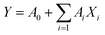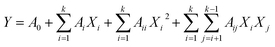Open Access Article
Open Access ArticleAcidolysis-dominated pretreatment elevates distillation yield and impacts composition, antioxidant and antifungal activities of essential oil from Cuminum cyminum seeds
Zhong Zhang ab,
Qiang Qinb,
Ruojun Dingb,
Yibing Xiab,
Libo Xiongb,
Yang Bi*ab and
Dov Pruskyb
ab,
Qiang Qinb,
Ruojun Dingb,
Yibing Xiab,
Libo Xiongb,
Yang Bi*ab and
Dov Pruskyb
aCollege of Horticulture, Gansu Agricultural University, Lanzhou 730070, China. E-mail: beyang62@163.com; foodgau@126.com
bCollege of Food Science and Engineering, Gansu Agricultural University, Lanzhou 730070, China
First published on 18th September 2018
Abstract
Proper pretreatment of herbal material containing essential oils (EOs) could enhance its volatile components release through either removing physical barriers or conquering chemical bonds and thereby improve hydrodistillation yield. In this regard, a trial pretreatment including pulverization, enzymolysis, short time microwave irradiation and acidolysis of Cuminum cyminum seeds was integrated into the essential oil (EO) preparation to elevate the EO yield. On the basis of Plackett–Burman design analysis, three parameters (acidolysis duration, HCl concentration of acidolysis and sieving mesh) were significant for the EO preparation. Box–Behnken design based optimization of the remaining factors concluded that the optimal pretreatment was pulverizing the seeds to 40 mesh and implementing 45 min acidolysis in 2.5 M L−1 HCl wherein the predicted EO yield of 3.78% was close to that of the experimental value 3.86%. This pretreatment produced an EO yield increase of 50.78% over the control sample of raw seeds (2.56%). In total 53 components were identified in the acidolysis-pretreated cumin EO (AEO) whilst 47 components were identified in the control cumin EO (CEO). In both AEO and CEO, cuminaldehyde was the predominant common component, but the AEO contained more phenols (0.51% vs. 0.18%) and alcohols (7.76% vs. 0.18%) than the CEO did. The compositional features gave the AEO mightier antioxidant potency and stronger antifungal efficacy against four postharvest fungi, viz. Alternaria alternata, Penicillium expansum, Trichothecium roseum and Fusarium sulphureum, as compared with the situations of CEO. In conclusion, the pretreatment elevates the hydrodistillation yield, modifies the EO chemical profiles and confers stronger antioxidant and antifungal activities upon cumin EO.
1. Introduction
Cumin (Cuminum cyminum) seed is the second most consumed spice after black pepper in the world and is mainly cultivated in Arabia, India, China and some countries adjoining the Mediterranean Sea.1 Traditionally the seed has been extensively used for various medicinal, culinary, and aromatic purposes,2 but to date more new bioactivities of its essential oil (EO) have been investigated for deleterious microorganism inhibition,1,3 grain-storage,4 and phytophagous pest control5 objectives. Moreover, cumin EO exerts anti-inflammatory effects in LPS-stimulated RAW264.7 cells.6 This effect is of great importance since inflammation is regarded as a cause of chronic disease7 which accounts for nearly two-thirds of deaths worldwide, and the emergence of chronic diseases as the predominant challenge to global health is undisputed.8As a value-added product of cumin seeds processing, the EO has a disappointing low yield, and elevating this yield is of significance for increasing the profitability of the EO production. Hydrodistillation, the typical EO extraction approach can obtain the highest yield, but the yield is still associated with the intrinsic nature of the raw materials.9 Modifications of this nature seem an accessible vehicle for fully tapping the latent EO components in the material, thereby elevating the yield within a short space of time. EO compounds are found in intracellular spaces, more than on the surface of the vegetal cell.10 For dry herbal materials like cumin seeds, pulverization can crumble the tissues of the materials and partly remove any physical barrier to EO releasing.11 But normal grinding can raise the product temperature to 42–95 °C and cause loss of volatile components up to 40%,12 thus the optimal extent of pulverization deserves investigation. Furthermore, EOs were accumulated in the secretory structures of plant tissues and cellulolytic degradation of such structures can facilitate releasing and diffusion of EO components.13 More importantly, particular amount of EO components are glycosidically bound14 and thereupon are generally hydrophilic, nonvolatile, and unobtainable with simple hydrodistillation. Hydrolytically breaking of such glycosidical bonds can liberate aglycone moieties that could be latent EO components, whereas the effect of acidolysis on cumin EO lacks reports. External microwave assistance with ionic water hydrodistillation shortens the extraction time without obvious influence on EO constituents,15 but this simultaneous application of microwave irradiation require more complex facilities. Interestingly, microwave-heated cumin seeds gain higher EO yield than conventionally roasted samples do in then hydrodistillation,16 but the effect of microwave irradiation, prior to distillation, on cumin EO yield was not explored.
In documented investigations into the effect of pretreatment on cumin EO preparation, the most researches focused on single factor introduced, whilst the application of hyphenated compound factors in the treatment of cumin seeds is less analyzed. Generally, these introduced treatments might have some influences on the compositional and bioactive attributes of the EO, thus evaluation of these influences is necessary when considering the feasibility of the application of these potential treatments in production. As stereotypical bioactivities of the EO, antioxidant and antimicrobial activities were assessed as basic aspects when the bioactive quality of different EOs was compared.17
In the present work, the Plackett–Burman design (PBD) and Box–Behnken design (BBD) based analyses were employed for the determination of key factors and their best function zones where an optimal EO yield could be procured. To evaluate the influence of the introduced treatments on the chemical attributes and bioactivities of the EO, the composition, in vitro antioxidant potency and antifungal activity of the EO were analyzed. To the best of our knowledge, this investigation was the first one that integrates acidolysis into the extraction of EOs with the intention of liberating the bound EO components.
2. Material and methods
2.1. Cumin seeds
Cumin (C. cyminum cv. Dunyu no. 1) seeds newly harvested were obtained from the grower Dunhuang Seed Co., Ltd. (Dunhuang, China).2.2. Chemicals and reagents
Cellulase from Trichodermavirid (Solarbio, Japan) was used for treatment of cumin seed slurry. Chromatographic level n-hexane for sample dilution and homologous series of C5–C24 n-alkanes for gas chromatography-mass spectrometry (GC-MS) analysis were purchased from Sigma-Aldrich (Steineheim, Germany). The 2,2-diphenyl-1-picrylhydrazyl(DPPH),2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)(ABTS), (2,4,6-trislz-pyridyl)-s-triazine(TPTZ), β-carotene and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were analytical grade and purchased from the Sinopharm Chemical Reagent (Shanghai, China).2.3. Essential oil preparation
| Run | Factors | Yield (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | ||
| a X1 = sieving mesh, X2 = enzyme concentration (104 U L−1), X3 = enzymolysis pH, X4 = enzymolysis temperature (°C), X5 = enzymolysis duration (min), X6 = microwave irradiation duration (s), X7 = HCl concentration of acidolysis (M L−1), X8 = acidolysis duration (min), X9 = sample to liquid ratio. For each factor, the small values represent its low level (−1) and the large values represent its high level (+1). | ||||||||||
| 1 | 20 | 1 | 4.5 | 47 | 30 | 60 | 3 | 75 | 1/6 | 2.80 ± 0.02 |
| 2 | 70 | 1 | 5.5 | 47 | 30 | 30 | 3 | 45 | 1/6 | 2.53 ± 0.01 |
| 3 | 70 | 3 | 5.5 | 47 | 30 | 60 | 1 | 45 | 1/6 | 2.29 ± 0.05 |
| 4 | 70 | 1 | 5.5 | 47 | 60 | 60 | 3 | 45 | 1/6 | 2.42 ± 0.08 |
| 5 | 20 | 1 | 5.5 | 43 | 30 | 60 | 3 | 45 | 1/8 | 2.49 ± 0.02 |
| 6 | 20 | 1 | 4.5 | 43 | 30 | 30 | 1 | 45 | 1/8 | 2.38 ± 0.06 |
| 7 | 20 | 1 | 5.5 | 47 | 30 | 30 | 3 | 45 | 1/8 | 2.57 ± 0.01 |
| 8 | 70 | 1 | 5.5 | 47 | 60 | 30 | 1 | 75 | 1/8 | 2.48 ± 0.01 |
| 9 | 70 | 1 | 4.5 | 43 | 60 | 60 | 3 | 75 | 1/8 | 2.46 ± 0.01 |
| 10 | 70 | 3 | 5.5 | 43 | 60 | 60 | 1 | 75 | 1/8 | 2.45 ± 0.02 |
| 11 | 70 | 3 | 4.5 | 43 | 30 | 30 | 3 | 45 | 1/6 | 2.56 ± 0.02 |
| 12 | 20 | 3 | 4.5 | 43 | 60 | 30 | 1 | 45 | 1/6 | 2.49 ± 0.04 |
2.3.3.1. Single factor screening. For screening the factors that significantly (P < 0.05) influence the EO yield, candidate factors (sieving mesh, cellulase concentration, temperature, pH and duration for enzymolysis, duration of microwave irradiation, acidolysis HCl concentration and duration, and ratio of cumin powder to liquid) were examined with the PBD analysis. Each variable was tested at low (−1) and high (+1) levels and twelve trials were performed without consideration of any interactive effects and every trial was conducted in triplicate. The values of these levels were represented in Table 1. The first order polynomial equation for PBD was as following:
where Y is the EO yield response, A0 is intercept, Ai is the linear regression coefficient for ith factor, Xi is the level of the independent factor. The analysis of variance (ANOVA) was used to determine main effects of parameters. The normalized results of the experimental design were evaluated at a 5% significant level and illustrated by standardized Pareto chart with a vertical line as an indicator beyond which extending bars represent the factors that are statistically significant and were considered for further experiments.
2.3.3.2. Full factorial design. Each remained factor was coded (Table 2) at three levels, i.e. low (−1), medium (0) and high (+1) and a total of 17 experimental runs (Table 3) were conducted.
| Factor | Low | Medium | High |
|---|---|---|---|
| X1 mesh size | 20 | 40 | 70 |
| X2 HCl concentration(M L−1) | 1.5 | 2 | 2.5 |
| X3 acidolysis duration (min) | 45 | 60 | 75 |
| Run | X1 | X2 | X3 | Yield (%) | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | −1 | 0 | 1 | 2.54 ± 0.01 | 2.51 |
| 2 | −1 | −1 | 0 | 2.36 ± 0.01 | 2.30 |
| 3 | 1 | −1 | 0 | 2.62 ± 0.02 | 2.53 |
| 4 | −1 | 1 | 0 | 2.76 ± 0.02 | 2.85 |
| 5 | 1 | 0 | 1 | 2.79 ± 0.03 | 2.80 |
| 6 | 1 | 1 | 0 | 2.80 ± 0.01 | 2.85 |
| 7 | 0 | 0 | 0 | 2.86 ± 0.02 | 2.95 |
| 8 | 0 | −1 | 1 | 2.90 ± 0.01 | 2.99 |
| 9 | 0 | 0 | 0 | 2.93 ± 0.03 | 2.95 |
| 10 | 1 | 0 | −1 | 2.95 ± 0.02 | 2.98 |
| 11 | 0 | 0 | 0 | 2.98 ± 0.01 | 2.95 |
| 12 | 0 | 0 | 0 | 2.99 ± 0.02 | 2.95 |
| 13 | 0 | 0 | 0 | 3.01 ± 0.02 | 2.95 |
| 14 | 0 | −1 | −1 | 3.04 ± 0.08 | 3.10 |
| 15 | −1 | 0 | −1 | 3.06 ± 0.08 | 3.05 |
| 16 | 0 | 1 | 1 | 3.23 ± 0.06 | 3.17 |
| 17 | 0 | 1 | −1 | 3.86 ± 0.06 | 3.78 |
Fit the BBD experimental data into second order polynomial equation, via regression analysis, as following:
2.4. GC-MS analysis
Chemical characteristic of EO was determined on a gas chromatograph model Trace 1310 with TG-WAX (60 m × 0.25 mm, 0.25 μm) capillary column in tandem with ISQ single quadrupole mass detector (Thermo Scientific, USA). Helium was carrier gas at a flow rate of 1 mL min−1; injector temperature was 250 °C. Column temperature was programmed at 60 °C isothermal for 4 min, and then increased to 240 °C at a rate of 4 °C min−1 and held isothermal for 15 min. The split ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, ionization voltage 70 eV; ion source temperature 200 °C; mass scan range: 40–500 mass units; detector temperature 280 °C. The injection volume was 1.0 μL after 1% (v/v) dilution of the EO with n-hexane. The percentage composition was calculated from the GC peak areas using the normalization method (without correction factors). The mixture of C5–C24 n-alkanes was injected using the above mentioned temperature program in order to calculate the retention indices.20 Identification of the components was based on computer matching their mass spectral fragmentation patterns with those in NIST05 and Wiley (Flavor & Fragrance Natural &Synthetic Compounds 1.2) Library.
20, ionization voltage 70 eV; ion source temperature 200 °C; mass scan range: 40–500 mass units; detector temperature 280 °C. The injection volume was 1.0 μL after 1% (v/v) dilution of the EO with n-hexane. The percentage composition was calculated from the GC peak areas using the normalization method (without correction factors). The mixture of C5–C24 n-alkanes was injected using the above mentioned temperature program in order to calculate the retention indices.20 Identification of the components was based on computer matching their mass spectral fragmentation patterns with those in NIST05 and Wiley (Flavor & Fragrance Natural &Synthetic Compounds 1.2) Library.
2.5. Antioxidant activity determination in vitro
In DPPH radical scavenging, ABTS radical scavenging and ferric reducing antioxidant power assaying experiments, Trolox in concentrations ranging from 50 to 800 μM L−1 was used to construct standard curve. The tested antioxidant powers were expressed as Trolox equivalent (TE) antioxidant capacity in mMTE L−1. All the antioxidant testing experiments were conducted in triplicate.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) (v/v) with ethanol to 0.035 mM with an absorbance of 0.7 ± 0.02 at 734 nm. Methanolic solutions of the EOs were mixed (1
100) (v/v) with ethanol to 0.035 mM with an absorbance of 0.7 ± 0.02 at 734 nm. Methanolic solutions of the EOs were mixed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (v/v) with the prepared ABTS˙+ solution. After reaction at room temperature for 6 min, the absorbance at 734 nm was measured via Spectrophotometer 1510 (Thermo Fisher scientific) using a 96-well plate. Lower absorbance of the reaction mixture indicates higher ABTS˙+ scavenging activity.
4) (v/v) with the prepared ABTS˙+ solution. After reaction at room temperature for 6 min, the absorbance at 734 nm was measured via Spectrophotometer 1510 (Thermo Fisher scientific) using a 96-well plate. Lower absorbance of the reaction mixture indicates higher ABTS˙+ scavenging activity.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v/v). The reaction was then performed by mixing 2.8 mL of FRAP solution with 0.2 mL of the methanol diluted EO samples. The mixture was incubated at 37 °C for 30 min. The absorbance at 593 nm was determined against methanol as a blank.
10 (v/v/v). The reaction was then performed by mixing 2.8 mL of FRAP solution with 0.2 mL of the methanol diluted EO samples. The mixture was incubated at 37 °C for 30 min. The absorbance at 593 nm was determined against methanol as a blank.| AA(%) = [(A0 − A1)/A0] × 100 |
2.6. Antifungal assays in vitro
Four postharvest pathogenic fungi were used in the bioassay. Among them, Alternaria alternata was separated from naturally infected pear fruit marked black spot; Penicillium expansum was isolated from naturally blue mold occurred apples; Trichothecium roseum was isolated from decayed muskmelon fruit typically with pink mold; Fusarium sulphureum Schlechlendah, causal fungus of dry rot of potato tuber, was provided by the Institute of Plant Protection, Gansu Academy of Agricultural Sciences. All the fungal species were maintained on potato dextrose agar (PDA) plate at 4 °C and conidia of the pathogens were obtained as described by Deng et al.25| GI(%) = [(Gc − Gt)/Gc] × 100 |
| MGI(%) = [(C − T)/C] × 100 |
The minimum fungicidal concentrations (MFCs) were determined by taking agar plugs from above MIC experiment plates showing no visible mycelial growth and reinoculating them upside down on unamended PDA medium. MFC was regarded as the lowest EO concentrations preventing growth of the pathogens after seven-day incubation. There were three replicates for each EO test at each concentration.
2.7. Statistical analysis
All the experimental data were expressed in terms of mean values and subjected to statistical analysis with Minitab v.17.1. Percentage values of inhibition of spore germination were subjected to arcsine square root transformation before analysis of variance. Mean separation was performed by the Duncan's multiple range test at P < 0.05. All the graphs were drawn using the Origin Pro9 (OriginLab, MA, USA).3. Results and discussion
3.1. Remaining significant factors
Among the variables tested, HCl concentration, sieving mesh and acidolysis duration had significant effects (P < 0.05) on EO yield (Table 4). The coefficient of determination (R2 = 0.9822) indicates that 98.22% of the variability of the response could be explained by the model, so the goodness of fitting of the model was confirmed. The EO-yield-predicting polynomial model obtained from PBD regression analysis is expressed in terms of coded factors as:| Y = 2.5331 − 0.0826X1 + 0.0175X2 + 0.0427X3 − 0.0207X4 − 0.0180X5 − 0.0576X6 + 0.0827X7 + 0.1353X8 + 0.0736X9 |
| Term | DF | Adj. SS | Adj. MS | F-value | P-value |
|---|---|---|---|---|---|
| Model | 9 | 0.156133 | 0.017346 | 20.48 | 0.047 |
| Linear | 9 | 0.156113 | 0.017346 | 20.48 | 0.047 |
| X1 | 1 | 0.044380 | 0.044380 | 30.05 | 0.032 |
| X2 | 1 | 0.000895 | 0.000895 | 0.61 | 0.518 |
| X3 | 1 | 0.005238 | 0.005238 | 3.55 | 0.200 |
| X4 | 1 | 0.000978 | 0.000978 | 0.66 | 0.501 |
| X5 | 1 | 0.002070 | 0.002070 | 1.40 | 0.358 |
| X6 | 1 | 0.024510 | 0.024510 | 16.59 | 0.055 |
| X7 | 1 | 0.037705 | 0.037705 | 25.53 | 0.037 |
| X8 | 1 | 0.060747 | 0.060747 | 41.13 | 0.023 |
| X9 | 1 | 0.012543 | 0.012543 | 8.49 | 0.100 |
| Error | 2 | 0.002954 | 0.001477 | ||
| Total | 11 | 0.159067 | |||
| S = 0.0384323 | |||||
| R-sq = 0.9822 | |||||
| Adj. R-sq = 0.9023 | |||||
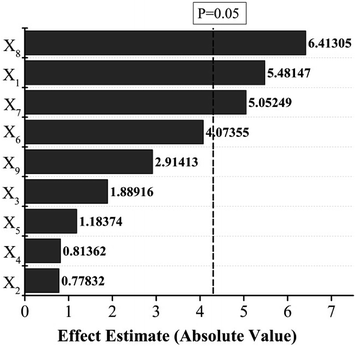
The Pareto chart (Fig. 1) illustrates that three parameters (acidolysis duration (X8), HCl concentration (X7) and sieving mesh (X1)) were significant factors. Microwave assistance applied in EO preparation in both solvent-free dry distillation29,30 and simultaneous combination with hydrodistillation11,31 can improve EO yield and shorten extraction time. Variations in microwave power and duration in the pretreatment of black cumin seeds can influence the oil pressing efficiency from the seeds.32 In the present work, short time microwave pretreatment was statistically excluded from the significant factors determining the EO yield. Enzymatic pretreatment with cellulase alone or allied with hemicellulase can increase the EO yields of both Thymus capitatus and Rosmarinus officinalis and elevate content of the predominant component carvacol for T. capitatus but a drop in the content of main component 1,8-cineole in the case of R. officinalis.33 In another case, spraying of the cumin seeds with cellulase solution increased its EO yield but changed the oil chemical profile.34 Back to the current exploration matrix, the influence of cellulase pretreatment upon EO yields was statistically regarded as insignificant.
3.2. BBD optimization of EO preparation
| Source | DF | Adj. SS | Adj. MS | F-value | P-value |
|---|---|---|---|---|---|
| a P < 0.01, highly significant; 0.01< P < 0.05, significant; P > 0.05, insignificant. | |||||
| Model | 9 | 1.586 | 0.176222 | 19.78 | <0.0001 |
| Linear | 3 | 0.65858 | 0.219526 | 24.64 | <0.0001 |
| X1 | 1 | 0.02498 | 0.024976 | 2.8 | 0.138 |
| X2 | 1 | 0.37368 | 0.37368 | 41.94 | <0.0001 |
| X3 | 1 | 0.25992 | 0.25992 | 29.17 | 0.001 |
| Square | 3 | 0.82068 | 0.273561 | 30.7 | <0.0001 |
| X12 | 1 | 0.58017 | 0.580166 | 65.11 | <0.0001 |
| X22 | 1 | 0.01087 | 0.010866 | 1.22 | 0.306 |
| X32 | 1 | 0.27175 | 0.271753 | 30.5 | 0.001 |
| 2-way interaction | 3 | 0.010674 | 0.035581 | 3.99 | 0.06 |
| X12 | 1 | 0.01254 | 0.012544 | 1.41 | 0.0274 |
| X13 | 1 | 0.03294 | 0.032942 | 3.7 | 0.096 |
| X23 | 1 | 0.06126 | 0.061256 | 6.87 | 0.034 |
| Error | 7 | 0.06237 | 0.00891 | ||
| Lack-of-fit | 3 | 0.04723 | 0.015743 | 4.16 | 0.101 |
| Pure error | 4 | 0.01514 | 0.003786 | ||
| Total | 16 | 1.64837 | |||
| S = 0.0943954 | |||||
| R2 = 0.9622 | |||||
| R2(Adj.) = 0.9135 | |||||
The significance of each coefficient was determined by the F-value and the P-value. In this case, X2, X3, X12, X32, X1X2 and X2X3 were significant model terms (P < 0.05). The most effective variables were HCl concentration and acidolysis duration in the process. The coefficients of independent variables determined by the second-order polynomial model are given below (in terms of coded variables):
| Y = 2.9534 + 0.0559X1 + 0.2161X2 − 0.1803X3 − 0.3712X12 + 0.0508X22 + 0.2541X32 − 0.0560X1X2 + 0.0907X1X3 − 0.1238X2X3 |
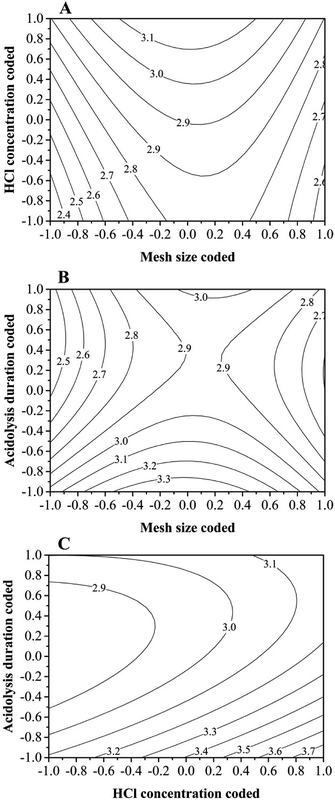
The influence of HCl on the EO yield at a given mesh size is likely to increase as the concentration is increased (Fig. 2A). But too high HCl concentration is impractical because of the detrimental effects on the EO quality and operator. Mesh size had the best performance at its near medium value. This is possibly due to the two opposite effects of grinding process. On one hand, grinding increases the specific surface area of the sample and elevates the efficiency of acidolysis and EO releasing; on the other hand, over grinding makes the sample temperature high and evaporation causes yield losses.
Effects of acidolysis duration and mesh size on EO yield at a fixed acid concentration of 2 M L−1 were shown in Fig. 2B. Acidolysis duration and mesh size appear to have a more complex and saddle-like pattern of effect on the oil yield, while the interaction between them was positively correlated with yield. The figure displays that for any acidolysis durations, the EO yields reached the maximal response when the mesh size was at medium value. By moving along the X and Y axes, it can be observed that at the coded acidolysis duration of 0.4 and at the medium mesh size there is a saddle point where the EO yield response was low. Below the saddle-point acidolysis duration value, the lower the acidolysis duration was, the higher the EO yield might be.
Fig. 2C displays the relationship and interaction between the acidolysis duration and HCl concentration in the preparation of EO. Contour lines indicate that the influence of acidolysis duration could diminish after some critical values, suggesting that extended acidolysis duration more than necessary means loss of EO yield. It should be emphasized that the acid concentration and acidolysis duration had a positive quadratic effect on the oil yield.
Theoretically it can be concluded from the regression equation that there is a combination zone of certain values of mesh size, HCl concentration and acidolysis duration where a highest EO yield response could be achieved. With the analyses of gained response contours and considering the damaging effects of too high HCl concentration on the EO quality, a compromise concentration was reached to be 2.5 M L−1. The optimized mesh size and acidolysis duration were 40 mesh and 45 min, respectively. Under above extraction conditions, the model response was 3.78%. This prediction was well validated by the value 3.86% achieved by confirmatory experiments under above optimized conditions.
Certain plant volatile compounds exist as glycosidically bound components14 and most of these glycosidic compounds contain aglycone moieties belonging to different classes of metabolites with a preponderance of phenols and terpenoids.35 Glycosylation of lipophilic volatile compounds keeps the labile cellular components from damage and protects plants themselves from toxicity.36 After being bound, these otherwise hydrophobic substances could achieve a good accumulation, transport, and storage.37 Subjected to acidolysis or enzymolysis, the glycosidic precursors can yield free volatiles.38 Seldom does conventional distillation without proper hydrolysis consider the liberating of bound volatiles in obtaining of EO. In the current work, acidolysis is assumed to break the glycosidical bound and assist the releasing of hydrophobic aglycones that can then be easily included in the EOs.
3.3. Impact on EO yield and composition
| Compounds | RT | KI | Peak area (%) | ||
|---|---|---|---|---|---|
| AEO | CEO | ||||
| a KI: Kovats retention indexes relative to C5–C24 n-alkanes on TG-WAX column. (−): not detected. | |||||
| Alkanes | 0.02 | ||||
| cis-Pinane | 7.96 | 1052 | — | 0.02 | |
| Alkenes | 34.68 | 47.07 | |||
| Cyclopentene | 6.75 | 987 | — | 0.01 | |
| Cyclene | 7.05 | 1007 | 0.02 | 0.01 | |
| α-Pinene | 7.29 | 1019 | 0.02 | 1.71 | |
| α-Thujene | 7.35 | 1022 | — | 1.47 | |
| 1-Isopropylcyclohex-1-ene | 7.38 | 1023 | 0.05 | — | |
| (+)-Camphene | 8.01 | 1054 | 0.05 | ||
| Camphene | 8.19 | 1063 | 0.26 | 0.04 | |
| 2,6-Dimethylocta-2,6-diene | 8.65 | 1085 | — | 0.03 | |
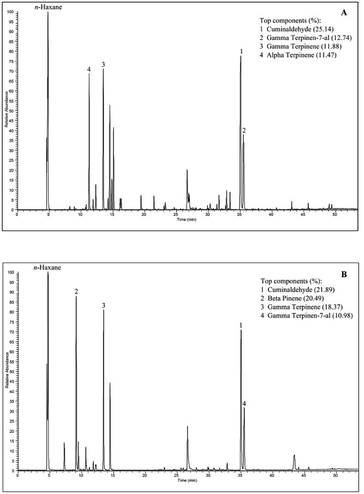
| β-Pinene | 9.18 | 1108 | 0.12 | 20.49 | |
| Sabinene | 9.52 | 1119 | — | 2.45 | |
| (−)-β-Pinene | 9.75 | 1127 | — | 0.02 | |
| (+)-p-Menth-1-ene | 9.89 | 1131 | — | 0.10 | |
| 1-Methyl-4-isopropyl-1-cyclohexene | 9.91 | 1132 | 0.05 | — | |
| Carene | 10.27 | 1144 | — | 0.12 | |
| Myrcene | 10.70 | 1158 | 0.16 | — | |
| α-Phellandrene | 10.78 | 1161 | 0.37 | 0.36 | |
| α-Terpinene | 11.23 | 1176 | 11.47 | 0.25 | |
| (±)-Limonene | 11.87 | 1197 | 0.74 | 0.71 | |
| (3E)-3,7-Dimethyl-1,3,6-octatriene | 13.06 | 1229 | 0.03 | — | |
| γ-Terpinene | 13.49 | 1241 | 11.88 | 18.37 | |
| Terpinolene | 14.81 | 1276 | 7.70 | 0.14 | |
| Bicyclo[4.2.0]oct-1-ene,7-endo-ethenyl | 16.13 | 1311 | 0.93 | — | |
| (4E,6Z)-2,6-Dimethylocta-2,4,6-triene | 18.61 | 1372 | 0.03 | — | |
| β-Cyclocitral | 24.44 | 1516 | — | 0.06 | |
| Cadina-1,4-diene < trans-> | 27.57 | 1595 | 0.14 | — | |
| (−)-Isosativene | 28.56 | 1633 | 0.04 | — | |
| trans-β-Farnesene | 29.88 | 1686 | 0.33 | 0.26 | |
| (−)-α-Acoradiene | 30.78 | 1711 | 0.15 | — | |
| α-Neocallitropsene | 30.8 | 1712 | — | 0.23 | |
| β-Cedrene | 30.92 | 1724 | 0.03 | 0.14 | |
| β-Bisabolene | 32.09 | 1738 | 0.11 | 0.06 | |
| (E)-Anethole | 36.86 | 1850 | — | 0.04 | |
| Phenols | 0.51 | 0.18 | |||
| (+/−)-cis-Piperitol | 33.37 | 1764 | — | 0.05 | |
| Thymol | 48.08 | 2200 | 0.10 | ||
| Carvacrol | 49.00 | 2229 | 0.06 | 0.03 | |
| p-Cumenol | 49.40 | 2241 | 0.35 | 0.10 | |
| Alcohols | 7.76 | 1.86 | |||
| Cineole | 12.28 | 1209 | 2.20 | 0.51 | |
| Cyclohexanol | 22.49 | 1468 | 0.10 | ||
| 1-(3,3-Dimethylcyclohexylidene)ethanol | 24.86 | 1527 | — | 0.02 | |
| (−)-cis-Caran-trans-(5)-ol | 25.62 | 1546 | 0.08 | — | |
| Linalool | 25.65 | 1547 | — | 0.20 | |
| Octan-1-ol | 26.03 | 1556 | 0.06 | — | |
| 1-Methyl-4-(1-methylethyl)-2-cyclohexen-1-ol | 26.45 | 1567 | 1.46 | 0.09 | |
| 1,3,3-Trimethylbicyclo[2.2.1]heptan-2-ol | 27.02 | 1582 | 1.31 | — | |
| 1,5,5-Trimethyl-norbornan-2-ol | 27.29 | 1588 | 0.18 | — | |
| (−)-Terpinen-4-ol | 28.05 | 1612 | 0.12 | 0.23 | |
| β-Terpineol | 29.11 | 1655 | 0.04 | — | |
| (+/−)-Isoborneol | 31.67 | 1730 | 1.40 | — | |
| α-Terpineol | 31.7 | 1733 | — | 0.19 | |
| (4-Prop-1-en-2-ylcyclohexyl)methanol | 37.87 | 1879 | — | 0.07 | |
| 2-(2,4-dimethylphenyl)ethanol | 43.10 | 2039 | 0.45 | — | |
| (4-Propan-2-ylcyclohexa-1,4-dien-1-yl)methanol | 44.1 | 2071 | — | 0.22 | |
| Cuminol | 45.70 | 2122 | 0.46 | 0.23 | |
| Aldehydes | 45.72 | 41.49 | |||
| Furfural | 23.22 | 1486 | 0.72 | — | |
| γ-Terpinen-7-al | 26.65 | 1572 | 5.43 | 7.95 | |
| Myrtenal | 29.27 | 1662 | — | 0.03 | |
| Phellandral | 32.88 | 1754 | 1.50 | 0.64 | |
| Cuminaldehyde | 35.08 | 1799 | 25.14 | 21.89 | |
| γ-Terpinen-7-al | 35.57 | 1813 | 12.74 | 10.98 | |
| 2-Methyl-3-phenylacrolein | 40.69 | 1964 | 0.19 | — | |
| Esters | 0.13 | 0.03 | |||
| Terpinylformate | 24.37 | 1514 | 0.06 | — | |
| trans-Pinocarveyl acetate | 29.63 | 1676 | — | 0.03 | |
| d-Sabinyl-acetate[trans-] | 48.87 | 2225 | 0.07 | — | |
| Ketones | 0.59 | 0.00 | |||
| trans-4-Caranone | 28.13 | 1615 | 0.03 | — | |
| (−)-cis-Carane-4-one | 28.72 | 1639 | 0.17 | — | |
| 3-(Isopropyl)-6-methylcyclohex-2-en-1-one; | 32.69 | 1750 | 0.31 | — | |
| 6,10,14-Trimethyl-2-pentadecanone | 45.88 | 2128 | 0.08 | — | |
| Miscellaneous | 10.63 | 9.25 | |||
| 1-Isopropyliden-3-methylencyclopentan | 8.85 | 1095 | — | 0.04 | |
| Ocimenequintoxide | 12.65 | 1218 | 0.05 | — | |
| p-Cymene | 14.49 | 1268 | 8.99 | 8.89 | |
| 2-p-Tolyl-1-propene | 21.43 | 1442 | 1.22 | — | |
| Daucene | 23.03 | 1481 | 0.33 | 0.23 | |
| (−)-Germacrene-D | 24.93 | 1529 | — | 0.04 | |
| Ionene | 29.66 | 1677 | 0.04 | — | |
| Isodaucene | 32.29 | 1742 | — | 0.05 | |
3.4. Antioxidant activity
As demonstrated (Table 7), the DPPH and ABTS radical scavenging activities of AEO were 2.41 and 4.11-fold of that of CEO, respectively. The ferric reducing antioxidant power of AEO was 73.75 times as strong as that of CEO. In β-carotene bleaching, the AEO achieved a value 3.20 times as much as CEO did. The four antioxidant testing methods employed in the current work evaluated the sample antioxidant capacities based on different chemical mechanisms. The AEO displayed highly significantly stronger antioxidant capacities in every assay conducted than the CEO (P < 0.01). This might be due to the relative higher content of phenols in AEO, and this type of compound has particular antioxidant properties to delay or stop the aerobic oxidation of organic matter. It has been generally recognized that phenolic compounds act as antioxidants due to their high reactivity with peroxyl radicals, which are quenched by formal hydrogen atom transfer.42 In contrast, the CEO included more non-phenolic terpenoids that can easily undergo autoxidation to form unsaturated lipids.43 Thus poor antioxidant protection can be expected from CEO because products arising from autoxidation of these components or their cooxidation with substances to be protected in the matrices are reactive species with potential of propagating the oxidative chain.44 When obtained from the same botanical material but with different extraction techniques, the EOs with more phenolic compounds might hold stronger antioxidant potency than rivals containing more non-phenolic terpenoids, particularly unsaturated ones.45,46| EOs | DPPH(mMTE L−1) | ABTS(mMTE L−1) | FRAP(mMTE L−1) | BCBT(%) |
|---|---|---|---|---|
| a TE: trolox equivalent; **: P < 0.01 values are means ± SD. | ||||
| AEO | 92.84 ± 11.93** | 231.34 ± 2.88** | 159.31 ± 0.76** | 55.17 ± 5.97** |
| CEO | 38.49 ± 11.92 | 56.27 ± 13.97 | 2.16 ± 1.03 | 17.24 ± 10.34 |
3.5. Antifungal activity analysis
| T. roseum | A. alternata | F. sulphureum | P. expansum | ||
|---|---|---|---|---|---|
| a Each value represents the mean of three replicates. Peer data in same row of a subgroup with differing superscript lower cases are significantly different (P < 0.05) between two concentrations of the same preparation. Peer data in same column with differing superscript capitals are significantly different (P < 0.05) between tested fungi at same EO concentration. P values with shoulder “*” and “**” indicating significant difference (P < 0.05) and highly significant difference (P < 0.01), respectively, between two preparations at same concentration. | |||||
| AEO(μL L−1) | 400 | 49.8cA | 13.79cBC | 31.75dB | 22.52dC |
| 800 | 55.17cA | 32.57bC | 43.80cB | 41.98cB | |
| 1200 | 67.05bAB | 45.98aC | 71.53bA | 61.83bB | |
| 1600 | 80.08aB | 53.64aC | 90.88aA | 75.19aB | |
| CEO(μL L−1) | 400 | 21.07dAB | 13.41dB | 24.45cA | 20.23dAB |
| 800 | 39.85cA | 22.61cB | 42.7bA | 27.86cB | |
| 1200 | 54.79bA | 37.55bB | 64.23aA | 56.87bA | |
| 1600 | 64.37aB | 50.19aC | 74.09aA | 72.14aAB | |
| P400 | 0.002** | 0.923 | 0.147 | 0.616 | |
| P800 | 0.018* | 0.025* | 0.766 | 0.008** | |
| P1200 | 0.054 | 0.119 | 0.259 | 0.182 | |
| P1600 | 0.002** | 0.355 | 0.007** | 0.345 | |
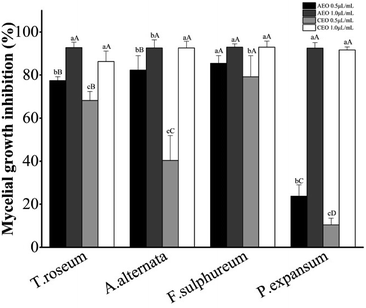
As to this spore static effect of both EOs at a particular concentration, a rough rule is that T. roseum, F. sulphureum and P. expansum are more sensitive to cumin EO than A. alternata. For each tested strain, there was at least one treatment concentration at which the two EOs showed significant difference in spore static efficacy. Particularly, this efficacy presented highly significant difference (P < 0.01) between the two EOs when assayed against T. roseum (400 μL L−1), F. sulphureum (1600 μL L−1) and P. expansum (800 μL L−1).
These differences in spore static capacities are connected with their compositional distinction. The relationship between the EO composition especially dominating components and its antimicrobial activities was well documented. Compounds, like phenols, possessing a system of delocalized electrons (aromatic ring) and a hydroxyl group display higher antimicrobial activities than other EO constituents.47 EOs containing more phenols or aldehydes manifested the highest antimicrobial activity followed by EOs containing terpene alcohols, whereas other EOs containing terpene hydrocarbons were generally ineffective.48–50 Among other constituents, the monoterpene phenols and aldehyde derivatives were found to be the most active constituents in the EOs, and other terpene alcohols and hydrocarbons like α-pinene, 1, 8-cineole, terpinene-4-ol and γ-terpinene were generally found to have moderate or weak antifungal activity.51
Using a volatile phase method, Zamani-Zadeh and coworkers39 evaluated the antifungal activity of cumin EO against pathogenic fungus Botrytis spp. and proved that the activity is dose-dependent but ineffective below the concentration of 3 μL mL−1. With the food poison technique, Behdani et al.52 determined the strong effect of cumin EO against B. cinerea mycelial growth on PDA culture even at the concentration of 0.25 μL mL−1.
| EOs | T. roseum | A. alternata | F. sulphureum | P. expansum | |
|---|---|---|---|---|---|
| a MIC: minimum inhibitory concentration; MFC: minimum fungicidal concentration. | |||||
| AEO | MIC | 1000 | 1000 | 1000 | 2000 |
| MFC | 2000 | 4000 | 4000 | 4000 | |
| CEO | MIC | 2000 | 4000 | 4000 | 4000 |
| MFC | >4000 | >4000 | >4000 | >4000 | |
Among the recorded MICs the least MIC (1000 μL L−1) was on the AEO against A. alternata, T. roseum and F. sulphureum, but this value against P. expansum was 2000 μL L−1. By comparison, the least MIC of 2000 μL L−1 was only measured on CEO against T. roseum and the value was two times folded when it came to other three fungi. The best fungicidal effect was demonstrated by AEO against T. roseum with an MFC value of 2000 μL L−1 while this value was 4000 μL L−1 against the remaining three fungi. In contrast, the MFCs (>4000 μL L−1) of CEO against all four fungi were outperformed by that of AEO. Simply put, the AEO had stronger antifungal activities than the CEO against four postharvest fungi tested. Interestingly, after transferring the inhibited Botrytis spp. mycelia disk from PDA treated with vapor phase cumin EO at MIC to non-treated PDA, Zamani-Zadeh et al.39 observed no mycelial growth and thereby determined the fungicidal nature of the cumin EO against the fungus.
Presumably the variance in the chemical composition of EOs like ‘AEO’ and ‘CEO’ could account for the differences in their bioactivities, but determination of the exact responsible components seems not easy. When we emphasized the key roles of certain compounds that can individually demonstrate noticeable activities, the holistic effect of different components of the EO should not be neglected. Milos and Makota53 investigated the antioxidant synergisms and antagonisms among thymol, carvacrol, thymoquinone and p-cymene, and concluded that majority of mixtures displayed some discrepancy in antioxidant capacity when compared to that of their individual constituents. Multiple components of EO partake in a bioactivity performance but they may have different function targets or mechanisms, and in some cases even exist offsetting between positive and negative effects. Nonetheless, the compositional information of EOs could adduce evidence in the rough comparison of some of their bioactivities. The relative antifungal activities of EOs might be tentatively judged according to their predominant components content as following: phenols > alcohols > aldehydes > ketones > ethers > hydrocarbons.54 All these views were well mirrored in the relationship of compositional profile and bioactivities of the AEO and CEO, two preparations from the same botanical material.
The seeking a higher yield of EO extraction never stops since our ancestors' invention of the earliest EO distillation process. Particularly in recent couple of decades, modern high technology and sophisticated equipment conferred researchers and industry more opportunities to elevate the EO yield to an even higher degree. But this dependence on fashionable technology and equipment often renders the extraction costly. Revisiting some traditional means may advance our understanding and application of these technical arsenals during the EO preparation. Acidolysis is an economical operation extensively utilized in biochemical industries especially for the pretreatment of raw materials, but until now, it was not reported in pretreatment of herbal materials for the EO distillation. To our knowledge, the present work is a fresh report on the application of acidolysis pretreatment to the EO extraction and thereby the influences on the EO yield, composition and bioactivities.
The current investigation intended to integrate acidolysis, pulverization, enzymolysis and microwave irradiation into the pretreatment of cumin seeds prior to distillation. After statistical analysis based on PBD and BBD designs, two relative new arsenals, enzymolysis and short time microwave irradiation were screened out, and the two conventional technical elements, acidolysis and pulverization remained and their best performing zone were also determined. When properly applied, the two technical elements offered the following hydrodistillation a more than 50% increase of EO yield, this elevation were brilliant in comparison to that made by most of other technical improvements documented. Due to the liberation of more otherwise bound phytochemicals, remarkable species of new components were procured in the EO, which imparted the EO impressive variation of chemical profile, and enhanced antioxidant and antifungal bioactivities.
4. Conclusion
Acidolysis and pulverization extent have significant effects on the EO yield of cumin seeds. The optimal pretreatment was: pulverizing the seeds to 40 mesh and conducting 45 min acidolysis in 2.5 M L−1 HCl, wherein the predicted EO yield 3.78% was near close with the experimental value 3.86%. The acidolysis-dominated pretreatment gave the seeds an EO-yield increase of 50.78% over the control sample (2.56%). AEO and CEO shared the predominant component cuminaldehyde, but they had different chemical profiles. The AEO showed significantly higher antioxidant capability in DPPH radical scavenging, ABTS radical scavenging and ferric reducing antioxidant power assays and β-carotene bleaching test than CEO did. Additionally, the antifungal activities of AEO against four postharvest pathogenic fungi, viz. A. alternata, P. expansum, T. roseum and F. sulphureum outperformed that of CEO in most circumstances. This surpassing of bioactivities of AEO is resulted from its chemical characteristics imparted by the acidolysis-dominated pretreatment. The application of this novel pretreatment might be trialed on other aromatic plant materials to achieve valorization of their EO exploitation.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work is financed by National Natural Science Foundation of China (31360416), Key Sci-tech Problem Tackling Project on Chinese Materia Media Industry of Gansu Province (2014) and Agricultural Sci-tech Innovation Program (GNCX-2012-42) of Gansu Province.References
- H. Hajlaoui, H. Mighri, E. Noumi, M. Snoussi, N. Trabelsi, R. Ksouri and A. Bakhrouf, Food Chem. Toxicol., 2010, 48, 2186–2192 CrossRef PubMed.
- S. Mnif and S. Aifa, Chem. Biodiversity, 2015, 12, 733–742 CrossRef PubMed.
- T. Allahghadri, I. Rasooli, P. Owlia, M. J. Nadooshan, T. Ghazanfari, M. Taghizadeh and S. D. A. Astaneh, J. Food Sci., 2010, 75, H54–H61 CrossRef PubMed.
- A. Kedia, B. Prakash, P. K. Mishra and N. K. Dubey, Int. J. Food Microbiol., 2014, 168–169, 1–7 CrossRef PubMed.
- R. M. O. F. Sousa, J. S. Rosa, L. Oliveira, A. Cunha and M. Fernandes-Ferreira, Ind. Crops Prod., 2015, 63, 226–237 CrossRef.
- J. Wei, X. Zhang, Y. Bi, R. Miao, Z. Zhang and H. Su, J. Evidence-Based Complementary Altern. Med., 2015 DOI:10.1155/2015/474509.
- A. Tsoupras, R. Lordan and I. Zabetakis, Nutrients, 2018, 10 DOI:10.3390/nu10050604.
- U. E. Bauer, P. A. Briss, R. A. Goodman and B. A. Bowman, Lancet, 2014, 384, 45–52 CrossRef.
- R. Ascrizzi, J. González-Rivera, C. S. Pomelli, C. Chiappe, P. Margari, F. Costagli, I. Longo, M. R. Tiné, G. Flamini and C. Duce, React. Chem. Eng., 2017, 2, 577–589 RSC.
- T. Fornari, G. Vicente, E. Vázquez, M. R. García-Risco and G. Reglero, J. Chromatogr. A, 2012, 1250, 34–48 CrossRef PubMed.
- M. M. A. Rashed, Q. Tong, A. Nagi, J. P. Li, N. U. Khan, L. Chen, A. Rotail and A. M. Bakry, Ind. Crops Prod., 2017, 100, 236–245 CrossRef.
- L. K. Sharma, D. Agarwal, S. S. Rathore, S. K. Malhotra and S. N. Saxena, J. Food Sci. Technol., 2016, 53, 2827–2834 CrossRef PubMed.
- R. Kowalski, G. Kowalska, J. Jamroz, A. Nawrocka and D. Metyk, Ultrason. Sonochem., 2015, 24, 214–220 CrossRef PubMed.
- B. Sgorbini, C. Cagliero, A. Pagani, M. Sganzerla, L. Boggia, C. Bicchi and P. Rubiolo, Phytochemistry, 2015, 117, 296–305 CrossRef PubMed.
- Y. Zhai, S. Sun, Z. Wang, J. Cheng, Y. Sun, L. Wang, Y. Zhang, H. Zhang and A. Yu, J. Sep. Sci., 2009, 32, 3544–3549 CrossRef PubMed.
- S. Behera, S. Nagarajan and L. Jagan Mohan Rao, Food Chem, 2004, 87, 25–29 CrossRef.
- H. Xiang, L. Zhang, L. Xi, Y. Yang, X. Wang, D. Lei, X. Zheng and X. Liu, Ind. Crops Prod., 2018, 111, 298–305 CrossRef.
- Pharmacopoeia Committee of People’s Republic China, Pharmacopoeia of People's Republic China, 2015 Search PubMed.
- T. Belwal, I. D. Bhatt, R. S. Rawal and V. Pande, Ind. Crops Prod., 2017, 95, 393–403 CrossRef.
- R. Rodríguez-Solana, J. M. Salgado, J. M. Domínguez and S. Cortés-Diéguez, Ind. Crops Prod., 2014, 60, 186–192 CrossRef.
- G. B. Avanço, F. D. Ferreira, N. S. Bomfim, P. A. de S. R. dos Santos, R. M. Peralta, T. Brugnari, C. A. Mallmann, B. A. de Abreu Filho, J. M. G. Mikcha and M. Machinski Jr, Food Control, 2016, 73, 1–8 Search PubMed.
- A. Carrasco, V. Ortiz-Ruiz, R. Martinez-Gutierrez, V. Tomas and J. Tudela, Ind. Crops Prod., 2015, 73, 16–27 CrossRef.
- O. Aissi, M. Boussaid and C. Messaoud, Ind. Crops Prod., 2016, 91, 56–65 CrossRef.
- I. Jallali, Y. Zaouali, I. Missaoui, A. Smeoui, C. Abdelly and R. Ksouri, Food Chem, 2014, 145, 1031–1038 CrossRef PubMed.
- J. Deng, Y. Bi, Z. Zhang, D. Xie, Y. Ge, W. Li, J. Wang and Y. Wang, Postharvest Biol. Technol., 2015, 106, 53–61 CrossRef.
- G. S. Chitarra, P. Breeuwer, M. J. R. Nout, A. C. Van Aelst, F. M. Rombouts and T. Abee, J. Appl. Microbiol., 2003, 94, 159–166 CrossRef PubMed.
- A. Ben Ghnaya, I. Amri, M. Hanana, S. Gargouri, B. Jamoussi, A. Romane and L. Hamrouni, Ind. Crops Prod., 2016, 83, 113–117 CrossRef.
- H. Boubaker, H. Karim, A. El Hamdaoui, F. Msanda, D. Leach, I. Bombarda, P. Vanloot, A. Abbad, E. H. Boudyach and A. Ait Ben Aoumar, Ind. Crops Prod., 2016, 86, 95–101 CrossRef.
- Q. Chen, Z. Hu, F. Y. D. Yao and H. Liang, LWT--Food Sci. Technol., 2016, 66, 538–545 CrossRef.
- B. Bayramoglu, S. Sahin and G. Sumnu, J. Food Eng., 2008, 88, 535–540 CrossRef.
- N. Jeyaratnam, A. H. Nour, R. Kanthasamy, A. H. Nour, A. R. Yuvaraj and J. O. Akindoyo, Ind. Crops Prod., 2016, 92, 57–66 CrossRef.
- H. Bakhshabadi, H. Mirzaei, A. Ghodsvali, S. M. Jafari, A. M. Ziaiifar and V. Farzaneh, Ind. Crops Prod., 2017, 97, 1–9 CrossRef.
- K. Hosni, I. Hassen, H. Chaâbane, M. Jemli, S. Dallali, H. Sebei and H. Casabianca, Ind. Crops Prod., 2013, 47, 291–299 CrossRef.
- H. B. Sowbhagya, P. Srinivas, K. T. Purnima and N. Krishnamurthy, Food Chem, 2011, 127, 1856–1861 CrossRef.
- P. Winterhalter and G. K. Skouroumounis, Adv. Biochem. Eng./Biotechnol., 1997, 55, 73–105 CrossRef PubMed.
- E. Stahl-Biskup, F. Intert, J. Holthuijzen, M. Stengele and G. Schulz, Flavour Fragrance J., 1993, 8, 61–80 CrossRef.
- W. Hoesel, Biochem. Plants, 1981, 7, 725–753 Search PubMed.
- J. N. Ren, Y. N. Tai, M. Dong, J. H. Shao, S. Z. Yang, S. Y. Pan and G. Fan, Food Chem, 2015, 185, 25–32 CrossRef PubMed.
- M. Zamani-Zadeh, S. Soleimanian-Zad, M. Sheikh-Zeinoddin and S. A. H. Goli, Postharvest Biol. Technol., 2014, 92, 149–156 CrossRef.
- R. Li and Z. T. Jiang, Flavour Fragrance J., 2004, 19, 311–313 CrossRef.
- P. N. Dubey, S. N. Saxena, B. K. Mishra, R. K. Solanki, M. K. Vishal, B. Singh, L. K. Sharma, S. John, D. Agarwal and A. Yogi, Ind. Crops Prod., 2017, 95, 50–59 CrossRef.
- M. C. Foti, J. Pharm. Pharmacol., 2007, 59, 1673–1685 CrossRef PubMed.
- U. Neuenschwander, F. Guignard and I. Hermans, ChemSusChem, 2010, 3, 75–84 CrossRef PubMed.
- R. Amorati, M. C. Foti and L. Valgimigli, J. Agric. Food Chem., 2013, 61, 10835–10847 CrossRef PubMed.
- M. M. Esquível, M. A. Ribeiro and M. G. Bernardo-Gil, J. Supercrit. Fluids, 1999, 14, 129–138 CrossRef.
- B. Marongiu, A. Piras, S. Porcedda, R. Casu and P. Pierucci, J. Essent. Oil Res., 2005, 17, 530–532 CrossRef.
- A. Ultee, M. H. J. Bennik and R. Moezelaar, Appl. Environ. Microbiol., 2002, 68, 1561–1568 CrossRef PubMed.
- A. Ait-Ouazzou, L. Cherrat, L. Espina, S. Lorán, C. Rota and R. Pagán, Innovative Food Sci. Emerging Technol., 2011, 12, 320–329 CrossRef.
- J. Carneiro de Barros, M. Lúcia da Conceição, N. J. Gomes Neto, A. C. Vieira da Costa, J. P. Siqueira, I. D. Basílio and E. Leite de Souza, LWT--Food Sci. Technol., 2009, 42, 1139–1143 CrossRef.
- M. M. Tajkarimi, S. A. Ibrahim and D. O. Cliver, Food Control, 2010, 21, 1199–1218 CrossRef.
- M. Nikkhah, M. Hashemi, M. B. Habibi Najafi and R. Farhoosh, Int. J. Food Microbiol., 2017, 257, 285–294 CrossRef PubMed.
- M. Behdani, M. Pooyan and S. Abbasi, Int. J. Agric. Crop Sci., 2012, 4, 1012–1016 Search PubMed.
- M. Milos and D. Makota, Food Chem, 2012, 131, 296–299 CrossRef.
- P. Matusinsky, M. Zouhar, R. Pavela and P. Novy, Ind. Crops Prod., 2015, 67, 208–215 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |