Open Access Article
Open Access ArticleIn vivo detection of salicylic acid in sunflower seedlings under salt stress†
Ye Huab,
Jing Zhaoac,
Haiyang Lia,
Xiaodong Wangab,
Peichen Houab,
Cheng Wangab,
Aixue Li *ab and
Liping Chen*ab
*ab and
Liping Chen*ab
aBeijing Research Center of Intelligent Equipment for Agriculture, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, P. R. China. E-mail: liax@nercita.org.cn
bBeijing Research Center for Information Technology in Agriculture, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, P. R. China. E-mail: chenlp@nercita.org.cn
cDepartment of Environmental Science and Engineering, Zhejiang Ocean University, Zhoushan, Zhejiang 316022, P. R. China
First published on 27th June 2018
Abstract
Salicylic acid (SA) is an important phytohormone. It plays an essential role in regulating many physiological processes of plants. Most of the conventional methods for SA detection are based on in vitro processes. More attention should be paid to develop in vivo methods for SA detection. In this work, Pt nanoflowers and GO were simultaneously electrodeposited and reduced on a Pt wire microelectrode in one step. The Pt nanoflowers/ERGO modified Pt microsensor demonstrated high sensitivity and selectivity for SA. SA could be detected from 100 pM to 1 μM with a detection limit of 48.11 pM. Then this microsensor was used to detect SA in the stem of sunflower seedlings under different salt stresses in vivo. The result showed that with the increasing concentration of salt, SA levels decreased. Our result was also confirmed by UPLC-MS and gene expression analysis. To the best of our knowledge, this is the first report of in vivo detection of SA in plants using the Pt nanoflowers/ERGO modified Pt microelectrode. It is foreseeable that our strategy could pave the way for the in vivo detection of phytohormones in plants.
1. Introduction
Salicylic acid (SA) is an important phytohormone, which widely exists in both monocot and dicot plants.1 As a signal molecule, it plays an essential role in regulating many physiological processes of plants, including flowering, seed germination, heat production, membrane permeability, defence responses, etc.2 The level of SA varies from ng to mg per gram of fresh weight in different plant species, and it will change under biotic and abiotic stresses for enhancing plants stress toleration.3Further studies on the synthesis, transport and molecular mechanisms of SA require the accurate quantitative analysis of SA. Nowadays, there are many technologies for detecting SA level in plants, such as high performance liquid chromatography (HPLC),4 gas chromatography-mass spectrometry (GC-MS),5 enzyme linked immunosorbent assay (ELISA) and fluorescence spectrometry6,7 etc. But in these methods, SA is measured in vitro using plant samples, such as roots, stems and leaves. The plant samples need to undergo complex pre-processing, where complex and expensive instruments are always required, and some biological information may be lost during this process. In addition, the collections of plant samples always result in great damage and even death, while many plant materials are very precious and rare. More importantly, an in vivo detection of SA would provide more instant and accurate information about the response mechanism of plants under different environmental conditions. Therefore, it is very important to develop in vivo method for the detection of SA.
To date, there are only a few reports for the in vivo detection of SA, such as colorimetry,8 bioluminescence,9 reverse iontophoresis10 and electrochemical sensors.11 Colorimetry and bioluminescence methods were mainly used to map the spatial distribution of SA in plant tissues. Reverse iontophoresis has been used to assay the level of SA in basil leaves in situ, but it needed an 8 hours extraction period for collecting the sap of the plants, which couldn't reflect the instant information of SA under changed environment. Sun et al.11 detected the change of SA in tomato leaves in vivo using a paper-based electrochemical sensor, which indicated that electrochemical sensor could be used for in situ and in vivo detection of SA in plants. However, it seems that this sensor is more suitable for the leaves, while not suitable for the stems or roots of the plants.
In recent years, microelectrode has attracted much interest due to its unique characters, such as smaller size, rapid response time, increased signal-to-noise ratio, and less requirement for support electrolyte, etc.12,13 Additionally, the small size of microelectrode makes it ideal for in vivo application in plants, especially for the stems or roots of plants. For example, Pt microelectrode has been successfully used to monitor the H2O2,14–17 NO and pH15 in plants in vivo. However, up to date, there are few reports about the application of microelectrode in detecting of phytohormone in plants in vivo.
As the environment for in vivo detection is very complex, complicated and complex method for fabrication of the microsensor is not suitable. It has been reported that electrochemically reduced graphene oxide (ERGO)–metal nanocomposite films can be obtained by direct electrodeposition method.18,19 This method is green and fast, and the prepared nanocomposite films exhibit excellent catalytic activity. In this work, Pt nanoflowers (PtNF) and ERGO were successfully fabricated on a Pt wire microelectrode by one step electrodeposition method. Then this PtNF/ERGO modified Pt microelectrode was used to detect SA in the stem of sunflower seedlings under salt stress in vivo. To the best of our knowledge, this is the first report for in vivo detection of SA in plants using the PtNF/ERGO modified Pt microelectrode. It may open the opportunity for application of microelectrode in detecting of phytohormone in vivo in plants. Our result was also confirmed by UPLC-MS and gene expression analysis.
2. Materials and methods
2.1. Chemicals and materials
Hexachloroplatinic acid hexahydrate (H2PtCl6·6H2O), SA, methyl jasmonate, malic acid, succinic acid, citric acid, indole acetic acid and abscisic acid were purchased from Sigma-Aldrich Co. LLC. The monolayer graphene oxide (GO) (diameter: 500 nm to 5 μm) was obtained from Nanjing XFNANO Materials Tech Co. Ltd. All other chemicals used were analytically grade and purchased from Beijing Chemical Works. All aqueous solutions were prepared with ultrapure water. The SA stock solution (0.01 M) was dissolved by 0.01 M PBS (pH = 7.0) with 0.2% ethanol. It was stored under 4 °C and diluted freshly by PBS before use.2.2. Preparation of the Pt nanoflowers/ERGO modified Pt (PtNF/ERGO/Pt) microelectrodes
Pt wire microelectrode (0.5 mm in diameter) was purchased from Tianjin Ida Heng Sheng Technology Development Co., Ltd (Tianjin, China). The microelectrode was immersed in concentrated nitric acid solution for 10 min to remove the impurities. Then, it was polished in turn with 0.3 and 0.05 μm alumina powder, and ultrasonic cleaning with deionized water and ethanol. After drying by N2, the Pt microelectrode was voltammetry cycled in 0.1 M sulfuric acid with the potential from −1.5 to 1.5 V for 30 cycles.The cleaned Pt wire microelectrode was potential scanned in GO aqueous suspension (0.1 M carbonate buffer at pH 9.0) containing H2PtCl6 under the potential window from 0 to −1.5 V at a scan rate of 25 mV s−1. The resulted PtNF/ERGO/Pt wire electrode was rinsed by deionized water and dried by nitrogen. Then it was wrapped with insulating tape, leaving 2 mm in length for sensing.
The ERGO/Pt and PtNF/Pt electrode was also prepared in 0.1 M carbonate buffer contain optimal concentrations of GO or H2PtCl6 for control.
2.3. Characterization
The surface morphology of the different modified Pt wire electrodes were characterized by scanning electron microscopic (SEM, JEOL JSM-6700F). The X-ray diffraction (XRD) analysis of the different modified Pt electrode was carried out on a SmartLab XRD system using Cu Kα as the radiation source at an operating voltage of 40 kV. Pt sheet was used as the substrate to simulate the Pt wire.2.4. Preparation of plant samples and homogenate
A homogenous seeds of sunflower (LD5009) were obtained from Beijing Kafry Technology Co., LTD. After seed germination, the seedlings with consistent growth were selected and transplanted into half-strength Hoagland solution. After 15 days hydroponic culture in an illuminating incubator (10 h/14 h of light/dark period, 25 ± 2 °C and 60% humidity), the sunflowers were randomized into three groups (each group contained 20 plant samples) and treated by different salt stress for 10 h through introducing different levels of NaCl (0, 50 and 100 mM) into the Hoagland solution.Homogenates of stems of sunflower seedling was prepared by homogenized the plant materials thoroughly with 0.5 g of crystalline silica. The homogenates were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (10 min, 4 °C), and the supernatant solutions were collected and transferred to new tubes. The homogenates were prepared freshly before each measurement.
000 rpm (10 min, 4 °C), and the supernatant solutions were collected and transferred to new tubes. The homogenates were prepared freshly before each measurement.
2.5. Electrochemical measurements
All the electrochemical experiments were performed on a CHI 760e electrochemical working station. A two-electrode system was employed for the measurements. The modified Pt microelectrode was used as working electrode. A Ag/AgCl wire was used as reference/counter electrode, which was prepared by chloridizing Ag wire at 0.6 V in 0.1 M hydrochloride acid for about 30 min. Cyclic voltammetry (CV) was conducted from 0.6 V to 1.4 V at 50 mV s−1. Differential pulse voltammetry (DPV) was conducted from 0.6 V to 1.4 V, and the parameters were: 0.02 V of increasing potential, 0.02 s of pulse width, 0.05 V of amplitude, 1 s of pulse period, and 0.02 s of sampling width.For in vivo detection of SA in the stem of sunflower seedlings, the stem of the seedlings were punched with a puncture needle to obtain two holes. The working electrode and Ag/AgCl electrode were placed into the holes respectively. For different plant, the electrode was placed approximately the same site as possible.
2.6. Ultra-performance liquid chromatography-mass spectrometric (UPLC-MS) measurements
For the UPLC-MS (Water Acquity I-Class, Waters Corporation, MA, US; Thermo Q-Exactive, Thermo Scientific, MA, US) measurements, the stem samples of the seedlings were collected and ground in liquid nitrogen immediately after 10 hours salt treatments. 1 g of ground sample was added 2 ml 4 °C precooled methanol (80%) and extracted overnight at 4 °C. The extract was mixed with 1 ml of CHCl3. Then the solution was vibrated at 900 rpm for 4 min at 4 °C. After centrifuging at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at 4 °C, the supernatant was collected and dried by nitrogen at room temperature. The prepared sample was dissolved in 1 mL of methanol and filtrated by a 0.22 μm microporous membrane before UPLC-MS measurements.
000 rpm for 5 min at 4 °C, the supernatant was collected and dried by nitrogen at room temperature. The prepared sample was dissolved in 1 mL of methanol and filtrated by a 0.22 μm microporous membrane before UPLC-MS measurements.
2.7. Gene expression analysis
To confirm the result of in vivo detection of SA, semi quantitative RT-PCR analysis was performed for SA-related gene def and HaAC1. Total RNA was extracted from stem samples of sunflower seedlings (0.1 g fresh weight) with OminiPlant RNA Kit (DNase I) (ComWin Biotech, Beijing, China) according to the protocols. BioRT cDNA First Strand Synthesis Kit (Bioer, Hangzhou, China) was used to synthesis the first-strand cDNAs from 2 μg total RNA. RT-PCR was performed with 1 μL generated cDNA. PCR amplifications conditions: initial denaturation, 95 °C for 5 min; 30 cycles of amplification, 94 °C (30 s), 58 °C (40 s), and 72 °C (40 s); final extension, 72 °C for 7 min. 6 μL of the PCR product was separated on agarose gel (1.5%).The primers used for def were: F: 5′-GTGAGAAGGCAAGCCAGACA-3′ and R: 5′-TCAAGGTTTGGCTGTCGCCT-3′ (AF364865, referred to Hu et al., 2003).20 The primers used for HaAC1 were: F: 5′-CACCACCAGGGAAATGATG-3′ and R: 5′-TGCGGACAACTGAGAACAACA-3′ (AF030301). G-Marker (EU112625) (F: 5′-GCTGTGGCCCTTGTGTTG-3′ and R: 5′- CGCAGACCGATAGGATACCATT-30) was used as control for the comparison of amounts of cDNA in each reaction.
3. Result and discussion
3.1. Characterization of the PtNF/ERGO/Pt microelectrodes
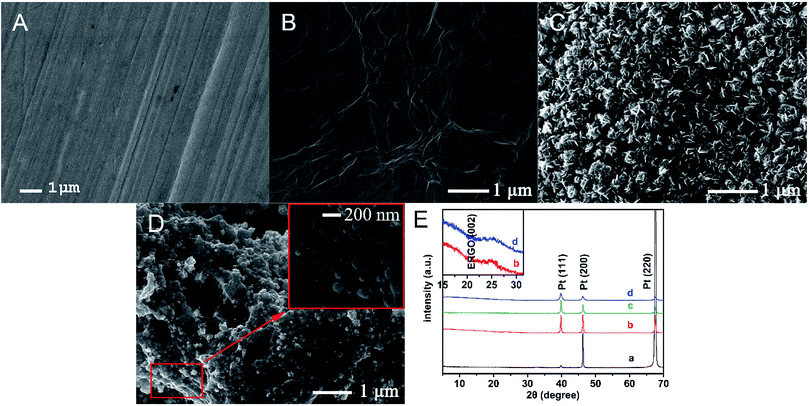
The surface morphology of electrodes was characterized by SEM. As shown in Fig. 1A, the surface of the bare Pt microelectrode is smooth and clean. When the ERGO was formed on the Pt microelectrode (Fig. 1B), the typical crumpled and wrinkled structure of ERGO can be observed. When the PtNF was formed on the Pt microelectrode (Fig. 1C), a densely and uniformly distributed flower-like Pt nanostructure with the diameter of 200–500 nm can be observed. When the Pt and GO was co-deposition and co-reduction on the Pt microelectrode, flower-like Pt nanostructure are dispersed in the lamellar ERGO, constructing a porous network structure (Fig. 1D). The diameters of the PtNF range from 50–100 nm. It has been reported that GO or RGO can be used to disperse and prevent the growth of the nanostructures, because they could be regarded as unconventional polymeric surfactant,21–23 so smaller size and better dispersion were observed for the PtNF with the attendance of ERGO. And ERGO can also provide anchoring site for metal nanostructures or its precursors.24,25 Moreover, the irregularly shaped PtNF and untidy ERGO can enhance the surface area and catalytic activity of the Pt microelectrode greatly.2,26The XRD pattern of (a) bare Pt, (b) EGRO/Pt, (c) Pt NF/Pt and (d) Pt NF/EGRO/Pt was shown in Fig. 1E. For the bare Pt (a), the peaks at 2θ of 39.8°, 46.3°, and 67.8° are assigned to the (111), (200), and (220) planes of the Pt particles. The peak at 2θ of 67.8° is very strong, which might be ascribed to the preferred orientation of block as we used the Pt sheet to simulate the Pt wire. For the EGRO/Pt (b), a weak peak originating from graphene sheets (002) is also appeared at 2θ of about 25°, which confirms the reduction of GO into ERGO.27 For the Pt NF/Pt (c), the intensity of peaks of 39.8°, 46.3°, and 67.8° are changed compare to the bare Pt sheet (a), indicating the formation of Pt crystals.28 As expected, a peak at 2θ of 25° also appears for the Pt NF/EGRO/Pt.
3.2. Feasibility of the PtNF/ERGO/Pt microelectrode for detecting SA
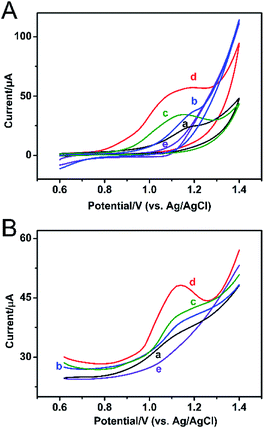
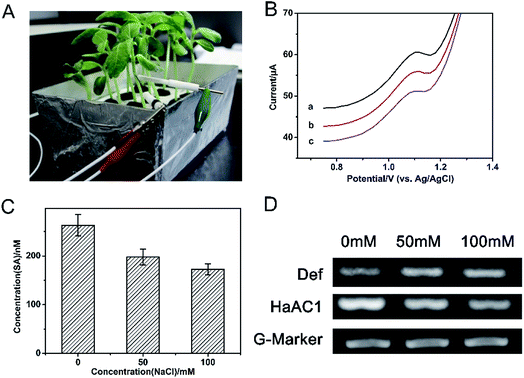
The feasibility of the modified PtNF/ERGO/Pt microelectrode for detecting SA was tested by cyclic voltammetry (CV) and differential pulse voltammetry (DPV) methods. For CV curve of 500 μM SA (Fig. 2A), a single oxidation peak appeared around 1.15 V by the bare Pt microelectrode in the PBS buffer (curve a), which is probably attributed to the electrochemically active phenol group of SA.11 The oxidation peak was increased when the ERGO/Pt microelectrode (curve b) and the PtNF/Pt microelectrode (curve c) were used, indicating the PtNF and ERGO can respectively enhanced the catalysis effect of SA. The oxidation peak of SA was significantly increased when the PtNF/ERGO/Pt microelectrode was used (curve d), indicating that the integration of high density Pt NF with the layered ERGO can induce more active sites and construct an electrical network, which greatly increased the catalytic capacity to SA. No oxidation peak was observed in the absence of SA using the PtNF/ERGO/Pt microelectrode (curve e). In the DPV curve (Fig. 2B), similar trends as that of CV curves were observed for the different modified electrodes. An oxidation peak with much higher peak height also appeared at the similar potential for the PtNF/ERGO/Pt microelectrode in the presence of SA (Fig. 2B, curve e).3.3. Optimization of conditions for detection in solution
The concentration of GO and H2PtCl6·6H2O and the scan cycles of electrodeposition were optimized as they are the key factors in the electrodeposition process. As shown in Fig. 3A, with the increase of the concentration of GO from 0 to 1 mg ml−1, the peak current of SA increases. While further increasing the concentration of GO, the peak current of SA decreases. So the optimal concentration of GO is 1 mg ml−1. The optimal conditions for H2PtCl6·6H2O and scan cycles are 10 mM and 45 cycles respectively as can be seen from Fig. 3B and C.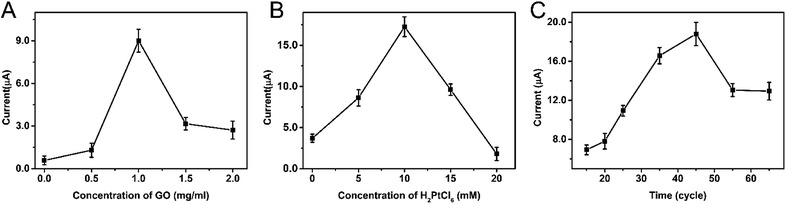 | ||
| Fig. 3 Optimization of the modification process with different concentrations of GO (A), H2PtCl6 (B), and different scan cycles for deposition (C). | ||
3.4. Performance of PtNF/ERGO/Pt microsensor
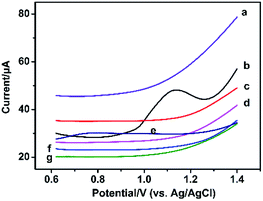
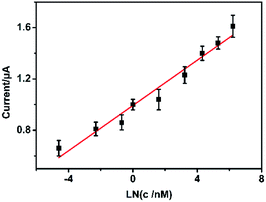
The modified electrode was used to detect different concentrations of SA standard solution using DPV method. As shown in Fig. 4A, with the increasing concentrations of SA, the peak current of SA increases correspondingly. There is a decent linear relation between the peak currents and the logarithm value of the concentrations of SA from 100 pM to 1 μM (Fig. 4B). And the equation is Ipeak (μA) = 0.45LNCSA (nM) + 1.05, the correlation coefficient is 0.9807. The detection limit of this sensor is 48.11 pM (S/N = 3.0). The sensing performance of the PtNF/ERGO/Pt microsensor for SA is compared with the previous reports. As shown in Table S1,† the developed sensor in this work is superior to most electrochemical sensors existed for SA.2,11,29–33Selectivity is an important parameter to evaluate the performance of the sensor. Therefore, the possible interference in the seedling stem sap was examined by the proposed sensor under the same condition. As shown in Fig. 5, an oxidation peak around 1.15 V is observed for SA. Besides, a weak peak near +0.8 V is observed for indole-3-acetic acid with the same concentration, which is consistent with previous report.11 However, it does not interfere with the detection of SA. Other components including malic acid, citric acid, succinic acid, abscisic acid and methyl jasmonate don't show any responses in the DPV curve. This result suggests the high selectivity of the proposed microsensor. The RSD was 15% for 8 different PtNF/ERGO/Pt microelectrodes in the presence of 500 μM SA. There is still 86% sensing ability remained for the microsensor after storage at 4 °C for three weeks, indicating that this microsensor is highly stable.
In order to obtain a standard curve which is more suitable to be applied in the living plants,34 the DPV responses of modified electrode for SA was also detected in 10% homogenate of sunflower stems diluted by 0.1 M PBS (Fig.S1†). As shown in Fig. 6, the linear equation is 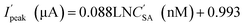 for SA from 10 pM to 500 nM. The detection limit is 3.3 pM (at S/N of 3.0), and correlation coefficient is 0.9811.
for SA from 10 pM to 500 nM. The detection limit is 3.3 pM (at S/N of 3.0), and correlation coefficient is 0.9811.
3.5. In vivo measurements of SA in sunflower seedlings under salt stress
Salt stress is one of the major abiotic environmental factors which adversely affect plant growth and development. Phytohormones play critical roles in regulating plant responses to salt stress.35 In this work, the modified PtNF/ERGO/Pt microelectrode was used to detect the SA levels in vivo in sunflower seedlings under different salt stress. Fig. 7A is the photograph for the in vivo detection of SA in sunflower seedlings. Fig. 7B shows the typical DPV results for the in vivo detection of SA in sunflower seedlings. The concentration of SA in the stem part of sunflowers is 256.1 ± 0.32 nM, 179.5 ± 0.25 nM, and 158.6 ± 0.15 nM for 0 mM, 50 mM and 100 mM of NaCl treatments (10 h), respectively, according to the standard curve in 10% homogenate of sunflower stems. This result suggests that with the increasing concentrations of salt, the SA levels decreases. This result is consistent with previous studies.31,36 In addition, the diameter of the microelectrode is only 0.5 mm, only tiny holes were left after the detection. And the electrochemical test is so fast that there is no much influences were observed on the following growth of the plants.In order to verify our result, UPLC-MS method was used to measure the SA levels in the extracted samples of the stem part of sunflower seedlings after different treatment of salt stress. The obtained result is shown in Fig. 7C, it could be found that the trend of SA levels is decreased with the increasing concentrations of salt, which is consistent with those obtained by the proposed electrochemical sensor.
The expression of SA related gene in sunflowers was also analyzed by RT-PCR method. The product of def is defensin (PR12 protein). It is a kind of antifungus, which can be impacted by SA.20 HaAC1 encodes aldoketo reductase, which also is a marker of SA pathway in sunflowers.37 As shown in Fig. 7D, the expression of HaAC1 gene is down-regulated with the increasing concentrations of salt. The expression of def gene is slightly up-regulated for the salt-treated groups, but for the 50 mM and 100 mM of salt treatment, the expression change of def gene is not obvious. Similar trend of SA levels and the expression of def and HaAC1gene are also observed for sunflowers when it was attacked by Orobanche Cumana.20
4. Conclusions
In summary, the PtNF/ERGO/Pt microelectrode was developed to detect SA in the stem of sunflower seedlings under different salt stress in vivo. Our result was consistent with previous report, and also confirmed by UPLC-MS and gene expression analysis. This is the first time for in vivo detection of SA in plants using microelectrode. The proposed SA microsensor is simple and practical, and showed high sensitivity and selectivity. It is foreseeable that our strategy could pave the way for the in vivo detection of phytohormone in plants.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for the fundings from Beijing Natural Science Foundation (2182022), Scientific and Technological Innovation Team of Beijing Academy of Agricultural and Forestry Sciences (JNKYT201604), and National Natural Science Foundation of China (Grant No. 61571443).Notes and references
- B. Lacombe and P. Achard, Curr. Opin. Plant Biol., 2016, 34, 1–8 CrossRef PubMed.
- Z. Wang, F. Ai, Q. Xu, Q. Yang, J. H. Yu, W. H. Huang and Y. D. Zhao, Colloids Surf., B, 2010, 76, 370–374 CrossRef PubMed.
- S. Hayat, A. Ahmad and M. N. Alyemeni, Salicylic acid: plant growth and development, Springer, Netherlands, 2013 Search PubMed.
- S. Croubels, M. An, K. Baert and P. D. Backer, Anal. Chim. Acta, 2005, 529, 179–187 CrossRef.
- R. A. Budi Muljono, A. M. G. Looman, R. Verpoorte and J. J. C. Scheffer, Phytochem. Anal., 2015, 9, 35–38 CrossRef.
- J. Sun, B. Wang, Z. Xue, Z. Li and X. Yang, Anal. Chem., 2016, 88, 1355–1361 CrossRef PubMed.
- M. M. Sena, M. G. Trevisan and R. J. Poppi, Talanta, 2006, 68, 1707–1712 CrossRef PubMed.
- P. J. Tseng, C. Y. Wang, T. Y. Huang, Y. Y. Chuang, S. F. Fu and Y. W. Lin, Anal. Methods, 2014, 6, 1759–1765 RSC.
- W. Huang, L. Huang, G. Preston, M. Naylor, J. Carr, Y. Li, A. Singer, A. Whiteley and H. Wang, Plant J., 2010, 46, 1073–1083 CrossRef PubMed.
- M. I. Gonzálezsánchez, P. T. Lee, R. H. Guy and R. G. Compton, Chem. Commun., 2015, 51, 16534–16536 RSC.
- L. Sun, Q. Feng, Y. Yan, Z. Pan, X. Li, F. Song, H. Yang, J. Xu, N. Bao and H. Gu, Biosens. Bioelectron., 2014, 60, 154–160 CrossRef PubMed.
- Y. Liu, G. Sun, C. Jiang, X. Zheng, L. Zheng and C. Li, Microchim. Acta, 2014, 181, 63–70 CrossRef.
- Y. Ning, Y. Chen, Y. Shen, Y. Tang, J. Guo, F. Fang and S. Liu, J. Electrochem. Soc., 2013, 160, H715–H719 CrossRef.
- Q. Xu, F. Wei, Z. Wang, Q. Yang, Y. D. Zhao and H. Chen, Sens. Actuators, B, 2009, 141, 599–603 CrossRef.
- Q. Q. Ren, X. R. Huang, G. C. Liu, J. Ou-Yang, M. T. Li, H. Chen, Y. D. Zhao and W. Chen, Sens. Actuators, B, 2015, 220, 743–748 CrossRef.
- Q. Xu, F. Wei, Z. Wang, Q. Yang, Y. D. Zhao and H. Chen, Phytochem. Anal., 2010, 21, 192–196 CrossRef PubMed.
- A. S. Lima, K. R. Prieto, C. S. Santos, V. H. Paula, E. Y. Garciaochoa, A. Huertarobles, M. J. Beltrangarcia, M. P. Di and M. Bertotti, Biosens. Bioelectron., 2018, 99, 108–114 CrossRef PubMed.
- A. L. Liu, G. X. Zhong, J. Y. Chen, S. H. Weng, H. N. Huang, W. Chen, L. Q. Lin, Y. Lei, F. H. Fu and Z. L. Sun, Anal. Chim. Acta, 2013, 767, 50–58 CrossRef PubMed.
- H. Gao, F. Xiao, B. C. Chi and H. Duan, ACS Appl. Mater. Interfaces, 2011, 3, 3049–3057 CrossRef PubMed.
- X. Hu, D. L. Bidney, N. Yalpani, J. P. Duvick, O. Crasta, O. Folkerts and G. Lu, Plant Physiol., 2003, 133, 170–181 CrossRef PubMed.
- C. Liu, K. Wang, S. Luo, Y. Tang and L. Chen, Small, 2011, 7, 1203–1206 CrossRef PubMed.
- J. Yang, C. Tian, L. Wang and H. Fu, J. Mater. Chem., 2011, 21, 3384–3390 RSC.
- M. Zhu, P. Chen and M. Liu, Langmuir, 2012, 28, 3385–3390 CrossRef PubMed.
- C. V. Rao, A. L. M. Reddy, Y. Ishikawa and P. M. Ajayan, Carbon, 2011, 49, 931–936 CrossRef.
- C. V. Rao, C. R. Cabrera and Y. Ishikawa, J. Phys. Chem. C, 2011, 115, 21963–21970 CrossRef.
- Y. Hu, P. Wu, H. Zhang and C. Cai, Electrochim. Acta, 2012, 85, 314–321 CrossRef.
- L. J. Cote, R. Cruzsilva and J. Huang, J. Am. Chem. Soc., 2009, 131, 11027–11032 CrossRef PubMed.
- Y. Si and E. T. Samulski, Chem. Mater., 2008, 20, 6792–6797 CrossRef.
- L. Lu, X. Zhu, X. Qiu, H. He, J. Xu and X. Wang, Int. J. Electrochem. Sci., 2014, 9, 8057–8066 Search PubMed.
- J. Park and C. Eun, Electrochim. Acta, 2016, 194, 346–356 CrossRef.
- L. Sun, X. Liu, L. Gao, Y. Lu, Y. Li, Z. Pan, N. Bao and H. Gu, Anal. Lett., 2015, 48, 1578–1592 CrossRef.
- Z. Wang, F. Wei, S. Y. Liu, Q. Xu, J. Y. Huang, X. Y. Dong, J. H. Yu, Q. Yang, Y. D. Zhao and H. Chen, Talanta, 2010, 80, 1277–1281 CrossRef PubMed.
- M. H. A. Zavar, S. Heydari and G. H. Rounaghi, Arabian J. Sci. Eng., 2013, 38, 29–36 CrossRef.
- W. Chen, Q. Yang, Q. Q. Ren, W. Wen, D. H. Jiang and Y. D. Zhao, Nanosci. Nanotechnol., 2013, 5, 694–698 Search PubMed.
- A. Molina and J. P. Donaire, New Phytol., 2010, 156, 409–415 CrossRef.
- M. G. Javid, A. Sorooshzadeh, F. Moradi, S. A. M. M. Sanavy and I. Allahdadi, Aust. J. Crop Sci., 2011, 5, 726–734 Search PubMed.
- P. Letousey, A. De Zélicourt, D. S. C. Vieira, S. Thoiron, F. Monteau, P. Simier, P. Thalouarn and P. Delavault, Plant Pathol., 2007, 56, 536–546 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03475c |
| This journal is © The Royal Society of Chemistry 2018 |