Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development and evaluation of an IgY based silica matrix immunoassay platform for rapid onsite SEB detection†
J.
Achuth
 a,
R. M.
Renuka
a,
K.
Jalarama Reddy
b,
M. S.
Shivakiran
c,
M.
Venkataramana
*a and
K.
Kadirvelu
*a
a,
R. M.
Renuka
a,
K.
Jalarama Reddy
b,
M. S.
Shivakiran
c,
M.
Venkataramana
*a and
K.
Kadirvelu
*a
aDRDO-BU-CLS, Bharathiar University Campus, Coimbatore, Tamilnadu-641046, India. E-mail: ramana.micro@gmail.com; Kadirvelukrishna@yahoo.com; Tel: +0422 2428162
bFreeze Drying and Animal Product Technology Division, Defence Food Research Laboratory, Siddarthanagar, Mysore, Karnataka- 570011, India
cDepartment of Biotechnology, Vignan's University, Guntur, Andhra Pradesh-522213, India
First published on 16th July 2018
Abstract
The present study involves immunoassay platform development based on a surface functionalized silica matrix for rapid onsite detection of Staphylococcal enterotoxin B (SEB). Silica matrix functionalization as well as the immunoassay parameters was experimentally designed and optimized through response surface methodology (RSM). Silica surface functionalization was carried out with hydrofluoric acid (HF), ammonia, 3-aminopropyl triethoxysilane (APTES) and glutaraldehyde (GA). The RSM optimized matrix functionalization parameters for HF, ammonia, APTES and GA were determined to be 10%, 40%, 20% and 10% (V/V), respectively. Antibodies for the study were generated against recombinant SEB toxin in rabbit (anti-SEB IgG) and chicken (anti-SEB IgY). Subsequently, antibodies were immobilized on the functionalized silica matrix and were further characterized by SEM and contact angle measurements to elucidate the surface uniformity and degree of hydrophilicity. The immunoassay platform was developed with anti-SEB IgG (capturing agent) and anti-SEB IgY (revealing partner). The limit of detection (LOD) of the developed platform was determined to be 0.005 μg mL−1 and no cross-reactivity with similar toxins was observed. Upon co-evaluation with a standard ELISA kit (Chondrex, Inc) against various field isolates, the platform was found to be on par and reliable. In conclusion, the developed method may find better utility in onsite detection of SEB from resource-poor settings.
1. Introduction
The study of biomolecular interactions in a multi protein screening of biological/medical samples through miniaturized array-based technology is a rapidly advancing field.1 The silica matrix can be tailored by various surface functionalizations as well as adhesion chemistries to accommodate biomolecules via adsorption and covalent immobilization. The chemistry involved in the background plays a decisive role in the stability and durability of the functionalized surface.2 Some of the existing platforms are based on the principles of bioaffinity recognition, physisorption and covalent immobilization of biomolecules on the base substrate.3,4 The non-covalent attachment of molecules increases slide noise and spot background resulting in false positive results. Hence, the stable linkage involving covalent bonding between the molecules is ideal for development of robust and durable detection systems.5,6 The optimization of conditions for surface functionalization often significantly influences the binding properties of molecules involved and additionally could also enhance the preservation of bioprobes' native conformation/orientation.1,7 Functionalization of the matrix surface with amine, sulfhydryl, carboxyl and amino N hydroxyl succinamide (NHS) ester or epoxide end groups is commonly used for covalent immobilization of proteins.3 Glass has the capability to adsorb considerably more water than its precursor material (silicon dioxide), thus ensuring a greater surface hydration that ultimately results in accelerated silanol group formation on the surface.8,9 Self-assembled monolayers condense at high temperature (curing) and stabilize into siloxane linkages over the surface by cross-linking with adjacent silanols due to the absence of local water molecules.3 The functionalized surface becomes hydrophobic due to the presence of non-reactive alkyl groups in silane.10 The free amino groups project outwards producing amine functionality while protonated acidic groups orient themselves towards the glass surface.11The surface activation of matrix necessitates the application of several functionalization agents that could successfully incorporate the desired functional groups. The determinations of optimum concentration for these agents under laboratory conditions are tedious and time consuming. Therefore, an in silico approach towards matrix functionalization overcomes such hurdles. In statistics, response surface methodology (RSM) explores the relationships between several explanatory variables and one or more response variables.13 RSM is a collection of mathematical and statistical techniques and will be useful for the modeling and analysis of problems in which a response of interest is influenced by several variables and the objective is to optimize this response.12,13 The main reason for the use of RSM encompasses the use of experimental design, generation of mathematical equations and graphics outcomes by employing multi-various factors, statistical experimental design fits into mathematical equations for prediction and optimization of factorial responses under study environment.14 The RSM analysis also reduces the cost and time of overall analysis by reaching the optimal values of variables with the smallest number of experiments in the shortest time duration.15,35 The first step involves identification of factors that affect experimental data followed by design of the experiment in order to minimize the effects of uncontrollable parameters and finally statistical analysis to separate the effects of the various factors.16 The criteria for the optimal design of experiments are mostly associated with the mathematical model of the process which is generally polynomials with unknown structure. The designs could be of full factorial design approach, central composite rotatable design and D optimal design, wherein the central composite rotatable design is selected in the present study.17 The objective of RSM in present study was to optimize the silica matrix functionalization factors and bioprobes in order to develop a sensitive, high responsive detection platform. Designed RSM models were further validated through laboratory protocols thus to develop cost-effective in vitro diagnostic platform for detection of SEB.
Staphylococcus aureus comes under the list of pathogenic organisms that poses a serious challenge during clinical infections. S. aureus produces a wide variety of exotoxins and related virulence factors such as cytolysins etc., that alter immune function during the local infection environment.18 The Staphylococcal SAgs secreted in late stationary phase results in serious human illnesses, such as TSS through their effects on T lymphocyte and APC cytokine production, also SFP (Staphylococcal food poisoning) is one of the most prevalent causes of gastroenteritis worldwide caused by SE's (Staphylococcal enterotoxins).19 The organism is also profoundly gaining resistance to antibiotics and with the likes of α-hemolysin, several enterotoxins (from SEA to SHV), TSST and other secretory proteins it poses a serious emerging threat.20–22 Furthermore, SEB is one of the most potent potential agents of bioterrorism, despite its ban after Biological and Toxin Weapons Convention (BWC) of 1972, it remains as serious concern that SEB could be used as bio-warfare agent.23 The SEB toxin could also be aerosolized and its superantigenic nature leads to incapacitation of enemy forces.24 It is therefore critical to develop countermeasures to prevent or treat the lethal and incapacitating effects of SEB.25
In the present study, an IgY based silica matrix immunoassay platform for rapid and onsite SEB detection was developed. SEB is a potent biothreat agent with LD50 and ED50 values of 20 ng kg−1 and 0.4 ng kg−1 body weights respectively.26 There are numerous detection platforms available that follows enzyme-linked immunosorbent assay and bio-nano transduction principle and sensitive enough to detect 2.9 ng mL−1 and 10 ng mL−1, respectively.27,28 However, for onsite detection of toxin and pathogens, detection systems developed by avoiding tedious laboratory settings and skilled manpower is requisite. Moreover, a cost effective detection platform will also ensure its deployment under resource-poor settings.29 Unfortunately, existing detection assays involve sample pre-incubation to reduce binding of SpA to IgG that makes it tedious and time-consuming.30 Avian IgY antibodies doesn't have an affinity towards Staphylococcal protein A making it suitable for immunoassays that involve S. aureus related toxins and antigens.31 The avian IgY and mammalian IgG are functionally equivalent but the former has added advantage of being non-invasive, economic, convenient, and also quantitatively higher than the latter.29 Hence, the study was intended to develop a cost-effective, sensitive onsite SEB detection platform from food and environmental sources. In brief, a theoretical design for surface functionalization was established through RSM and its characterization was carried out by scanning electron microscopic analysis and contact angle measurements. This was further employed in development of SEB detection platform. The platform was evaluated for its sensitivity, specificity and reliability for onsite detection by assessing several pure cultures as well as naturally contaminated food samples.
2. Experimental
2.1 Materials
Microscopic glass slides and potassium dichromate were obtained from HiMedia (Mumbai, India). Hydrofluoric acid (HF), sulphuric acid, ammonia and glutaraldehyde (GA) were obtained from Merck Millipore (Bengaluru, India). 3′-Aminopropyl triethoxysilane (APTES) was obtained from Sigma Aldrich (USA). Tetra methylene benzidine–hydrogen peroxide (TMB/H2O2) was obtained from Aristogene Pvt Ltd (Bengaluru, India). Freund's complete and incomplete adjuvant, horse anti-rabbit IgG HRP and donkey anti-chicken IgY HRP were procured from Sigma Aldrich (US). Other chemicals used in the study were fine grade and obtained from Merck Millipore (Bengaluru, India).2.2 Preparation and evaluation of bioprobes for immunoassay
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min and stored at −20 °C until further use. The anti-SEB IgG titer was determined onto rSEB (10 μg per well) coated immunoassay plates followed by standard indirect ELISA protocols. (Ethical statement: ANUCPS/IAEC/AH/Protocol/2/2014: Dt 15/07/2014).
000 rpm for 5 min and stored at −20 °C until further use. The anti-SEB IgG titer was determined onto rSEB (10 μg per well) coated immunoassay plates followed by standard indirect ELISA protocols. (Ethical statement: ANUCPS/IAEC/AH/Protocol/2/2014: Dt 15/07/2014).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8000) in sera, IgY was purified from eggs by PEG precipitation method.33 The anti-SEB IgY titer was determined onto rSEB (10 μg per well) coated immunoassay plates followed by standard indirect ELISA protocols.32
8000) in sera, IgY was purified from eggs by PEG precipitation method.33 The anti-SEB IgY titer was determined onto rSEB (10 μg per well) coated immunoassay plates followed by standard indirect ELISA protocols.32
2.3 Preparation and characterization of silica matrix platform for immunoassay
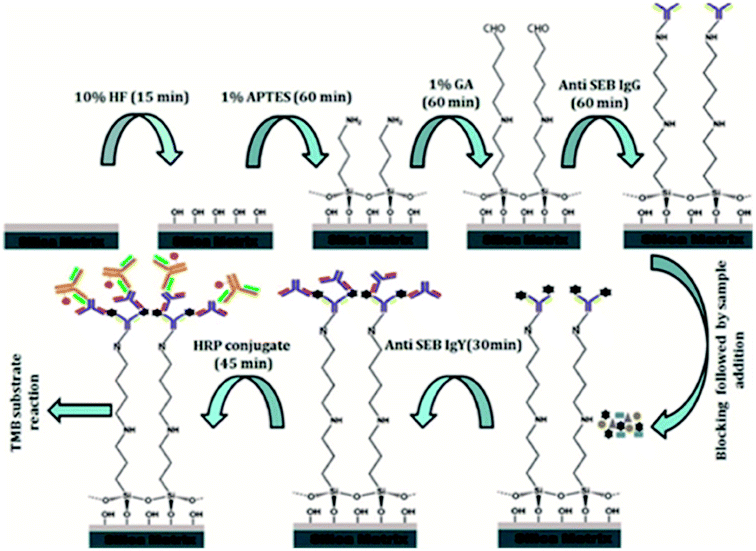
Detailed flowchart for the assay development was depicted in Fig. 1.| Runa | Factors | Response | |||
|---|---|---|---|---|---|
| HFb, % | Ammoniac, % | APTESd, % | GAe, % | Optical valuef | |
| a Run Order. b HF (1–40%). c Ammonia (1–25%). d APTES (1–100%). e GA (1–25%). f Optical density (nm), HF-hydrofluoric acid, AN-ammonia, APS-3 aminopropyl triethoxy silane, GA-glutaraldehyde. | |||||
| 1 | 20.5 | 13 | 50.5 | 13 | 0.812 |
| 2 | 20.5 | 13 | 1 | 13 | 0.89 |
| 3 | 10.75 | 19 | 75.25 | 19 | 0.26 |
| 4 | 20.5 | 13 | 50.5 | 13 | 0.58 |
| 5 | 1 | 13 | 50.5 | 13 | 0.2 |
| 6 | 20.5 | 13 | 50.5 | 13 | 0.8 |
| 7 | 30.25 | 7 | 75.25 | 7 | 0.15 |
| 8 | 30.25 | 19 | 25.75 | 7 | 0.52 |
| 9 | 30.25 | 7 | 75.25 | 19 | 0.155 |
| 10 | 20.5 | 13 | 50.5 | 25 | 0.325 |
| 11 | 40 | 13 | 50.5 | 13 | 0.128 |
| 12 | 10.75 | 19 | 75.25 | 7 | 0.35 |
| 13 | 10.75 | 7 | 75.25 | 7 | 0.18 |
| 14 | 30.25 | 7 | 25.75 | 19 | 0.248 |
| 15 | 30.25 | 19 | 75.25 | 19 | 0.187 |
| 16 | 20.5 | 25 | 50.5 | 13 | 0.684 |
| 17 | 20.5 | 13 | 50.5 | 13 | 0.725 |
| 18 | 10.75 | 19 | 25.75 | 7 | 1.301 |
| 19 | 20.5 | 1 | 50.5 | 13 | 0.35 |
| 20 | 30.25 | 19 | 25.75 | 19 | 0.253 |
| 21 | 10.75 | 7 | 25.75 | 19 | 0.487 |
| 22 | 10.75 | 7 | 25.75 | 7 | 1.05 |
| 23 | 30.25 | 7 | 25.75 | 7 | 0.39 |
| 24 | 10.75 | 19 | 25.75 | 19 | 0.622 |
| 25 | 30.25 | 19 | 75.25 | 7 | 0.159 |
| 26 | 20.5 | 13 | 50.5 | 13 | 0.759 |
| 27 | 20.5 | 13 | 50.5 | 13 | 0.85 |
| 28 | 10.75 | 7 | 75.25 | 19 | 0.12 |
| 29 | 20.5 | 13 | 100 | 13 | 0.102 |
| 30 | 20.5 | 13 | 50.5 | 1 | 0.82 |
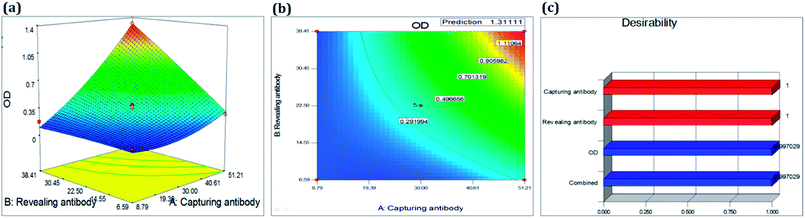
| Runsa | Factors | Responses | |
|---|---|---|---|
| Capturing antibodyb | Revealing antibodyb | Optical valuec | |
| a Run Order. b Capturing and revealing antibody (0–60 min). c Optical density (nm). | |||
| 1 | 30.00 | 22.50 | 0.35 |
| 2 | 30.00 | 22.50 | 0.37 |
| 3 | 51.21 | 38.41 | 1.32 |
| 4 | 51.21 | 6.59 | 0.27 |
| 5 | 30.00 | 22.50 | 0.38 |
| 6 | 30.00 | 0.00 | 0.01 |
| 7 | 30.00 | 45.00 | 0.66 |
| 8 | 0.00 | 22.50 | 0.01 |
| 9 | 8.79 | 38.41 | 0.18 |
| 10 | 60.00 | 22.50 | 1.11 |
| 11 | 8.79 | 6.59 | 0.15 |
| 12 | 30.00 | 22.50 | 0.39 |
| 13 | 30.00 | 22.50 | 0.40 |
The response from the results for the central composite rotatable designs was used to fit second-order polynomial equation. The regression analysis of the response i.e. reduction percentage was carried out by fitting with suitable models represented by (1) and (2). All variables of the polynomial regression at a significance level of p < 0.05 were included in the model, and the coefficient of determination (r2) was generated in order to assess the accuracy of the model. The response surfaces were generated from the equation of the second order polynomial, using the values of each independent variable to the maximum quadratic response.34,45
First order linear eqn (1)
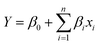 | (1) |
Second-order polynomial eqn (2)
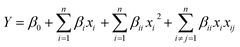 | (2) |
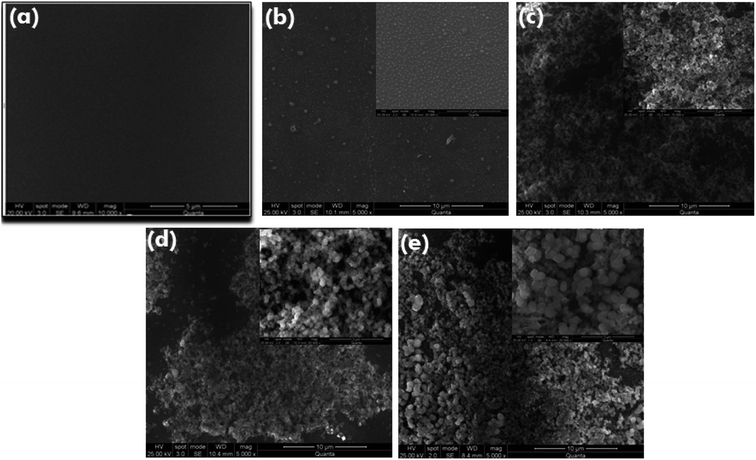
2.4 Characterization of immunoassay matrix
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) glass surfaces were obtained using FEI Quanta 200 system at 25 kV in low vacuum mode with magnification of 500× and 1000×. The substrates before analysis were sprayed with gold particles and various portions of the glass slides were analyzed to observe the uniformity in surface modification.
1000) glass surfaces were obtained using FEI Quanta 200 system at 25 kV in low vacuum mode with magnification of 500× and 1000×. The substrates before analysis were sprayed with gold particles and various portions of the glass slides were analyzed to observe the uniformity in surface modification.
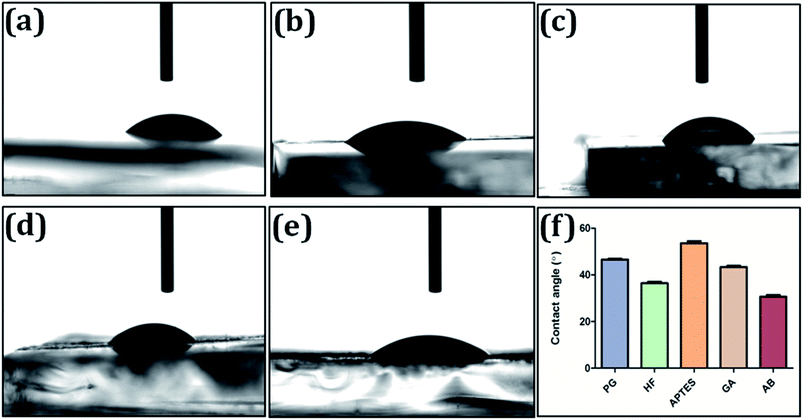
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) glass surfaces were analyzed and average of the results obtained for five different locations on the substrate is reported as final contact angle value.
1000) glass surfaces were analyzed and average of the results obtained for five different locations on the substrate is reported as final contact angle value.
2.5 Development of surface functionalized silica based immunoassay for detection of SEB
Following successful characterization of the functionalized silica matrix, a simple to use, cost-effective, sandwich ELISA method was developed for onsite SEB detection from food and environmental samples.The specificity and sensitivity of the functionalized immunoassay matrix was carried out with anti-SEB IgG as the capturing antibody and anti-SEB IgY as its revealing partner. The specificity was evaluated on different toxins produced by S. aureus strains as well as the cross-reacting cell wall surface protein A and the sensitivity was evaluated onto different rSEB toxin concentration (10 μg mL−1 to 0.001 μg mL−1).
3. Results
3.1 Preparation and evaluation of antibodies for immunoassay
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28
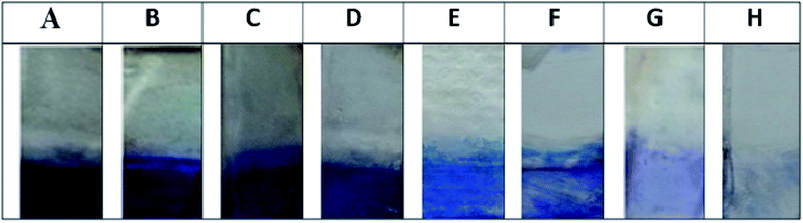
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and furthermore no significant reactivity were observed for the pre-immunized sera (ESI Fig. 1A†).
000 and furthermore no significant reactivity were observed for the pre-immunized sera (ESI Fig. 1A†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32
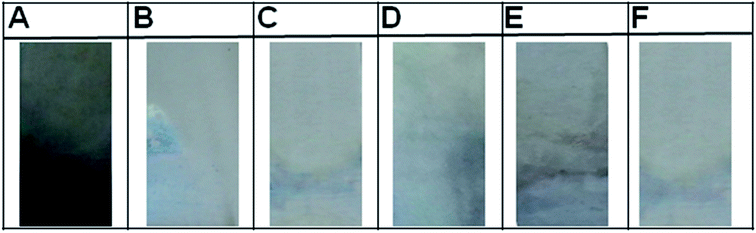
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, whereas no reactivity was observed for the IgY extracted from pre-immunized sera as determined through indirect ELISA (ESI Fig. 1B†).
000, whereas no reactivity was observed for the IgY extracted from pre-immunized sera as determined through indirect ELISA (ESI Fig. 1B†).
3.2 Preparation and characterization of silica matrix platform for immunoassay
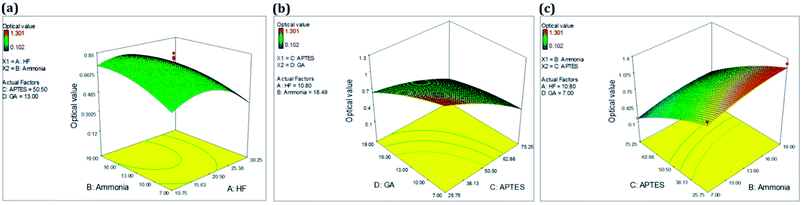 | ||
| Fig. 2 RSM analysis of silica matrix functionalization. 3D plot for optimized conditions of (a) ammonia & HF, (b) GA & APTES and (c) APTES and ammonia. | ||
Silanization
| (Silica matrix) OH− + H2N(CH2)3Si(OC2H5)3 → OHSi(O)3(CH2)3NH2 |
Cross-linking
| OHSi(O)3(CH2)3NH2 + CH2(CH2CHO)2 → OHSi(O)3(CH2)3N–CH–(CH2)3CHO |
Biomolecule immobilization
| OHSi(O)3(CH2)3N–CH–(CH2)3CHO + H2N–B (Biomolecule) → OHSi(O)3(CH2)3N–CH–(CH2)3–CH–N–B |
Multiple regression equations generated for responses are represented as follows,
Final equation in terms of actual factors (silica matrix functionalization):
| Optical value = +0.90057 + 0.025415 × HF + 0.078404 × ammonia − 0.013572 × APTES − 0.031323 × GA − 5.55556 × 10−4 × HF × ammonia + 4.63610 × 10−4 × HF × APTES + 1.08547 × 10−3 × HF × GA − 7.15488 × 10−5 × Ammonia × APTES − 4.30556 × 10−4 × Ammonia × GA + 6.45623 × 10−4 × APTES × GA − 1.61451 × 10−3 × HF2 − 1.81192 × 10−3 × Ammonia2 − 1.15056 × 10−4 × APTES2 − 42650 × 10−3 × GA2 |
Final equation in terms of actual factors (bioprobe incubation period standardization)
| OD = +0.20234 − 0.013515 × capturing antibody − 4.53964 × revealing antibody + 7.46667 × capturing antibody × revealing antibody + 2.22639 × capturing antibody2 − 4.96296 × revealing antibody2 |
The contour plots in Fig. 2 were suitable to represent optimization process since they allow defining the optimal conditions for achieving the maximum percentage of optical response. From response surfaces, it can be observed that lower APTES and GA concentration resulted in better responses. However, a higher concentration of ammonia and medium HF levels resulted in a better response, as summarized in Table 5.
| Parameters | RSM | |||||
|---|---|---|---|---|---|---|
| Matrix optimization | Bioprobe optimization | |||||
| HF | AN | APTES | GA | CA | RV | |
| a a-concentration in percentage, b-concentration in dilutions, HF-hydrofluoric acid, AN-ammonia, APS-3 aminopropyl triethoxy silane, GA-glutaraldehyde. CA-capturing antibody (anti-SEB IgG), RA-revealing antibody (anti SEB IgY). | ||||||
| Concentration (a and b) | 10.80a | 18.50a | 25.75a | 7.00a | — | — |
| Time (in min) | — | — | — | — | 51.21 | 38.27 |
3.3 Characterization of immunoassay matrix
3.4 Specificity and sensitivity of developed ELISA assay
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 for 60 min) capturing and anti-SEB IgY (1
1000 for 60 min) capturing and anti-SEB IgY (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
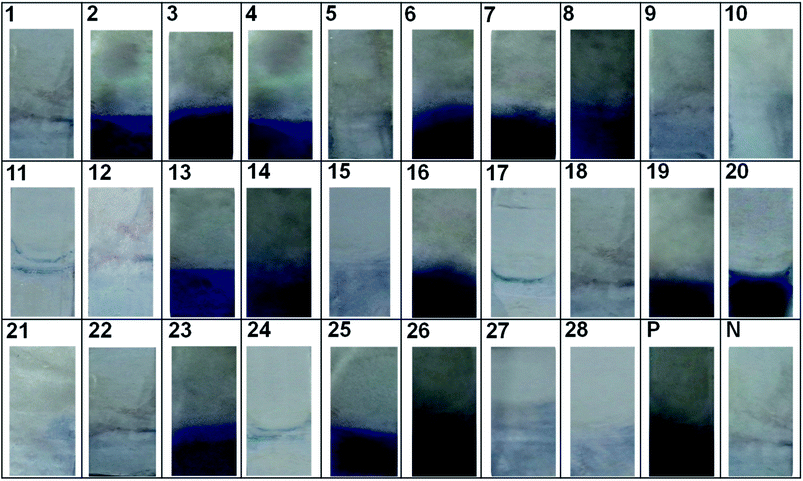
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 for 30 min) as revealing probe (Fig. 6). The lowest detection value of the developed sandwich platform was determined as 0.005 μg mL−1 of SEB antigen which is well below the LD50 value. The developed method showed no cross-reactivity with other related enterotoxins and protein A (Fig. 7).
100 for 30 min) as revealing probe (Fig. 6). The lowest detection value of the developed sandwich platform was determined as 0.005 μg mL−1 of SEB antigen which is well below the LD50 value. The developed method showed no cross-reactivity with other related enterotoxins and protein A (Fig. 7).
3.5 Evaluation of developed sandwich ELISA on food samples
The evaluation of individual bioprobes onto natural samples and standard cultures by indirect ELISA (with anti-SEB IgG and anti-SEB IgY) as well as sandwich ELISA are given in ESI Fig. 3.† The indirect ELISA by anti-SEB IgG resulted in false positive reactions in food isolates and standard cultures that are negative for SEB as shown in ESI Fig. 3A (MI 01), B (MT01 and 05), C (CK 02, 03, 08 and 09) and D (B-S. aureus ATCC 19095).† These results point to the fact that mammalian IgG cross-reacts with Staphylococcal protein A producing false positive results in non SEB positive Staphylococcus aureus cultures as well as SEB negative food isolates. The indirect ELISA with anti-SEB IgY and sandwich ELISA detects only the SEB positive samples specifically.To check the reliability and field usage, developed method was evaluated on to various contaminated food samples and reference cultures. The results of the developed immunoassay were on par with the standard ELISA kit method (Table 6 and Fig. 8).
| S. no | Source | Chondrex standard ELISA kit | Developed IgY based silica matrix platform |
|---|---|---|---|
| 1 | Milk isolate 01 | − | − |
| 2 | Milk isolate 02 | + | + |
| 3 | Milk isolate 03 | + | + |
| 4 | Milk isolate 04 | + | + |
| 5 | Meat isolate 01 | − | − |
| 6 | Meat isolate 02 | + | + |
| 7 | Meat isolate 03 | + | + |
| 8 | Meat isolate 04 | + | + |
| 9 | Meat isolate 05 | − | − |
| 10 | Cake isolate 01 | − | − |
| 11 | Cake isolate 02 | − | − |
| 12 | Cake isolate 03 | − | − |
| 13 | Cake isolate 04 | + | + |
| 14 | Cake isolate 05 | + | + |
| 15 | Cake isolate 06 | − | − |
| 16 | Cake isolate 07 | + | + |
| 17 | Cake isolate 08 | − | − |
| 18 | Cake isolate 09 | − | − |
| 19 | Cake isolate 10 | + | + |
| 20 | S. aureus ATCC-29213 | + | + |
| 21 | S. aureus ATCC-19095(SEC positive) | − | − |
| 22 | S. epidermidis ATCC-12228 | − | − |
| 23 | S. aureus NCIM-5021 | + | + |
| 24 | Salmonella typhimurium ATCC-14028 | − | − |
| 25 | S. aureus NCIM-2657 | + | + |
| 26 | S. aureus NCIM-2654 | + | + |
| 27 | Escherichia coli ATCC-10536 | − | − |
| 28 | Klebsiella pneumonia ATCC-10031 | − | − |
Additionally, the inter as well as intra assay coefficient of variance was determined wherein it was found to between 8.9–12.6% and 3–8.4%, respectively (Table 7). Therefore, this suggests that the present method is well suited and applicable for detection of SEB from diverse sample types.
| S. no | Sample | Intra assay coefficient of variation (%) | Inter assay coefficient of variation (%) | ||||
|---|---|---|---|---|---|---|---|
| IgGa | IgYa | Sandwich | IgGa | IgYa | Sandwich | ||
| a Indirect ELISA. | |||||||
| 1 | Milk isolate 01 | 7.1 | 5.9 | 3.6 | 11.6 | 10.5 | 9.3 |
| 2 | Milk isolate 02 | 7.9 | 6.7 | 4.4 | 12.4 | 11.3 | 10.1 |
| 3 | Milk isolate 03 | 7.1 | 5.9 | 3.6 | 11.3 | 10.2 | 9 |
| 4 | Milk isolate 04 | 7.6 | 6.4 | 4.1 | 11.5 | 10.4 | 9.2 |
| 5 | Meat isolate 01 | 7.3 | 6.1 | 3.8 | 11.25 | 10.15 | 8.95 |
| 6 | Meat isolate 02 | 7.8 | 6.6 | 4.3 | 11 | 9.9 | 8.7 |
| 7 | Meat isolate 03 | 8.1 | 6.9 | 4.6 | 12.34 | 11.24 | 10.04 |
| 8 | Meat isolate 04 | 8.4 | 7.2 | 4.9 | 12.6 | 11.5 | 10.3 |
| 9 | Meat isolate 05 | 7.5 | 6.3 | 4 | 11.4 | 10.3 | 9.3 |
| 10 | Cake isolate 01 | 7.8 | 6.6 | 4.3 | 11.8 | 10.7 | 9.5 |
| 11 | Cake isolate 02 | 7.7 | 6.5 | 4.2 | 11.65 | 10.54 | 9.34 |
| 12 | Cake isolate 03 | 7.3 | 6.1 | 3.8 | 11.3 | 10.2 | 9 |
| 13 | Cake isolate 04 | 8.3 | 7.1 | 4.8 | 12.4 | 11.3 | 10.1 |
| 14 | Cake isolate 05 | 6.1 | 4.9 | 2.6 | 11.12 | 10.02 | 8.92 |
| 15 | Cake isolate 06 | 6.3 | 5.2 | 2.9 | 11.2 | 10.1 | 8.9 |
| 16 | Cake isolate 07 | 6.8 | 5.6 | 3.3 | 11.7 | 10.6 | 9.4 |
| 17 | Cake isolate 08 | 6.5 | 5.3 | 3 | 11.5 | 10.4 | 9.2 |
| 18 | Cake isolate 09 | 6.8 | 5.6 | 3.3 | 11.6 | 10.5 | 9.3 |
| 19 | Cake isolate 10 | 6.2 | 5 | 3 | 11.2 | 10.1 | 8.9 |
4. Discussion
Immobilization of biomolecules onto various surfaces have been of prime focus to many researchers for development of novel, durable, ready to use and portable detection systems. To accomplish this, there are several parameters that influence surface activation strategies such as function stabilization, structure/functional group conservation and proper binding orientation of the biomolecules.42 The covalent attachment of biomolecules can be accomplished through variety of functionalization chemistry that imparts groups such as NH2, SH, COOH, NHS ester as well as epoxide and often this is achieved on a glass or oxide surface through self-assembly of silanes.43 The utilization of single silane ensures functional uniformity whereas a mixture of silanes could possibly result in uncharacteristic functional groups.11 The multilayer formation leads to an unstable silane layer, hence are vulnerable to get washed away during common washing steps of immunoassay.3 Therefore, the matrix functionalization in the present work is accomplished through silanization using (3-aminopropyl) triethoxysilane (APTES) on silica matrix to produce a thin and stable silane layer after hydrofluoric acid etching, upon which biomolecule immobilization was achieved via glutaraldehyde linker. Thus, this functionalization strategy satisfies the parameters of efficient bioprobe immobilization and non hindrance with its biological function therewith enhancing the sensitivity of assay.44,45 The previous studies reported till now majorly focus on glass surface activation for biomolecule immobilization3,9,37–39,41,42 and some pertains to assay development,46,47 however, none have quite evolved into a sensitive detection platform for routine laboratory application and onsite screening. Thus, the present study focuses on the development of silica functionalized matrix based onsite SEB detection platform.Immunodetection platforms are cost effective, more suitable, robust and portable for onsite detection due to its less technical expertise requirement compared to PCR assays.48 The PCR assays are more sensitive as well as specific in detection of toxin associated genes but more clinical relevance could only be established through immunoassays. Moreover, the main drawback associated with PCR is their inability to correlate with the toxin expression by the organism in the samples.32 Therefore, the bioligands were generated with high sensitivity in rabbit and chicken systems. The sandwich immunoassay strategy was employed, wherein the anti-rabbit SEB IgG was used as the capturing probe and chicken anti-SEB IgY as its revealing partner. The anti-SEB IgG were then permanently immobilized on to the immunoassay matrix prepared through silane glutaraldehyde chemistry. Characterization by scanning electron microscopy and contact angle measurements revealed the successful matrix functionalization and bioligands immobilization onto the matrix.
The chicken egg yolk antibodies are more hygienic, cost-efficient and convenient compared with the traditional antibodies obtained from mammalian serum.49 The maintenance costs for keeping hens are also lower than those for mammals such as rabbits and even more viable in ethical aspect due to the non invasive purification of antibodies. Moreover, one immunized chicken could generate yield more than 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mg of IgY per year that is equivalent to the production by 4.3 rabbits over a year.50 Furthermore, an added advantage arises because of the phylogenetic distance as well as genetic background between birds and mammals that improve the likelihood of an immune response against antigens or epitopes that may be non-immunogenic in mammals. The mammalian immunoglobulins may have deleterious effects on the performance of different immunoassay formats, particularly in their use as bioactive molecules to capture or detect the analyte, that are affected by heterophilic antibodies as well as high levels of non-specific binding (e.g. Staphylococcal protein A).51 The various approaches to eradicate heterophilic antibody interference includes removal or inactivation of interfering immunoglobulins through precipitation with PEG, buffer additives and proteolytic Fc fragments cleavage, however, these are practicably unviable for onsite detection system.50 This was further confirmed through indirect ELISA, wherein anti-SEB IgY antibody was not found to produce any cross reactivity unlike its mammalian counterpart anti-SEB IgG antibody without sample pre-treatment. Thus considering these factors, chicken IgY antibodies offer several advantages over mammalian counterparts as they do not interact with rheumatoid factor (RF), human anti-mouse IgG antibodies (HAMA), complement components or mammalian Fc receptors31 and thereby enhances the aptness of the developed platform for onsite applications.
500 mg of IgY per year that is equivalent to the production by 4.3 rabbits over a year.50 Furthermore, an added advantage arises because of the phylogenetic distance as well as genetic background between birds and mammals that improve the likelihood of an immune response against antigens or epitopes that may be non-immunogenic in mammals. The mammalian immunoglobulins may have deleterious effects on the performance of different immunoassay formats, particularly in their use as bioactive molecules to capture or detect the analyte, that are affected by heterophilic antibodies as well as high levels of non-specific binding (e.g. Staphylococcal protein A).51 The various approaches to eradicate heterophilic antibody interference includes removal or inactivation of interfering immunoglobulins through precipitation with PEG, buffer additives and proteolytic Fc fragments cleavage, however, these are practicably unviable for onsite detection system.50 This was further confirmed through indirect ELISA, wherein anti-SEB IgY antibody was not found to produce any cross reactivity unlike its mammalian counterpart anti-SEB IgG antibody without sample pre-treatment. Thus considering these factors, chicken IgY antibodies offer several advantages over mammalian counterparts as they do not interact with rheumatoid factor (RF), human anti-mouse IgG antibodies (HAMA), complement components or mammalian Fc receptors31 and thereby enhances the aptness of the developed platform for onsite applications.
Several SEB detection assays,23,24,48 as well as systems with IgY based strategies have been reported previously.52–54 Despite establishment of such novel approaches and numerous improvements within these described assays, the commercially available kits still utilize polyclonal/monoclonal IgG antibodies based ELISA as represented in Table 8.
| Kit name | Manufacturer | Sensitivity (ng mL−1) | Assay time | Enrichment/extraction | Detection method |
|---|---|---|---|---|---|
| a ELISA-Enzyme Linked Immunosorbent Assay. b RPLA-Reversed Passive Latex Agglutination. c ELFA-Enzyme Linked Immunofluorescent Assay. d LFA-Lateral Flow Assay. | |||||
| Ridascreen® SET | R-Biopharm | 0.25 (liquid sample), 0.375 (solid sample), 0.25 (culture supernatant) | Within 4 h | Yes | ELISAa |
| Tecra visual immunoassay (VIA™) | 3M | 1 | Within 4 h | Yes | ELISA |
| SET-RPLA toxin detection kit | Oxoid | 0.5 | 24 h | Yes | RPLAb |
| Transia PLATE staphylococcal enterotoxins | Biocontrol | 0.02 | Within 2 h | Yes | ELISA |
| Vidas SET2 | Biomerieux | 0.025 | Max 80 min | Yes | ELFAc (automated system) |
| BADD SEB | AdVnt's | 10 | Within 15 min | No | LFAd |
| SEB antibody assay kit | Chondrex | 1 (only liquid samples) | Within 2 h | Yes | ELISA |
| Developed silica platform | — | 5 | Max 90 min | No | ELISA |
The limit of detection of these represented assays range between 0.02 ng mL−1 to 10 ng mL−1. However, some of these assays are either time consuming (sample processing includes; pre-enrichment/extraction step), require sophisticated instrumentation (ELISA reader, automated system), or even labor-intensive (require technical expertise for analysis). Notwithstanding, specificity of these commercial kits is relatively low since the likelihood of false positives occurring due to matrix components (e.g., protein A) with the Fc fragment (and, to a lesser extent, Fab fragments) in immunoglobulin G from several animal species (e.g., mouse or rabbit, but not rat or goat) is reasonably high.31 Moreover, SEB being a potent bio-threat agent necessitates the detection platform to be onsite/field deployable.
Considering the above mentioned aspects, the present study has developed IgY based silica matrix platform for SEB detection. Herein, the matrix functionalization (HF, ammonia, APTES and GA) optimized through RSM technique accomplished uniform reactive groups throughout the activated surface thereby increasing the bioprobes proportion. This significantly improvised the sensitivity of the assay with a limit of detection up-to 0.005 μg mL−1. Likewise, application of anti-SEB IgY as revealing bioprobe enhanced specificity of the assay as no cross reactivity towards any closely associated toxins as well as other interfering factors was observed. Further, its onsite feasibility was established through SEB detection from various food matrices. Besides this, inter and intra assay coefficient of variance confirmed reproducibility of the platform. Therefore, these attributes render the developed platform to be highly specific, easy to operate, low cost, and sensitive assay for the rapid and reproducible on-site detection of SEB toxin.
5. Conclusion
In collective, the study presents silica matrix functionalization strategy through RSM approach, and further development of an IgY based rapid onsite SEB detection platform. The functionalization chemistry was optimized to self-assemble silane monolayers uniformly, which in turn was critical for successful homogenous biomolecule immobilization. This was further substantiated through SEM and contact angle characterization. The LOD of the developed platform was estimated to be 5 ng mL−1 with total assay duration of 90 min without sample processing. The robustness and on site portability of the system was verified through SEB detection from different food matrices, wherein inter and intra assay coefficient of variance was observed to be below 15% and 10%, respectively. In addition to this, the developed platform was found to be on par upon co-evaluation with commercial SEB detection kit. Therefore, the developed platform possesses high sensitivity and nil cross reactivity, thereupon confirming its significant potential for the rapid and sensitive onsite detection of SEB toxin.Conflicts of interest
There is no financial conflict of interest.Ethical statement
All animal experiments were reviewed and approved by animal ethical committee at the Acharya Nagarjuna University, Guntur, India and were conducted in accordance with the Institutional Animal Ethical Committee (IAEC) guidelines. ANUCPS/IAEC/AH/Protocol/2/2014: Dt 15/07/2014.Acknowledgements
Financial (JRF to Mr Achuth J) and laboratory facilities was provided by DRDO BU Center for Life Sciences funded by the Ministry of Defense, State government of Tamil Nadu and Bharathiar University under Phase II project.References
- Q. Xu and K. S. Lam, BioMed Res. Int., 2003, 2003, 257–266 Search PubMed.
- D. K. Agarwal, N. Maheshwari, S. Mukherji and V. R. Rao, RSC Adv., 2016, 6, 17606–17616 RSC.
- N. S. K. Gunda, M. Singh, L. Norman, K. Kaur and S. K. Mitra, Appl. Surf. Sci., 2014, 305, 522–530 CrossRef CAS.
- S. A. Bhakta, E. Evans, T. E. Benavidez and C. D. Garcia, Anal. Chim. Acta, 2015, 872, 7–25 CrossRef CAS PubMed.
- T. Kamra, S. Chaudhary, C. Xu, N. Johansson, L. Montelius, J. Schnadt and L. Ye, J. Colloid Interface Sci., 2015, 445, 277–284 CrossRef CAS PubMed.
- S. L. Seurynck-Servoss, A. M. White, C. L. Baird, K. D. Rodland and R. C. Zangar, Anal. Biochem., 2007, 371, 105–115 CrossRef CAS PubMed.
- K. Sterzynska, J. Budna, E. Frydrych-Tomczak, G. Hreczycho, A. Malinska, H. Maciejewski and M. Zabel, Folia Histochem. Cytobiol., 2014, 52, 250–255 CrossRef PubMed.
- M. Hashizume, S. Fukagawa, S. Mishima, T. Osuga and K. Iijima, Langmuir, 2016, 32, 12344–12351 CrossRef CAS PubMed.
- J. J. Cras, C. A. Rowe-Taitt, D. A. Nivens and F. S. Ligler, Biosens. Bioelectron., 1999, 14, 683–688 CrossRef CAS.
- W. Kusnezow, A. Jacob, A. Walijew, F. Diehl and J. D. Hoheisel, Proteomics, 2003, 3, 254–264 CrossRef CAS PubMed.
- J. H. Seo, L.-J. Chen, S. V. Verkhoturov, E. A. Schweikert and A. Revzin, Biomaterials, 2011, 32, 5478–5488 CrossRef CAS PubMed.
- Q. Zhang, T. Chen, S. Yang, X. Wang and H. Guo, Appl. Microbiol. Biotechnol., 2013, 97, 4149–4158 CrossRef CAS PubMed.
- S. P. Montgomery, J. S. Drouillard, J. J. Sindt, M. A. Greenquist, B. E. Depenbusch, E. J. Good, E. R. Loe, M. J. Sulpizio, T. J. Kessen and R. T. Ethington, J. Anim. Sci., 2005, 83, 2440–2447 CrossRef CAS PubMed.
- P. Barmpalexis, F. I. Kanaze and E. Georgarakis, J. Pharm. Biomed. Anal., 2009, 49, 1192–1202 CrossRef CAS PubMed.
- S. Tsapatsaris and P. Kotzekidou, Int. J. Food Microbiol., 2004, 95, 157–168 CrossRef CAS PubMed.
- N. G. Margaritelis, C. K. Markopoulou and J. E. Koundourellis, Anal. Methods, 2013, 5, 3334–3346 RSC.
- K. J. Reddy, M. C. Pandey, P. T. Harilal and K. Radhakrishna, Int. Food Res. J., 2013, 20, 3101 Search PubMed.
- A. Imani Fouladi, A. Choupani and J. Fallah Mehrabadi, Kowsar Med. J., 2011, 16, 21–25 CAS.
- E. Cook, X. Wang, N. Robiou and B. C. Fries, Clin. Vaccine Immunol., 2007, 14, 1094–1101 CrossRef CAS PubMed.
- A. J. Brosnahan and P. M. Schlievert, FEBS J., 2011, 278, 4649–4667 CrossRef CAS PubMed.
- D. V. Kamboj, A. K. Goel and L. Singh, Def. Sci. J., 2006, 56, 495 CrossRef.
- M. Llewelyn and J. Cohen, Lancet Infect. Dis., 2002, 2, 156–162 CrossRef CAS PubMed.
- Y. Xu, B. Huo, X. Sun, B. Ning, Y. Peng, J. Bai and Z. Gao, RSC Adv., 2018, 8, 16024–16031 RSC.
- A. Sharma, V. K. Rao, D. V. Kamboj, S. Upadhyay, M. Shaik, A. R. Shrivastava and R. Jain, RSC Adv., 2014, 4, 34089–34095 RSC.
- H. Karauzum, G. Chen, L. Abaandou, M. Mahmoudieh, A. R. Boroun, S. Shulenin, V. S. Devi, E. Stavale, K. L. Warfield, L. Zeitlin and others, J. Biol. Chem., 2012, 287, 25203–25215 CrossRef CAS PubMed.
- E. Cook, X. Wang, N. Robiou and B. C. Fries, Clin. Vaccine Immunol., 2007, 14, 1094–1101 CrossRef CAS PubMed.
- M. L. Hale, in Bioterrorism, InTech, 2012 Search PubMed.
- M. Venkataramana, M. D. Kurkuri and others, Sens. Actuators, B, 2016, 222, 1201–1208 CrossRef.
- K. E. Sapsford, J. Francis, S. Sun, Y. Kostov and A. Rasooly, Anal. Bioanal. Chem., 2009, 394, 499–505 CrossRef CAS PubMed.
- P. N. Reddy, S. Nagaraj, M. H. Sripathy and H. V. Batra, Ann. Microbiol., 2015, 65, 1915–1922 CrossRef CAS.
- A. S. Araújo, Z. I. P. Lobato, C. Chávez-Olórtegui and D. T. Velarde, Toxicon, 2010, 55, 739–744 CrossRef PubMed.
- V. Mudili, S. S. Makam, N. Sundararaj, C. Siddaiah, V. K. Gupta and P. V. L. Rao, Sci. Rep., 2015, 5, 15151 CrossRef CAS PubMed.
- A. Polson, M. B. von Wechmar and M. H. V. Van Regenmortel, Immunol. Commun., 1980, 9, 475–493 CrossRef CAS PubMed.
- R. H. Myers, D. C. Montgomery, G. G. Vining and T. J. Robinson, Generalized linear models: with applications in engineering and the sciences, John Wiley & Sons, 2012, vol. 791 Search PubMed.
- H. N. Sin, S. Yusof, N. S. A. Hamid and R. A. Rahman, J. Food Eng., 2006, 73, 313–319 CrossRef CAS.
- G.-J. Oh, J.-H. Yoon, V. T. Vu, M.-K. Ji, J.-H. Kim, J.-W. Kim, E.-K. Yim, J.-C. Bae, C. Park, K.-D. Yun and others, J. Nanosci. Nanotechnol., 2017, 17, 2645–2648 CrossRef CAS.
- C. Gruian, E. Vanea, S. Simon and V. Simon, Biochim. Biophys. Acta, Proteins Proteomics, 2012, 1824, 873–881 CrossRef CAS PubMed.
- E. Metwalli, D. Haines, O. Becker, S. Conzone and C. G. Pantano, J. Colloid Interface Sci., 2006, 298, 825–831 CrossRef CAS PubMed.
- C. R. Vistas, A. C. P. Águas and G. N. M. Ferreira, Appl. Surf. Sci., 2013, 286, 314–318 CrossRef CAS.
- S. Chaudhary, T. Kamra, K. M. A. Uddin, O. Snezhkova, H. S. N. Jayawardena, M. Yan, L. Montelius, J. Schnadt and L. Ye, Appl. Surf. Sci., 2014, 300, 22–28 CrossRef CAS.
- G. D. Nagare and S. Mukherji, Appl. Surf. Sci., 2009, 255, 3696–3700 CrossRef CAS.
- M. González-González, R. Bartolome, R. Jara-Acevedo, J. Casado-Vela, N. Dasilva, S. Matarraz, J. García, J. A. Alcazar, J. M. Sayagues, A. Orfao and others, Anal. Biochem., 2014, 450, 37–45 CrossRef PubMed.
- J. H. Seo, D.-S. Shin, P. Mukundan and A. Revzin, Colloids Surf., B, 2012, 98, 1–6 CrossRef CAS PubMed.
- A. Gang, G. Gabernet, L. D. Renner, L. Baraban and G. Cuniberti, RSC Adv., 2015, 5, 35631–35634 RSC.
- O. Majumder, A. K. S. Bankoti, T. Kaur, A. Thirugnanam and A. K. Mondal, RSC Adv., 2016, 6, 107344–107354 RSC.
- J. H. Seo, L.-J. Chen, S. V. Verkhoturov, E. A. Schweikert and A. Revzin, Biomaterials, 2011, 32, 5478–5488 CrossRef CAS PubMed.
- A. Antoniou, G. Herlem, C. André, Y. Guillaume and T. Gharbi, Talanta, 2011, 84, 632–637 CrossRef CAS PubMed.
- D. Pauly, S. Kirchner, B. Stoermann, T. Schreiber, S. Kaulfuss, R. Schade, R. Zbinden, M.-A. Avondet, M. B. Dorner and B. G. Dorner, Analyst, 2009, 134, 2028–2039 RSC.
- Y. Xu, X. Li, L. Jin, Y. Zhen, Y. Lu, S. Li, J. You and L. Wang, Biotechnol. Adv., 2011, 29, 860–868 CrossRef CAS PubMed.
- R. Schade, E. G. Calzado, R. Sarmiento, P. A. Chacana, J. Porankiewicz-Asplund, H. R. Terzolo and others, Altern. Lab. Anim., 2005, 33, 129–154 CrossRef CAS PubMed.
- E. Spillner, I. Braren, K. Greunke, H. Seismann, S. Blank and D. du Plessis, Biologicals, 2012, 40, 313–322 CrossRef CAS PubMed.
- P. Reddy, S. Ramlal, M. H. Sripathy and H. V. Batra, J. Immunol. Methods, 2014, 408, 114–122 CrossRef CAS PubMed.
- A. C. Vinayaka, S. P. Muthukumar and M. S. Thakur, Bionanoscience, 2013, 3, 232–240 CrossRef.
- W. Jin, K. Yamada, M. Ikami, N. Kaji, M. Tokeshi, Y. Atsumi, M. Mizutani, A. Murai, A. Okamoto, T. Namikawa and others, J. Microbiol. Methods, 2013, 92, 323–331 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03574a |
| This journal is © The Royal Society of Chemistry 2018 |