4) | Base (K2CO3) | Solvent (DMSO) | Yield (%) | |
|---|---|---|---|---|---|
| 6nb (E) | 6nb′ (Z) | ||||
| a Reaction conditions: aryl iodides 1n (0.5 mmol), ethyl propiolate 4b (1 mmol), Pd/CuFe2O4 (4 mol%), K2CO3 (1 mmol) and DMSO (1 mL) at 120 °C.b Isolated yields of products 6. | |||||
| 1 | 4 mol% | 2 eq. | 1 mL | 57 | 39 |
| 2 | — | 2 eq. | 1 mL | 32 | 16 |
| 3 | — | 2 eq. | — | 35 | 18 |
| 4 | 4 mol% | 2 eq. | — | 53 | 35 |
| 5 | — | — | 1 mL | — | — |
| 6 | 4 mol% | — | 1 mL | — | — |
| 7 | — | — | — | — | — |
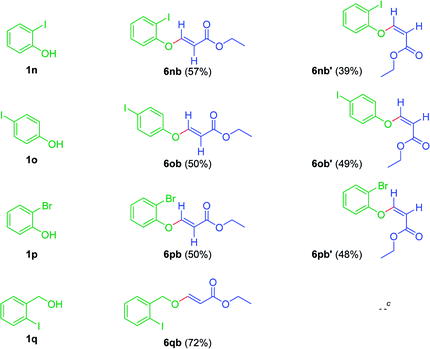
To further confirm this sort of reactivity, the reaction was explored with different halophenols (1n, 1o, 1p & 1q) with ethyl propiolate 4b, under standard reaction conditions. As anticipated, furnished the corresponding arylvinyl ethers as Z and E diastereomeric mixture, as minor and major isomers, respectively (Table 9). Surprisingly, when 2-iodobenzylalcohol 1q was used as the nucleophile, exclusively, gave the E isomer in 72% yield (Table 9).
Conclusions
In summary, we have demonstrated a facile route for the synthesis of biphenyls, diphenylacetylene and 2-aryl/alkyl benzofuran derivatives via ligand-free Suzuki, Sonogashira coupling reactions using the catalyst Pd/CuFe2O4 nanowires. The Suzuki cross-coupling reaction furnished the biphenyls with excellent to near quantitative yields. Further, the optimized conditions were applied for Sonogashira coupling followed by the intramolecular nucleophilic attack for the formation benzofurans. It was also demonstrated for the unusual formation arylvinyl ethers via nucleophilic attack of the hydroxyl group of phenol onto the triple bond of ethyl propiolate.Conflicts of interest
There is no conflict of interest.Acknowledgements
B. L. Narayana would like to thanks to University Grant Commission (UGC), New Delhi, for awarding Junior & Senior Research Fellowship (JRF & SRF).References
- R. Hudson, Y. Feng, R. S. Varma and A. Moores, Green Chem., 2014, 16, 4493–4505 RSC.
- R. S. Varma, Green Chem., 2014, 16, 2027–2041 RSC.
- J. A. Gladysz, Chem. Rev., 2002, 102, 3215–3216 CrossRef PubMed.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef PubMed.
- M. B. Gawande, R. K. Pandey and R. V. Jayaram, Catal. Sci. Technol., 2012, 2, 1113–1125 Search PubMed.
- S. N. Shelke, S. R. Bankar, G. R. Mhaske, S. S. Kadam, D. K. Murade, S. B. Bhorkade and R. S. Varma, ACS Sustainable Chem. Eng., 2014, 2, 1699–1706 CrossRef.
- M. B. Gawande, P. S. Branco, K. Parghi, J. J. Shrikhande, R. K. Pandey, C. A. A. ghumman, N. Bundaleski, O. Teodoro and R. V. Jayam, Catal. Sci. Technol., 2011, 1, 1653–1664 Search PubMed.
- G. A. Somorjai, H. Feri and J. Y. Park, J. Am. Chem. Soc., 2009, 131, 16589–16605 CrossRef PubMed.
- M. Beller, H. Fischer, K. Kuhlein, C. P. Reisinger and W. A. Herrmann, J. Organomet. Chem., 1996, 520, 257–259 CrossRef.
- M. L. Kantam, S. Roy, M. Roy, B. Sreedhar and B. M. Choudary, Adv. Synth. Catal., 2005, 347, 2002–2008 CrossRef.
- X. R. Ye, Y. H. Lin, C. M. Wang, M. H. Engelhard, Y. Wang and C. M. Wai, J. Mater. Chem., 2004, 14, 908–913 RSC.
- B. Lakshminarayana, L. Mahendar, P. Ghosal, G. Satyanarayana and Ch. Subrahmanyam, ChemistrySelect, 2017, 2, 2703–2710 CrossRef.
- R. Narayanan and M. A. El-Sayed, J. Am. Chem. Soc., 2003, 125, 8340–8347 CrossRef PubMed.
- S. Shylesh, V. Schunemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef PubMed.
- K. H. Lee, B. Lee, K. R. Lee, M. H. Yi and N. H. Hur, Chem. Commun., 2012, 48, 4414–4416 RSC.
- A. T. Nguyen, L. T. Pham, N. T. Phan and T. Truong, Catal. Sci. Technol., 2014, 4, 4281–4288 Search PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef.
- G. Ding, W. Wang, T. Jiang and B. Han, Green Chem., 2013, 15, 3396–3403 RSC.
- C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef PubMed.
- L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef PubMed.
- A. Fihri, M. Bouhrara, B. Nekoueishahraki, J. M. Basset and V. Polshettiwar, Chem. Soc. Rev., 2011, 40, 5181–5203 RSC.
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef PubMed.
- A. Radadiya and A. Shah, Eur. J. Med. Chem., 2015, 97, 356–376 CrossRef PubMed.
- R. J. Nevagi, S. N. Dighe and S. N. Dighe, Eur. J. Med. Chem., 2015, 97, 561–581 CrossRef PubMed.
- H. Khanam and Shamsuzzaman, Eur. J. Med. Chem., 2015, 97, 483–504 CrossRef PubMed.
- P. Stehrer-Schmid and H. U. Wolf, Mutat. Res., Rev. Genet. Toxicol., 1995, 339, 61–72 CrossRef.
- R. Naik, D. S. Harmalkar, X. Xu, K. Jang and K. Lee, Eur. J. Med. Chem., 2015, 90, 379–393 CrossRef PubMed.
- J. Knoll, CNS Drug Rev., 2001, 7, 317–345 CrossRef PubMed.
- M. J. Bosiak, M. Rakowiecki, A. Wolan, J. Szlachta, E. Stanek, D. Cycoń and K. Skupień, Dyes Pigm., 2015, 121, 79–87 CrossRef.
- M. J. Bosiak, J. A. Jakubowska, K. B. Aleksandrzak, S. Kamiński, A. Kaczmarek-Kędziera, M. Ziegler-Borowska, D. Kędziera and J. Adams, Tetrahedron Lett., 2012, 53, 3923–3926 CrossRef.
- M. J. Bosiak, M. Rakowiecki, K. J. Orłowska, D. Kędziera and J. Adams, Dyes Pigm., 2013, 99, 803–811 CrossRef.
- K. Kadac, M. J. Bosiak and J. Nowaczyk, Synth. Met., 2012, 162, 1981–1986 CrossRef.
- L. Duan, R. Fu, B. Zhang, W. Shi, S. Chen and Y. Wan, ACS Catal., 2016, 6, 1062–1074 CrossRef.
- L. Mahendar and G. Satyanarayana, J. Org. Chem., 2015, 80, 7089–7098 CrossRef PubMed.
- E. Speckmeier, C. Padié and K. Zeitler, Org. Lett., 2015, 17, 4818–4821 CrossRef PubMed.
- S. C. Yin, Q. Zhou, X. Y. Zhao and L. X. Shao, J. Org. Chem., 2015, 80, 8916–8921 CrossRef PubMed.
- R. Karmakar, P. Pahari and D. Mal, Chem. Rev., 2014, 114, 6213–6284 CrossRef PubMed.
- L. De Luca, G. Giacomelli and G. Nieddu, J. Comb. Chem., 2008, 10, 517–520 CrossRef PubMed.
- O. Miyata, N. Takeda and T. Naito, Org. Lett., 2004, 6, 1761–1763 CrossRef PubMed.
- D. Rosario-Amorin, M. Gaboyard, R. Clerac, L. Vellutini, S. Nlate and K. Heuze, Chem.–Eur. J., 2012, 18, 3305–3315 CrossRef PubMed.
- Y. Jang, J. Chung, S. Kim, S. W. Jun, B. H. Kim, D. W. Lee, B. M. Kim and T. Hyeon, Phys. Chem. Chem. Phys., 2011, 13, 2512–2516 RSC.
- M. Y. Zhu and G. W. Diao, J. Phys. Chem. C, 2012, 116, 1626 Search PubMed.
- Y. T. Liao, L. S. He, J. Huang, J. Y. Zhang, L. Zhuang, H. Shen and C. Y. Su, ACS Appl. Mater. Interfaces, 2011, 2, 2333–2338 Search PubMed.
- M. Zeltner, A. Schatz, M. L. Hefti and W. J. Stark, J. Mater. Chem., 2011, 21, 2991–2996 RSC.
- A. Schatz, T. R. Long, R. N. Grass, W. J. Stark, P. R. Hanson and O. Reiser, Adv. Funct. Mater., 2010, 20, 4323–4328 CrossRef PubMed.
- R. Li, P. Zhang, Y. Huang, P. Zhang, H. Zhong and Q. Chen, J. Mater. Chem., 2012, 22, 22750–22755 RSC.
- B. Lakshminarayana, L. Mahendar, P. Ghosal, B. Sreedhar, G. Satyanarayana and C. h. Subrahmanyam, New J. Chem., 2018, 42, 1646–1654 RSC.
- B. Lakshminarayana, L. Mahendar, J. Chakraborty, G. Satyanarayana and C. h. Subrahmanyam, J. Chem. Sci., 2018, 130, 47 CrossRef.
- M. Nikoorazm, A. Ghorbani-Choghamarani, M. Ghobadi and S. Massahi, Appl. Organomet. Chem., 2017, 31, e3848 CrossRef.
- N. Seyedi, K. Saidi and H. Sheibani, Catal. Lett., 2018, 148, 277–288 CrossRef.
- M. Gholinejad, F. Zareh and C. Nájera, Appl. Organomet. Chem., 2018, 32, 1–14 CrossRef.
- R. N. Baig, M. N. Nadagouda and R. S. Varma, Green Chem., 2014, 16, 4333–4338 RSC.
- F. Mäsing, H. Nüsse, J. Klingauf and A. Studer, Org. Lett., 2018, 20, 752–755 CrossRef PubMed.
- A. Khazaei, M. Khazaei and M. Nasrollahzadeh, Tetrahedron, 2017, 73, 5624–5633 CrossRef.
- H. A. Patel, L. A. Patel and A. V. Bedekar, New J. Chem., 2016, 40, 8935–8945 RSC.
- M. Nasrollahzadeh and S. M. Sajadi, J. Colloid Interface Sci., 2016, 465, 121–127 CrossRef PubMed.
- H. Liu, P. Wang, H. Yang, J. Niu and J. Ma, New J. Chem., 2015, 39, 4343–4350 RSC.
- M. R. Kuram, M. Bhanuchandra and A. K. Sahoo, Angew. Chem., Int. Ed., 2013, 125, 4705–4710 CrossRef.
- C. E. Castro, E. J. Gaughan and D. C. Owsley, J. Org. Chem., 1966, 31, 4071–4078 CrossRef.
- A. Arcadi, F. Marinelli and S. Cacchi, Synthesis, 1986, 9, 749–751 CrossRef.
- R. C. Larock, E. K. Yum, M. J. Doty and K. K. C. Sham, J. Org. Chem., 1995, 60, 3270–3271 CrossRef.
- R. Sanz, M. P. Castroviejo, Y. Fernandez and F. J. Fananas, J. Org. Chem., 2005, 70, 6548–6551 CrossRef PubMed.
- G. S. Gill, D. W. Grobelny, J. H. Chaplin and B. L. Flynn, J. Org. Chem., 2008, 73, 1131–1134 CrossRef PubMed.
- N. akai, N. Uchida and T. Konakahara, Tetrahedron Lett., 2008, 49, 3437–3440 CrossRef.
- J. R. Wang and K. Manabe, J. Org. Chem., 2010, 75, 5340–5342 CrossRef PubMed.
- R. Wang, S. Mo, Y. Lu and Z. Shen, Adv. Synth. Catal., 2011, 353, 713–718 CrossRef.
- Z. Zhou, G. Liu, Y. Shen and X. Lu, Org. Chem. Front., 2014, 1, 1161–1165 RSC.
- C. H. Yeh, W. C. Chen, P. Gandeepan, Y. C. Hong, C. H. Shih and C. H. Cheng, Org. Biomol. Chem., 2014, 12, 9105–9108 Search PubMed.
- D. H. Lee, K. H. Kwon and C. S. Yi, J. Am. Chem. Soc., 2012, 134, 7325–7328 CrossRef PubMed.
- V. S. Thirunavukkarasu, M. Donati and L. Ackermann, Org. Lett., 2012, 14, 3416–3419 CrossRef PubMed.
- S. Mochida, M. Shimizu, K. Hirano, T. Satoh and M. Miura, Chem.–Asian J., 2010, 5, 847–851 CrossRef PubMed.
- K. Tsuchikama, Y. K. Hashimoto, K. Endo and T. Shibata, Adv. Synth. Catal., 2009, 351, 2850–2854 CrossRef.
- J. Liao, P. Guo and Q. Chen, Catal. Commun., 2016, 77, 22–25 CrossRef.
- J. Bonnamour, M. Piedrafita and C. Bolm, Adv. Synth. Catal., 2010, 352, 1577–1581 CrossRef.
- R. Zhu, J. Wei and Z. Shi, Chem. Sci., 2013, 4, 3706–3711 RSC.
- W. Zeng, W. Wu, H. Jiang, L. Huang, Y. Sun, Z. Chen and X. Li, Chem. Commun., 2013, 49, 6611–6613 RSC.
- M. W. Khan, M. J. Alam, M. A. Rashid and R. Chowdhury, Bioorg. Med. Chem., 2005, 13, 4796–4805 CrossRef PubMed.
- A. Gómez-Suárez, Y. Oonishi, A. R. Martin and S. P. Nolan, Beilstein J. Org. Chem., 2016, 12, 172–178 CrossRef PubMed.
- A. Gopi Krishna Reddy, J. Krishna and G. Satyanarayana, Tetrahedron Lett., 2012, 53, 5635–5640 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Details of catalyst synthesis, characterization and experimental studies. 1H, 13C-NMR spectra of all isolated products. See DOI: 10.1039/c8ra03697g |
| This journal is © The Royal Society of Chemistry 2018 |